Nonlinear Statistical Analysis of Normal and Pathological Infant Cry Signals in Cepstrum Domain by Multifractal Wavelet Leaders
Abstract
:1. Introduction
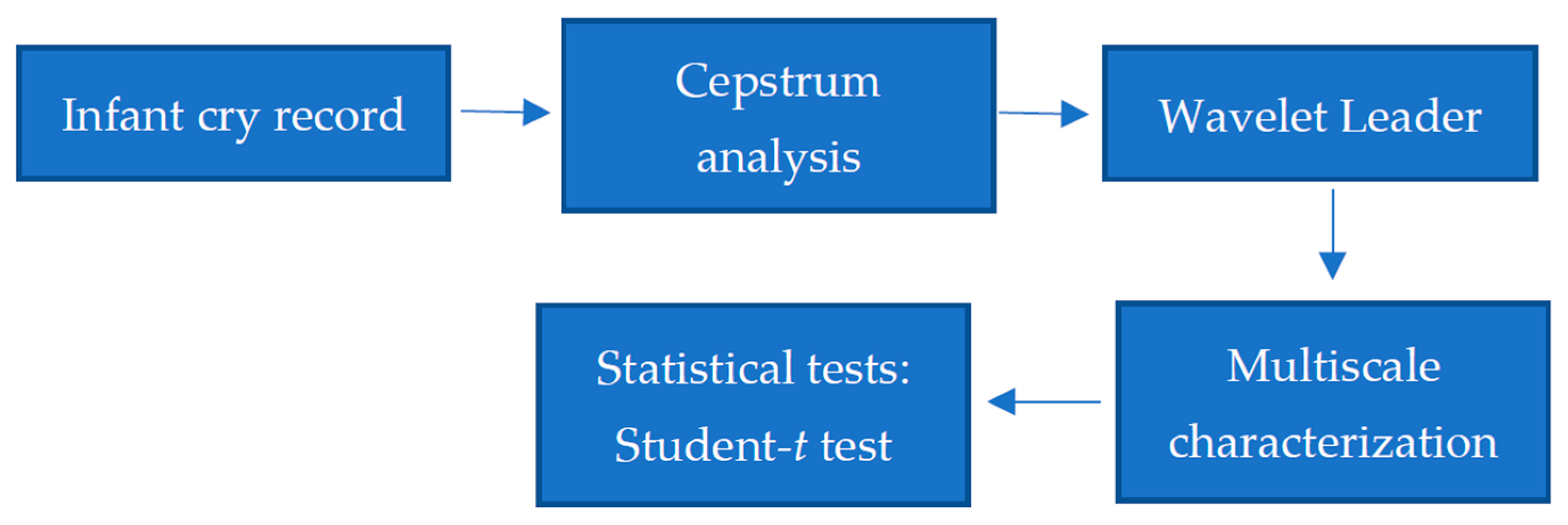
2. Materials and Methods
2.1. Cepstrum Analysis
2.2. Wavelet Leaders
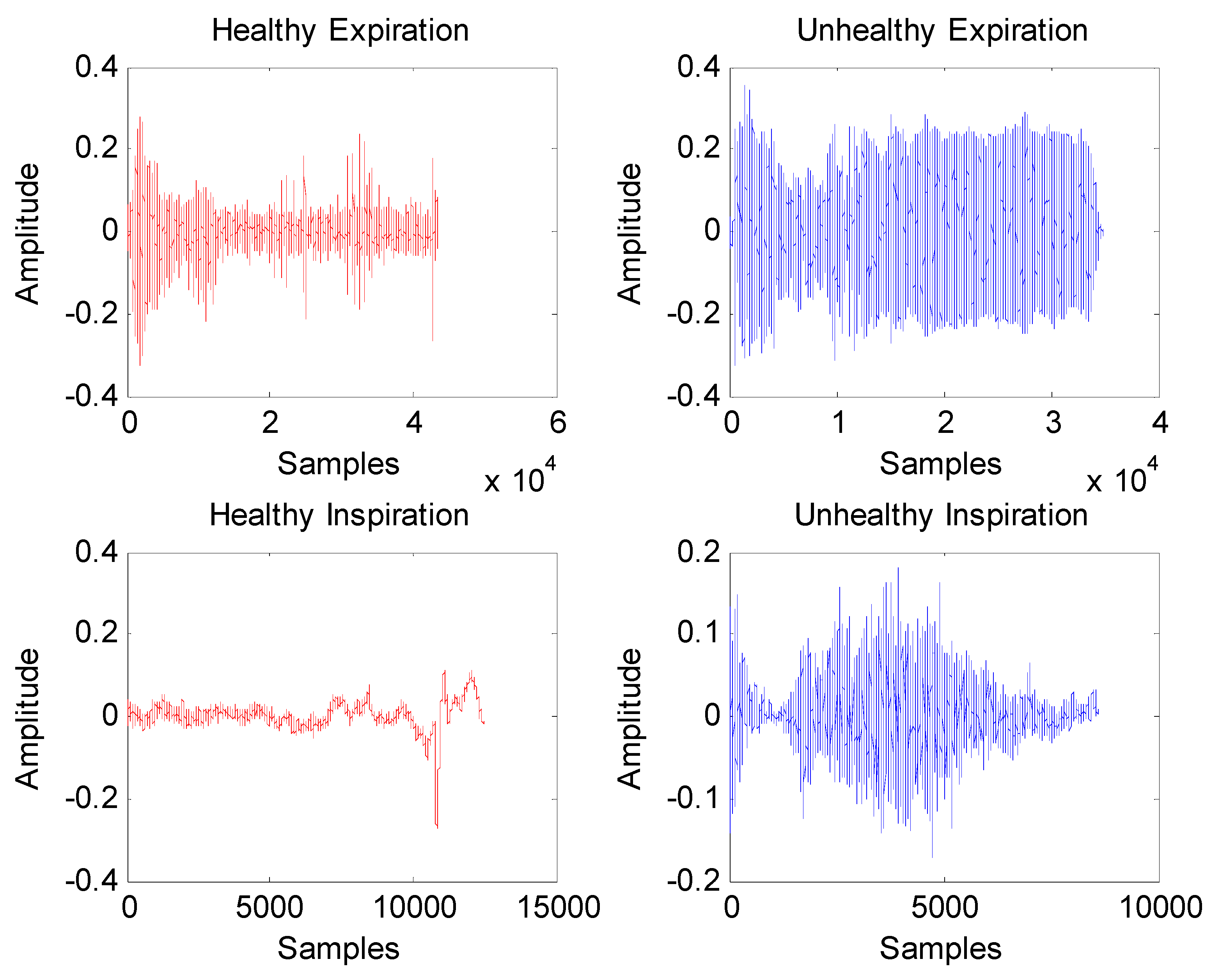
3. Results
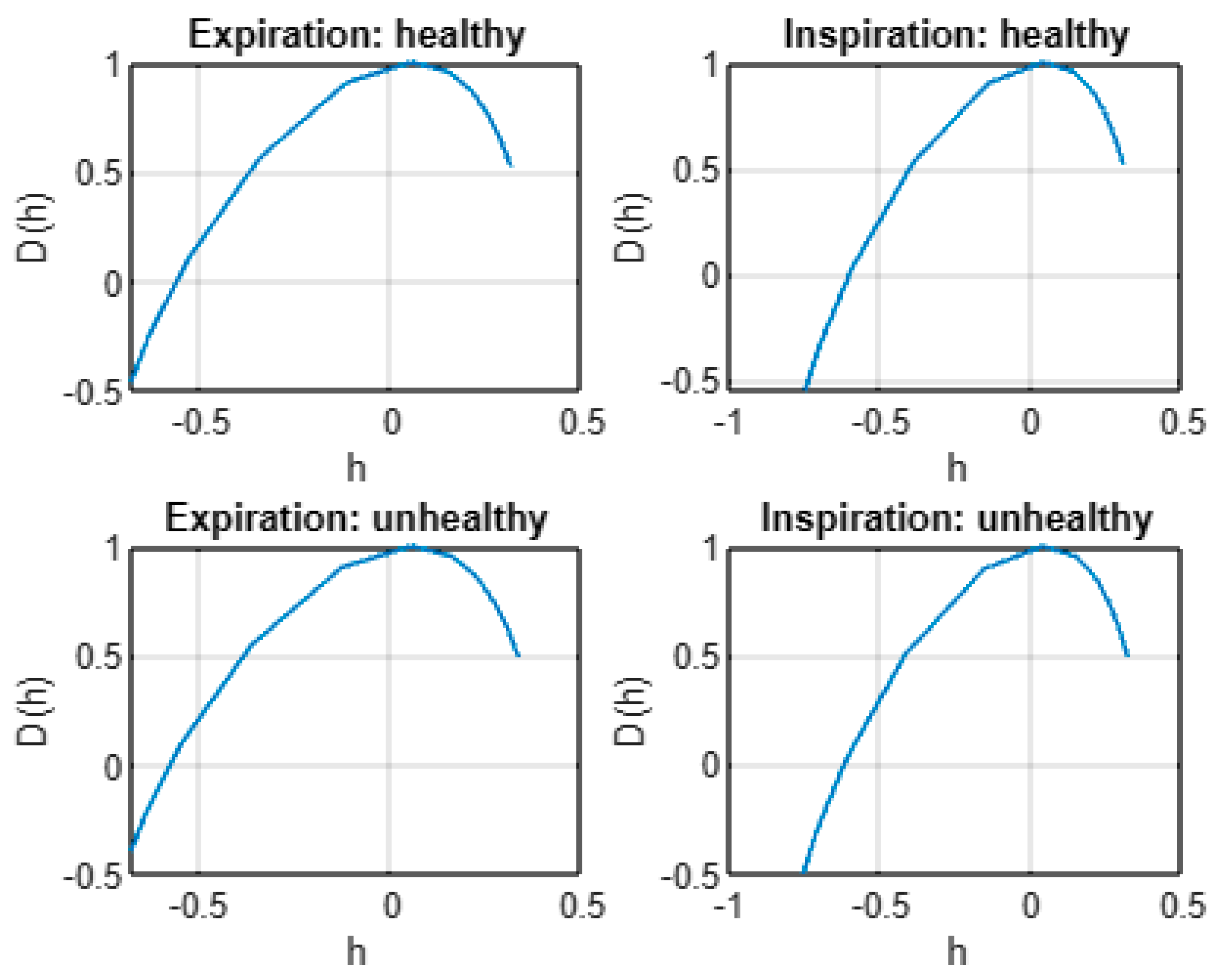
- All infant cry records exhibit evidence of multifractal properties according to estimated multifractal spectra D(h) and scaling exponent functions ζ(q).
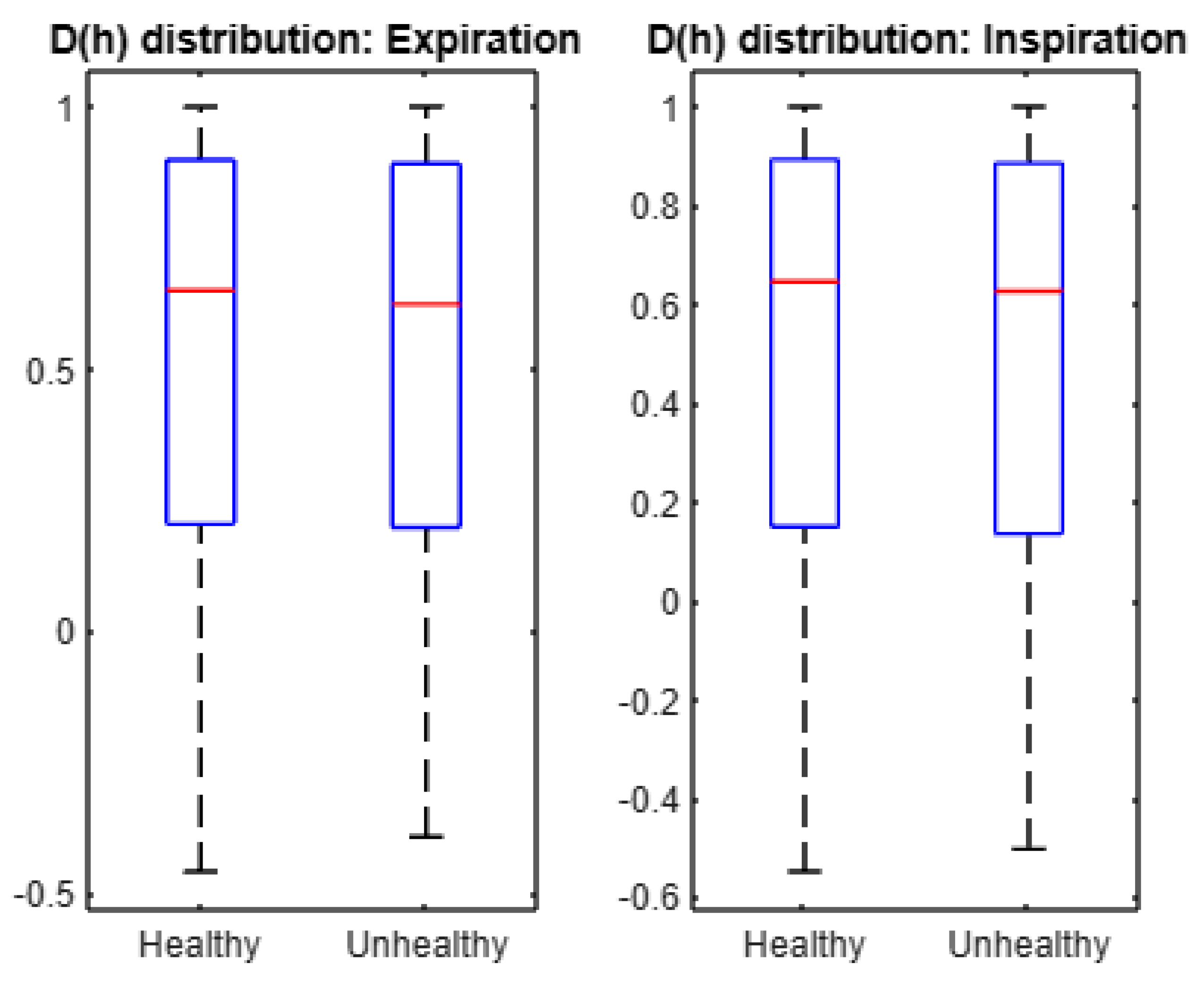
- The mean of spectrum D(h) is larger under healthy conditions than under unhealthy conditions. In addition, expiration and inspiration signals exhibit more complexity under healthy conditions than under unhealthy conditions.
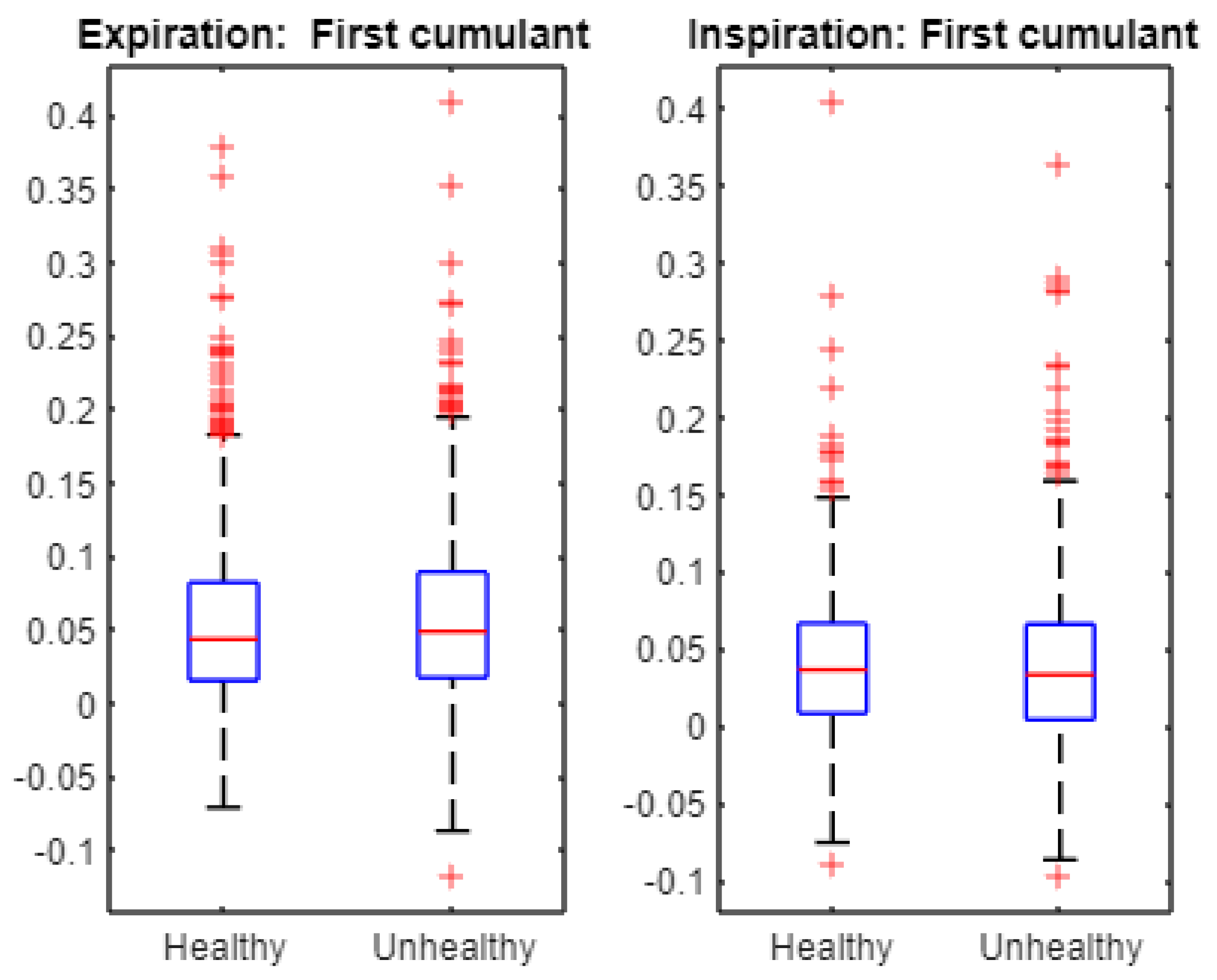
- Multifractal characteristics as represented by the first, second, and third cumulants in expiration, and inspiration signals are statistically different across healthy and unhealthy conditions.
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Müller, A.; Kraemer, J.F.; Penzel, T.; Bonnemeier, H.; Kurths, J.; Wessel, N. Causality in physiological signals. Physiol. Meas. 2016, 37, R46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peitgen, H.-O.; Jürgen, H.; Saupe, D. Chaos and Fractals: New Frontiers of Science; Springer-Verlag: New York, NY, USA, 1992. [Google Scholar]
- Souza França, L.G.; Vivas Miranda, J.G.; Leite, M.; Sharma, N.K.; Walker, M.C.; Lemieux, L.; Wang, Y. Fractal and Multifractal Properties of Electrographic Recordings of Human Brain Activity: Toward Its Use as a Signal Feature for Machine Learning in Clinical Applications. Front. Physiol. 2018, 9, 1767. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zorick, T.; Mandelkern, M.A. Multifractal detrended fluctuation analysis of human EEG: Preliminary investigation and comparison with the wavelet transform modulus maxima technique. PLoS ONE 2013, 8, e68360. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhou, W.; Yuan, S. Multifractal Analysis and Relevance Vector Machine-Based Automatic Seizure Detection in Intracranial EEG. Int. J. Neural Syst. 2015, 25, 1550020. [Google Scholar] [CrossRef]
- Lahmiri, S. An accurate system to distinguish between normal and abnormal electroencephalogram records with epileptic seizure free intervals. Biomed. Signal Processing Control 2018, 40, 312–317. [Google Scholar] [CrossRef]
- Lahmiri, S.; Shmuel, A. Accurate classification of seizure and seizure-free intervals of intracranial EEG signals from epileptic patients. IEEE Trans. Instrum. Meas. 2018, 68, 791–796. [Google Scholar] [CrossRef]
- Lahmiri, S. Generalized Hurst exponent estimates differentiate EEG signals of healthy and epileptic patients. Phys. A 2018, 490, 378–385. [Google Scholar] [CrossRef]
- Gaurav, G.; Anand, R.S.; Kumar, V. EEG based cognitive task classification using multifractal detrended fluctuation analysis. Cogn. Neurodyn. 2021, 15, 999–1013. [Google Scholar] [CrossRef]
- Zebende, G.F.; Oliveira Filho, F.M.; Leyva Cruz, J.A. Auto-correlation in the motor/imaginary human EEG signals: A vision about the FDFA fluctuations. PLoS ONE 2017, 12, e0183121. [Google Scholar] [CrossRef] [Green Version]
- Gadhoumi, K.; Do, D.; Badilini, F.; Pelter, M.M.; Hu, X. Wavelet leader multifractal analysis of heart rate variability in atrial fibrillation. J. Electrocardiol. 2018, 51, S83–S87. [Google Scholar] [CrossRef]
- Orozco-Duque, A.; Novak, D.; Kremen, V.; Bustamante, J. Multifractal analysis for grading complex fractionated electrograms in atrial fibrillation. Physiol. Meas. 2015, 36, 2269–2284. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Molina, A.A.; Angulo-Brown, F.; Muñoz-Diosdado, A. Multifractal Spectrum Curvature of RR Tachograms of Healthy People and Patients with Congestive Heart Failure, a New Tool to Assess Health Conditions. Entropy 2019, 21, 581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reyes-Manzano, C.F.; Lerma, C.; Echeverría, J.C.; Martínez-Lavin, M.; Martínez-Martínez, L.A.; Infante, O.; Guzmán-Vargas, L. Multifractal Analysis Reveals Decreased Non-linearity and Stronger Anticorrelations in Heart Period Fluctuations of Fibromyalgia Patients. Front. Physiol. 2018, 9, 1118. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.C.; Sharif, A. Common multifractality in the heart rate variability and brain activity of healthy humans. Chaos 2010, 20, 023121. [Google Scholar] [CrossRef] [PubMed]
- Lahmiri, S. Glioma detection based on multi-fractal features of segmented brain MRI by particle swarm optimization techniques. Biomed. Signal Processing Control 2017, 31, 148–155. [Google Scholar] [CrossRef]
- Lahmiri, S.; Boukadoum, M. New approach for automatic classification of Alzheimer’s disease, mild cognitive impairment and healthy brain magnetic resonance images. Healthc. Technol. Lett. 2014, 1, 32–36. [Google Scholar] [CrossRef] [Green Version]
- Rohini, P.; Sundar, S.; Ramakrishnan, S. Differentiation of early mild cognitive impairment in brainstem MR images using multifractal detrended moving average singularity spectral features. Biomed. Signal Processing Control 2020, 57, 101780. [Google Scholar] [CrossRef]
- Gerasimova-Chechkina, E.; Toner, B.; Marin, Z.; Audit, B.; Roux, S.G.; Argoul, F.; Khalil, A.; Gileva, O.; Naimark, O.; Arneodo, A. Comparative Multifractal Analysis of Dynamic Infrared Thermograms and X-Ray Mammograms Enlightens Changes in the Environment of Malignant Tumors. Front. Physiol. 2016, 7, 336. [Google Scholar] [CrossRef] [Green Version]
- Gerasimova-Chechkina, E.; Toner, B.; Marin, Z.; Audit, B.; Roux, S.G.; Argoul, F.; Khalil, A.; Gileva, O.; Naimark, O.; Arneodo, A. Wavelet-based multifractal analysis of dynamic infrared thermograms to assist in early breast cancer diagnosis. Front. Physiol. 2014, 5, 176. [Google Scholar]
- Borowska, M.; Bębas, E.; Szarmach, J.; Oczeretko, E. Multifractal characterization of healing process after bone loss. Biomed. Signal Processing Control 2019, 52, 179–186. [Google Scholar] [CrossRef]
- Wang, J.; Shao, W.; Kim, J. Combining MF-DFA and LSSVM for retina images classification. Biomed. Signal Processing Control 2020, 60, 101943. [Google Scholar] [CrossRef]
- Scala, I.; Rosi, G.; Nguyen, V.-H.; Vayron, R.; Haiat, G.; Seuret, S.; Jaffard, S.; Naili, S. Ultrasonic characterization and multiscale analysis for the evaluation of dental implant stability: A sensitivity study. Biomed. Signal Processing Control 2018, 42, 37–44. [Google Scholar] [CrossRef]
- Oprić, D.; Stankovich, A.D.; Nenadović, A.; Kovačević, S.; Obradović, D.D.; de Luka, S.; Nešović-Ostojić, J.; Milašin, J.; Ilić, A.Ž.; Trbovich, A.M. Fractal analysis tools for early assessment of liver inflammation induced by chronic consumption of linseed, palm and sunflower oils. Biomed. Signal Processing Control 2020, 61, 101959. [Google Scholar] [CrossRef]
- Wendt, H.; Abry, P. Multifractality Tests Using Bootstrapped Wavelet Leaders. IEEE Trans. Signal Processing 2007, 55, 4811–4820. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.K.; Buldyrev, S.V.; Havlin, S.; Simons, M.; Stanley, H.E.; Goldberger, A.L. Mosaic organization of DNA nucleotides. Phys. Rev. E 1994, 49, 1685–1689. [Google Scholar] [CrossRef] [Green Version]
- Di Matteo, T. Multi-scaling in finance. Quant. Financ. 2007, 7, 21–36. [Google Scholar] [CrossRef]
- Matikolaie, F.S.; Tadj, C. On the use of long-term features in a newborn cry diagnostic system. Biomed. Signal Processing Control 2020, 59, 101889. [Google Scholar] [CrossRef]
- Alaie, H.F.; Abou-Abbas, L.; Tadj, C. Cry-based infant pathology classification using GMMs. Speech Commun. 2016, 77, 28–52. [Google Scholar] [CrossRef] [Green Version]
- Hariharan, M.; Sindhu, R.; Vijean, V.; Yazid, H.; Nadarajaw, T.; Yaacob, S.; Polat, K. Improved binary dragonfly optimization algorithm and wavelet packet based non-linear features for infant cry classification. Comput. Methods Programs Biomed. 2018, 155, 39–51. [Google Scholar] [CrossRef]
- Orlandi, S.; Garcia, C.A.R.; Bandini, A.; Donzelli, G.; Manfredi, C. Application of Pattern Recognition Techniques to the Classification of Full-Term and Preterm Infant Cry. J. Voice 2016, 30, 656–663. [Google Scholar] [CrossRef] [Green Version]
- Kheddache, Y.; Tadj, C. Identification of diseases in newborns using advanced acoustic features of cry signals. Biomed. Signal Processing Control 2019, 50, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Kheddache, Y.; Tadj, C. Resonance frequencies behavior in pathologic cries of newborns. J. Voice 2015, 29, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childers, D.G.; Skinner, D.P.; Kemerait, R.C. The Cepstrum: A Guide to Processing. Proc. IEEE 1977, 65, 1428–1443. [Google Scholar] [CrossRef]
- Oppenheim, A.W. Discrete-Time Signal Processing; Pearson: London, UK, 2010. [Google Scholar]
- Travieso, C.M.; Alonso, J.B.; del Pozo, M.; Ticay, J.R.; Castellanos-Dominguez, G. Building a Cepstrum-HMM kernel for Apnea identification. Neurocomputing 2014, 132, 159–165. [Google Scholar] [CrossRef]
- Lahmiri, S.; Tadj, C.; Gargour, C. Biomedical diagnosis of infant cry signal based on analysis of cepstrum by deep feedforward artificial neural networks. IEEE Instrum. Meas. Mag. 2021, 24, 24–29. [Google Scholar] [CrossRef]
- Lahmiri, S.; Tadj, C.; Gargour, C.; Bekiros, S. Deep learning systems for automatic diagnosis of infant cry signals. Chaos Solitons Fractals 2022, 154, 111700. [Google Scholar] [CrossRef]
- Shekatkar, S.M.; Kotriwar, Y.; Harikrishnan, K.P.; Ambika, G. Detecting abnormality in heart dynamics from multifractal analysis of ECG signals. Sci. Rep. 2017, 7, 15127. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lahmiri, S.; Tadj, C.; Gargour, C. Nonlinear Statistical Analysis of Normal and Pathological Infant Cry Signals in Cepstrum Domain by Multifractal Wavelet Leaders. Entropy 2022, 24, 1166. https://doi.org/10.3390/e24081166
Lahmiri S, Tadj C, Gargour C. Nonlinear Statistical Analysis of Normal and Pathological Infant Cry Signals in Cepstrum Domain by Multifractal Wavelet Leaders. Entropy. 2022; 24(8):1166. https://doi.org/10.3390/e24081166
Chicago/Turabian StyleLahmiri, Salim, Chakib Tadj, and Christian Gargour. 2022. "Nonlinear Statistical Analysis of Normal and Pathological Infant Cry Signals in Cepstrum Domain by Multifractal Wavelet Leaders" Entropy 24, no. 8: 1166. https://doi.org/10.3390/e24081166
APA StyleLahmiri, S., Tadj, C., & Gargour, C. (2022). Nonlinear Statistical Analysis of Normal and Pathological Infant Cry Signals in Cepstrum Domain by Multifractal Wavelet Leaders. Entropy, 24(8), 1166. https://doi.org/10.3390/e24081166






