Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity
Abstract
:1. Introduction
1.1. Second Law Analysis Focusing the Organisms
1.2. Intermittent Fasting
1.3. Chronic Liver Diseases
1.4. Entropic Age
2. Materials and Methods
2.1. Intermittent Fasting Diet Plans
2.2. Fatty Liver Disease Diet Plans
2.3. Entropy Generation and Lifespan Estimation
2.3.1. Intermittent Fasting
2.3.2. Fatty Liver Disease
3. Results and Discussions
3.1. Intermittent Fasting Diet
3.2. NAFLD Diets
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Nomenclature
| Enthalpy of formation of the products at the body temperature (J/mole) | |
| Enthalpy of formation of the nutrients at the standard conditions (J/mole) | |
| Enthalpy of heat generation rate due to heat released from the metabolic reaction of a nutrient (J/mole) | |
| Mole number rates of the products exiting from the system (moles/s) | |
| Mole number of the reactants entering the system (moles/s) | |
| Rate of heat release from the body (J/s) | |
| Entropy of the nutrients while being uptaken by a person (J/mole K) | |
| Entropy of the excreted molecules (J/mole K) | |
| η | Metabolic efficiency of the nutrients |
| Glossary of medical and biological terms | |
| ATP | Adenosine triphosphate, an energy-carrying molecule found in the cells of organisms |
| Autopagy | A cellular maintenance process that causes the degradation of the damaged components, such as misfolded proteins or damaged organelles |
| BCAA | Branched-chain amino acid |
| BMR | Basal metabolic rate: the number of calories that the body needs to accomplish its most basic, life-sustaining functions |
| Child–Pugh Scoring System | Also known as the Child–Pugh–Turcotte Score, it predicts mortality in cirrhosis patients to guide the selection of patients who would benefit from surgery. A—good hepatic function, B—moderately impaired hepatic function, and C—advanced hepatic dysfunction |
| Cirrhosis | Appearance of the scars in the liver usually in the form of fibrous connective tissue caused by long-term liver damage |
| CR | Calorie restriction: means providing less calories to a subject |
| EEP | Excess entropy production |
| IF | Intermittent fasting: skipping meals without increasing the calories of the others |
| MUFA | Monounsaturated fatty acids |
| NAFLD | Non-alcoholic fatty liver disease: there is fat but no damage in the liver |
| NASH | Non-alcoholic steatohepatitis: inflammation of the liver with concurrent fat accumulation |
| SFA | Saturated fatty acids |
| Steatosis | A largely harmless build-up of fat in the liver cells |
| Ubiquitination | An enzymatic process that involves the bonding of a ubiquitin protein to a substrate protein |
References
- Özilgen, M.; Yılmaz, B.; Bilgin, V.A.; Yildiz, C. Organisms live at far-from-equilibrium with their surroundings while maintaining homeostasis, importing exergy and exporting entropy. Int. J. Exergy 2020, 31, 287. [Google Scholar] [CrossRef]
- Aoki, I. Entropy production in human life span: A thermodynamical measure for aging. Age 1994, 17, 29–31. [Google Scholar] [CrossRef]
- Caliskan, H. Energetic and exergetic comparison of the human body for the summer season. Energy Convers. Manag. 2013, 76, 169–176. [Google Scholar] [CrossRef]
- Mady, C.E.K.; Ferreira, M.S.; Yanagihara, J.I.; de Oliveira, S. Human body exergy analysis and the assessment of thermal comfort conditions. Int. J. Heat Mass Transf. 2014, 77, 577–584. [Google Scholar] [CrossRef]
- Mete, F.; Kilic, E.; Somay, A.; Yilmaz, B. Effects of heat stress on endocrine functions & behaviour in the pre-pubertal rat. Indian J. Med. Res. 2012, 135, 233–239. [Google Scholar] [PubMed]
- Molliet, D.S.; Mady, C.E.K. Exergy analysis of the human body to assess thermal comfort conditions: Comparison of the thermal responses of males and females. Case Stud. Therm. Eng. 2021, 25, 100972. [Google Scholar] [CrossRef]
- Özilgen, M.; Kayali, D.; Yilmaz, B.; Yavuz, Y. Entropic Assessment of Sleeping Comfort. Int. J. Thermodyn. 2022, 25, 64–73. [Google Scholar] [CrossRef]
- Öngel, M.E.; Yıldız, C.; Akpınaroğlu, C.; Yilmaz, B.; Özilgen, M. Why women may live longer than men do? A telomere-length regulated and diet-based entropic assessment. Clin. Nutr. 2020, 40, 1186–1191. [Google Scholar] [CrossRef]
- Yildiz, C.; Öngel, M.E.; Yilmaz, B.; Özilgen, M. Diet-dependent entropic assessment of athletes’ lifespan. J. Nutr. Sci. 2021, 10, E83. [Google Scholar] [CrossRef]
- Öngel, M.E.; Yildiz, C.; Yilmaz, B.; Özilgen, M. Nutrition and disease-related entropy generation in cancer. Int. J. Exergy 2021, 34, 411. [Google Scholar] [CrossRef]
- Ulu, G.; Öngel, M.E.; Yilmaz, B.; Özilgen, M. Thermodynamic Assessment of the Impact of Pregnancy and Lactation on the Longevity of Women. Int. J. Thermodyn. 2022, 25, 45–54. [Google Scholar] [CrossRef]
- Yildiz, C.; Özilgen, M. Why brain functions may deteriorate with aging: A thermodynamic evaluation. Int. J. Exergy 2022, 37, 87–101. [Google Scholar] [CrossRef]
- Cotter, T.G.; Rinella, M. Nonalcoholic Fatty Liver Disease 2020: The State of the Disease. Gastroenterology 2020, 158, 1851–1864. [Google Scholar] [CrossRef] [PubMed]
- Sepanlou, S.G.; Safiri, S.; Bisignano, C.; Ikuta, K.S.; Merat, S.; Saberifiroozi, M.; Poustchi, H.; Tsoi, D.; Colombara, D.V.; Abdoli, A.; et al. The global, regional, and national burden of cirrhosis by cause in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 245–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beard, D.A.; Qian, H. Thermodynamic-based computational profiling of cellular regulatory control in hepatocyte metabolism. Am. J. Physiol. Metab. 2005, 288, E633–E644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.H.; Joo, Y.; Kim, M.-S.; Choe, H.K.; Tong, Q.; Kwon, O. Effects of Intermittent Fasting on the Circulating Levels and Circadian Rhythms of Hormones. Endocrinol. Metab. 2021, 36, 745–756. [Google Scholar] [CrossRef]
- Patterson, R.E.; Sears, D.D. Metabolic effects of intermittent fasting. Annu. Rev. Nutr. 2017, 37, 371–393. [Google Scholar] [CrossRef] [Green Version]
- Fisher, F.M.; Maratos-Flier, E. Understanding the Physiology of FGF21. Annu. Rev. Physiol. 2016, 78, 223–241. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, B.; Gilmore, D.; Wilson, C. Inhibition of the pre-ovulatory LH surge in the rat by central noradrenergic mediation: Involvement of an anaesthetic (urethane) and opioid receptor agonists. Biogenic Amines 1996, 12, 423–435. [Google Scholar]
- Kutlu, S.; Yilmaz, B.; Canpolat, S.; Sandal, S.; Ozcan, M.; Kumru, S.; Kelestimur, H. Mu opioid modulation of oxytocin secretion in late pregnant and parturient rats. Involvement of noradrenergic neurotransmission. Neuroendocrinology 2004, 79, 197–203. [Google Scholar] [CrossRef]
- Aydin, M.; Oktar, S.; Yonden, Z.; Ozturk, O.H.; Yilmaz, B. Direct and indirect effects of kisspeptin on liver oxidant and antioxidant systems in young male rats. Cell Biochem. Funct. 2010, 28, 293–299. [Google Scholar] [CrossRef]
- Canpolat, S.; Tug, N.; Seyran, A.D.; Kumru, S.; Yilmaz, B. Effects of raloxifene and estradiol on bone turnover parameters in intact and ovariectomized rats. J. Physiol. Biochem. 2010, 66, 23–28. [Google Scholar] [CrossRef] [PubMed]
- De Cabo, R.; Mattson, M.P. Effects of intermittent fasting on health, aging, and disease. N. Engl. J. Med. 2019, 381, 2541–2551, Corrigendum in 2020, 382, 978. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Koya, D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021, 17, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Cortez-Pinto, H.; Jesus, L.; Barros, H.; Lopes, C.; Moura, M.C.; Camilo, M.E. How different is the dietary pattern in non-alcoholic steatohepatitis patients? Clin. Nutr. 2006, 25, 816–823. [Google Scholar] [CrossRef] [PubMed]
- Eslam, M.; Newsome, P.N.; Sarin, S.K.; Anstee, Q.M.; Targher, G.; Romero-Gomez, M.; Zelber-Sagi, S.; Wong, V.W.-S.; Dufour, J.-F.; Schattenberg, J.M.; et al. A new definition for metabolic dysfunction-associated fatty liver disease: An international expert consensus statement. J. Hepatol. 2020, 73, 202–209. [Google Scholar] [CrossRef]
- Kalaitzakis, E.; Bosaeus, I.; Öhman, L.; Björnsson, E. Altered postprandial glucose, insulin, leptin, and ghrelin in liver cirrhosis: Correlations with energy intake and resting energy expenditure. Am. J. Clin. Nutr. 2007, 85, 808–815. [Google Scholar] [CrossRef] [Green Version]
- Tsoris, A.; Marlar, C.A. Use of the Child Pugh Score in Liver Disease; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- AHA. Saturated Fat. 2021. Available online: https://www.heart.org/en/healthy-living/healthy-eating/eat-smart/fats/saturated-fats (accessed on 8 September 2021).
- Zivkovic, A.M.; German, J.B.; Sanyal, A.J. Comparative review of diets for the metabolic syndrome: Implications for nonalcoholic fatty liver disease. Am. J. Clin. Nutr. 2007, 86, 285–300. [Google Scholar] [CrossRef] [Green Version]
- Jornayvaz, F.R.; Jurczak, M.J.; Lee, H.-Y.; Birkenfeld, A.L.; Frederick, D.W.; Zhang, D.; Zhang, X.-M.; Samuel, V.T.; Shulman, G.I. A high-fat, ketogenic diet causes hepatic insulin resistance in mice, despite increasing energy expenditure and preventing weight gain. Am. J. Physiol. Endocrinol. Metab. 2010, 299, E808–E815. [Google Scholar] [CrossRef]
- Ouyang, X.; Cirillo, P.; Sautin, Y.; McCall, S.; Bruchette, J.L.; Diehl, A.M.; Johnson, R.J.; Abdelmalek, M.F. Fructose consumption as a risk factor for non-alcoholic fatty liver disease. J. Hepatol. 2008, 48, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Asrih, M.; Jornayvaz, F.R. Diets and nonalcoholic fatty liver disease: The good and the bad. Clin. Nutr. 2014, 33, 186–190. [Google Scholar] [CrossRef] [PubMed]
- USDA. Dietary Guidelines for Americans 2020–2025. 2020. Available online: https://www.dietaryguidelines.gov/sites/default/files/2021-03/Dietary_Guidelines_for_Americans-2020-2025.pdf (accessed on 9 September 2021).
- Musso, G.; Gambino, R.; De Michieli, F.; Cassader, M.; Rizzetto, M.; Durazzo, M.; Fagà, E.; Silli, B.; Pagano, G. Dietary habits and their relations to insulin resistance and postprandial lipemia in nonalcoholic steatohepatitis. Hepatology 2003, 37, 909–916. [Google Scholar] [CrossRef] [PubMed]
- Levy, J.R.; Clore, J.N.; Stevens, W. Dietary n-3 polyunsaturated fatty acids decrease hepatic triglycerides in Fischer 344 rats. Hepatology 2004, 39, 608–616. [Google Scholar] [CrossRef]
- Zelber-Sagi, S.; Nitzan-Kaluski, D.; Goldsmith, R.; Webb, M.; Blendis, L.; Halpern, Z.; Oren, R. Long term nutritional intake and the risk for non-alcoholic fatty liver disease (NAFLD): A population based study. J. Hepatol. 2007, 47, 711–717. [Google Scholar] [CrossRef]
- Bisschop, P.H.; De Metz, J.; Ackermans, M.T.; Endert, E.; Pijl, H.; Kuipers, F.; Meijer, A.J.; Sauerwein, H.P.; Romijn, J.A. Dietary fat content alters insulin-mediated glucose metabolism in healthy men. Am. J. Clin. Nutr. 2001, 73, 554–559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yildiz, C.; Semerciöz, A.S.; Yalçınkaya, B.H.; Ipek, T.D.; Isik, E.O.; Özilgen, M. Entropy generation and accumulation in biological systems. Int. J. Exergy 2020, 33, 444. [Google Scholar] [CrossRef]
- Hayflick, L. Biological Aging Is No Longer an Unsolved Problem. Ann. N. Y. Acad. Sci. 2007, 1100, 1–13. [Google Scholar] [CrossRef]
- Hershey, D.; Lee, W.E. Entropy, aging and death. Syst. Res. 1987, 4, 269–281. [Google Scholar] [CrossRef]
- Semerciöz, A.S.; Yılmaz, B.; Özilgen, M. Thermodynamic assessment of allocation of energy and exergy of the nutrients for the life processes during pregnancy. Br. J. Nutr. 2020, 124, 742–753. [Google Scholar] [CrossRef]
- Fine, E.J.; Feinman, R.D. Thermodynamics of weight loss diets. Nutr. Metab. 2004, 1, 15. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, K.; Silva, C. Entropy Stress and Scaling of Vital Organs over Life Span Based on Allometric Laws. Entropy 2012, 14, 2550–2577. [Google Scholar] [CrossRef]
- Kuddusi, L. Thermodynamics and life span estimation. Energy 2015, 80, 227–238. [Google Scholar] [CrossRef]
- Harris, J.A.; Benedict, F.G. A Biometric Study of Human Basal Metabolism. Proc. Natl. Acad. Sci. USA 1918, 4, 370–373. [Google Scholar] [CrossRef] [Green Version]
- NIH. Expert panel on the identification, evaluation, and treatment of overweight and obesity in adults clinical guidelines on the identification, evaluation, and treatment of overweight and obesity in adults—The evidence report. NIH Obes. Res. 1998, 6, 51S–209S. [Google Scholar]
- Teiusanu, A.; Andrei, M.; Arbanas, T.; Nicolaie, T.; Diculescu, M. Nutritional status in cirrhotic patients. Maedica 2012, 7, 284–289. [Google Scholar] [PubMed]
- Montano-Loza, A.J.; Angulo, P.; Meza-Junco, J.; Prado, C.M.M.; Sawyer, M.B.; Beaumont, C.; Esfandiari, N.; Ma, M.; Baracos, V.E. Sarcopenic obesity and myosteatosis are associated with higher mortality in patients with cirrhosis. J. Cachex-Sarcopenia Muscle 2015, 7, 126–135. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersen, T.; Gluud, C.; Franzmann, M.-B.; Christoffersen, P. Hepatic effects of dietary weight loss in morbidly obese subjects. J. Hepatol. 1991, 12, 224–229. [Google Scholar] [CrossRef]
- Plauth, M.; Cabré, E.; Riggio, O.; Assis-Camilo, M.; Pirlich, M.; Kondrup, J.; Ferenci, P.; Holm, E.; Dahl, S.V.; Müller, M.; et al. ESPEN Guidelines on Enteral Nutrition: Liver disease. Clin. Nutr. 2006, 25, 285–294. [Google Scholar] [CrossRef]
- Hanje, A.J.; Fortune, B.; Song, M.; Hill, D.; McClain, C. The Use of Selected Nutrition Supplements and Complementary and Alternative Medicine in Liver Disease. Nutr. Clin. Pract. 2006, 21, 255–272. [Google Scholar] [CrossRef] [Green Version]
- Feher, J. Quantitative Human Physiology; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar] [CrossRef]
- Silva, C.; Annamalai, K. Entropy generation and human aging: Lifespan entropy and effect of physical activity level. Entropy 2008, 10, 100–123. [Google Scholar] [CrossRef] [Green Version]
- Davidson, H.I.; Richardson, R.; Sutherland, D.; Garden, O.J. Macronutrient preference, dietary intake, and substrate oxidation among stable cirrhotic patients. Hepatology 1999, 29, 1380–1386. [Google Scholar] [CrossRef] [PubMed]
- Özilgen, M.; Sorgüven, E. Biothermodynamics; CRC Press Inc.: Boca Raton, FL, USA, 2016. [Google Scholar]
- Pinter, M.; Trauner, M.; Peck-Radosavljevic, M.; Sieghart, W. Cancer and liver cirrhosis: Implications on prognosis and management. ESMO Open 2016, 1, e000042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goodrick, C.L.; Ingram, D.K.; Reynolds, M.A.; Freeman, J.R.; Cider, N.L. Effects of Intermittent Feeding Upon Growth and Life Span in Rats. Gerontology 1982, 28, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ulu, G.; Semerciöz, A.S.; Özilgen, M. Energy storage and reuse in biological systems: Case studies. Energy Storage 2021, 3, e253. [Google Scholar] [CrossRef]
- Yang, L.; Licastro, D.; Cava, E.; Veronese, N.; Spelta, F.; Rizza, W.; Bertozzi, B.; Villareal, D.T.; Hotamisligil, G.S.; Holloszy, J.O.; et al. Long-Term Calorie Restriction Enhances Cellular Quality-Control Processes in Human Skeletal Muscle. Cell Rep. 2016, 14, 422–428. [Google Scholar] [CrossRef] [Green Version]
- Koyuncu, S.; Loureiro, R.; Lee, H.J.; Wagle, P.; Krueger, M.; Vilchez, D. Rewiring of the ubiquitinated proteome determines ageing in C. elegans. Nature 2021, 596, 285–290. [Google Scholar] [CrossRef]
- Morrow, M. Minimally invasive surgery for breast cancer. BMJ 2009, 338, b557. [Google Scholar] [CrossRef]
- Ravussin, E.; Gilmore, L.A.; Redman, L.M. Calorie Restriction in Humans. In Molecular Basis of Nutrition and Aging; Academic Press: Cambridge, MA, USA, 2016; pp. 677–692. [Google Scholar] [CrossRef]
- Mattison, J.A.; Colman, R.J.; Beasley, T.M.; Allison, D.B.; Kemnitz, J.W.; Roth, G.S.; Ingram, D.K.; Weindruch, R.; de Cabo, R.; Anderson, R.M. Caloric restriction improves health and survival of rhesus monkeys. Nat. Commun. 2017, 8, 14063. [Google Scholar] [CrossRef] [Green Version]
- Willcox, B.J.; Willcox, D.C.; Todoriki, H.; Fujiyoshi, A.; Yano, K.; He, Q.; Curb, J.D.; Suzuki, M. Caloric Restriction, the Traditional Okinawan Diet, and Healthy Aging: The Diet of the World’s Longest-Lived People and Its Potential Impact on Morbidity and Life Span. Ann. N. Y. Acad. Sci. 2007, 1114, 434–455. [Google Scholar] [CrossRef] [Green Version]
- Yıldız, C.; Yılmaz, B.; Özilgen, M. Fraction of the metabolic ageing entropy damage to a host may be flushed out by gut microbiata. Int. J. Exergy 2021, 34, 179. [Google Scholar] [CrossRef]
- Çatak, J.; Develi, A.; Sorgüven, E.; Özilgen, M.; İnal, H.S. Lifespan entropy generated by the masseter muscles during chewing: An indicator of the life expectancy? Int. J. Exergy 2015, 18, 46. [Google Scholar] [CrossRef]
- Fabbrini, E.; Sullivan, S.; Klein, S. Obesity and nonalcoholic fatty liver disease: Biochemical, metabolic, and clinical implications. Hepatology 2010, 51, 679–689. [Google Scholar] [CrossRef] [Green Version]
- Durand, F.; Valla, D. Assessment of the prognosis of cirrhosis: Child–Pugh versus MELD. J. Hepatol. 2005, 42, S100–S107. [Google Scholar] [CrossRef]
- Schneeweiss, B.; Graninger, W.; Ferenci, P.; Eichinger, S.; Grimm, G.; Schneider, B.; Laggner, A.N.; Lenz, K.; Kleinberger, G. Energy metabolism in patients with acute and chronic liver disease. Hepatology 1990, 11, 387–393. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.A.; Annamalai, K. Entropy Generation and Human Aging: Lifespan Entropy and Effect of Diet Composition and Caloric Restriction Diets. J. Thermodyn. 2009, 2009, 186723. [Google Scholar] [CrossRef] [Green Version]
- Annamalai, K. Oxygen deficient (OD) combustion and metabolism: Allometric laws of organs and Kleiber’s law from OD metabolism? Systems 2021, 9, 54. [Google Scholar] [CrossRef]
- Sinclair, D.A. Toward a unified theory of caloric restriction and longevity regulation. Mech. Ageing Dev. 2005, 126, 987–1002. [Google Scholar] [CrossRef] [PubMed]
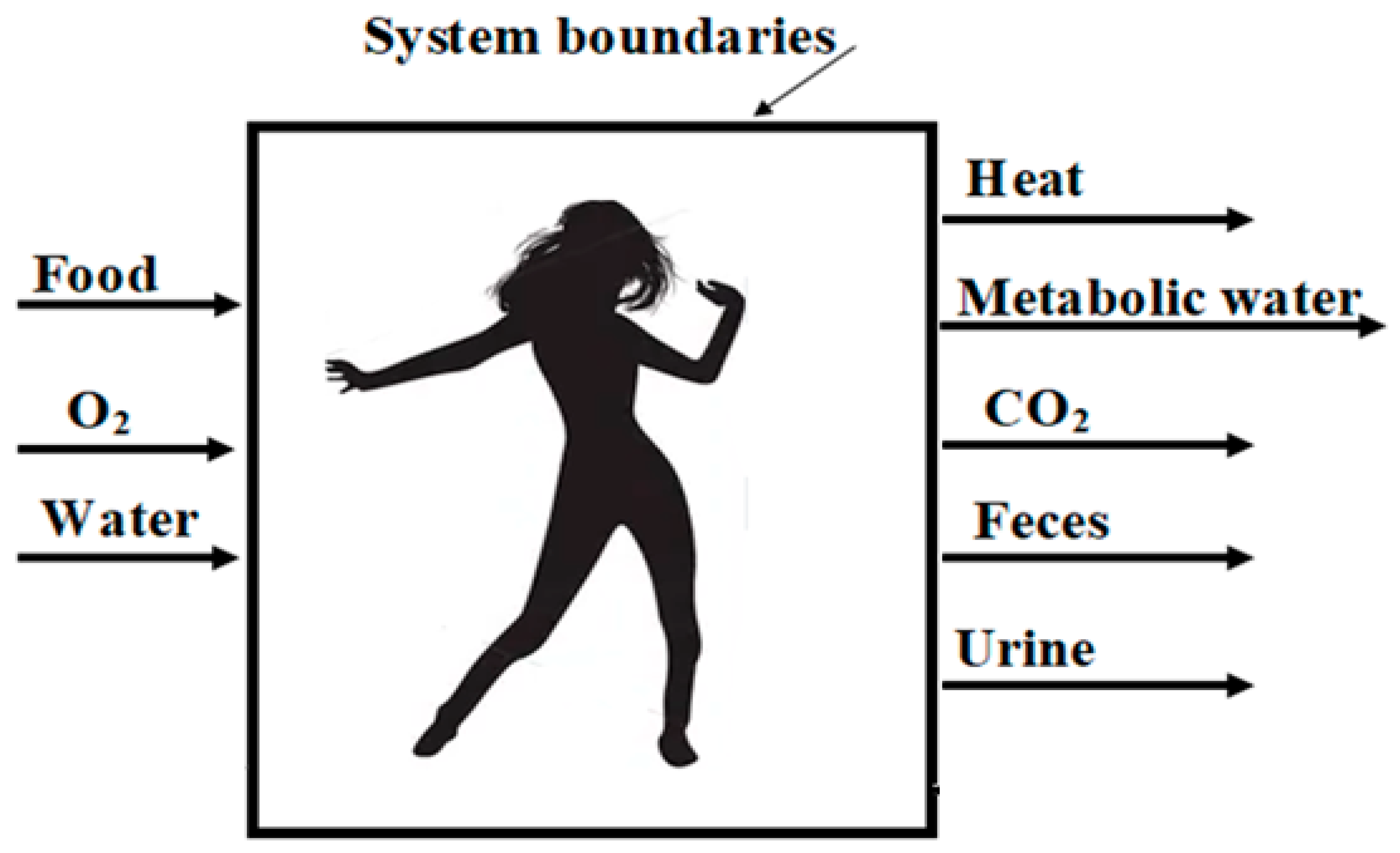
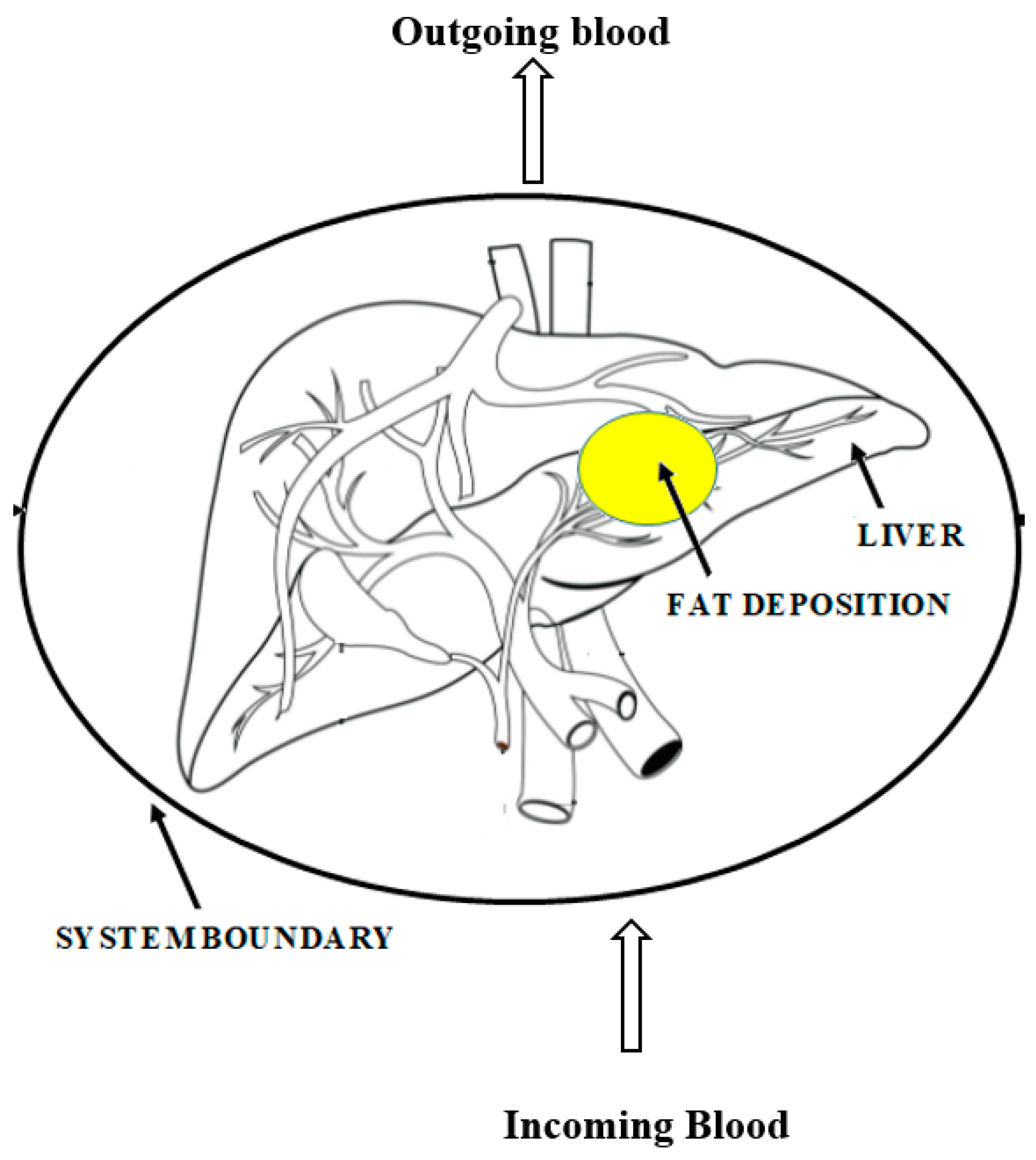
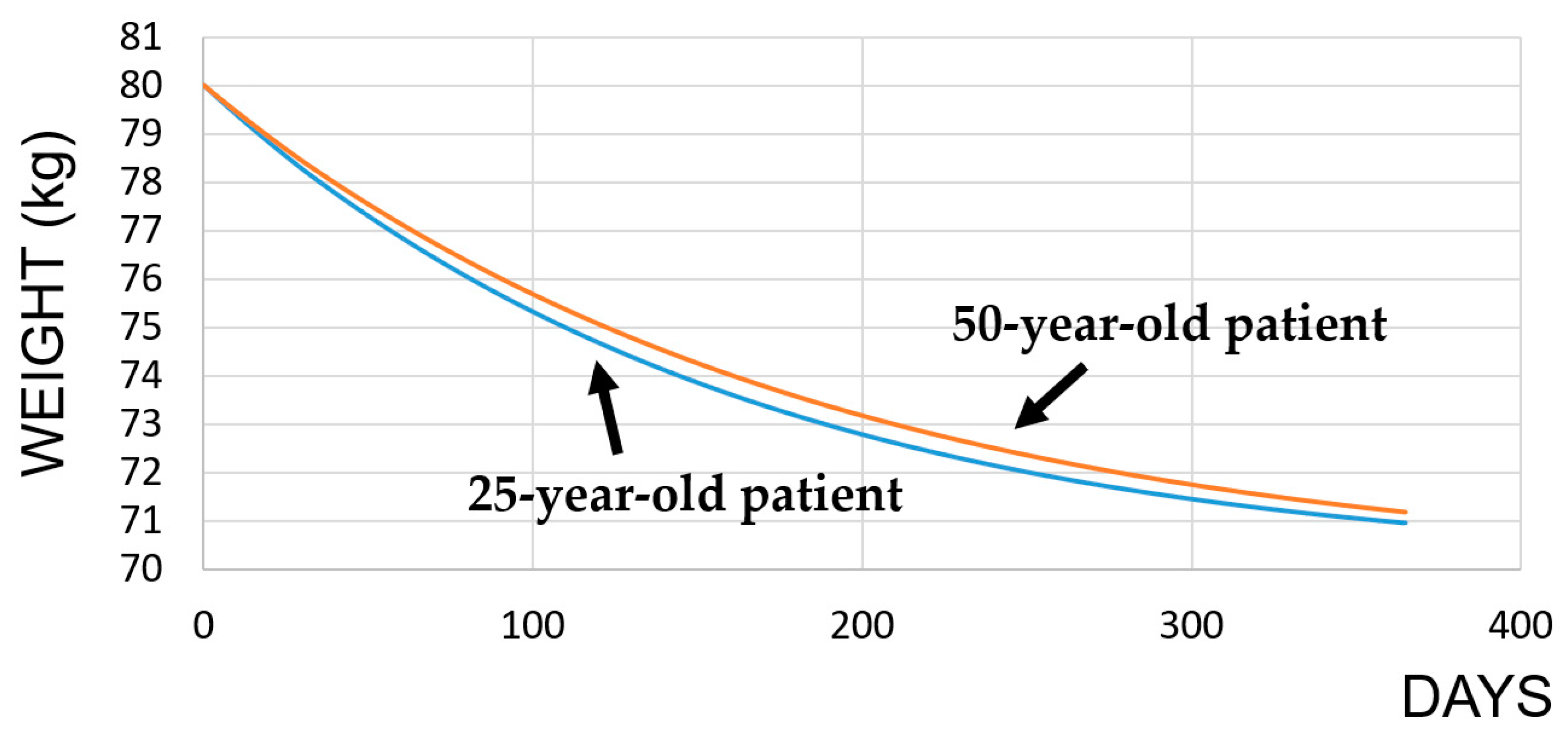
| Subjects | Initial BMR | After IF BMR |
|---|---|---|
| 25-year-old men | 1885 kcal/day | 1680 kcal/day |
| 7879 kJ/day | 7022 kJ/day | |
| 50-year-old men | 1618 kcal/day | 1478 kcal/day |
| 6763 kJ/day | 6178 kcal/day |
| Type of Diet | Calorie (kcal) | Carbohydrates (g) | Protein (g) | Lipid (g) |
|---|---|---|---|---|
| Weight-maintaining IF diet for a 25-year-old, 80 kg person | 2210 | 318 | 99 | 59 |
| Weight-maintaining IF diet for a 50-year-old, 80 kg person | 2010 | 295 | 86 | 54 |
| Weight gain IF diet for a 25-year-old, 80 kg person | 1912 | 276 | 94 | 48 |
| Weight gain IF diet for a 50-year-old, 80 kg person | 1711 | 257 | 74 | 43 |
| Weight loss IF diet for a 25-year-old, 80 kg person | 1663 | 242 | 68 | 47 |
| Weight loss IF diet for a 50-year-old, 80 kg person | 1510 | 225 | 67 | 38 |
| O2 (g/day) | H2O (g/day) | CO2 (g/day) | Urine (g/day) | Dry Feces (g/day) | |
|---|---|---|---|---|---|
| Weight-maintaining IF diet for a 25-year-old, 80 kg person | 532.9 | 257.6 | 652.0 | 894.7 | 1646.5 |
| Weight-maintaining IF diet for a 50-year-old, 80 kg person | 475.7 | 232.0 | 584.6 | 677.0 | 1856.1 |
| Weight gain diet for a 25-year-old, 80 kg person | 613.2 | 296.6 | 748.4 | 942.3 | 1601.8 |
| Weight gain diet for a 50-year-old, 80 kg person | 560.0 | 271.7 | 684.6 | 786.8 | 1751.9 |
| Weight loss IF diet for a 25-year-old, 80 kg person | 463.7 | 224.7 | 565.2 | 647.2 | 1883.6 |
| Weight loss IF diet for a 50-year-old, 80 kg person | 420.0 | 204.5 | 515.7 | 613.0 | 19,016.9 |
| Chemical | Enthalpies (h) and Entropies of the Macronutrients (s) of O2, CO2, and H2O at 1 atm and 298 K and 310 K | |||
|---|---|---|---|---|
(kJ/kmol) | s at (298 K) (kJ/kmol K) | (kJ/kmol) | s at (310 K) (kJ/kmol K) | |
| C6H12O6 (glucose) | −1260 × 103 | 212 | - | |
| C16H32O2 (palmitic acid) | −835 × 103 | 452 | - | |
| C4.57H9.03N1.27O2.25S0.046 (average of the 20 amino acids) | −385 × 103 | 1.401 × 119 | - | |
| O2 | 8682 | 218 | 220 | |
| H2O | 10,302 | 219 | ||
| CO2 | 9807 | 243 | ||
| 25-Year-Old Individual | 50-Year-Old Individual | |
|---|---|---|
| Total entropy generation until the age of 25 or 50 and BMR | 2560 kJ/kg K 7879 kJ/day | 4651 kJ/kg K 6763 kJ/day |
| Annual entropy generation due to consumption of the weight gain IF diet (kJ/kg K year) | 102.4 | 135 |
| Annual entropy generation rate due to IF weight-maintaining diet (kJ/kg K year) | 81.1 | 121 |
| Annual entropy generation rate due to weight loss IF diet (kJ/kg K year) | 74.1 | 91 |
| Estimated lifespan (years) when they continue consuming the IF weight gain diet until the end of their lifespan | 110 | 100 |
| Estimated lifespan (years) when they continue consuming IF weight-maintaining diet until the end of their lifespan | 133 | 106 |
| Estimated lifespan of the subjects if they continue consuming the weight loss IF diet until the end of their lifespan | 143 years 7022 kJ/day | 135 years 6178 kcal/day |
| Child–Pugh Scores | Child–Pugh Score A | Child–Pugh Score B | Child–Pugh Score C | Healthy Obese Person |
|---|---|---|---|---|
| kcal/day | 3000 | 3200 | 3300 | 3100 |
| Carbohydrate (g/day) | 428 | 416 | 470 | 430 |
| Protein (g/day) | 130 | 160 | 165 | 150 |
| Fat (g/day) | 92 | 110 | 79 | 98 |
| Total (g) | 650 | 686 | 714 | 687 |
| Healthy Obese Person | Child–Pugh Score A | Child–Pugh Score B | Child–Pugh Score C | |
|---|---|---|---|---|
| O2 (g/day) | 898 | 574 | 619 | 626 |
| H2O (g/day) | 428 | 294 | 307 | 320 |
| CO2 (g/day) | 1081 | 728 | 763 | 793 |
| Dry feces (g/day) | 1078 | 2326 | 2221 | 233 |
| Urine (g/day) | 1428 | 377 | 513 | 394 |
| Healthy Obese Person | Child–Pugh Score A Patient | Child–Pugh Score B Patient | Child–Pugh Score C Patient | |
|---|---|---|---|---|
| The annual entropy generation rate until age 40 (kJ/kg K year) | 119.9 | 119.9 | 119.9 | 119.9 |
| Total entropy generation in 40 years Shealthy (kJ/kg K) | 4796 | 4796 | 4796 | 4796 |
| The annual entropy generation rate until age 40 in the case of no diet change (kJ/kg K year) | 119.9 | 126.2 | 149.9 | 272.5 |
| The annual entropy generation rate after the age of 40 in the case of a change of diet (kJ/kg K year) | 122 | 76.4 | 87.2 | 98.8 |
| Expected lifespan in the case of no diet change (years) | 95 | 92 | 84 | 64 |
| Expected lifespan in the case of the change of the diet (years) | - | 127 | 116 | 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öngel, M.E.; Yildiz, C.; Başer, Ö.; Yilmaz, B.; Özilgen, M. Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity. Entropy 2023, 25, 227. https://doi.org/10.3390/e25020227
Öngel ME, Yildiz C, Başer Ö, Yilmaz B, Özilgen M. Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity. Entropy. 2023; 25(2):227. https://doi.org/10.3390/e25020227
Chicago/Turabian StyleÖngel, Melek Ece, Cennet Yildiz, Özge Başer, Bayram Yilmaz, and Mustafa Özilgen. 2023. "Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity" Entropy 25, no. 2: 227. https://doi.org/10.3390/e25020227
APA StyleÖngel, M. E., Yildiz, C., Başer, Ö., Yilmaz, B., & Özilgen, M. (2023). Thermodynamic Assessment of the Effects of Intermittent Fasting and Fatty Liver Disease Diets on Longevity. Entropy, 25(2), 227. https://doi.org/10.3390/e25020227







