Effects of Nb Additions and Heat Treatments on the Microstructure, Hardness and Wear Resistance of CuNiCrSiCoTiNbx High-Entropy Alloys
Abstract
:1. Introduction
2. Materials and Methods
3. Thermodynamic Parameters and Considerations for Phase Formation in HEAs
4. Results and Discussion
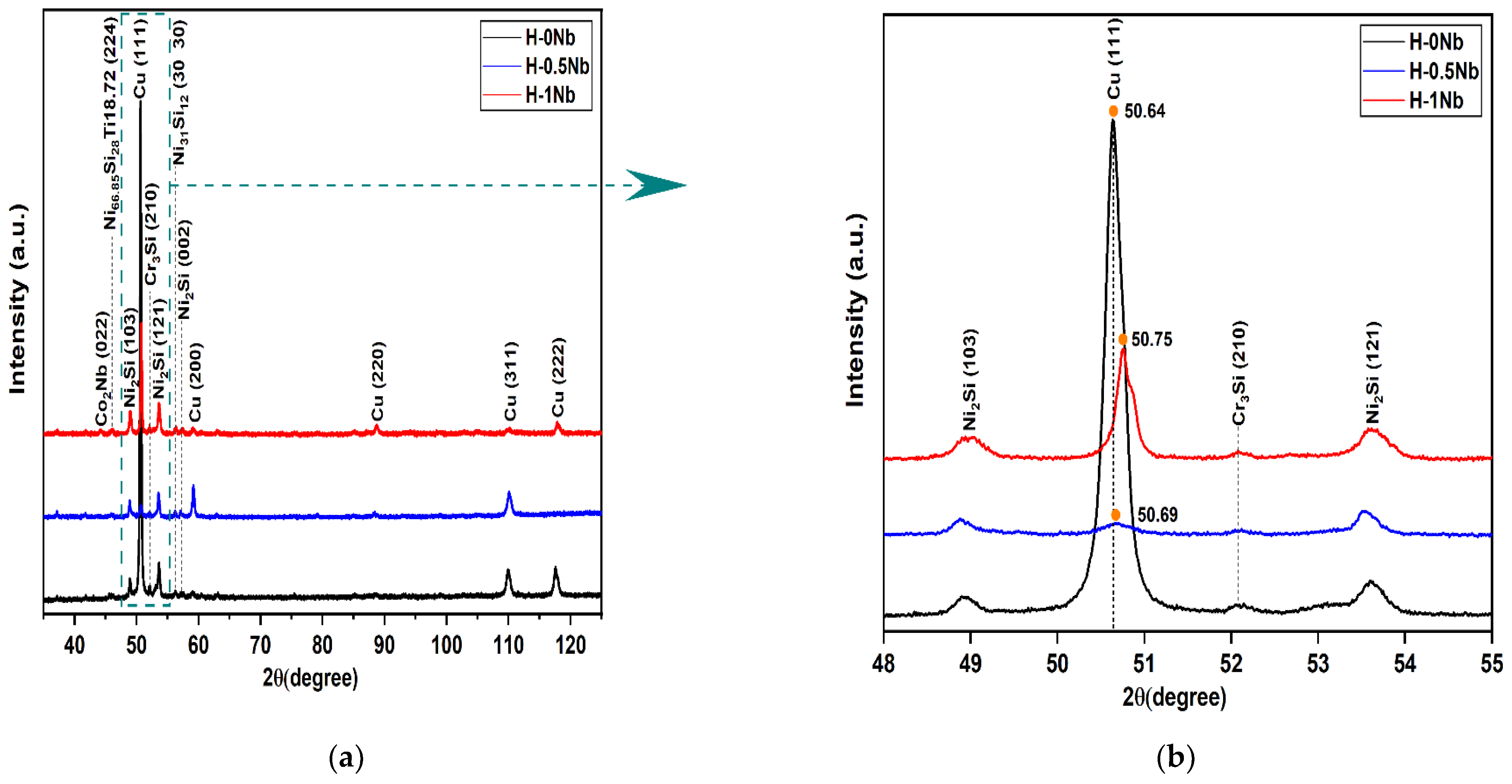
4.1. Phase Structure by XRD before Heat Treatment
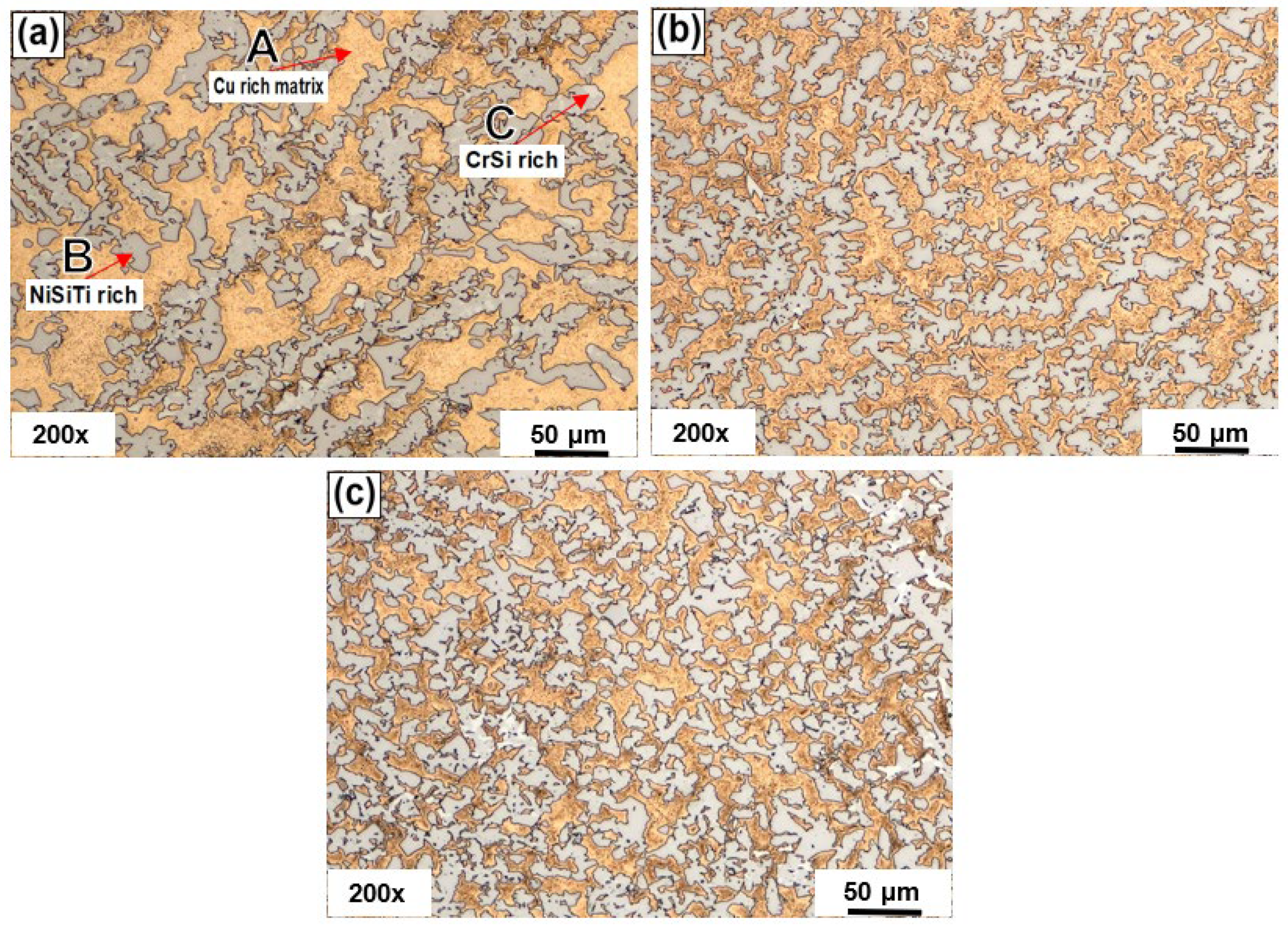
4.2. Microstructural Characterization before Heat Treatments
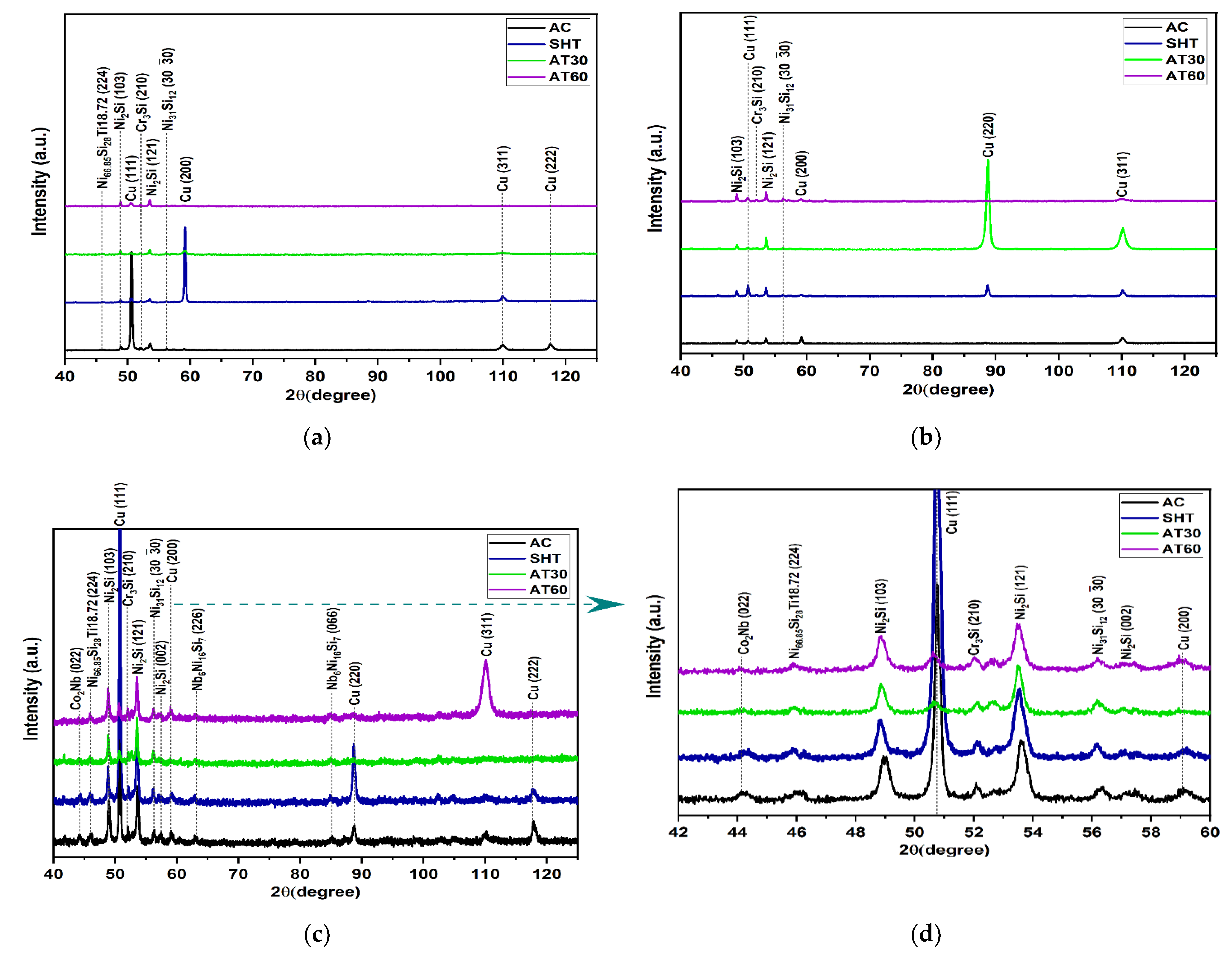
4.3. Phase Structure by XRD after Heat Treatments
4.4. Microstructural Characterization after Heat Treatments
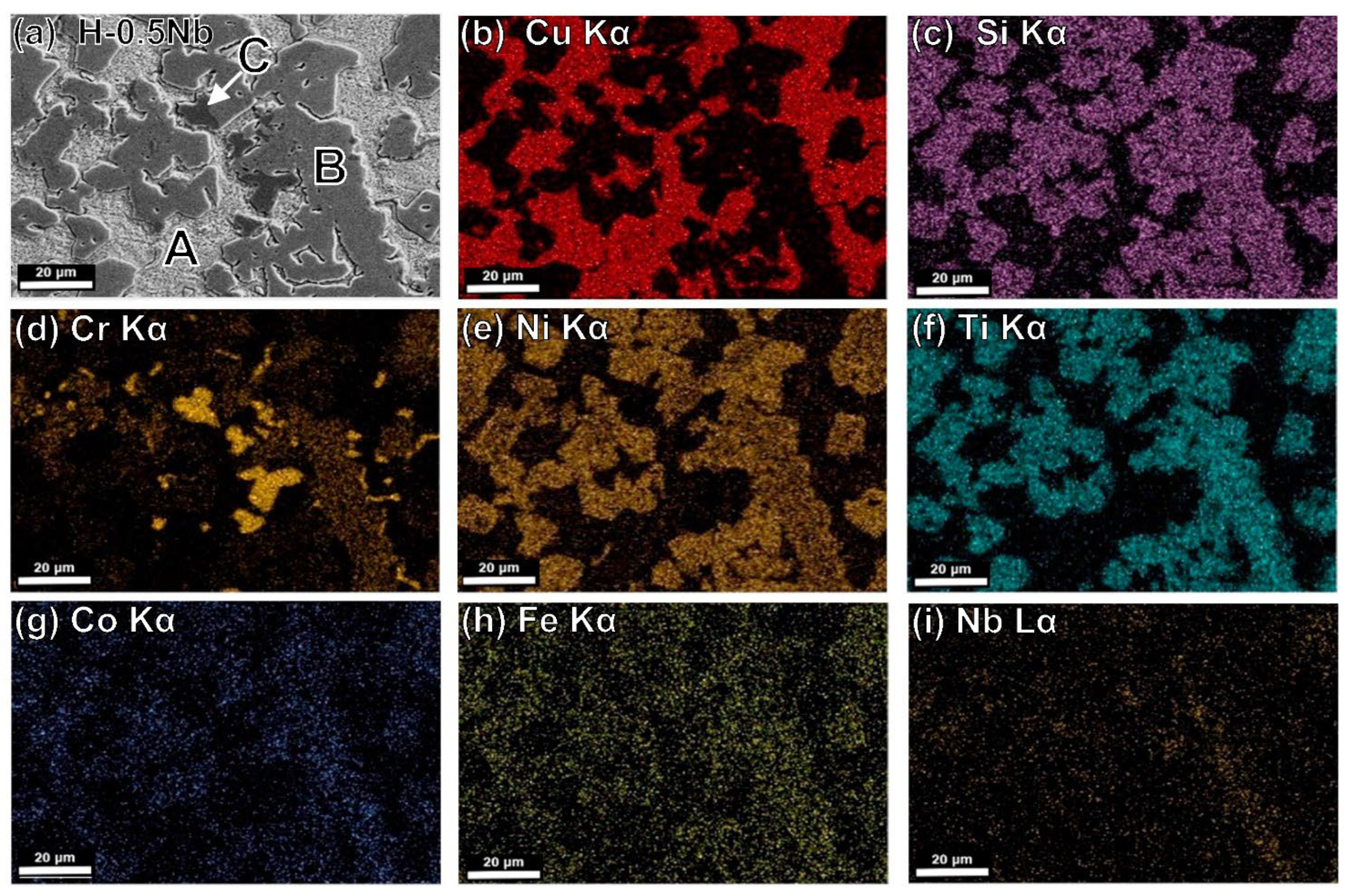
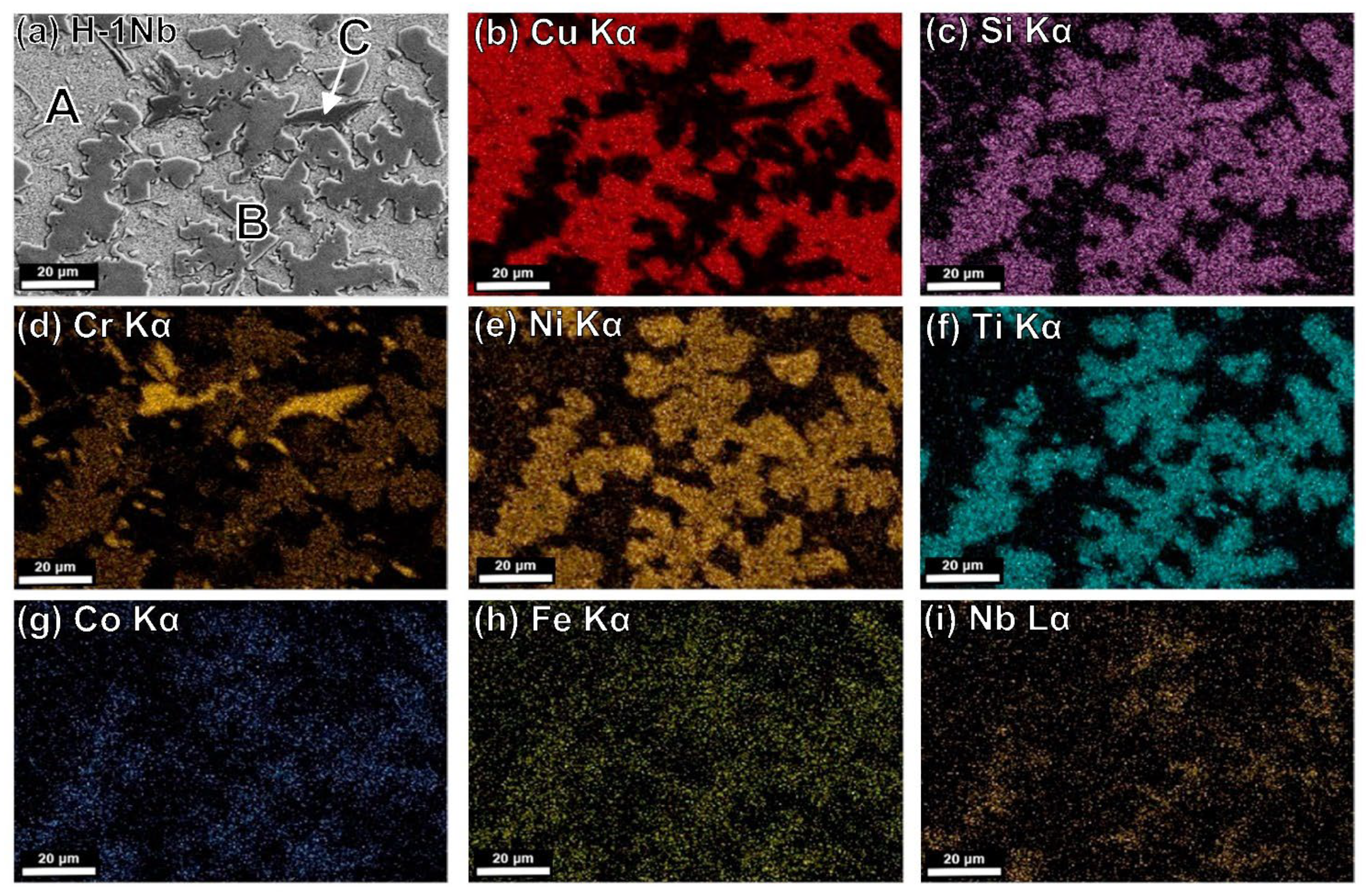
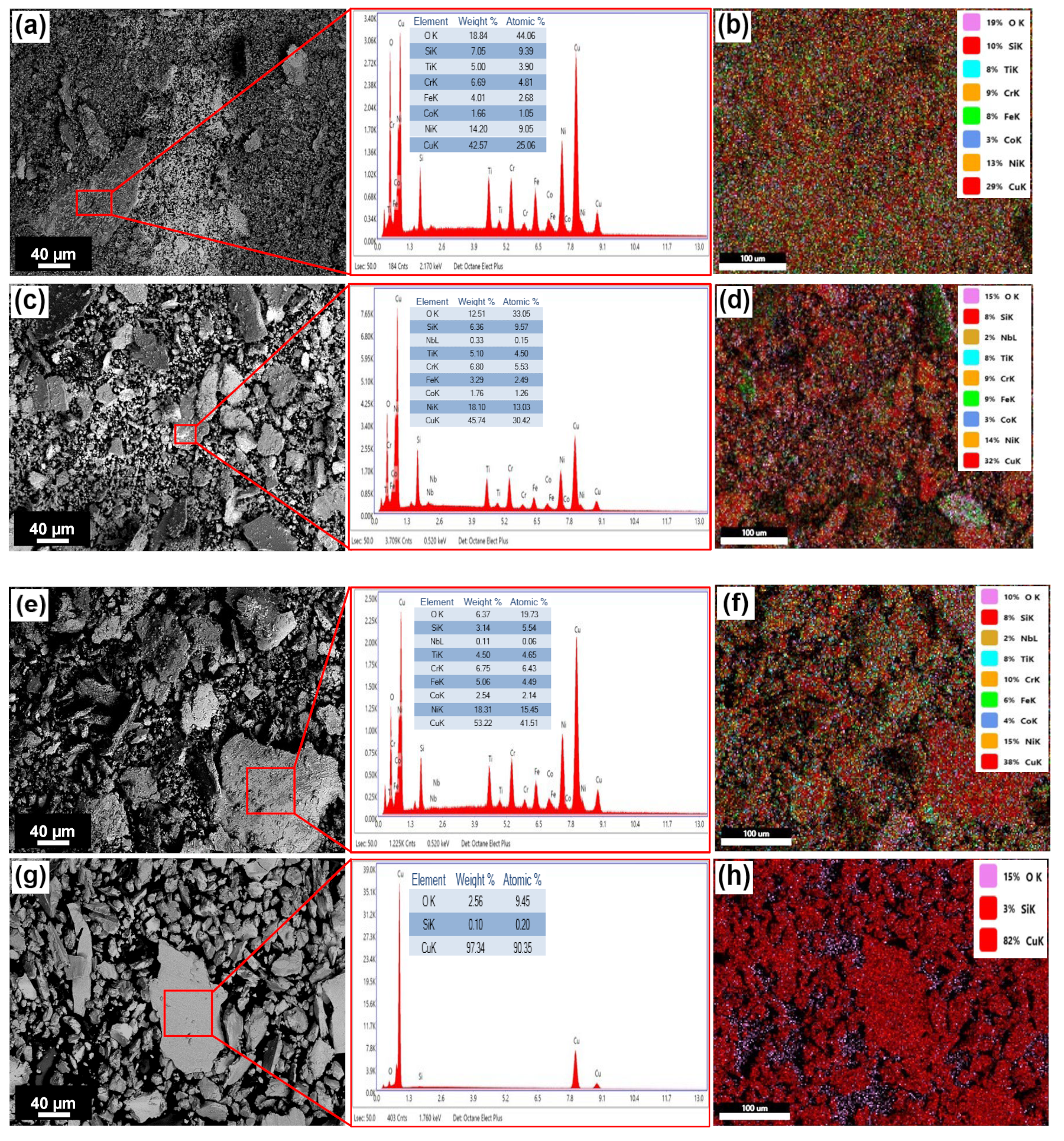
4.5. Microstructural Characterization by SEM-EDS
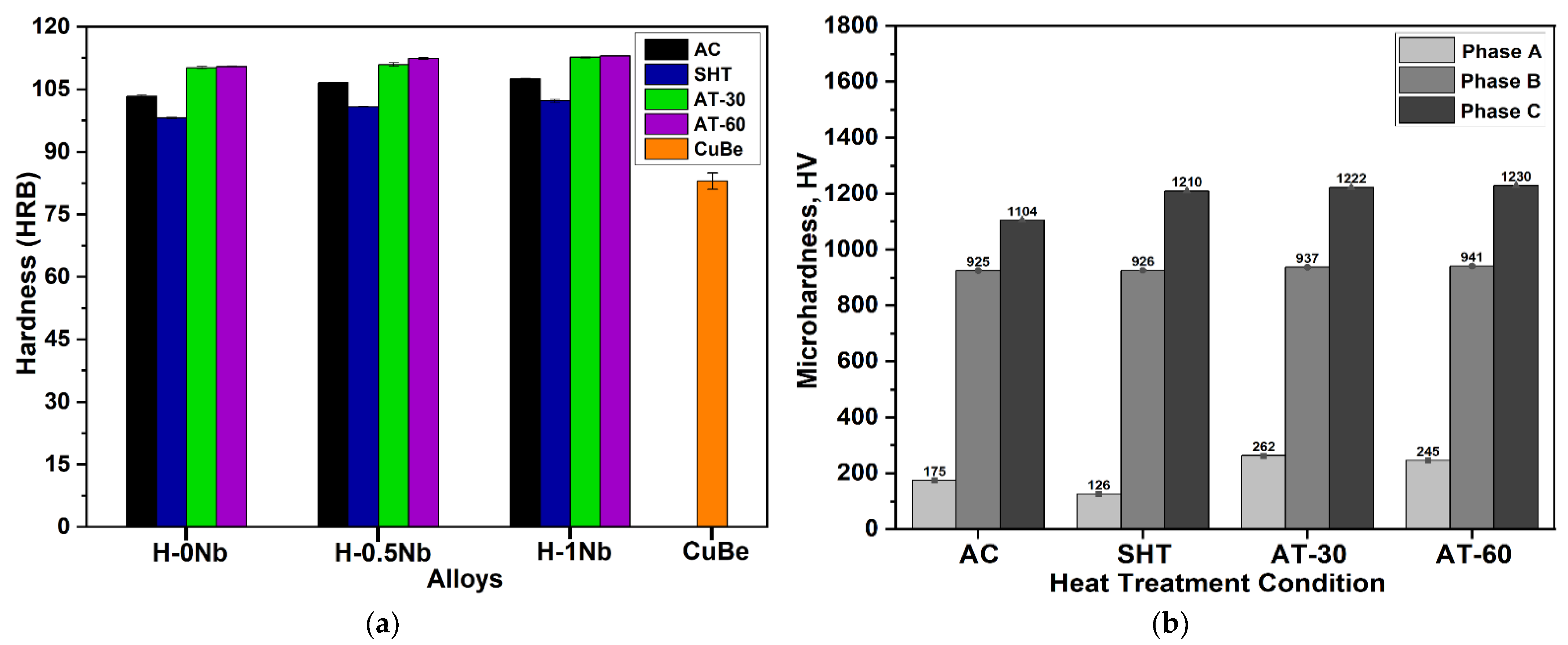
4.6. Macrohardness Rockwell B and Microhardness Vickers Test Results
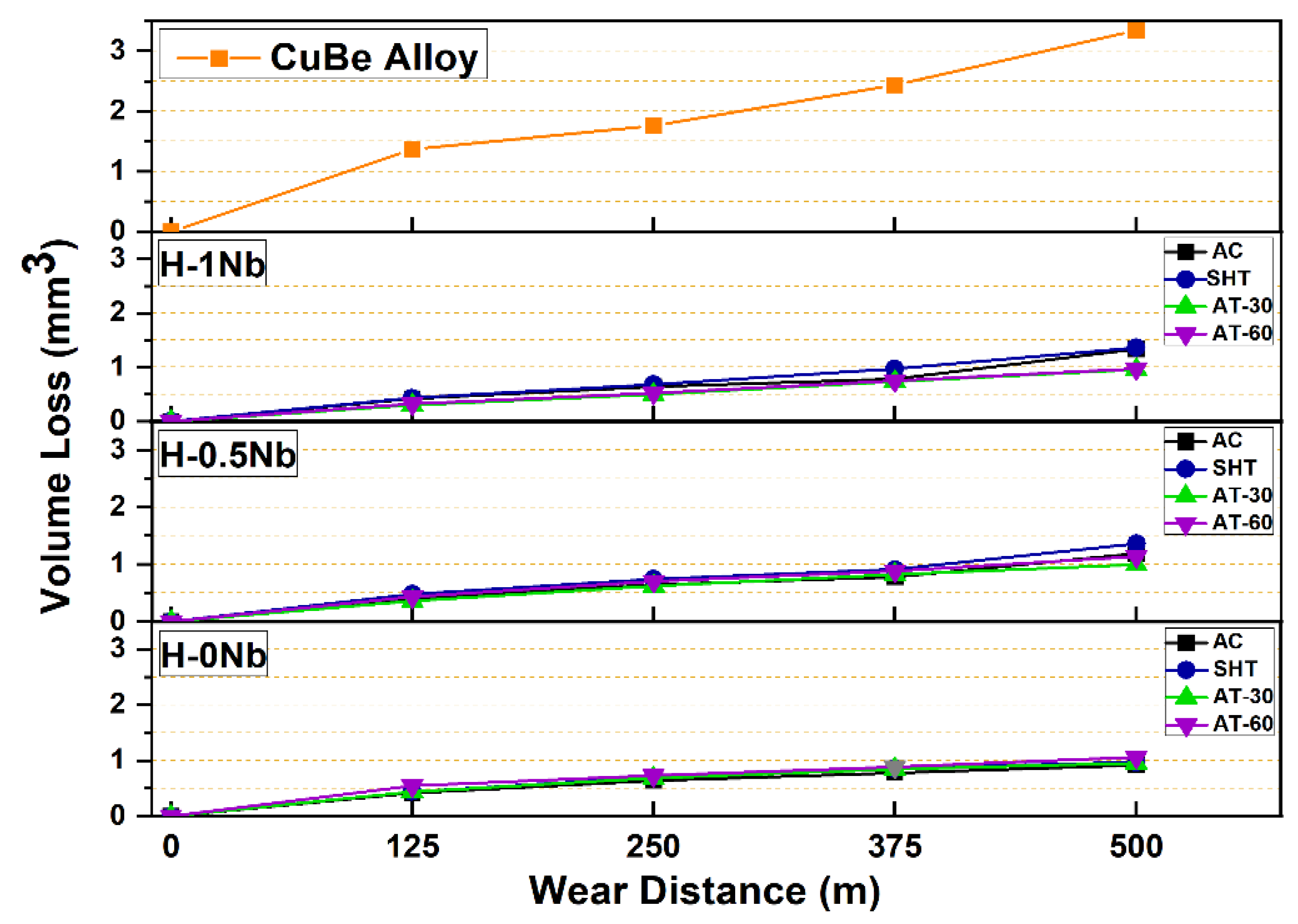
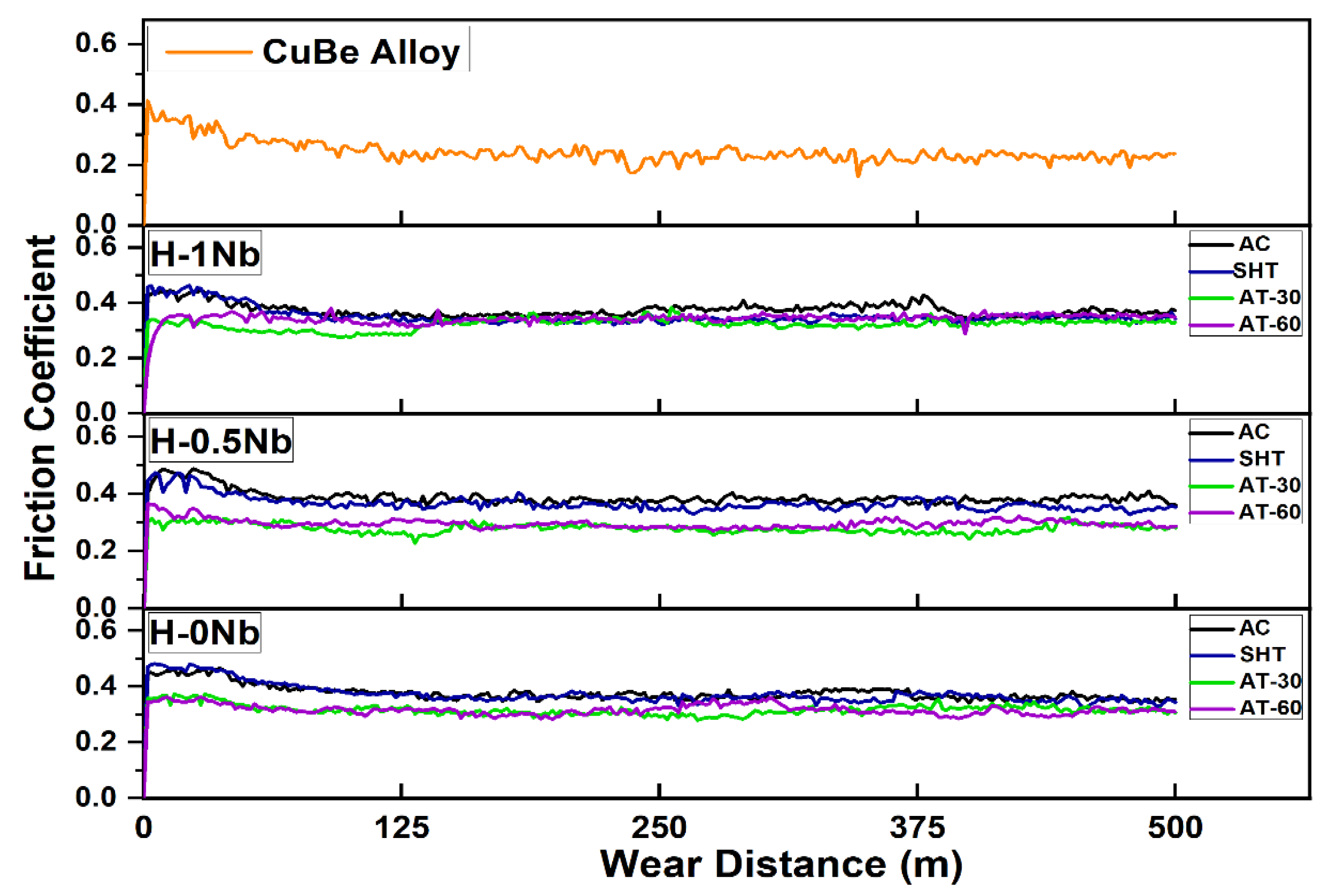
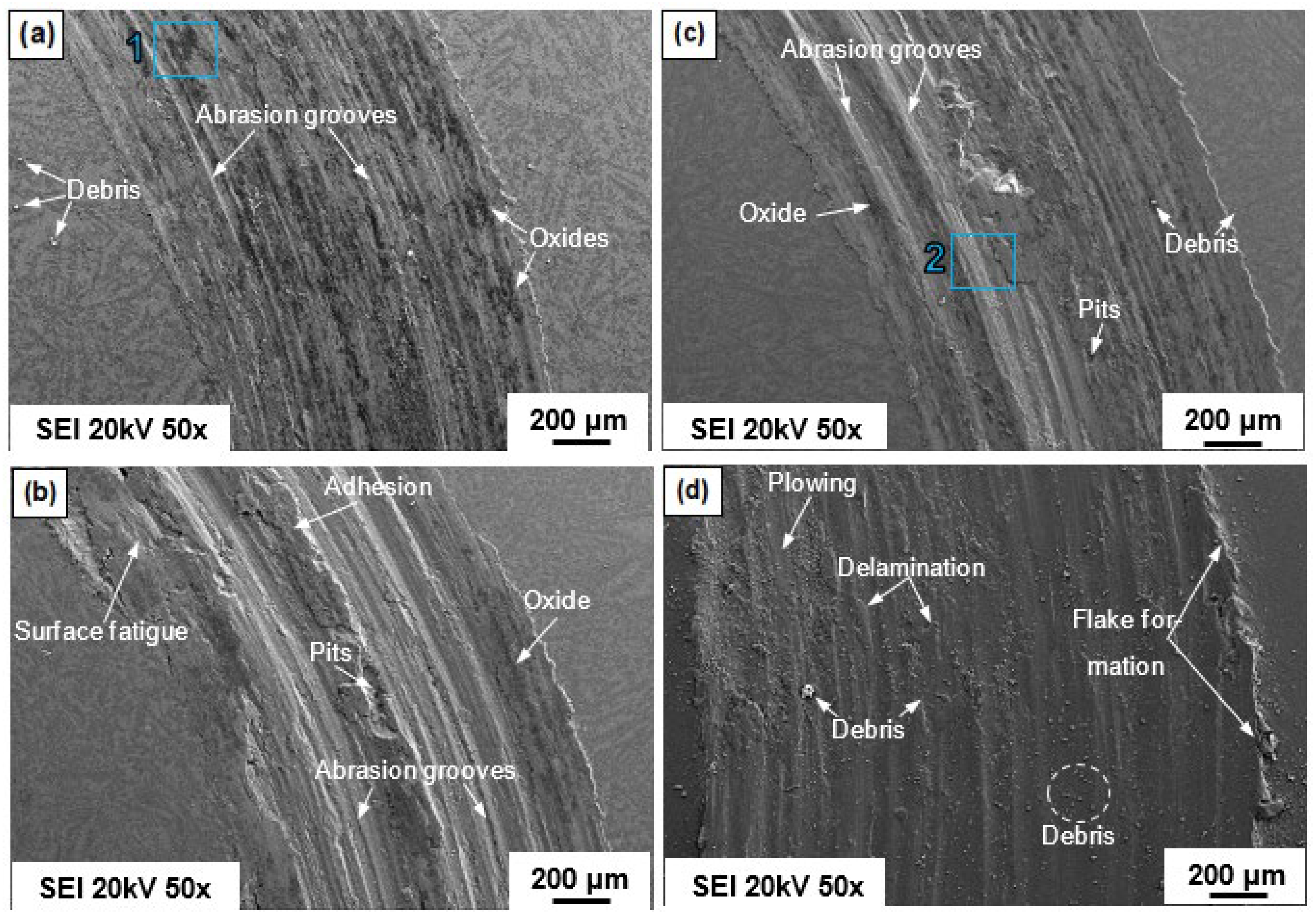
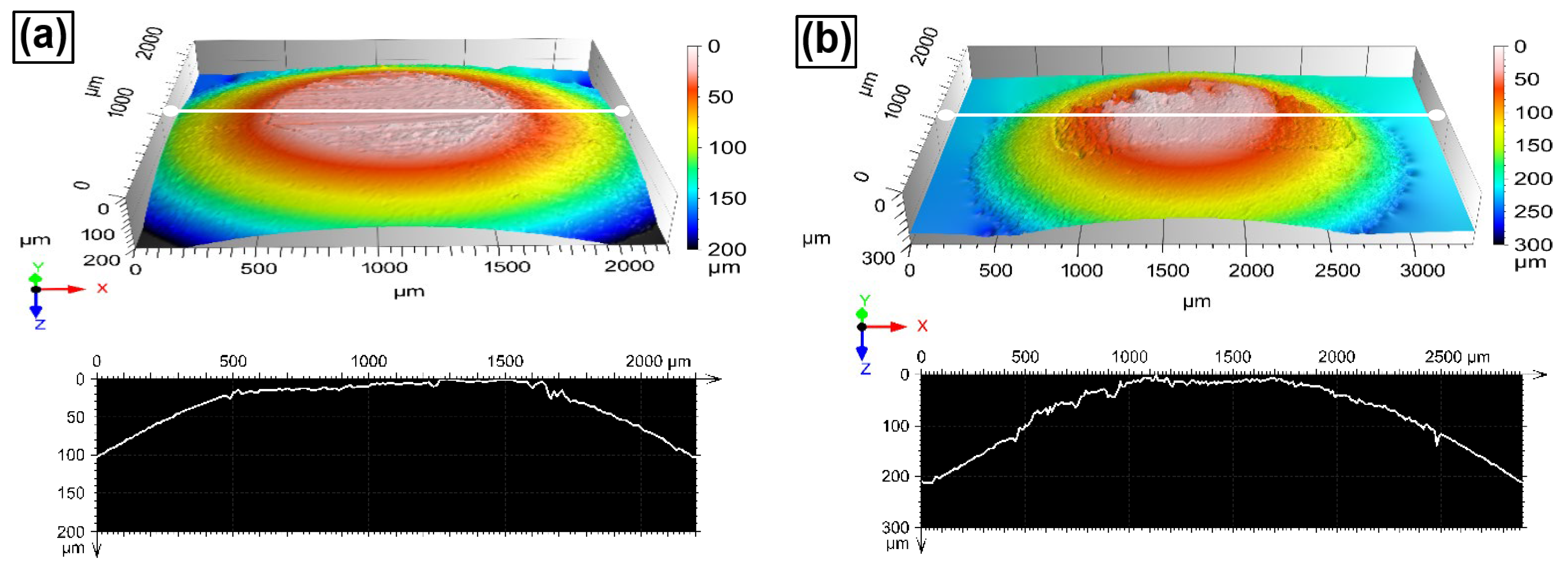
4.7. Wear and Friction Behaviors of the HEAs
5. Conclusions
- (1)
- Nb addition resulted in a gradual increase in configurational entropy from 12.14 J/mol·K 12.36 J/mol·K in the H-0Nb alloy, and to 12.55 J/mol·K in both the H-0.5Nb and H-1Nb alloys, resulting in the formation of FCC and BCC solid solutions.
- (2)
- DRX results revealed the presence of FCC and BCC phases as well as Co2Nb, N2Si, Cr3Si and Nb6Ni16Si7 compounds. In addition, calculations of the lattice constant showed a reduction based on the displacement of the (111) peak to higher angles as Nb levels increased in the alloys.
- (3)
- Microstructure transformation was influenced by Nb additions and heat treatment producing the precipitation of interdendritic phases and microstructure refinement.
- (4)
- The increase in hardness and microhardness of the HEAs were attributed to a higher content of NiSiTi and CrSi-rich phases of high hardness as well as to the precipitation of particles during AT, resulting in higher hardness values, compared with the CuBe commercial alloy.
- (5)
- The H-0Nb alloy exhibited better wear performance for all experimental conditions. However, wear losses also increased with Nb addition. This was attributed to microstructure fragilization due to a high-density Cr3Si precipitation of high hardness. The best wear performance was exhibited by the H-0Nb alloy in the AT-30 condition, with a volume loss of 0.92 mm3, which very low when compared to the value of 3.34 mm3 obtained for the CuBe commercial alloy.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yeh, J.-W.; Chen, S.K.; Lin, S.-J.; Gan, J.-Y.; Chin, T.-S.; Shun, T.-T.; Tsau, C.-H.; Chang, S.-Y. Nanostructured High-Entropy Alloys with Multiple Principal Elements: Novel Alloy Design Concepts and Outcomes. Adv. Eng. Mater. 2004, 6, 299–303. [Google Scholar] [CrossRef]
- Cantor, B.; Chang, I.T.H.; Knight, P.; Vincent, A.J.B. Microstructural development in equiatomic multicomponent alloys. Mater. Sci. Eng. A. 2004, 375–377, 213–218. [Google Scholar] [CrossRef]
- Yeh, J. Alloy Design Strategies and Future Trends in High-Entropy Alloys. JOM 2013, 65, 1759–1771. [Google Scholar] [CrossRef]
- Zhang, W.; Liaw, P.K.; Zhang, Y. Science and technology in high-entropy alloys. Sci. CHINA Mater. 2018, 61, 2–22. [Google Scholar] [CrossRef]
- Miracle, B.D.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Gao, M.C.; Liaw, P.K.; Yeh, J.W.; Zhang, Y. High-Entropy Alloys: Fundamentals and Applications; Springer: Cham, Switzerland, 2016; pp. 1–21. [Google Scholar] [CrossRef]
- Ye, Y.F.; Wang, Q.; Lu, J.; Liu, C.T.; Yang, Y. High-entropy alloy: Challenges and prospects. Mater. Today. 2016, 19, 349–362. [Google Scholar] [CrossRef]
- Diao, H.Y.; Feng, R.; Dahmen, K.A.; Liaw, P.K. Fundamental deformation behavior in high-entropy alloys: An overview. Curr. Opin. Solid State Mater. Sci. 2017, 21, 252–266. [Google Scholar] [CrossRef]
- Yeh, J.W.; Chen, Y.L.; Lin, S.J.; Chen, S.K. High-Entropy Alloys—A New Era of Exploitation. Mater. Sci. Forum. 2007, 560, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Zhao, Y.; Qiao, J.; Ma, S.; Gao, M.; Yang, H.; Chen, M.; Zhang, A. A hexagonal close-packed high-entropy alloy: The effect of entropy. Mater. Des. 2016, 96, 10–15. [Google Scholar] [CrossRef]
- Birbilis, N.; Choudhary, S.; Scully, J.R.; Taheri, M.L. A perspective on corrosion of multi-principal element alloys. NPJ Mater. Degrad. 2021, 5, 14. [Google Scholar] [CrossRef]
- Pickering, E.J.; Jones, N.G. High-entropy alloys: A critical assessment of their founding principles and future prospects. Int. Mater. Rev. 2016, 61, 183–202. [Google Scholar] [CrossRef]
- Lu, Z.; Wang, H.; Chen, M.; Baker, I.; Yeh, J.; Liu, C.; Nieh, T. An assessment on the future development of high-entropy alloys: Summary from a recent workshop. Intermetallics 2015, 66, 67–76. [Google Scholar] [CrossRef]
- Yeh, J.; Chen, S.; Gan, J.; Lin, S.; Chin, T. Communications: Formation of Simple Crystal Structures in Cu-Co-Ni-Cr-Al-Fe-Ti-V Alloys with Multiprincipal Metallic Elements. Metall. Mater. Trans. 2004, 35, 2533–2536. [Google Scholar] [CrossRef]
- Chuang, M.H.; Tsai, M.H.; Wang, W.R.; Lin, S.J.; Yeh, J.W. Microstructure and wear behavior of AlxCo 1.5CrFeNi1.5Tiy high-entropy alloys. Acta Mater. 2011, 59, 6308–6317. [Google Scholar] [CrossRef]
- Gómez-Esparza, C.D.; Camarillo-Cisneros, J.; Estrada-Guel, I.; Cabañas-Moreno, J.G.; Herrera-Ramírez, J.M.; Martínez-Sánchez, R. Effect of Cr, Mo and Ti on the microstructure and Vickers hardness of multi-component systems. J. Alloy. Compd. 2015, 615, 638–644. [Google Scholar] [CrossRef]
- He, J.Y.; Wang, H.; Huang, H.L.; Xu, X.D.; Chen, M.W.; Wu, Y.; Liu, X.J.; Nieh, T.G.; An, K.; Lu, Z.P. A precipitation-hardened high-entropy alloy with outstanding tensile properties Acta Materialia A precipitation-hardened high-entropy alloy with outstanding tensile properties. Acta Mater. 2016, 102, 187–196. [Google Scholar]
- Zou, Y.; Ma, H.; Spolenak, R. Ultrastrong ductile and stable high-entropy alloys at small scales. Nat. Commun. 2015, 6, 7748. [Google Scholar] [CrossRef]
- Li, D.; Li, C.; Feng, T.; Zhang, Y.; Sha, G.; Lewandowski, J.J.; Liaw, P.K. High-entropy Al0.3CoCrFeNi alloy fibers with high tensile strength and ductility at ambient and cryogenic temperatures. Acta Mater. 2016, 123, 285–294. [Google Scholar] [CrossRef]
- Tang, Z.; Yuan, T.; Tsai, C.W.; Yeh, J.W.; Lundin, C.D.; Liaw, P.K. Fatigue behavior of a wrought Al0.5CoCrCuFeNi two-phase high-entropy alloy. Acta Mater. 2015, 99, 247–258. [Google Scholar] [CrossRef]
- Wang, X.; Guo, W.; Fu, Y. High-Entropy Alloys: Emerging Materials for Advanced Functional Applications. J. Mater. Chem. A. 2020, 9, 663–701. [Google Scholar] [CrossRef]
- Martienssen, W.; Warlimont, H. Springer Handbook of Condensed Matter and Materials Data, 1st ed.; Springer: Berlin, Germany, 2005. [Google Scholar]
- Nunes, R.; Adams, J.H.; Ammons, M. Introduction to Copper and Copper Alloys. Properties and Selection: Nonferrous Alloys and Special-Purpose Materials; SM International: Materials Park, OH, USA, 1992; Volume 2, pp. 760–790. [Google Scholar]
- Li, J.; Huang, G.; Mi, X.; Peng, L.; Xie, H.; Kang, Y. Effect of Co addition on microstructure and properties of Cu-Ni-Si alloy. In Advances in Materials Processing; Springer: Singapore, 2018; pp. 353–360. [Google Scholar]
- Ozawa, A.; Watanabe, C.; Monzen, R. Influence of Co on Strength of Cu-Ni-Co-Si Alloy. Mater. Sci. Forum. 2014, 783, 2468–2473. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Y.; Tian, B.; Jia, Y.; Liu, Y. Co effects on Cu-Ni-Si alloys microstructure and physical properties. J. Alloy. Compd. 2019, 797, 1327–1337. [Google Scholar] [CrossRef]
- Geng, Y.; Ban, Y.; Wang, B.; Li, X.; Song, K.; Zhang, Y.; Jia, Y.; Tian, B.; Liu, Y.; Volinsky, A.A. A review of microstructure and texture evolution with nanoscale precipitates for copper alloys. J. Mater. Res. Technol. 2020, 9, 11918–11934. [Google Scholar] [CrossRef]
- Wang, H.; Chen, H.; Gu, J.; Hsu, C.; Wu, C. Effects of heat treatment processes on the microstructures and properties of powder metallurgy produced Cu–Ni–Si–Cr alloy Huei-Sen. Mater. Sci. Eng. A. 2014, 619, 221–227. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, X.; Liaw, P.K. Alloy design and properties optimization of high-entropy alloys. Jom 2012, 64, 830–838. [Google Scholar] [CrossRef]
- Gao, M.; Chen, Z.; Kang, H.; Li, R.; Wang, W.; Zou, C.; Wang, T. Effects of Nb addition on the microstructures and mechanical properties of a precipitation hardening Cu-9Ni-6Sn alloy. Mater. Sci. Eng. A 2018, 715, 340–347. [Google Scholar] [CrossRef]
- Lee, E.; Han, S.; Euh, K.; Lim, S.; Kim, S. Effect of Ti addition on tensile properties of Cu-Ni-Si alloys. Met. Mater. Int. 2011, 17, 569–576. [Google Scholar] [CrossRef]
- Avila-Salgado, D.A.; Juárez-Hernández, A.; Medina-Ortíz, F.; Banda, M.L.; Hernández-Rodríguez, M.A.L. Influence of B and NB additions and heat treatments on the mechanical properties of Cu–Ni– Co–Cr–Si alloy for high pressure die casting applications. Metals 2020, 10, 602. [Google Scholar] [CrossRef]
- Avila-Salgado, D.A.; Juárez-Hernández, A.; Cabral-Miramontes, J.; Camacho-Martínez, L. Strengthening properties and wear resistance of the cu-xni-yco-cr-si alloy by varying Ni/Co and Zr addition. Lubricants 2021, 9, 96. [Google Scholar] [CrossRef]
- ASTM G99-95A; Standard Test Method for Wear Testing with a Pin-on-Disk. ASTM International: West Conshohocken, PA, USA, 2000; pp. 1–5. [CrossRef]
- Zhang, Y.; Zhou, J.Y.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Guo, S.; Liu, C.T. Phase stability in high entropy alloys: Formation of solid-solution phase or amorphous phase. Prog. Nat. Sci. Mater. Int. 2011, 21, 433–446. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Y. Prediction of high-entropy stabilized solid-solution in multi-component alloys. Mater. Chem. Phys. 2012, 132, 233–238. [Google Scholar] [CrossRef]
- Guo, S.; Ng, C.; Lu, J.; Liu, C.T. Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys Effect of valence electron concentration on stability of fcc or bcc phase in high entropy alloys. J. Appl. Phys. 2011, 109, 103505. [Google Scholar] [CrossRef]
- Nong, Z.S.; Zhu, J.C.; Cao, Y.; Yang, X.W.; Lai, Z.H.; Liu, Y. Stability and structure prediction of cubic phase in as cast high entropy alloys. Mater. Sci. Technol. 2014, 30, 363–369. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-entropy alloys: A critical review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Sen Wang, H.; Chen, H.G.; Gu, J.W.; Hsu, C.E.; Wu, C.Y. Improvement in strength and thermal conductivity of powder metallurgy produced Cu–Ni–Si–Cr alloy by adjusting Ni/Si weight ratio and hot forging. J. Alloy. Compd. 2015, 633, 59–64. [Google Scholar] [CrossRef]
- ASTM standard 407-07; Standard Practice for Microetching Metals and Alloys. ASTM International: West Conshohocken, PA, USA, 2007; pp. 1–21. [CrossRef]
- Erdogan, A.; Doleker, K.M.; Zeytin, S. Effect of Al and Ti on High-Temperature Oxidation Behavior of CoCrFeNi-Based High-Entropy Alloys. Jom 2019, 71, 3499–3510. [Google Scholar] [CrossRef]
- Xu, Z.; Li, D.Y.; Chen, D.L. Effect of Ti on the wear behavior of AlCoCrFeNi high-entropy alloy during unidirectional and bi-directional sliding wear processes. Wear 2021, 476, 203650. [Google Scholar] [CrossRef]




| n | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ΔSConf | 0 | 0.69R | 1.1R | 1.39R | 1.61R | 1.79R | 1.95R | 2.08R | 2.2R | 2.3R | 2.4R |
| Alloy | Elemental Constituents (wt%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Cu | Ni | Cr | Si | Co | Ti | Fe | Nb | |
| H-0Nb | 56.92 | 19.50 | 6.50 | 6.50 | 2.00 | 6.50 | 2.08 | 0.00 |
| H-0.5Nb | 56.18 | 19.50 | 6.50 | 6.50 | 2.00 | 6.50 | 2.32 | 0.50 |
| H-1Nb | 55.44 | 19.50 | 6.50 | 6.50 | 2.00 | 6.50 | 2.56 | 1.00 |
| Element (Atomic Size, NM) | Cu | Ni | Cr | Si | Co | Ti | Fe | Nb |
|---|---|---|---|---|---|---|---|---|
| Cu (0.135) | - | 4 | 12 | −19 | 6 | −9 | 13 | 3 |
| Ni (0.135) | - | - | −7 | −40 | 0 | −35 | −2 | −30 |
| Cr (0.140) | - | - | - | −37 | −4 | −7 | −1 | −7 |
| Si (0.110) | - | - | - | - | −38 | −66 | −35 | −56 |
| Co (0.135) | - | - | - | - | - | −28 | −1 | −25 |
| Ti (0.14) | - | - | - | - | - | - | −17 | 2 |
| Fe (0.14) | - | - | - | - | - | - | - | −16 |
| Nb (0.145) | - | - | - | - | - | - | - | - |
| Alloy | ΔSConf (J/mol·K) | ΔHMix (k·J/mol) | Ω | Tm (K) | δ (%) | Δχ (%) | VEC | CS |
|---|---|---|---|---|---|---|---|---|
| H-0Nb | 12.14 | −13.66 | 1.35 | 1516.15 | 5.44 | 0.110 | 8.93 | FCC/BCC |
| H-0.5Nb | 12.36 | −13.81 | 1.36 | 1519.15 | 5.49 | 0.111 | 8.90 | FCC/BCC |
| H-1Nb | 12.55 | −13.95 | 1.37 | 1522.15 | 5.53 | 0.111 | 8.87 | FCC/BCC |
| Alloy | Phase | Elemental Constituents (At%) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cu | Si | Cr | Ni | Ti | Co | Fe | Nb | ||
| H-0Nb | Nominal | 50.00 | 12.93 | 6.98 | 18.54 | 7.58 | 1.89 | 2.08 | 0.00 |
| A | 82.85 | 8.57 | 0.00 | 6.86 | 0.23 | 0.24 | 1.25 | 0.00 | |
| B | 10.21 | 24.42 | 5.06 | 39.94 | 12.31 | 3.97 | 4.09 | 0.00 | |
| C | 1.48 | 23.02 | 70.30 | 1.14 | 0.42 | 0.00 | 3.64 | 0.00 | |
| H-0.5Nb | Nominal | 49.40 | 12.94 | 6.98 | 18.56 | 7.59 | 1.90 | 2.33 | 0.30 |
| A | 85.74 | 6.37 | 0.00 | 6.56 | 0.26 | 0.28 | 0.79 | 0.00 | |
| B | 4.59 | 23.92 | 5.58 | 43.80 | 14.44 | 3.90 | 3.33 | 0.44 | |
| C | 0.87 | 22.21 | 70.53 | 1.63 | 0.65 | 0.63 | 3.45 | 0.03 | |
| H-1Nb | Nominal | 48.81 | 12.96 | 6.99 | 18.59 | 7.60 | 1.90 | 2.55 | 0.60 |
| A | 84.49 | 6.09 | 0.00 | 7.34 | 0.53 | 0.35 | 1.20 | 0.00 | |
| B | 5.44 | 23.12 | 16.40 | 30.90 | 14.02 | 4.68 | 4.30 | 1.14 | |
| C | 1.31 | 21.35 | 70.00 | 2.15 | 0.20 | 0.66 | 3.74 | 0.59 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avila-Salgado, D.A.; Juárez-Hernández, A.; Lara Banda, M.; Bedolla-Jacuinde, A.; Guerra, F.V. Effects of Nb Additions and Heat Treatments on the Microstructure, Hardness and Wear Resistance of CuNiCrSiCoTiNbx High-Entropy Alloys. Entropy 2022, 24, 1195. https://doi.org/10.3390/e24091195
Avila-Salgado DA, Juárez-Hernández A, Lara Banda M, Bedolla-Jacuinde A, Guerra FV. Effects of Nb Additions and Heat Treatments on the Microstructure, Hardness and Wear Resistance of CuNiCrSiCoTiNbx High-Entropy Alloys. Entropy. 2022; 24(9):1195. https://doi.org/10.3390/e24091195
Chicago/Turabian StyleAvila-Salgado, Denis Ariel, Arturo Juárez-Hernández, María Lara Banda, Arnoldo Bedolla-Jacuinde, and Francisco V. Guerra. 2022. "Effects of Nb Additions and Heat Treatments on the Microstructure, Hardness and Wear Resistance of CuNiCrSiCoTiNbx High-Entropy Alloys" Entropy 24, no. 9: 1195. https://doi.org/10.3390/e24091195
APA StyleAvila-Salgado, D. A., Juárez-Hernández, A., Lara Banda, M., Bedolla-Jacuinde, A., & Guerra, F. V. (2022). Effects of Nb Additions and Heat Treatments on the Microstructure, Hardness and Wear Resistance of CuNiCrSiCoTiNbx High-Entropy Alloys. Entropy, 24(9), 1195. https://doi.org/10.3390/e24091195







