Influence of Mo on the Microstructure and Corrosion Behavior of Laser Cladding FeCoCrNi High-Entropy Alloy Coatings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Powders by Gas Atomization
2.2. Preparation of Laser Cladding Coatings
2.3. Microstructure Characterization of Coatings
2.4. Electrochemical Measurements
3. Results
3.1. Microstructure of Powder
3.2. Phase Structure of Powder and Laser Cladding Coatings
3.3. Microstructure of Laser Cladding High-Entropy Alloy Coatings
3.4. Corrosion Behavior of Laser Cladding High-Entropy Alloy Coatings
3.4.1. Potentiodynamic Polarization Curves
3.4.2. Electrochemical Impedance Spectroscopy
3.4.3. Surface Morphologies of Corroded Coatings
3.4.4. Passive Film Analysis
4. Discussion
4.1. Influence of Mo Doping on the Microstructure of Powders and Coatings
4.2. Influence of Mo Doping on the Corrosion Resistance of Coatings
5. Conclusions
- (1)
- Both FeCoCrNi and FeCoCrNiMo0.2 high-entropy alloy powders prepared by gas atomization are single-phase FCC structures. Due to the remelting and multiple heat treatments during the preparation of the laser cladding coating, a small amount of σ phase and μ phase appeared in the FeCoCrNiMo0.2 coating;
- (2)
- The microstructure of the two coatings from the bonding area to the top layer is planar, columnar and equiaxed grains, respectively. After adding the Mo element, the region of the plane grain in FeCoCrNiMo0.2 coating becomes larger. The size of dendrites in the middle of the FeCoCrNiMo0.2 coating increased significantly and showed obvious orientation characteristics;
- (3)
- The corrosion current densities of FeCoCrNi and FeCoCrNiMo0.2 coatings are 0.78 and 0.45 times that of 304 stainless steel, respectively. This indicates that both coatings exhibit excellent corrosion resistance and passivation performance in 3.5 wt.% NaCl solution. Compared with FeCoCrNi coating, the passivation film on the surface of FeCoCrNiMo0.2 coating contains high content of Cr and Mo oxides. The addition of Mo element enhances the compactness and pitting resistance of the passivation film.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liang, M.; Melchers, R.; Chaves, I. Corrosion and pitting of 6060 series aluminium after 2 years exposure in seawater splash, tidal and immersion zones. Corros. Sci. 2018, 140, 286–296. [Google Scholar] [CrossRef]
- Thee, C.; Hao, L.; Dong, J.; Mu, X.; Wei, X.; Li, X.; Ke, W. Atmospheric corrosion monitoring of a weathering steel under an electrolyte film in cyclic wet–dry condition. Corros. Sci. 2014, 78, 130–137. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.; Knight, P.; Vincent, A. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, S.K.; Lin, S.J.; Gan, J.Y.; Chin, T.S.; Shun, T.T.; Tsau, C.H.; Chang, S.Y. Nanostructured high-entropy alloys with multiple principal elements: Novel alloy design concepts and outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. JOM 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Gao, M.C. Progress in high-entropy alloys. JOM 2013, 65, 1749–1750. [Google Scholar] [CrossRef] [Green Version]
- Nutor, R.K.; Cao, Q.; Wei, R.; Su, Q.; Du, G.; Wang, X.; Li, F.; Zhang, D.; Jiang, J.-Z. A dual-phase alloy with ultrahigh strength-ductility synergy over a wide temperature range. Sci. Adv. 2021, 7, eabi4404. [Google Scholar] [CrossRef]
- Dai, C.; Zhao, T.; Du, C.; Liu, Z.; Zhang, D. Effect of molybdenum content on the microstructure and corrosion behavior of FeCoCrNiMox high-entropy alloys. Mater. Sci. Technol.-Lond. 2020, 46, 64–73. [Google Scholar] [CrossRef]
- Sathiyamoorthi, P.; Basu, J.; Kashyap, S.; Pradeep, K.; Kottada, R.S. Thermal stability and grain boundary strengthening in ultrafine-grained CoCrFeNi high entropy alloy composite. Mater. Des. 2017, 134, 426–433. [Google Scholar] [CrossRef]
- Zhang, S.; Wu, C.; Zhang, C.; Guan, M.; Tan, J. Laser surface alloying of FeCoCrAlNi high-entropy alloy on 304 stainless steel to enhance corrosion and cavitation erosion resistance. Opt. Laser Technol. 2016, 84, 23–31. [Google Scholar] [CrossRef]
- Eißmann, N.; Mühle, U.; Gaitzsch, U.; Walther, G.; Weißgärber, T.; Kieback, B. Precipitation hardening of high entropy alloy CoCrFeMnNi containing titanium. J. Alloy Compd. 2021, 857, 157610. [Google Scholar] [CrossRef]
- Liu, W.; Lu, Z.; He, J.; Luan, J.; Wang, Z.; Liu, B.; Liu, Y.; Chen, M.; Liu, C. Ductile CoCrFeNiMox high entropy alloys strengthened by hard intermetallic phases. Acta Mater. 2016, 116, 332–342. [Google Scholar] [CrossRef]
- Feng, K.; Zhang, Y.; Li, Z.; Yao, C.; Yao, L.; Fan, C. Corrosion properties of laser cladded CrCoNi medium entropy alloy coating. Surf. Coat. Technol. 2020, 397, 126004. [Google Scholar] [CrossRef]
- Kong, D.; Ni, X.; Dong, C.; Zhang, L.; Man, C.; Yao, J.; Xiao, K.; Li, X. Heat treatment effect on the microstructure and corrosion behavior of 316L stainless steel fabricated by selective laser melting for proton exchange membrane fuel cells. Electrochim. Acat 2018, 276, 293–303. [Google Scholar] [CrossRef]
- Milanti, A.; Matikainen, V.; Koivuluoto, H.; Bolelli, G.; Lusvarghi, L.; Vuoristo, P. Effect of spraying parameters on the microstructural and corrosion properties of HVAF-sprayed Fe–Cr–Ni–B–C coatings. Surf. Coat. Technol. 2015, 277, 81–90. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Gao, Z.; Wu, M.; Weng, F.; Liu, T.; Zhan, X. Influence of specific energy on microstructure and properties of laser cladded FeCoCrNi high entropy alloy. Metals 2020, 10, 1464. [Google Scholar] [CrossRef]
- Ye, Q.F.; Feng, K.; Li, Z.G.; Lu, F.G.; Li, R.F.; Huang, J.; Wu, Y.X. Microstructure and corrosion properties of CrMnFeCoNi high entropy alloy coating. Appl. Surf. Sci. 2017, 396, 1420–1426. [Google Scholar] [CrossRef]
- Wang, W.-R.; Qi, W.; Zhang, X.-L.; Yang, X.; Xie, L.; Li, D.-Y.; Xiang, Y.-H. Superior corrosion resistance-dependent laser energy density in (CoCrFeNi)95Nb5 high entropy alloy coating fabricated by laser cladding. Int. J. Miner. Metall. Mater. 2021, 28, 888–897. [Google Scholar] [CrossRef]
- Liang, X.B.; Guo, W.; Chen, Y.X.; Wang, L.L. Microstructure and mechanical properties of FeCrNiCoCu(B) high-entropy alloy coatings. Mater. Sci. 2011, 694, 502–507. [Google Scholar] [CrossRef]
- Laktionova, M.A.; Ta Bc Hnikova, E.D.; Tang, Z.; Liaw, P.K. Mechanical properties of the high-entropy alloy Ag0.5CoCrCuFeNi at temperatures of 4.2-300 K. Low Temp. Phys. 2013, 39, 630–632. [Google Scholar] [CrossRef]
- Chen, T.K.; Wong, M.S. Structure and properties of reactively-sputtered AlxCoCrCuFeNi oxide films. Thin Solid Films 2007, 516, 141–146. [Google Scholar] [CrossRef]
- Huang, Y.S.; Chen, L.; Lui, H.W.; Cai, M.H.; Yeh, J.W. Microstructure, hardness, resistivity and thermal stability of sputtered oxide films of AlCoCrCu0.5NiFe high-entropy alloy. Mater. Sci. Eng. A-Struct. 2007, 457, 77–83. [Google Scholar] [CrossRef]
- Yao, C.Z.; Peng, Z.; Meng, L.; Li, G.R.; Ye, J.Q.; Peng, L.; Tong, Y.X. Electrochemical preparation and magnetic study of Bi–Fe–Co–Ni–Mn high entropy alloy. Electrochim. Acta 2008, 53, 8359–8365. [Google Scholar] [CrossRef]
- Liu, X.-T.; Lei, W.-B.; Li, J.; Ma, Y.; Wang, W.-M.; Zhang, B.-H.; Liu, C.-S.; Cui, J.-Z. Laser cladding of high-entropy alloy on H13 steel. Rare Met. 2014, 33, 727–730. [Google Scholar] [CrossRef]
- Li, Y.; Dong, S.; He, P.; Yan, S.; Li, E.; Liu, X.; Xu, B. Microstructure characteristics and mechanical properties of new-type FeNiCr laser cladding alloy coating on nodular cast iron. J. Mater. Process. Technol. 2019, 269, 163–171. [Google Scholar] [CrossRef]
- Zhang, H.; Pan, Y.; He, Y.-Z. Synthesis and characterization of FeCoNiCrCu high-entropy alloy coating by laser cladding. Mater. Des. 2011, 32, 1910–1915. [Google Scholar] [CrossRef]
- Guo, W.; Zhang, H.; Zhao, S.; Ding, Z.; Liu, B.; Li, W.; Xu, H.; Liu, H. Corrosion Behavior of the CoNiCrAlY-Al2O3 Composite Coating Based on Core-Shell Structured Powder Design. Materials 2021, 14, 7093. [Google Scholar] [CrossRef]
- Shun, T.-T.; Chang, L.-Y.; Shiu, M.-H. Age-hardening of the CoCrFeNiMo0.85 high-entropy alloy. Mater. Charact. 2013, 81, 92–96. [Google Scholar] [CrossRef]
- Niu, Z.; Wang, Y.; Geng, C.; Xu, J.; Wang, Y. Microstructural evolution, mechanical and corrosion behaviors of as-annealed CoCrFeNiMox (x = 0, 0.2, 0.5, 0.8, 1) high entropy alloys. J. Alloy. Compd. 2020, 820, 153273. [Google Scholar] [CrossRef]
- Dai, C.-D.; Fu, Y.; Guo, J.-X.; Du, C.-W. Effects of substrate temperature and deposition time on the morphology and corrosion resistance of FeCoCrNiMo 0.3 high-entropy alloy coating fabricated by magnetron sputtering. Int. J. Miner. Metall. Mater. 2020, 27, 1388–1397. [Google Scholar] [CrossRef]
- He, F.; Wang, Z.; Wu, Q.; Li, J.; Wang, J.; Liu, C. Phase separation of metastable CoCrFeNi high entropy alloy at intermediate temperatures. Scr. Mater. 2017, 126, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Pan, Y.; He, Y.Z.; Wu, J.L.; Yue, T.M.; Guo, S. Application Prospects and Microstructural Features in Laser-Induced Rapidly Solidified High-Entropy Alloys. JOM 2014, 66, 2057–2066. [Google Scholar] [CrossRef]
- Lee, C.P.; Chang, C.C.; Chen, Y.Y.; Yeh, J.W.; Shih, H.C. Effect of the aluminium content of AlxCrFe1.5MnNi0.5 high-entropy alloys on the corrosion behaviour in aqueous environments. Corros. Sci. 2008, 50, 2053–2060. [Google Scholar] [CrossRef]
- Shi, Y.; Yang, B.; Xie, X.; Brechtl, J.; Dahmen, K.A.; Liaw, P.K. Corrosion of Al xCoCrFeNi high-entropy alloys: Al-content and potential scan-rate dependent pitting behavior. Corros. Sci. 2017, 119, 33–45. [Google Scholar] [CrossRef]
- Xu, Z.; Zhang, H.; Du, X.; He, Y.; Luo, H.; Song, G.; Mao, L.; Zhou, T.; Wang, L. Corrosion resistance enhancement of CoCrFeMnNi high-entropy alloy fabricated by additive manufacturing. Corros. Sci. 2020, 177, 108954. [Google Scholar] [CrossRef]
- Shuang, S.; Ding, Z.Y.; Chung, D.; Shi, S.Q.; Yang, Y. Corrosion resistant nanostructured eutectic high entropy alloy. Corros. Sci. 2020, 164, 108315. [Google Scholar] [CrossRef]
- Ray, M.; Singh, V. Effect of sulfuric acid on corrosion and passivation of 316 SS in organic solution. J. Electrochem. Soc. 2011, 158, C359–C368. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, B.; Guo, W.; Fu, A.; Duan, H.; Li, W. Corrosion behavior and mechanism of FeCrNi medium entropy alloy prepared by powder metallurgy. J. Alloys Compd. 2021, 867, 159094. [Google Scholar] [CrossRef]
- Wang, J.; Li, W.; Yang, H.; Huang, H.; Ji, S.; Ruan, J.; Liu, Z. Corrosion behavior of CoCrNi medium-entropy alloy compared with 304 stainless steel in H2SO4 and NaOH solutions. Corros. Sci. 2020, 177, 108973. [Google Scholar] [CrossRef]
- Luo, H.; Li, Z.; Mingers, A.M.; Raabe, D. Corrosion behavior of an equiatomic CoCrFeMnNi high-entropy alloy compared with 304 stainless steel in sulfuric acid solution. Corros. Sci. 2018, 134, 131–139. [Google Scholar] [CrossRef]
- Benoit, M.; Bataillon, C.; Gwinner, B.; Miserque, F.; Orazem, M.E.; Sánchez-Sánchez, C.M.; Tribollet, B.; Vivier, V. Comparison of different methods for measuring the passive film thickness on metals. Electrochim. Acta 2016, 201, 340–347. [Google Scholar] [CrossRef] [Green Version]
- Maurice, V.; Peng, H.; Klein, L.H.; Seyeux, A.; Zanna, S.; Marcus, P. Effects of molybdenum on the composition and nanoscale morphology of passivated austenitic stainless steel surfaces. Faraday Discuss. 2015, 180, 151–170. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, Y.; Wang, J.; Li, M.; Shen, J. Passivation behavior of Fe-based amorphous metallic coating in NaCl and H2SO4 solutions. Acta Met. Sin. 2015, 51, 49–56. [Google Scholar]
- Tian, H.; Cheng, X.; Wang, Y.; Dong, C.; Li, X. Effect of Mo on interaction between α/γ phases of duplex stainless steel. Electrochim. Acta 2018, 267, 255–268. [Google Scholar] [CrossRef]
- Lu, Y.; Clayton, C.; Brooks, A. A bipolar model of the passivity of stainless steels—II. The influence of aqueous molybdate. Corros. Sci. 1989, 29, 863–880. [Google Scholar] [CrossRef]
- Olsson, C.-O.A.; Hörnström, S.E. An AES and XPS study of the high alloy austenitic stainless steel 254 SMO® tested in a ferric chloride solution. Corros. Sci. 1994, 36, 141–151. [Google Scholar] [CrossRef]
- Zhao, T.; Liu, Z.; Du, C.; Dai, C.; Li, X.; Zhang, B. Corrosion fatigue crack initiation and initial propagation mechanism of E690 steel in simulated seawater. Mater. Sci. Eng. A 2017, 708, 181–192. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, H. Corrosion behaviors of a γ-toughened Cr13Ni5Si2/Cr3Ni5Si2 multi-phase ternary metal silicide alloy in NaCl solution. Electrochim. Acta 2008, 54, 421–429. [Google Scholar] [CrossRef]
- Jinlong, L.; Hongyun, L. Electrochemical investigation of passive film in pre-deformation AISI 304 stainless steels. Appl. Surf. Sci. 2012, 263, 29–37. [Google Scholar] [CrossRef]
- Wei, L.; Wang, Z.; Wu, Q.; Shang, X.; Li, J.; Wang, J. Effect of Mo Element and Heat Treatment on Corrosion Resistance of Ni2CrFeMox High-Entropy Alloyin NaCl Solution. Acta Met. Sin. 2019, 55, 840–848. [Google Scholar]
- Zheng, C.-B.; Cai, L.; Tang, Z.-J.; Shen, X.-L. The inhibition effect of the molybdate on hydrogen permeation of 2205 duplex stainless steel. Surf. Coat. Technol. 2016, 287, 153–159. [Google Scholar] [CrossRef]
- Shang, C.; Axinte, E.; Sun, J.; Li, X.; Li, P.; Du, J.; Qiao, P.; Wang, Y. CoCrFeNi (W1−xMox) high-entropy alloy coatings with excellent mechanical properties and corrosion resistance prepared by mechanical alloying and hot pressing sintering. Mater. Des. 2017, 117, 193–202. [Google Scholar] [CrossRef]
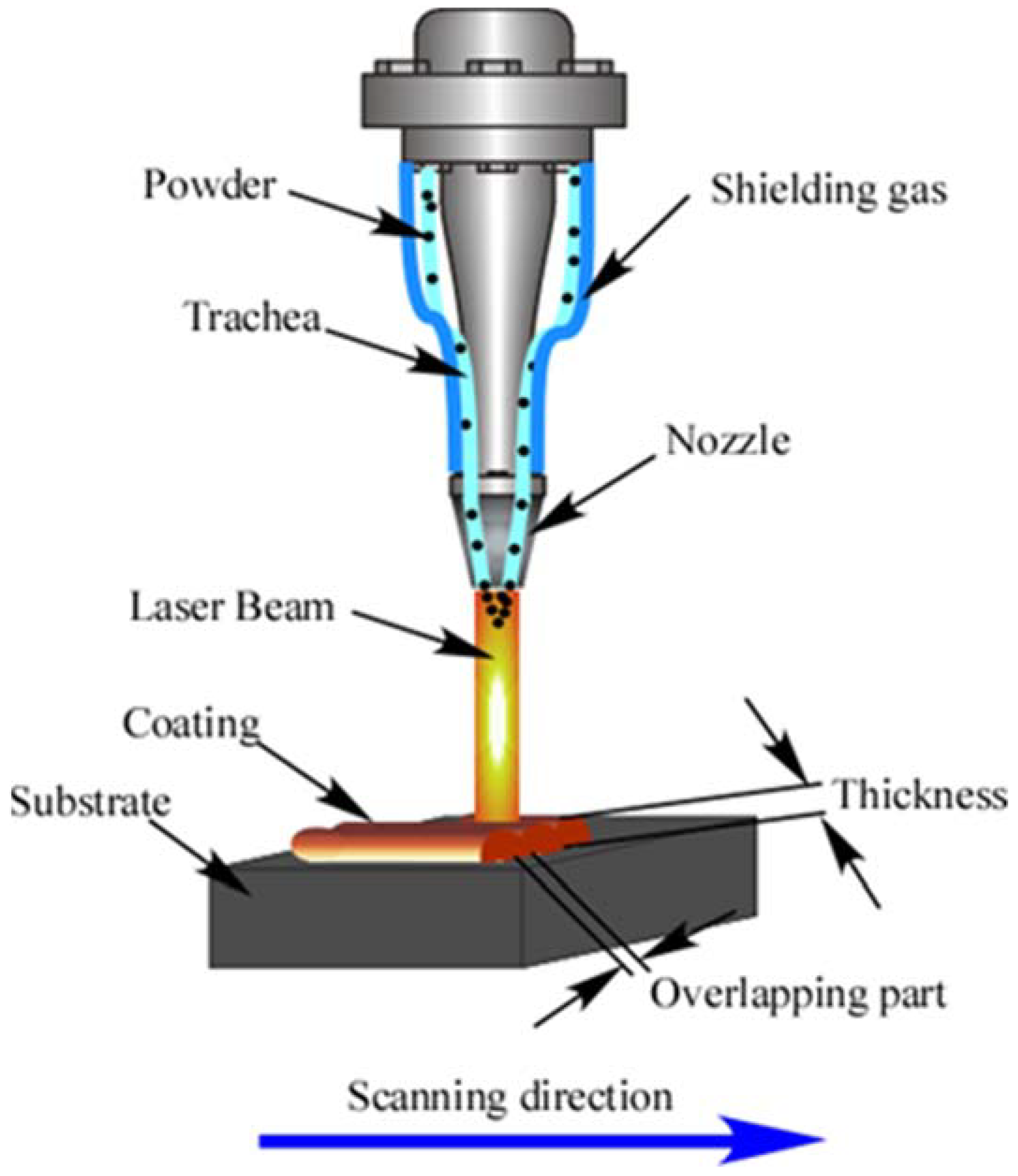



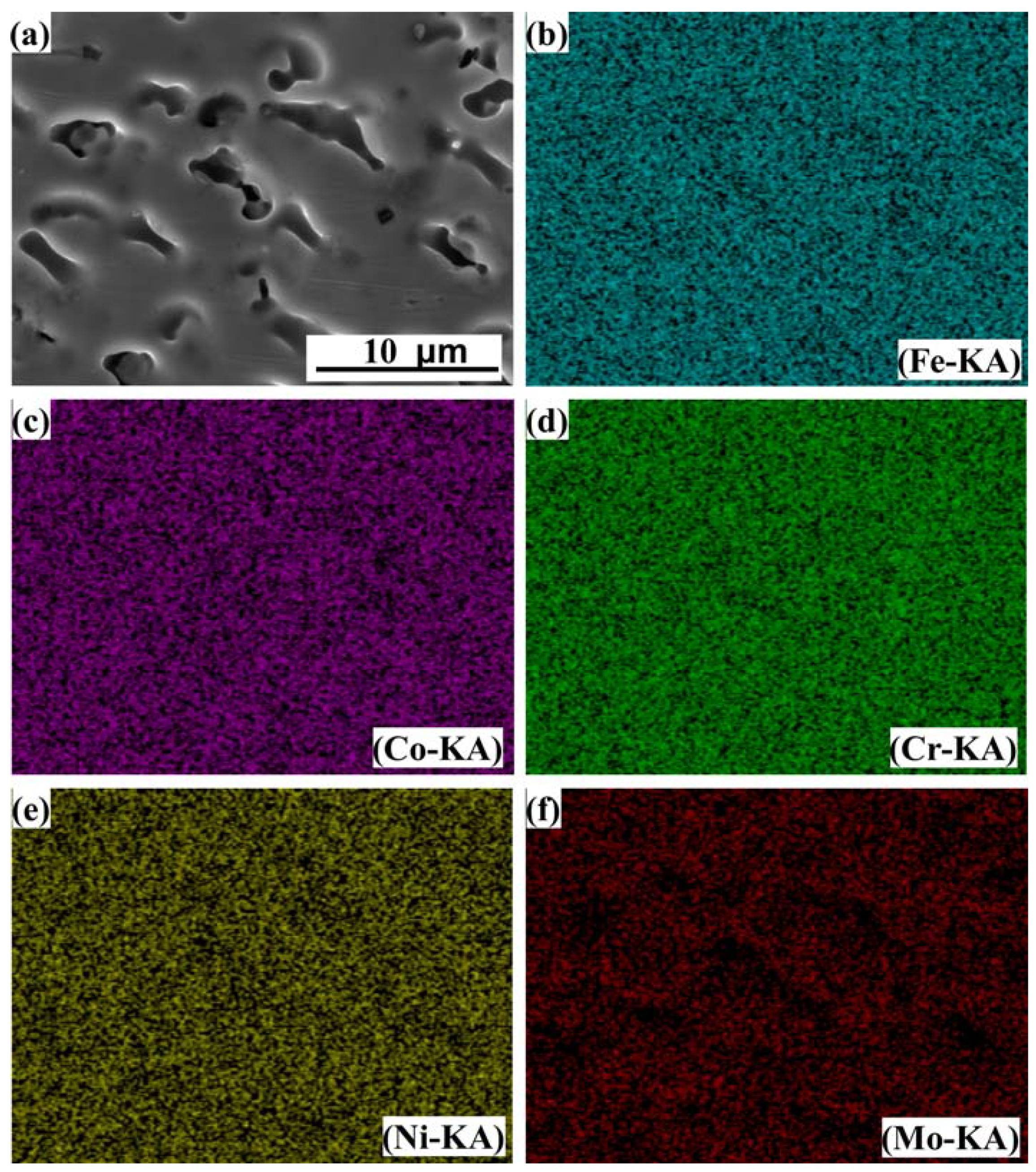








| Co | Cr | Ni | Fe | Mo | |
|---|---|---|---|---|---|
| FeCoCrNi | 25 | 25 | 25 | 25 | -- |
| FeCoCrNiMo0.2 | 23.81 | 23.81 | 23.81 | 23.81 | 4.76 |
| Cr | Ni | Mn | Si | P | S | C | Fe |
|---|---|---|---|---|---|---|---|
| 18~20 | 8~11 | 2 | 1 | 0.045 | 0.03 | 0.08 | Bal |
| Sample | Laser Power (W) | Spot Diameter (mm) | Overlap Rate (%) | Scanning Velocity (mm/min) | Feeding Rate (g/min) |
|---|---|---|---|---|---|
| FeCoCrNi | 2100 | 2 | 35 | 400 | 16 |
| FeCoCrNiMo0.2 | 2100 | 2 | 35 | 300 | 18 |
| Fe | Co | Cr | Ni | Mo | O | |
|---|---|---|---|---|---|---|
| 1 | 20.89 | 20.47 | 21.76 | 20.15 | 4.93 | 11.80 |
| 2 | 23.62 | 23.61 | 21.74 | 22.81 | -- | 8.22 |
| Ecorr (VSHE) | Icorr (A·cm−2) | Ebr (VSHE) | |
|---|---|---|---|
| FeCoCrNiMo0.2 | −0.01 | 0.94 × 10−7 | 1.17 |
| FeCoCrNi | −0.19 | 1.64 × 10−7 | 1.20 |
| SUS304 | −0.11 | 2.10 × 10−7 | 0.60 |
| Al0.5CoCrFeNi [35] | −0.015 | 2.52 × 10−7 | -- |
| Rs (Ω·cm2) | Qfilm (Ω−1·cm−2·s−n) | Rfilm (Ω·cm2) | N | χ2 | |
|---|---|---|---|---|---|
| FeCoCrNiMo0.2 | 8.64 | 2.18 × 10−5 | 5.59 × 105 | 0.77 | 6.45 × 10−3 |
| FeCoCrNi | 7.78 | 6.09 × 10−5 | 1.25 × 104 | 0.88 | 1.94 × 10−3 |
| Fe | Ni | Co | O | Mo | Cr | |
|---|---|---|---|---|---|---|
| 1 | 20.81 | 19.89 | 18.28 | 22.66 | 3.41 | 14.94 |
| 2 | 21.63 | 15.76 | 14.42 | 27.16 | 2.89 | 18.15 |
| 3 | 22.79 | 22.16 | 21.44 | 15.20 | -- | 18.42 |
| 4 | 6.75 | 7.06 | 7.50 | 31.74 | -- | 37.70 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, W.; Guo, W.; Zhang, H.; Xu, H.; Chen, L.; Zeng, J.; Liu, B.; Ding, Z. Influence of Mo on the Microstructure and Corrosion Behavior of Laser Cladding FeCoCrNi High-Entropy Alloy Coatings. Entropy 2022, 24, 539. https://doi.org/10.3390/e24040539
Li W, Guo W, Zhang H, Xu H, Chen L, Zeng J, Liu B, Ding Z. Influence of Mo on the Microstructure and Corrosion Behavior of Laser Cladding FeCoCrNi High-Entropy Alloy Coatings. Entropy. 2022; 24(4):539. https://doi.org/10.3390/e24040539
Chicago/Turabian StyleLi, Wenjuan, Wenmin Guo, Hongling Zhang, Huanhuan Xu, Liang Chen, Junshan Zeng, Bin Liu, and Zhibing Ding. 2022. "Influence of Mo on the Microstructure and Corrosion Behavior of Laser Cladding FeCoCrNi High-Entropy Alloy Coatings" Entropy 24, no. 4: 539. https://doi.org/10.3390/e24040539
APA StyleLi, W., Guo, W., Zhang, H., Xu, H., Chen, L., Zeng, J., Liu, B., & Ding, Z. (2022). Influence of Mo on the Microstructure and Corrosion Behavior of Laser Cladding FeCoCrNi High-Entropy Alloy Coatings. Entropy, 24(4), 539. https://doi.org/10.3390/e24040539






