2. Some Essential Background(s)
The implementation of thermodynamic principles, such as entropy and exergy, in order to improve our understanding of the functionality, efficiency, adaptation, evolution, development, variation, and selection in both biological systems in general and ecosystems specifically has been advocated for several decades now. If we accept Lotka’s seminal papers from 1922 [
2,
3] and take his presentations on this issue as a starting point, we can view his statements about organisms’
competition for free energy and
maximization of power as some of the first examples of the application of a thermodynamic interpretation to biological systems [
8]. Thus, while working on this paper, we are in fact celebrating a 100-year anniversary of this topic in the biological sciences.
Over the years, applications have occurred at almost all levels of the biological hierarchy resulting in many interesting but also, from time to time, apparently conflicting statements and findings. Unfortunately, the results and conclusions from analyses at various levels have been based on very different systems that use many different approaches to thermodynamics. The area thus ranges from mere energetic, first-law analysis, to increasingly advanced methods based on the second law. Here, we find the applications of concepts, such as entropy and exergy, and other ideas emerging from differing world views.
Energetic ways of analysis have been popular among biologists with a physiological and autecological orientation and, in most cases, concentrate on mapping how energy is taken up and invested by organisms. Odum’s and Pinkerton’s [
4] interpretation of Lotka’s
maximum power principle [
2] brings us close to the border between the two laws and thus to a possible interface (e.g., [
9]). With the introduction of the second law, which follows, among others, the ideas of Lotka around competition for free energy as sketched above, but also reflects the concepts of Schrödinger [
10], Brillouin [
11,
12], Odum [
13], Prigogine [
14,
15], Jørgensen [
16], Ulanowicz and Hannon [
17] Schneider and Kay, [
18], Weber and Depew [
19], and many others, it is now clear that the development and evolution in biological systems are not only dependent on energy quantity; but also
energy form, it’s quality, and thus the formation of entropy; i.e., dissipation. That is, energy quality is of maybe even greater importance than just mere energy.
Meanwhile, where the application of the first law from a backward perspective appears to have been almost trivial, this can hardly be said about the transfer of second law approaches, such as entropy, to biological systems in a broader sense. The problems one meets when working in the area arise from issues that are deeply rooted in physics. Here, we deal with the fact that entropy, strictly speaking, only relates to ideal gases and systems at near-equilibrium conditions. Such conditions are hard to meet since all biological systems are operating under far-from-equilibrium (FFE) conditions [
20]. Furthermore, Prigogine and coworkers’ expansion of thermodynamics to FFE conditions based on Onsager’s work [
21,
22] continues to assume a linear relationship between forces and fluxes [
23], which actually is not the case [
24].
In brief, the implementation of entropy in biology brings as an implicit consequence that one works with a concept that has only a vague definition, if any at all, under the conditions normal to biological systems [
14]. This puts an extra demand on authors in the area to explain their use of these terms with stringency and consistency.
The basic equations used for entropy, the Boltzmann equation [
25] or its extended version, the so-called Boltzmann–Gibbs equation [
26], have been widely applied at almost all levels of the biological hierarchy. One problem now becomes clear, namely that the configurational description of the various systems in terms of their respective ontological particles, which the equations are now used for, is far from the original description of possible microstates in a gas that consists merely of atoms or molecules. It cannot be treated as such, although some authors seemingly accept the metaphor [
27]. At almost all levels of the biological hierarchy, the ontological units or particles used in such entropy calculations differ greatly between levels that are often referred to as
levels of organization [
28]. This raises some questions that need to be answered. When we shift between hierarchical levels, we, at the same time, accept a shift in the ontological units used in calculations or, as we will call them here, “particles”, which come to differ between the various levels under consideration. But are we then really talking about classical, conventional entropy [
29]? Or is it “something” else? In the latter case, this “something” needs to be defined. And, in the end, are we just observing systems with similar behavior, which may present an analogous way of understanding without being the “real”, classical entropy of physics any longer? And how may observations on such systems be translated into thermodynamics and entropy? Or are we dealing with entropy at all? If not, how do our observations then relate to thermodynamics? A recent review [
1] identified some of these different uses of entropy and, in addition, a particular use of entropy that appears to depend on the background of the authors or the area of implementation; for example, biodiversity studies or comparisons of landscapes [
1].
Another problem relating to the fundamental physical understanding of the systems is whether we use entropy to describe the state of systems or whether we use it to study changes within or differences between certain systems through time and space as a consequence of flows and processes, i.e., entropy production.
The present paper examines how these different understandings and uses of entropy vary within physics and chemistry and adds up to impose a quite complex picture on biological systems. Here lies a great part of the crux of a semantic problem.
Meanwhile, the problem is not only a question of semantics. Introducing entropy as a concept in biology will affect communications even further, as many of the classical terms will change or even lose their definitions with respect to the various levels of the biological hierarchy under consideration. This is valid, especially when considering how the almost arbitrary choice of the ontological “elementary particles” used in “entropy” calculations varies and as a consequence influences any discussions about “entropy” and the respective reference levels.
We will not make any attempt to resolve all the issues in the area. In many ways, it is much too late for this since many confusing statements in the field have already been made. Usually, the reason for this confusion is an imprecise usage of terms and the associated statements about various phenomenologies arising from this usage. Therefore, we will try to indicate the sources of confusion in the field of the application of thermodynamics to biological systems in general. This is performed to raise the awareness of new readers in the area. As a more constructive contribution, we will conclude with some modest, initial proposals toward a clarifying adjustment of present terminology. This is seen as a necessary first step toward a possible resolution. We find it astonishing that this is necessary after more than 100 years of usage.
3. Sketching Entropy Controversies
As a starting point, two major issues seem to be forming the core of controversies in the area. At the core, we can identify, first, the important statements by Lotka in the papers mentioned above concerning the relationship between the size of structures, energy flows, and usage in biological systems, although the papers take a clear first-law stance and tend to ignore entropy formation and dissipation. From this an additional discussion arises, namely whether in nature there is a
final goal or
telos or whether there is at least an
orientor indicating a
direction toward an
extremum state of certain system property acting as an attractor, such as the storage, utilization, or efficiency of any of the thermodynamic functions [
30,
31,
32]. A second perspective to this point is that there also seems to be a major bifurcation between the ways in which thermodynamic concepts, such as entropy, are used. In physics and engineering, we find entropy being used both to describe the
states of systems (alone) and also to study
entropy formation together with process and path dependency. This deals with the necessity to involve such aspects as how changes in and between thermodynamic states take place, i.e., to involve process-oriented thermodynamics. Both directions have been taken and can be observed in the literature, but most often in situations where they have been applied separately. Already, at this time, we must make the remark that most probably, to fully comprehend biological and ecological systems, both approaches, “both sides of the coin”, are needed to give a truly holistic understanding of biological systems.
- (A)
What do biological systems actually do?
In ecology, the respective processes are investigated by succession theory, which tries to describe and understand the continuous unidirectional sequential change in the species composition of natural communities [
33], which is often accompanied by typical changes in ecological state variables. Based upon the initial abiotic conditions, typical sequences of species can be observed, which, under specific external conditions, can strive toward a typical climax community [
34]. Meanwhile, also at lower levels of hierarchy, we may observe similar developmental trends among the constituent components, namely the organisms’ continuous adaptation toward optimal exploitation of the resources offered by the surrounding environment.
Lotka’s papers [
2,
3] seem, somehow, to be at the crux of a debate on how biological systems (probably primarily organisms) distribute and utilize the available energy that is offered to them as outside–inwards gradients relative to the surrounding environment. Lotka [
2] states:
“In every instance considered, natural selection will so operate as to increase the total mass of the organic system, to increase the rate of circulation of matter through the system, and to increase the total energy flux through the system, so long as there is presented an un-utilized residue of matter and available energy”.
We interpret this as meaning that organisms develop to build up more biomass, which in turn increases both the inflow and outflow of energies from the system; these energies include both chemically bound transfers as opposed to destroyed dissipated energies. The fundamental importance of solar radiation in driving the ecosystems receives only little attention. The increase in biomass and flows may occur as long as useful energies are abundant:
“If sources are presented, capable of supplying available energy in excess of that actually being tapped by the entire system of living organisms, then an opportunity is furnished for suitably constituted organisms to enlarge total energy flux through the system”.
This seems implicitly to mean that the development will be heavily influenced and eventually constrained by the amounts of energy supplied to it. Likewise, he makes important statements concerning selection and evolution:
“This may be expressed by saying that natural selection tends to make the energy flux through the system a maximum, so far as compatible with the constraints to which the system is subject”.
Meanwhile:
“It is not lawful to infer immediately that evolution tends thus to make this energy flux a maximum. For in evolution two kinds of influences are at work: selecting influences and generating influences. The former selects, the latter furnish the material for selection”.
This passage clearly demonstrates an awareness of the problems of extremum behavior and probably also a special arrangement of causes between levels of hierarchy.
All in all, Lotka saw both the storages and processes and thus the changes to be of importance in being responsible for shaping the behavior, development, and evolution of biological systems. In an additional paper, he asserts that the outcome of evolutionary processes would be a consequence of the competition for free energy [
2].
- (B)
Do we talk about endpoints or just directions (orientors)?
Immediately upon presenting such views, one is forced to ask the question: Where does the development and/or evolution of biological systems end, and what will be the best indicator to tell us when this final state has been reached? In classical or conventional thermodynamics, we actually find relevant statements only in the form of references to the imperfect conversion of energy ending up in
heat loss, as first described by Carnot [
35], which led to the formulation of the second law by Clausius [
36], Boltzmann [
37], and Gibbs [
26], according to which an isolated system will develop toward an equilibrium state. Here, we have to be aware of the fact that some approaches to ecosystem theory [
38,
39] and criticize the conventional application of the physical equilibrium term in ecology because several approaches investigating ecological systems show that the exact opposite, the disequilibrium, not a balanced state, is a focal parameter of all living systems.
This conventionally comprehended equilibrium state in thermodynamics is represented in classical thermodynamics by an equiprobable distribution of its constituting components, atoms, or simple molecules. Meanwhile, some physicists see the two formulations—entropy as heat or entropy as a measure of distributions of microstates—as quite different from each other [
29]. In isolated systems, this equilibrium, over time, will assume a state of maximum entropy, a term coined by Clausius in the mid-1860s, which later gave rise to the understanding of entropy as defining the direction of time’s arrow. In the physics literature, the time it takes for a given system to return to its equilibrium state is often referred to as
relaxation time. Much of the earlier work was performed in order to understand steam engines [
35,
40], where pressure and volume differences were important factors in the process of getting work out of a system. The interpretation made on the basis of molecules that formed the core of thermodynamic interpretations of the dispersion of water/vapor particles, and pressure loss was based on the observation of the above-mentioned imperfect and irreversible conversion of energy. It is from these original considerations about the distribution of atoms and molecules that the problems emerge when transferring or reducing thermodynamic principles to other systems.
From a biological point of view, it is obvious that biological systems are far from being simple assemblages of atoms or small molecules and that they cannot be compared with steam engines either (although many mechanistic interpretations may be found in the literature). Biology is represented by systems that use other ontological units as their basis, rather than mere atoms or molecules, and they are constrained by other types of boundaries (other than adiabatic, etc.). Likewise, they are far from being in an equilibrium state where the actual situation can be represented by just assuming a random distribution of the ontological particles throughout the time-space phases of the systems.
Notwithstanding, much of the existing terminology has been established on the basis of simple isolated systems consisting of gas(es). We have thus inherited these terms as the basis for the many attempts natural scientists have made with the aim of formulating hypotheses and theories based on “thermodynamics” to gain a better understanding of the development and evolution of biological and ecological systems. In particular, the entropy concept has been found to be widely accepted as a framework for such discussions. At the same time, there has been a tendency to use the concept in quite diverse and undefined ways.
Meanwhile, the use of the term entropy has also become quite controversial, not only due to the many ways in which it can be used (on other ontological particles) but also because confusion arises from the many attempts made to establish a connection between thermodynamic entropy and the use of the same term in connection with various concepts of information, starting with Shannon [
41]. This latter conflict will be excluded from the current discussion so as not to introduce any additional and unnecessary confusion. Meanwhile, it should be noted that many works in the area place themselves exactly on this border between the two theoretical directions, e.g., [
42,
43,
44,
45]. We will try to focus on what we consider to be within the thermodynamic domain only.
- (C)
What types of systems dominate in biology?
Above, we briefly touched upon the different types of boundaries used to describe various systems, and a short clarification is now necessary. We will here apply the commonly used terminology by giving a distinction between types of thermodynamic systems; this is agreed upon by most authors and textbooks:
- (1)
Isolated systems, where no exchange of energy or matter to/from the surroundings can occur;
- (2)
Closed systems, which may exchange energy (both receive from and give back to) with the surroundings but with no exchange of matter, i.e., open to energy flows only;
- (3)
Open systems, which may exchange both energy and matter with the environment in which they are embedded.
From the discussion so far, it is clear that all systems in biology belong to the domain of open systems, which leads, as we shall see, to some distinctive and important consequences.
- (D)
What are the consequences of types of systems on the boundaries
While the above distinction between types of systems on the basis of the characteristics of their respective boundaries may seem quite trivial, the resulting consequences to the potential behavior of these systems are not. While isolated systems only possess one possible direction of development namely, that of evolving toward or returning to their equilibrium state (internal equiprobable distribution of particles), the other two types of systems can, and most likely will, take another direction. The time it takes for the system to come to (thermodynamic) equilibrium is, by definition, its “relaxation time”.
In the case of closed systems, where only energy flow from the outside is possible (but eventually may be supplied by different types of energy), it will be possible to use the inflow to structure the matter already enclosed in the system. If the structure receives a continuous energy input, it can, in principle, exist, as long as this input or gradient is maintained. But the system will not exhibit one important major feature—the capacity to grow. It may be organized in various ways according to internal and external constraints but will never increase in size.
In contrast, open systems, which are subject to gradients (flows of energy and matter in an inwards direction), will be able not only to form a structure that may grow both in terms of size but also in terms of the complexity of its organization, e.g., Ulanowicz’s distinction between “growth and development” in his first book on ascendency [
46]. Thus, the most important feature of life seems to be its propensity [
47] to increase in size or mass, expressed as a simple increase in biomass but, in addition to this, to evolve and develop new ways of investing these gains in new structures, new compartments, and new components that seem to possess a drive to exploit these gradients and their possibilities as efficiently as possible, e.g., Lotka’s papers.
- (E)
Can systems be described in terms of both states and/or processes?
We have already discussed the developmental trend of at least one indicator of state, namely the inevitable evolution toward increasing and finally maximum entropy in the distribution of particles of an isolated system. We have already commented on the aspect of the
relaxation time as well, as this expresses the rate of entropy production during the development toward equilibrium and, therefore, it might be important in future discussions; for example, concerning the importance of this term in the dynamics of biological systems with a hypothetical propensity [
47] to stay at or return to some (dynamic) equilibrium or balanced condition, which might apply at any or all of the various levels of biological hierarchy.
Meanwhile, for the other two types of systems,
closed and
open, we may be able to foretell only a little. Both may be seen as important pre-conditions for the emergence of life. Closed systems can establish structures as a result of imposed (energy) gradients alone. Most common examples come from physics, e.g., Benard cells, but also the structures arising as a result of chemical reactions, such as the Belousov–Zhabotinsky (BZ) reaction, which can be seen as belonging to this type of system. Meanwhile, for a system to establish the BZ reaction, we will need the presumption that it has been opened at a certain time prior to the reaction to allow for the provision of the right mixture of chemicals. The two systems are often mentioned in discussions in relation to dissipative structures, efficiency in the breakdown of gradients, etc. Both ways of establishing structures could have played a role in the emergence of life. The whole may be considered as emergent properties, the understanding of which was proposed by E.P. Odum [
48] to be a research strategy to increase our understanding of ecosystems.
The real difference between these two cases of structures and structures established in open systems becomes evident, as it is realized that closed systems are constrained by the gradients imposed on them either from outside or inside. Eventually, they will die out when these gradients are removed and/or dissipated, e.g., relaxation time, and will certainly not be able to exhibit growth and storage.
So, the most important difference between the systems in this context will be that living, biological systems, which belong to the class of open systems, will be able not only to persist but also to grow, building up increasingly complex molecules that take on various roles during their lifetimes, as remarked also by Lotka in the papers quoted above. They also tend to be highly dynamic systems in which energies and matter are transformed and exchanged throughout time and space.
Having recognized this difference in the properties of boundaries, together with the associated consequences to the structures, the next question arising is: is there a better way to describe the situation in open, living systems? When using entropy as a concept, are we better off describing states only (using path-independent state variables from thermodynamics) or is a process-based approach better, i.e., do we need to use changes in the state variables (usually path-dependent)?
- (F)
What special issues emerge that need attention?
A combination of the issues raised above is responsible for the emergence of most of the major items of confusion in the area. To summarize, there are basically five issues that we consider to be the most important:
- (1)
The interpretation of the Boltzmann–Gibbs equation and the shift in respective ontological units or “particles” while applying thermodynamics to different levels in the biological hierarchy;
- (2)
The confusing concept of negentropy, indicating that entropy can be negative, which is impossible, although dissipative processes in biological systems can result in them developing into states of increasing improbability/decreasing probability;
- (3)
The use of entropy to mean both state and/or a flow/process variable, as indicated in several places above and issue b) in this list;
- (4)
The use of entropy forms (or free energy, availability, exergy) as extremum principles. Should the system move toward a maximum or minimum? And a maximum/minimum of what? And should we speak about rates/acceleration or densities?
- (5)
The choice of reference level(s): when moving to levels of more complex systems, we may face a situation where situations “close to a true thermodynamic equilibrium” are not at all relevant any longer. Rather, we must define relevant “dis-equilibrium” conditions of an environment corresponding to the respective levels in the biological hierarchy;
- (6)
The use of entropy as information, a misconception introduced by Shannon under the influence of von Neumann in the works on information theory (e.g., genome calculations) has also added up to confusion in the area.
However, we have chosen to exclude the discussion of the possible connection to information entropy because this is not considered relevant to the issues we wish to raise here. Meanwhile, the situation is understandable since when looking at the similarities between the equations (isomorphism) used in various calculations of entropy, information, and biodiversity in the sense of the Shannon, Wiener, or Weaver indices [
49], it seems inevitable that the connection will not be made at some point. As a result, much confusion in the area has already emerged, and ignoring this perspective in the current discussion does not remove the already existing confusion. We will return to this point when dealing with hierarchical orientors.
In the following, we will concentrate on the use of the Boltzmann entropy as a central starting point, in principle, following the suggestions of Davison and Shiner [
50] but carrying it slightly further by making use of the expanded version in the form of the Boltzmann–Gibbs equation, which is partially isomorphic to the biodiversity index, as mentioned above. Recently, it has been recognized that this form of entropy could be more accurately named “landscape entropy” [
51], and we interpret this as an indication of an increased awareness of some of the controversies raised here.
- (a)
The classic entropy equations
Boltzmann [
25], in a search for a Hamiltonian function (
H) of thermodynamic systems, came to the conclusion that this was most likely to be proportional to the possible number of (micro)states of the system (
ω) and that the relationship would probably also be some kind of logarithmic dependency indicated by an
l, so that:
This later took the more familiar form (
W replacing ω probably due to typography):
where
SB now designates the Boltzmann entropy,
kB is the Boltzmann constant, and
W is the number of possible microstates of a given system, i.e., the number of possible configurations of “particles” that may be exhibited by that system. Most often, the natural logarithm is used. Which type of logarithm is used only matters when comparisons of systems are made and when conversions are performed easily by multiplication with a constant depending on the types to be converted.
When reformulating the equation in terms of the probability of finding a certain state of the system, we obtain:
where the symbols refer to the same conditions as Equation (2) above, and the probability of identifying one microstate 1/
W is replaced with
p.
Meanwhile, this equation is valid for a system consisting of particles indistinguishable from each other with the same probability. In this case, where we are considering different and distinguishable particles that do not share the same probability, we end up with the Gibbs version of entropy
SG:
where the index
i indicates the types of distinguishable particles.
In basic thermodynamics, one normally works with a given number of particles in the form of atoms or molecules, which belong, for instance, to the set of physical elements. In real classic thermodynamics, this might even be delimited to ideal gases.
In applying thermodynamic analysis in the form of entropy to biological systems and ecosystems, we first need to recognize that the basic ontological units of these systems are far from those of ideal gases, i.e., molecules or atoms, as used in classical thermodynamics. In fact, many physicists will insist that we no longer talk about entropy as this term does not have any relevant definition under normal conditions of life. Below, while deriving other principles of phenomenological behavior for various levels of the biological hierarchy, we will, to start, simply refer to these ontological units as “particles” using the word in its widest possible sense, sensu lato.
Another major source of confusion was introduced in the seminal paper of Schrödinger on “What is Life” [
10], where he introduced the proposal that living organisms feed on
negentropy. This formulation leaves, at least implicitly, the impression that it is possible for entropy to be negative. In spite of this term being an oxymoron, it continues to be used in literature, although what happens will be better understood as mutual constraints. The statement was believed to present a possible resolution to the observation that living organisms seem to be anything but randomly arranged systems. Rather, they exhibit an entropy state that is lower, i.e., less probable than that of a random distribution. Hence, “something” has to make it possible to lower the entropy state. The conclusion was obvious that this “something” was making it possible to change the distribution in the direction of a less randomized, less probable state, i.e., driving the living systems away from maximum entropy in a decreasing, negative direction; hence, this “driving factor” was (mis-)named negentropy.
What we are really talking about here is that for systems containing or receiving energy (e.g., paragraphs on closed vs. open systems), we can identify situations where the systems display a lower probability in their entropy distribution than equiprobability, i.e., maximum entropy. This means that:
where
Sstate is the entropy state observed and
Smax is the entropy of a state representing a random, equiprobable distribution of particles.
One measure that expresses this difference was introduced by Evans [
52], stating that:
where Ex is the exergy of a system and
T is the temperature. As
Smax is always higher than
Sstate, the expression is always positive. The difference is sometimes referred to as
thermodynamic information and due to its formulation, implies some possibly interesting links to information theory and also thermodynamics. As already stated, we will avoid discussions about this topic here. In the late 1970s, this equation was used as the starting point for the first applications of the exergy concept to ecosystems [
53,
54].
- (c)
Entropy as states of statistical distribution(s) or dissipative processes
Now, we may agree upon using the above expression (Equation (4)) as a measure of the distribution of particles in systems. Taking the classical case of an ideal gas under isolated conditions, i.e., surrounded by a boundary impenetrable to both energy and matter, the system will end up in a state where all particles are equally distributed. This is dictated by the laws of statistical dynamics, kinetic gas theory, and the models of random molecular dispersal demonstrated in basic physics. In such a system, the entropy Sstate will develop toward its maximum, Smax, a state in which all particles are randomly dispersed. Thus, these conditions at the same time refer to a state of the system where thermodynamic information and exergy (work potential), according to Equation (6), equals 0 (zero).
But as said, neither biological systems in general nor ecosystems deal with atoms and molecules as fundamental particles and basal ontological units. Even in cases of extreme reductionism, we are unlikely to consider or accept this possibility. In other words, we have left the realm or domain of classical thermodynamics. The hierarchical levels of organisms must be accepted as realistic levels to be used as the basis for calculations utilizing the previous equations. We only need a shift in the ontological particles. This has happened several times in the history of the application of thermodynamics to biological systems. Meanwhile, the results emerging from these calculations cannot be considered to be representing classical entropy anymore.
In addition, we are now dealing with “entropy” states which, from time to time, may approach some stable, steady state, or homeostatic conditions, but in the intervening time, biological systems may often exhibit periods of high dynamicity. It is of course interesting to identify what influences (constrains) these dynamics, why and how the systems move from one state to another, and how the changes are reflected by the thermodynamic functions applied during these changes. Therefore, we also need to account for the path-dependent processes between states and what exactly their role is in bringing in the resulting changes. That is the two views that imply a major difference in units by introducing a time dependency for the processes. A thermodynamic description most likely needs to understand both states as well as the flows and processes involving the formation of entropy.
- (d)
Maximum, minimum, or …?
Two major directions of
thermodynamic extremum principles can easily be identified in the scientific literature, namely a
maximum entropy [
55,
56,
57,
58,
59] or a principle of
minimum entropy [
23,
60,
61,
62,
63,
64,
65]. These two labels seem to imply a self-contradictory situation. How can entropy be maximized and minimized at the same time? Or are we dealing with a situation where shifts occur in the relative importance of these two principles in time and space? Are we discussing entropy as a state variable or as a process? And are both situations possible? Perhaps instead we need to talk about a compromise where different forms of entropies are
optimized, i.e., balanced with each other aiming at increased efficiency regarding the prevalent and respective situations that a given system is placed in, for instance with respect to its life cycle. This situation is envisaged in several works in the field [
66,
67,
68,
69].
Thus, for various reasons, the discussion seems to have taken two fundamentally different directions: one (1) that follows the idea of maximization of an entropy-like function, which seems to be founded in the works of Ziegler and later Jaynes on inference problems [
70], and another (2) based on the minimization of entropy for FFE systems, as formulated by Prigogine and co-workers [
23,
71]. Both directions seem, at present, to have reached a state where they are represented by various flavors that relate to the issues mentioned in the sections above, i.e., whether the expression under consideration is used to describe an entropy of state or the actual entropy production.
This leads to further questions within the paradigm of maximization of entropy. Many papers have pointed out that at least two or three basic understandings have been applied to these systems since they tend to refer to abbreviations or acronyms, such as first “MaxEnt” and “MEP”; both abbreviations seem to stand for
maximum entropy principle and later a third term, “MEPP”, the
maximum entropy production principle, was introduced. This is a rather simplified summary, as intermediate explanations are also found in the literature. The usage of these terms is far from being consistent between respective authors, and it is not always easy to interpret if it is real entropy, i.e., is it classical thermodynamic entropy that is referred to? Or is it actually a concept resembling entropy which is perhaps closer to information theory? Some remarks on the confusion that arises when implementing these principles can be found in the editorial by Kleidon et al. [
57], as well as in Harte’s general introduction to the use in ecology [
42]. Proceeding to the
minimization principles presented in the literature, further confusion arises. As already indicated, one finds statements that seem to be exactly opposite to the ones just presented. Most prominent is the minimum entropy production principle (which unfortunately would come to share acronyms with one of the maximization principles). For this reason, we will refer to it as the Priogine–Wiame principle [
23]. As stated, this principle refers to a rate, but unfortunately, it is not always clear whether the concept relates to an absolute, cumulative value or whether it should be normalized with respect to density, i.e., as a minimum of J/K per unit time and unit mass of the system under consideration. Regarding a corresponding principle stating a minimization of the entropy state of a system, we may identify Jørgensen and Mejer’s maximum exergy principle [
53], which was based on Evans [
52] expression (Equation (6)) for thermodynamic information:
where Ξ is exergy,
Tk is temperature in Kelvin, and
Itherm is the thermodynamic information given according to the above:
where
Sref is the probability distribution of particles at a reference state that needs to be defined and
Sstate is the observed particle distribution of the system. This value can be a cumulative expression of the major constituent elements of the ecosystem, e.g., C, H, N, O, P, and S, which for instance, as stated by Morowitz [
72], make up more than 99% of the elementary composition in biological systems. When the system moves toward a state of increasing exergy, it builds up more and more chemical elements. Thus, the deviation from maximum “entropy” increases accordingly, corresponding to a decrease in the “entropic” state of the particles.
A simplified set of acronyms is needed to illustrate the various situations described in the literature and investigate how they have been used. For this reason, we suggest that future contributions in the field should be as stringent as possible in implementing, for instance, the following suggested terms:
ME state to describe situations where the principle used must be understood as ontological particles developing toward a situation of maximum heterogeneity and equiprobable distribution;
ME prod will be used to describe situations where the system displays a maximum production entropy, an increase in exergy/energy destruction, or degradation;
JM principle, referring to Jørgensen/Mejer, will be used where the system observed, together with its ontological particles, which will tend to deviate as much as possible from thermodynamic equilibrium; for instance, thermodynamic information and exergy or a properly chosen reference state (see section f below);
PW principle, referring to Prigogine/Wiame—will refer to the principle of the minimum entropy production rate sensuo lato, not necessarily explicit time or density dependencies.
- (e)
The choice of reference levels
All biological systems exploit a gradient imposed on them from the external environment, to which they, in turn, must return all dissipations both in terms of energy (usually heat) and matter (usually small organic or inorganic molecules) [
73]. The point here is that even this external environment deviates from the concept of a surrounding reservoir used in basic thermodynamics. In other words, the real thermodynamic equilibrium is not an operational reference state with respect to life conditions, i.e., the biosphere.
Therefore, we need to define an environment where it is still possible for life to exist. Most works in this area refer to some state of an inorganic solution exemplified by conditions in an “Oparin Ocean” [
74] and have assumed, as a reference point, a hydrosphere where the first simple organic molecules have emerged, leading to a further increase in complexity and eventually to the first forms of life; this is a more rigid physicochemical approach that basically corresponds to a media where dissolved inorganic nutrients are found in the most oxidized states at (bio)geochemical equilibrium concentrations [
54,
75].
- (f)
The use of entropy in analyzing data inference problems
In general, the maximum entropy principle seems to have been applied mostly as a method based on information theory for investigating so-called inference problems in biology and ecology [
76,
77,
78]. As previously suggested, we will omit the area of entropy as information from the discussions here and bring this statement only to indicate the connections that exist between the ME-based principles and the Shannon information/entropy concept, which seems to be tight in this area. One way of circumventing this dilemma is to accept various kinds of relationships between thermodynamics and entropy, for instance through the introduction of a distinction between structural and symbolic information, as suggested by Feistel and Ebeling [
79]. However, this approach seems to physically disconnect the two entities, although it recognizes that there is a connection between information treatment and the flows of energy and entropy [
80].
- (g)
The use of thermodynamic orientors in ecosystem theory
In ecosystem theory, several extremum principles have been observed. Based upon the above-mentioned approaches of succession, information theory, network theory, and applied thermodynamics with different viewpoints have been used to work out lists of ecosystem features that function as so-called orientors [
30] or goal functions [
81], which are optimized throughout undisturbed successional dynamics (see also [
82,
83]):
Community orientors:
- -
Biodiversity
- -
Niche diversity
- -
Life span
- -
Body mass
- -
Biomass
- -
Symbiotic relations
- -
Functional redundancy
Structural orientors:
- -
Information
- -
Heterogeneity
- -
Complexity
- -
Connectedness
- -
Gradient emergence and maintenance
- -
Gradient degradation
- -
Specialization
Thermodynamic orientors:
- -
Exergy capture
- -
Exergy flows
- -
Exergy storage
- -
Total entropy production
- -
Emergy
- -
Power
- -
Ascendency
- -
Mutual information
- -
“conditional” entropy
Ecophysiological orientors:
- -
Loss reduction
- -
Nutrient retention
- -
Storage capacity
- -
Flux density and internal flows
- -
Cycling
- -
Respiration
- -
Transpiration
- -
Total system throughput
Network orientors:
- -
Indirect effects
- -
Average trophic levels
- -
Trophic chain length
- -
Residence times
- -
Network synergism
It is noteworthy that most of the descriptors used in the above = mentioned fields are directly or indirectly connected to equations with roots in “thermodynamics” sensu lato.
Of course, the production of entropy or the description of an entropic state should also be included among these orientor functions. While the state of ecosystems usually developed in an entropy-minimizing manner, we interpret this as the maintenance of disordered conditions—the succession in non-disturbed conditions increases the overall order of the system.
This long list of ecological orientors may demonstrate that the search for a correct valuation of entropy in ecology is based on fundamentals, disciplinary data, and knowledge, and the diversity of indicators may illustrate the demand to integrate developmental experience, e.g., within a basic thermodynamic theory.
6. Thermodynamics in Biological Hierarchies
We now seem to be ready to take a look at some of the many implementations of thermodynamics and the various uses of the entropy concept that can be observed in the current literature [
100]. However, we will concentrate on illustrating some of the issues raised above rather than an exhaustive literature review.
The above concepts have. over time. been applied in various ways to many different levels of the biological hierarchy. Thus, applications have demonstrated the use of. For instance. the concept of entropy over a wide range of ontological particles, e.g., [
101]. This is by no means strange since the application of the equations above to a particular focal level immediately implies that the ontological units at the level immediately below may be used for calculations; see
Table 2 [
102]. Meanwhile, this does not necessarily imply that the entropies we talk about are homologous.
Moving to the biological hierarchy, things are happening that force us to stress these issues. From traditional
hierarchy theory, we know that we are changing scales in terms of space and time [
103,
104]. In addition, we may now see that at a certain state, we move from conditions where systems are embedded in each other to a state that is a composite (the ecosystem), consisting of components that, on one hand, belong to the set of organisms but, on the other hand, display differences in size and function that result in them working on quite a variety of scales of time and space, even though they exist within the same system. This gives an additional complexity to the ecosystem.
Nevertheless, as stated in earlier papers, a shift occurs in the structure of the biological hierarchy when moving up toward the level of ecosystems. We observe a change from systems physically embedded in each other to a situation where the hierarchy is constructed by researchers and is increasingly adapted to address epistemological issues (see
Table 2). The table is constructed on the basis of the traditional view of the biological hierarchy as consisting of cells, tissues, organisms, populations, communities, and the ecosystem [
102].
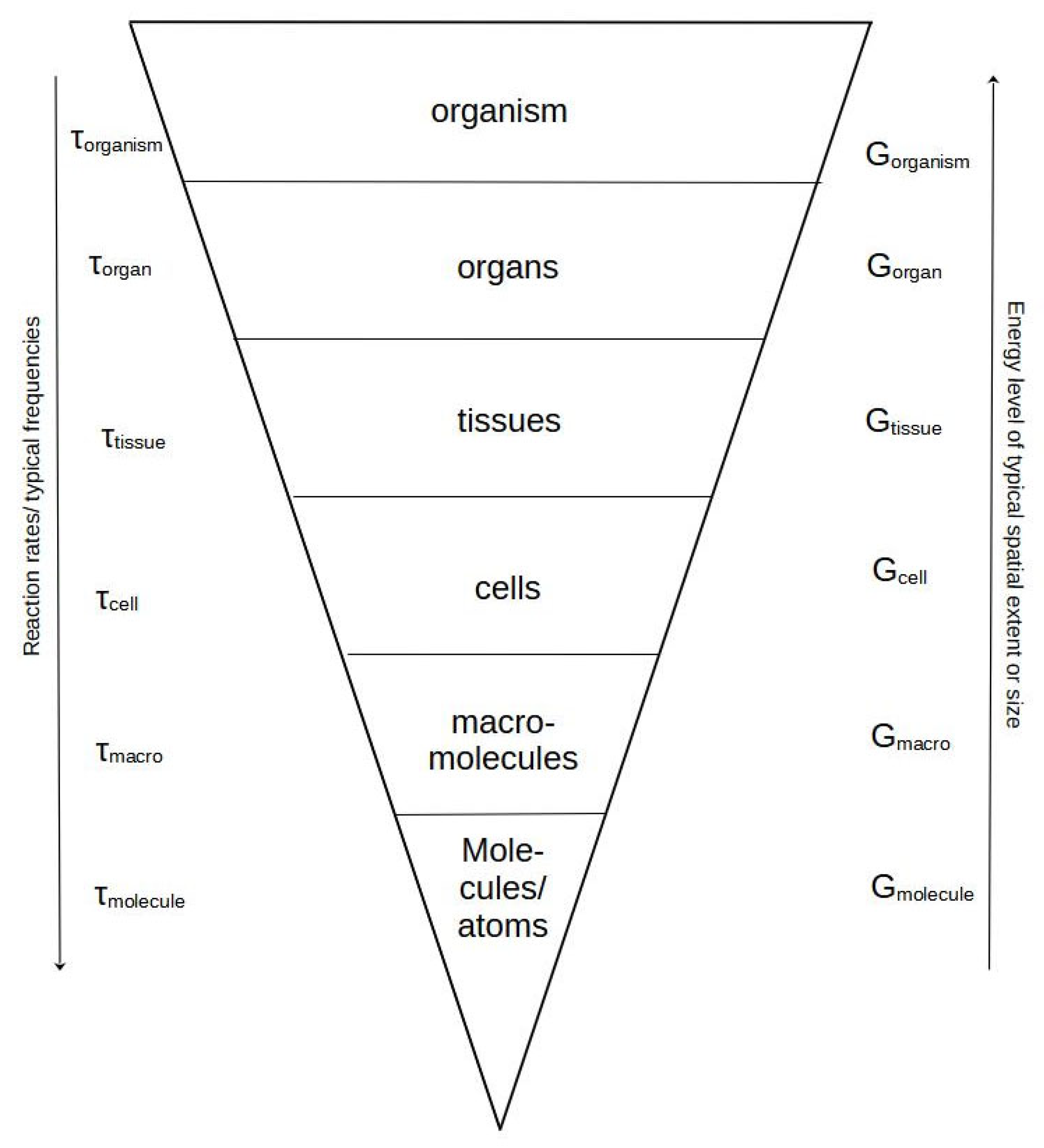
This fundamental shift takes place around the organism/population level. Up to the level of organisms, all lower levels are embedded in the upper levels and delimited with a physical boundary, e.g., membranes, connective tissues, skin, or exoskeleton. This embeddedness has the consequence that all organisms share almost the same basic functions, regulation of time dependencies, and inner biochemical relations. An attempt to illustrate this point is given in
Figure 1.
Consequently, organisms belonging to the same species share, to a large extent, the same respective functionality and physiological time scales. This relationship is considered valid to the level of (meta-) population, but with communities or societies, the situation gets more complicated.
Moving even further up the hierarchy to the ecosystem, all living constituent components or “particles” can indeed be assigned to organisms (or rather composites hereof, such as populations). However, even for a simple system, these may vary considerably in their observed time constants in accordance with hierarchy theory [
105]. Often, the entropy production rate of organisms is found to scale with the organism’s mass (M) to the power of ¾ [
105], and combining this scaling with the frequently observed M
¼ scaling of the organism’s physiological eigentime, PET (e.g., expressed by the number of heartbeats), results in a total entropy production per unit mass, which is constant over the PET and universal to all organisms. At the same time, this also represents a necessary condition for maximum efficiency, i.e., minimum overall entropy production. It must be remarked that the PET is not equal to the observed time scale.
In the construction of an ecosystem in terms of thermodynamic relations, the difference in functional role between the respective species, e.g., being an autotroph, a heterotroph, or decomposer, seems to be of greater importance than the various “roles” with their respective functions. They must all act together as a whole (system) despite their different time scale values.
In “entropic” terms, the expansion to higher levels has two core consequences related to classical discussions in ecology about time and space scale relationships. Increasingly higher levels include more and more particles that most often are dispersed and belong to an increasing number of compartments. The increase in possibilities of spatial distribution serves to create an initial increase in “entropy”. After this, specialization comes into play and may allow for the importance of efficiencies to emerge, whereby evolutionary organization and optimization principles come into play. Thus, cells are not necessarily individual cells any longer but will, for instance, through epigenesis, develop in certain directions, taking on
habits and becoming part of tissues or organs like the muscles, kidneys, liver, etc. It has been argued that such an organization maintains the lowest level of entropy [
106]. Eventually, the organs in combination form the organisms at the ultimate highest level, including all the embodied systems below (
Figure 1).
The jump to ecosystems consisting of organisms is defined as living systems in their environment [
107]. We must, according to hierarchy theory, consider organisms, populations, or functional units as our basic ontological entities. Eventually, when moving up to even higher levels, we rarely consider single individuals any longer but view them as assemblies, e.g., populations that have (almost) similar properties. Therefore, time relationships also change in their relative importance. Where an organism, according to hierarchy theory, has a longer time scale compared to all lower levels, their inclusion determines a top-down control of the system. That is, they must all adapt to or subsist within a common overall time constant.
The situation is much more complex in the ecosystem, as its development, adaptation, and evolution are dependent on sets of interconnected functional groups, usually referred to as
trophic levels, which all possess their own characteristic intrinsic time scales and, according to one hypothesis, a greater content of free energy; see
Figure 2. The situation is rarely so simple.
A certain trophic level in a network may be occupied by organisms belonging to quite different species and thus vary in functionality and spatio-temporal scales (see
Figure 3). All these different time scales must act together as a whole for the ecosystem to work properly. Together, they may serve as parts to co-determine the functional time scale of the ecosystem itself. Likewise, the role of the respective contents of available energies becomes less clear. In particular, the variable structure where upper levels are feeding on several lower levels, and the addition of a recycling element adds up to some unforeseen properties, e.g., Patten [
108].
The organisms are now expanded into an ecosystem network with increased entanglement. Thus, the previous relationships are becoming more blurred. The fastest reactions are demonstrated by decomposers of dead organic materials, and this is common to all kinds of detritus, i.e., not depending on the origins from respective compartments of the ecosystem. At the same time, the time and energy dependencies become difficult to explain when the upper levels are interacting with several of the lower levels, e.g., using them as feed. At the same time, this type of diagram reveals nothing about any regulations, cybernetics, or semiotics, for instance as a result of competition or communicative processes.
The basic processes at all of the forthcoming scales seem to be rather similar. Additionally, due to external flows of exergy inputs and entropy outputs, we need an autocatalytic sequence of chemical reactions, which produce a certain meta-stability of the single compounds. Investing in these reactions will provide some self-organized “lifespan” for the attained structure and will produce some entropic decay, which is “leaked” and has to be absorbed by the environment.
As a system develops more and more complex reaction networks through time, it will become more statistically ordered. That process is accelerated by the creation of gradients and organized inclinations between locations of low and high concentrations along a certain spatial distance. These gradients are found to gain a certain meta-stability already in the non-living appearances of microspheres and coacervates (Oparin). Their functional reliability will increase with the assistance of the above-mentioned characteristics of living systems. The gradients are responsible for an efficient specialization by defining certain process spaces, organizing the flows between these units, and maintaining their structures even over multi-generational time horizons.
Typical biological expressions of gradients are the organelles at the cell level, epithelia, mucous membranes, or the skins of organisms. The transition areas of ecotones, boundary layers between ecosystem compartments, or the frontiers between the subsystems of landscapes are ecological gradients. Additional gradients are created by microclimatic distinctions and by the accumulation of chemical compositions, e.g., within phyto-mass or along the enormous concentration variations of the soils.
Each of these environmental gradients operates at a certain spatio-temporal scale. Hierarchy theory pronounces the combination of spatial extensions and temporal rhythms: broad-scale units with big spatial sizes operate at relatively low frequencies under steady-state conditions. For example, the modification of geological features needs a long time and produces relatively uniform patterns. On the other hand, small-scale units, like the microflora of a forest, are small in extent and display fast dynamics. The most interesting feature about these scale interrelations is that the small units have historically created the bigger process bundles, and as they have been produced, the broad-scale holons sensu Koestler [
109,
110] provide constraints for the initial small-scale processes, restricting their degrees of freedom, i.e., constraining the system for example through mutual information [
91,
111,
112]. The two mentioned differ from other types of network complexity studies that are mainly qualitative, e.g., [
113], by being quantitatively founded in either energetic or material flows.
Coming back to our gradient viewpoint, it can be stated that in all self-organized systems, an input of exergy, e.g., solar radiation in the case of our ecosystems, is used to build up a complex system of internal gradients [
38,
39]. The resulting concentration profiles can be understood as components of internal order: they are surmounting the initial, high entropic, and equal distribution patterns of the systems, thus increasing their exergy storage. Normally this creation of gradients is a long-lasting, long-term process. It is based upon several single reactions, steps, and activities, which are accompanied by the slow development of ecological successions.
At the same time, the maintenance of the stabilized interwoven autocatalytic cycles leads to an increasing energy demand, which can be detected by an increasing entropy production. This output process can be observed, e.g., by measuring CO2 emissions, the evapotranspiration of water, or nutrient loss by seepage and erosion. Those degrading processes can occur very rapidly and with spontaneously high magnitudes and amplitudes if the system is approaching instability. But high process rates of entropy production are also necessary to keep a very complex structure alive. Therefore, the discussed form of entropy production can be used both as an indicator for the conservation of complexity and as an indicator of functional vulnerabilities.
Another interesting aspect of these eco-thermodynamic theories arises from the fact that the ecological target state is not the equilibrium, as required by all the basic physical comprehensions of entropy, but is at a disequilibrium. The more complex the gradients of a developing system are, the smaller will be the uniformity and similarity of the subsystems. Ecological succession, therefore, seems to reduce the embodied entropy of ecosystems, while entropy export is supported, as well as exergy storage.
In the following sections, we will attempt to deal with specific aspects of various levels found in current literature.
Biological cells represent the level of integration where we are closest to understanding the system on the basis of thermodynamics alone, for instance by applying a strictly thermo-chemical viewpoint. Several attempts can be identified where cells have been viewed as factories or machines with a network of processes consisting of possible conversions between both simple and more complex organic molecules. The processes are often separated functionally in the organelles of the cell. The conversions are taking place in accordance with free energy and supplies of ATP. This information may be enough for understanding the basic functions of the cell, but it is not enough to understand life. This problem may be illustrated by papers where thermodynamics have been coupled to explanations of the emergence and evolution of life [
18,
114,
115,
116]. Cells are close to the simplest biological systems we have today, but some protobiological subsystems have probably existed. Still, cells are FFE systems, and this already triggers considerations and questions as to whether the classic concept of entropy applies under such “simple” conditions.
Within the cells, processes take place in organelles, which make up the sub-level components of the cells. Their very existence makes it possible for further ordering the processes so that specific processes that may not coexist can take place in separate organelles. The reasons for this spatial separation range from mere physicochemical considerations to a necessary separation of processes that would have too high a risk of interfering with each other or are not able to take place within the same compartment, e.g., in some processes, oxygen is necessary, while it is toxic to others. This compartmentalization of subsystems initially leads to an increase in “entropy” but may later serve to open possibilities for a further separation of processes. In the end, events involving compartmentalization tend to further increase complexity at the cellular level. At the same time, it seems that this tendency is accompanied by a decrease in entropy formation [
117,
118].
Eventually, a cell is not just a cell and, even at this simple level, we find a few basic differences that are important to our understanding of the processes going on at higher levels in the biological hierarchy. We must point out here the role of autotrophic components. In the widest sense, organisms are, in principle, able to live on their own, either using light as a primary energy source (photoautotrophs) or obtaining primary energy from materials and chemical compounds (chemoautotrophs). In both cases, oxidants are needed to synthesize the organic molecules necessary to ensure life and the existence of the cell or (simple) organism. Organic molecules are easily described by the free energy content (usually ΔG). The more complex the molecule, the higher its free energy, and the more the molecule deviates from equilibrium or another reference state. This is also reflected in the determination of Gibb’s free Energy from the calculation of equilibrium constants in thermochemistry.
Due to the restricted space, the thermodynamic relationships in the cells are difficult to measure. Most studies have been carried out using models of biochemical cycling of the systems in order to understand the macroscopic functions. Meanwhile, there is a risk that such models, for instance the one presented by Demirel [
119], will be too reductionist to give us a simple overview in terms of a holistic thermodynamic balance.
- (b)
Collection of cells, tissues, and organs “sensu lato”
Although some mono-cellular organisms (e.g., phytoplankton) are abundant (sometimes as colonies), life soon developed into multicellular organisms both in the autotrophic and heterotrophic branches of the evolutionary tree. Some major differences can be observed if we consider mono- versus multicellular organisms. The difference is most likely to be found in the way the necessary material inputs of nutrients and respiratory elements to be used as oxidizers are supplied to the respective cells, i.e., in the relations with the external environment. In the first case, matter, often in the form of simpler molecules, is supplied through the relatively simple processes of diffusion or phagocytosis. The principles behind the organization of internal functions are surprisingly similar. Early in the development of multicellular organisms, cells became differentiated to carry out different higher-level functions, forming tissues and developing further into digestive tracts, excretory organs, transporting “organs”, etc. These “organs” have been developed and refined during evolution throughout the animal and plant kingdom.
The thermodynamic effect of this specialization has only been analyzed in very few cases, and research studies using modeling seem inconclusive in this context [
120]. In more complex organisms, the organs appear as highly structured and specialized tissues with individual functional contributions to the life of the organism, which at first glance may appear to be costly in a thermodynamic sense. However, it is only meaningful to evaluate their true value at the next level of complexity, namely the organism.
Organisms represent the ultimate level of nested systems, i.e., where all lower levels are somehow included or physically embedded in the
focal level. All basal units are considered to belong to a class or set of organisms. One focal level n has the elements from level n-1 as ontological units. (
Figure 1). Thus, at this point, the physically embedded type of scalar hierarchy ends, and we as observers need to construct the hierarchical levels and their respective ontological components, which we consider making up the system in accordance with other principles that we need to define ourselves.
The function of the individual organisms, also in the thermodynamic sense, is for all types of organisms determined by the dynamics of the inner components, the organs. Whatever the type of organism (e.g., autotroph vs. heterotroph, homeotherm vs. heterotherm), these parts are stated by many authors to interact and eventually reach a balance between the organism and its environment, which is often referred to as homeostasis [
121]. Most likely, this overall state is established as a consequence of an average time scale, including the consequences of limiting functions similar to
Liebig’s law, which hypothetically implies that the slowest reactions of the systems of the hierarchy below the focal level will determine the overall rate of the organism.
Meanwhile, in a thermodynamic sense, the entropy arising from all metabolic processes ends up as heat and/or excretory products. Both may be seen as dissipations, either of energy or matter, and need to be exported from the organism, passing over its boundaries and eventually ending up in the environment.
Taking into consideration the energetic balances of organisms as presented in (eco-)physiological textbooks and combining this with Odum and Pinkerton’s considerations on the maximization of power [
4], the picture becomes increasingly complicated. When calculating thermodynamic balances, we really need to know which state of a given organism’s life we are working on.
One generally observed trend goes in parallel with productivity: organisms tend to increase their entropy production during epigenesis at a relatively high rate while they are young. Subsequently, the rate of entropy formation decreases [
69,
122]. The same seems to be the case when considering the relative complexity of organisms in the biological hierarchy, as the specific entropy is found to decrease with the level in the hierarchy from yeast to birds [
123].
While the maximum entropy principles are often argued to be valid also for organisms, the conclusions are often drawn from the fact that larger organisms have a higher dissipation. This is in accordance with Lotka’s descriptions, where larger and more complex structures exhibit a higher throughput. Meanwhile, the previous authors seem to confirm that even though the dissipation is larger, it happens at a lower cost per unit, in short with an increasing efficiency. The findings of Vallino and Huber [
124] that the microbial populations in a meromictic pond develop according to the principle of maximum entropy is equivalent to the results of Ulanowicz [
125,
126], where the recycling component is allowed to have higher dissipation than the average of the component organisms and yet contributes to an increase in the
ascendency of the system.
The relatively new discipline of dynamic energy budget modeling for organisms may open new possibilities for thermodynamic analysis at the organism level; this will be interesting to follow in the future [
127]. The same is valid if we consider applying recent findings from the relatively new discipline of bio-semiotics [
128] or even ecosystem semiotics [
129]; this consists of including the role of communication, in its widest sense, among constituent organisms and the role of this communication in shaping the thermodynamic properties of the upper-level systems. For instance, investment in pheromone emissions has been found to facilitate foraging, “maximizing energy gains”, and increasing efficiency toward stabilized conditions [
130].
- (d)
Collections of organisms—populations and communities
The next levels of the biological hierarchy met in ecology are the population and community levels. Populations are collections of organisms usually belonging to the same species, whereas a community refers to a functional grouping, e.g., a microbial community, plankton community, plant community, and so forth. Both serve to illustrate the consequence of taking thermodynamics to a level where it is necessary for the observer to construct the boundaries of the system.
The boundary of a population may be quite virtual but is often defined in bio-geographical terms. This means that a boundary depends on an area that is normally exploited by the organisms. No need to say that this is much easier to define for plants than animals. In either case, the dynamics are determined by the intrinsic characteristics of the organisms, their life cycles, and physiological time scales.
It follows that the concept of communities is much more complex and may not be very useful for a thermodynamic approach. First, the fact that the constituent organisms at this “level” do not necessarily belong to the same species introduces multiple possible variations in their respective time constants. Second, they do not necessarily share exactly the same functions and hence may act differently with respect to the exploitation of both matter and energy gradients. Taking the example of microbial communities often mentioned in the literature, for a given soil or sediment, we will experience a set of quite different oxidizers and corresponding (inorganic) nutrients, as well as dominant processes with corresponding differences in energy consumption and dissipations.
Much of this complexity is transferred to the next level of hierarchy—the ecosystem. The question is whether we can assume a general trend from cells to ecosystems at the slowest physiological time at lower levels of hierarchy, which dictates recycling and hence turnover/development at the upper levels (and the ecological time scale-related version of the Liebig laws)
What happens in a thermodynamic sense when organisms of a certain species gather into populations or communities? Does this new structure simply act as an additive feature so that entropy production can simply be summed over the number of organisms, or does it present a relative increase or decrease in entropy production due to additional emergent properties?
The ecosystem level differs since by definition it is a composite of organisms, populations, and abiotic entities, which may all vary considerably with respect to their time constants and space scales. The variation exhibited is now likely to be even larger than in the above-mentioned example of communities. In addition to simple bio-geochemical functions, we have to add the perspective of the ecosystem’s food chain or network [
131]. According to van Dyne [
102], “the functions of an ecosystem include transformation, circulation and accumulation of matter and flow of energy through the medium of living organisms and their activities and through natural physical processes”. All in all, it seems feasible to apply a thermodynamic viewpoint to interpret the behavior of ecosystems. One question left is whether we really need to discussions if the ecosystem is a “superorganism” or if it is just a sum of individual behaviors [
132,
133]. This issue has often been dealt with under discussions about the emergent properties of ecosystems. Such properties appear already at lower levels, and their importance only seems to increase with complexity.
The most common way of constructing a hierarchical view is to concentrate on the pattern given by “who eats who”—usually referred to as the
trophic chain. This all starts at the level of the primary producers and continues through herbivores, primary and secondary carnivores, etc., forming a functional, scalar hierarchy where each upper level feeds on the level immediately below. In some rare cases, the ecosystems are so simple that they are composed of a single trophic chain, but usually, they consist of more or less entangled networks, as already pointed out by Lindemann [
131]. One focal level feeds may even feed on several of the levels below. In addition, depending on the respective time scales of the organisms as they die off, they will enter one or several pools of dead organic matter (detritus) and, in association with these pools, we will find decomposers (bacteria) whose function is to break down the dead matter and thus ensure the circularity/closedness of the system.
In all cases, in thermodynamic terms, the ecosystem must be considered to be an open system, as it needs energy either in the form of (1) solar radiation or (2) bound in matter, or most often a combination of the two, to exist. In both cases, they are constrained by this energy; in the first case by energy captured by photoautotrophs through photosynthesis and in the second by external supplies of allochthonous material or the internal recycling of nutrients made a possible detrital component.
Ecosystems have been analyzed from almost all types of second-law thermodynamic perspectives including entropy [
134,
135,
136], maximum entropy and maximum entropy production [
137,
138], minimum entropy [
139] and exergy [
53,
54], and eco-exergy [
81]. The latter perspective includes the suggestion of integration with a complexity measure of the ecosystem components and thus represents a possible link to information and energy-based indicators.
While in basic physics, gas molecules are used as basic units, we need other basic particles when we move out of the organism scale. Of course, it is easy to say that we need to account for all the organisms as units. When doing so, we have to be aware (i) that viruses and bacteria belong in this class, and we do not have sufficient knowledge about their appearance, activity, and structure of their community. In addition to this point, real ecosystem comprehension must also account for (ii) the abiotic processes, the flows, storages, and effects of energy, water, and matter transitions. If all of these components are considered in a holistic manner, we will find an enormous complexity of elements, subsystems, and scales, which hinders us from defining the “particles” or discovering an easily understandable and measurable common dimension. Furthermore, we can arrange the collection of basic units on the basis of functional features, e.g., referring to the focal pools and flows of water, energy, carbon, and nutrients. In that respect, the particles would also include soil horizons, groundwater storages, or specific microbial biomasses and soil organic matter (SOM). A holistic approach must integrate all these functional aspects, as well as the structural subsystems discussed above.
10. Suggestions for Connections and Possible Initial Steps toward Resolution
When evaluating the above attempts to analyze the wide range of applications of the concept of entropy, it must be concluded that the term does not have a uniform, unambiguous usage. In general, most of the problems can be reduced to questions of a usage describing states versus processes combined with situations of stability and linear–nonlinear dependencies between forces and flows. All of this leads to problems when analyzing biological systems from a thermodynamic angle. In the following, we assume that it will be possible to resolve questions about how far-from-equilibrium we can consider entropy to be valid and examine linear vs. nonlinear dependency.
Firstly, many applications refer to physical systems, which may be treated as (quasi-) isolated or closed systems. As a result, the treatments often involve exchanges of energy with the environment and do not involve exchanges of material. This is well-illustrated by the classical equation from Prigogine that is often used by scientists to describe the entropy balance of systems both from the direction of maximum or minimum entropy production, i.e.,
where
diS and
deS describe the internally formed entropy and entropy exchanges with the surroundings, respectively, and
dtotS is the universal change in entropy, which must be positive. The problems in describing a negative change in entropy with the environment have been discussed above. All in all, this equation ignores the results of the processes, which may be valid in isolated and closed systems where the emergence of dissipative structures only exists as the result of energetic gradients imposed on the system, i.e., the structure disappears soon after the disappearance or exhaustion of the gradient. It is clear that the equation is insufficient when it comes to describing an open system where it is possible to build up a material structure that lasts for a longer time.
Secondly, therefore, we need a description that accepts that the system is in fact an open one, receiving energy in the form of matter (chemical energy). At some places in the discussions by Lotka [
2,
3] on the energetic relations between structures, it is possible to identify a partial identification and possible solution to the problem, in which a unifying principle must include at least three elements: (a) the driving power, i.e., input of energy, (b) the build-up of structure (measured as energy deviation from a reference state), and (c) the dissipations by metabolization of energy (exergy destruction) or export to other compartments of the system (see
Figure 4).
At the same time, it would be desirable to couple this description to some basic (first-law) energy balances as normally used in physiology but reformulated in terms of exergies, e.g.:
where
Eximported covers import of radiative fluxes by plants and ingestion in animals and the digestion of matter by bacteria,
Exstored is represented by both the growth of tissues and reproductive investments,
Exrespired is energy loss (dissipation) through any metabolic pathway (anabolism, catabolism),
Exexcreted is loss through matter (exchange of solutes), and
Exdefecated is the loss of the undigested part of ingestion (only animals). In this way, the traditional formulation has been adapted to cover both plants and animals, as well as simpler organisms.
When compared to Lotka’s model, Eximported is comparable to the maximum power gradient available to the system, and Exstored, over time, represents the build-up of a physical structure (biomass), including reproductive investments. The remaining elements represent dissipative pathways, where Exrespired can be considered to represent the costs of building up and/or maintaining the structure.
Now, it becomes important how we estimate the growth in structure. One possible way goes through Evans’ [
52] equation (Equation (6)). From this, we could estimate a build-up of biomass (structure) over time from t1 to t2 by replacing the
Sstate in the Equation with the entropy states at either of the two times, and refer to them as
Sstate,1 and
Sstate,2, respectively. In this situation, the change in structure could be estimated by a change in Exergy,
Ex1,2 as:
where
Sref is described by an appropriately chosen reference situation and the system is in thermal balance with the surroundings. It is assumed that the state of the system is continuously evolving toward less entropy, and thus the difference in the last bracket is positive. When seasonal variations are considered, there is a high possibility for ecosystems that neither of the latter two assumptions hold.
The equation could be translated into differences in free energies (availability) following the layout given by Lotka. When combining the two equations, it becomes clear that it is possible for a system’s state entropy to decrease while the sum of the dissipative losses derived from the exergy equation still ensures that the second law holds.
It should be noted that the above equations neither mention nor assume the minimization or maximization of any relationships, but rather deal with the evolutionary trends (propensities) of a system, so they describe the directional tendency of the system, which is given by its intrinsic possibilities (e.g., adaptation) together with the prevailing situations of the system’s environment. Eventually, it has been suggested that the two directions are “different viewpoints of the same aspect: the first (minimization, authors’ comment) is related to the system, while the second (maximization, authors’ comment) is related to the interaction between the system and the environment” [
149]. However, this statement does not necessarily imply an optimal use of resources.
In fact, several authors mention the issue of the application of extremum or optimum principles in biological or ecological systems. In most cases, they point to the constraints of the systems, both internal as well as external, which, when taken together, seemingly prevent the systems from reaching such extreme states. Rather, the state achieved will represent some sort of compromise. So, the optimal path, as indicated in
Figure 4, will be followed as closely as possible in accordance with both internal and external constraints. This is probably an internal defensive mechanism developed to counteract the typical properties of FFE systems. According to Prigogine [
14], “Far from equilibrium, systems enter into the nonlinear range and display a multiplicity of solutions to the equations describing their evolution”. He continues his remarks by stating that the systems become sensitive to fluctuations and will bifurcate to states, which may not be predicted from deterministic equations [
14].
Nevertheless, the vast majority of the limits to development belong either to the input of available energy or the way in which energy transformation takes place and how efficiently it is used. The actual evolution and development of the system is the result of the dialectic interactions between the two. We should, therefore, rather talk about a Balanced Exergy Entropy Principle (BEEP), where systems tend to avoid going over the ”edge of chaos”. Continuous refinement and fine-tuning of the function in ecosystems are known potentially to lead to crashes (e.g., ascendency studies) or breakdown as a consequence of the system’s lack of flexibility and passivity due to the high costs of complexity, which leads to hypothetically repetitive Holling cycling [
150]. Such destructive dynamics would rapidly increase entropy production and reduce exergy storage, a situation that is not desirable. Therefore, ecologists can be happy that we seem to observe far fewer discontinuities than we might expect.
Coming back to our initial questions, we can finally summarize the discussions within the following points:
- (1)
The use of the entropy concept, in general, has been continuously introduced in a wide range of areas of natural sciences, and much confusion has been introduced with these multiple adoptions. The reason for this disorientation is the produced mix of viewpoints of the disciplines from which different aspects of entropies have been derived, e.g., mathematics, statistics, physics, engineering and biology, or ecology. An additional reason for the “entropy in comprehending entropy” arises from the different types of systems where different varieties of the concept have been used. That diversity of systems is highly correlated with a diversity of scales, and we have seen that the outcome of entropy analyses can be extremely different if different levels of the analytical hierarchy are used.
- (2)
One major distinction between the various uses can be derived from the basic starting points of the analyses. On one hand, we find entropy comprehensions, which, in their basis, are related to the distribution of ontological particles of the system and thus potentially describe the state properties. On the other hand, we find entropy concepts that are more closely related to material and energetic flows and conversions, and thus are describing the dissipative processes of the system.
- (3)
As ontological particles vary throughout the reductionist hierarchy, it must be concluded that even if the phenomenological behaviors are similar, the entropies are not the same. Hence, we need to be very careful when describing such entropies, in particular ensuring that a clear definition is given and that the basic conceptual understanding used as described above is clearly described.
- (4)
To each entropy type, a new name of classification should be given. Together with this, a consistent set of internal relations and constraints need to be described, and proper reference levels ought to be harmonized with respect to both types of entropies. Also, the organizational level of the respective system has to be fixed and delineated.
- (5)
Two thermodynamic extremum principles are often used, namely either a maximum or a minimum entropy applied to biological systems. These views, which seem to be totally contradictory at first glance, stem from the differentiated starting points of structural versus functional entropy. Are we looking statically at the information-related distribution patterns of parts or are we observing the production of heat and wasted energy in systems of energy and matter flows? Both approaches also utilize different time scales, and the outcome of an undisturbed development can be extremely different.
- (6)
At first glance, these results seem incompatible but introducing clearer definitions, as mentioned in #2, will be of great benefit. Through the clarification of the distinction between imposing a state view and a process view upon the systems, it could be demonstrated that it is possible to give a proposal for an initial merging of the extremes. What exactly happens is most likely not a maximization or minimization of a resulting state. Rather, it is likely to be a compromise, and an optimal stable state emerging from the internal and external constraints imposed on the respective system level.
As a result of these discussions, we can in fact ask for more terminological strictness for a higher accuracy in describing the starting points of argumentations and more detailed characteristics of the utilized entropy type, including denominations of the investigated scales. In the forthcoming steps, the interesting task of integrating the approaches within a holistic model, which is connecting structures and functions, can be developed further. From an ecological viewpoint, such a unified approach is urgently needed and extremely relevant if we observe the extreme entropy production of our societies and economies, which are now modifying the climatic constraints of our living conditions and the entropy of several spatial and informational patterns.