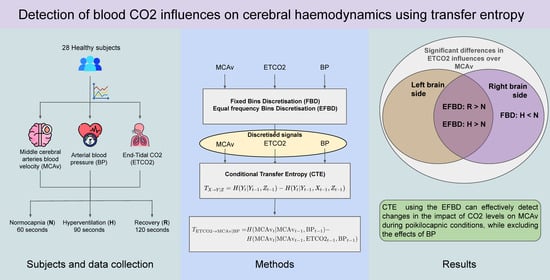
Detection of Blood CO2 Influences on Cerebral Hemodynamics Using Transfer Entropy
Abstract
1. Introduction
2. Materials and Methods
2.1. Subjects and Signals
2.2. Transfer Entropy
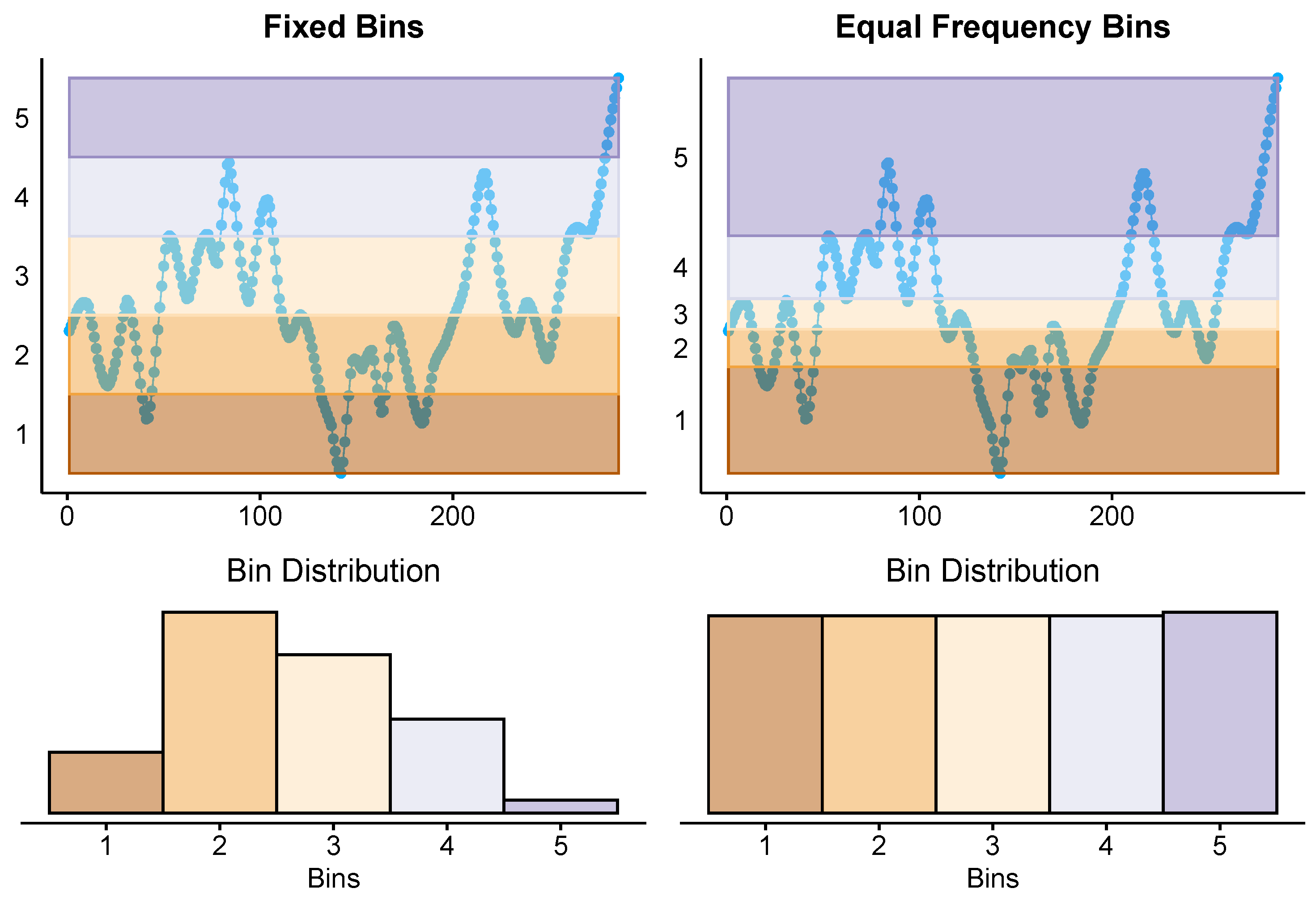
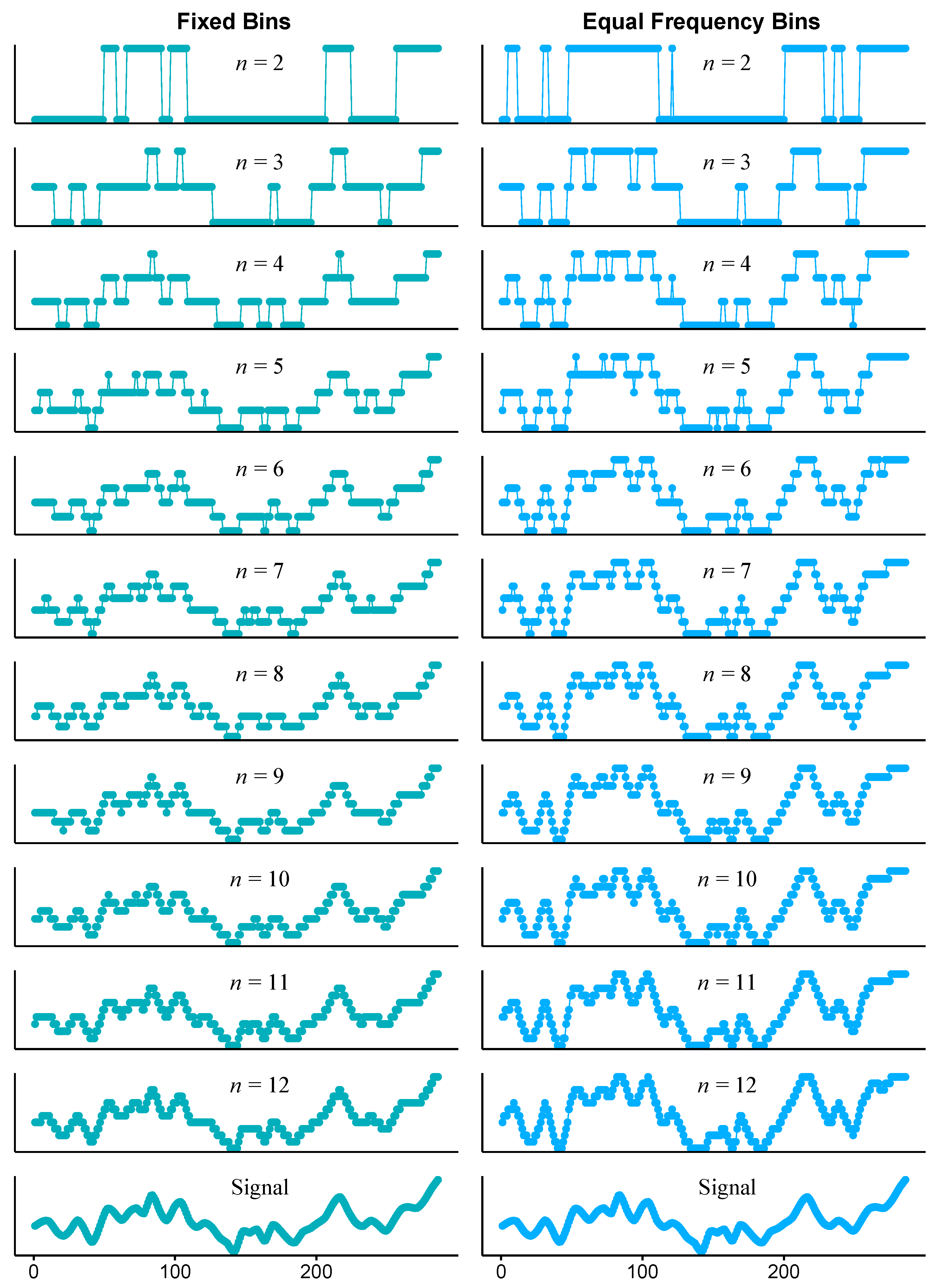
2.3. Time Series Discretisation
2.4. Procedure
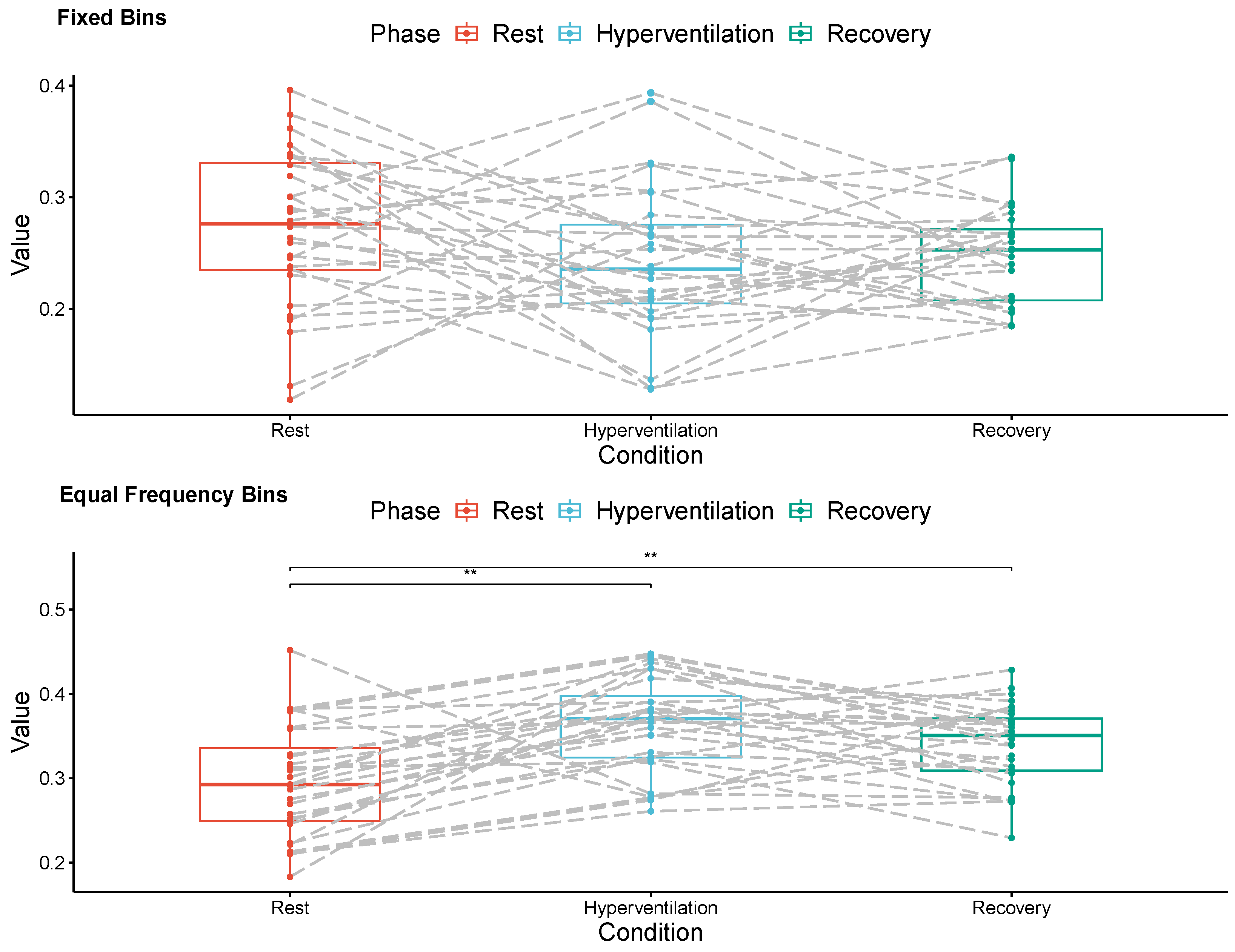
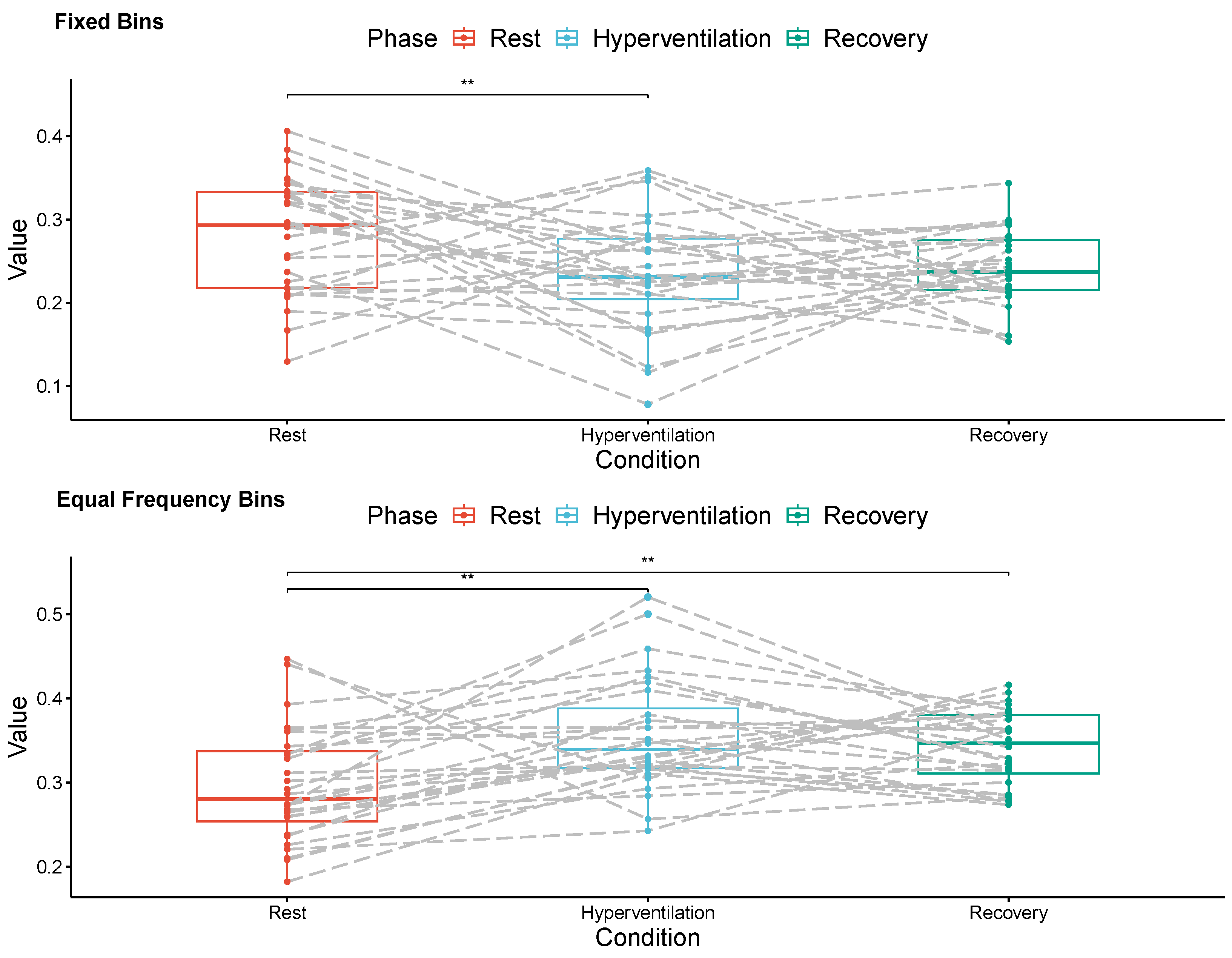
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BP | Blood pressure |
| CO2 | Carbon dioxide |
| EFD | Equal frequency bin discretization |
| ETCO2 | End-tidal carbon dioxide |
| FBD | Fixed bin discretization |
| MCAv | Middle cerebral artery blood flow velocity |
| Transfer entropy from variable X to variable Y | |
| Conditional transfer entropy from variable X to variable Y, conditioned by the interaction of the variable Z |
References
- Payne, S. Cerebral Autoregulation: Control of Blood Flow in the Brain; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Gao, J.; Hu, J.; Tung, W.W. Entropy measures for biological signal analyses. Nonlinear Dyn. 2012, 68, 431–444. [Google Scholar] [CrossRef]
- Schreiber, T. Measuring information transfer. Phys. Rev. Lett. 2000, 85, 461. [Google Scholar] [CrossRef]
- Barnett, L.; Barrett, A.B.; Seth, A.K. Granger causality and transfer entropy are equivalent for Gaussian variables. Phys. Rev. Lett. 2009, 103, 238701. [Google Scholar] [CrossRef]
- Lee, J.; Nemati, S.; Silva, I.; Edwards, B.A.; Butler, J.P.; Malhotra, A. Transfer entropy estimation and directional coupling change detection in biomedical time series. Biomed. Eng. Online 2012, 11, 1–17. [Google Scholar] [CrossRef]
- Luo, D.; Pan, W.; Li, Y.; Feng, K.; Liu, G. The interaction analysis between the sympathetic and parasympathetic systems in CHF by using transfer entropy method. Entropy 2018, 20, 795. [Google Scholar] [CrossRef]
- Harmah, D.J.; Li, C.; Li, F.; Liao, Y.; Wang, J.; Ayedh, W.M.; Bore, J.C.; Yao, D.; Dong, W.; Xu, P. Measuring the non-linear directed information flow in schizophrenia by multivariate transfer entropy. Front. Comput. Neurosci. 2020, 13, 85. [Google Scholar] [CrossRef]
- Ainslie, P.N.; Duffin, J. Integration of cerebrovascular CO2 reactivity and chemoreflex control of breathing: Mechanisms of regulation, measurement, and interpretation. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1473–R1495. [Google Scholar] [CrossRef]
- Hanby, M.F.; Panerai, R.B.; Robinson, T.G.; Haunton, V.J. Is cerebral vasomotor reactivity impaired in Parkinson disease? Clin. Auton. Res. 2017, 27, 107–111. [Google Scholar] [CrossRef][Green Version]
- Jara, J.; Morales-Rojas, C.; Fernández-Muñoz, J.; Haunton, V.J.; Chacón, M. Using complexity–entropy planes to detect Parkinson’s disease from short segments of haemodynamic signals. Physiol. Meas. 2021, 42, 084002. [Google Scholar] [CrossRef]
- Bossomaier, T.; Barnett, L.; Harr, M.; Lizier, J.T. An Introduction to Transfer Entropy: Information Flow in Complex Systems; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar]
- Stramaglia, S.; Cortes, J.M.; Marinazzo, D. Synergy and redundancy in the Granger causal analysis of dynamical networks. New J. Phys. 2014, 16, 105003. [Google Scholar] [CrossRef]
- Lizier, J.T.; Prokopenko, M.; Zomaya, A.Y. Information modification and particle collisions in distributed computation. Chaos Interdiscip. J. Nonlinear Sci. 2010, 20, 037109. [Google Scholar] [CrossRef]
- Montalto, A.; Faes, L.; Marinazzo, D. MuTE: A MATLAB toolbox to compare established and novel estimators of the multivariate transfer entropy. PLoS ONE 2014, 9, e109462. [Google Scholar] [CrossRef]
- Gourévitch, B.; Eggermont, J.J. Evaluating information transfer between auditory cortical neurons. J. Neurophysiol. 2007, 97, 2533–2543. [Google Scholar] [CrossRef]
- Duan, P.; Yang, F.; Chen, T.; Shah, S.L. Direct causality detection via the transfer entropy approach. IEEE Trans. Control Syst. Technol. 2013, 21, 2052–2066. [Google Scholar] [CrossRef]
- Shovon, M.H.I.; Nandagopal, N.; Vijayalakshmi, R.; Du, J.T.; Cocks, B. Directed connectivity analysis of functional brain networks during cognitive activity using transfer entropy. Neural Process. Lett. 2017, 45, 807–824. [Google Scholar] [CrossRef]
- Kale, P.; Acharya, J.V.; Acharya, J.; Subramanian, T.; Almekkawy, M. Normalized transfer entropy as a tool to identify multisource functional epileptic networks. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 17–21 July 2018; pp. 1218–1221. [Google Scholar]
- Benedetto, F.; Mastroeni, L.; Quaresima, G.; Vellucci, P. Does OVX affect WTI and Brent oil spot variance? Evidence from an entropy analysis. Energy Econ. 2020, 89, 104815. [Google Scholar] [CrossRef]
- Dash, R.; Paramguru, R.L.; Dash, R. Comparative analysis of supervised and unsupervised discretization techniques. Int. J. Adv. Sci. Technol. 2011, 2, 29–37. [Google Scholar]
- Dumville, J.; Panerai, R.; Lennard, N.; Naylor, A.; Evans, D. Can cerebrovascular reactivity be assessed without measuring blood pressure in patients with carotid artery disease? Stroke 1998, 29, 968–974. [Google Scholar] [CrossRef]
- Panerai, R.B.; Evans, D.H.; Naylor, A.R. Influence of arterial blood pressure on cerebrovascular reactivity. Stroke 1999, 30, 1293–1295. [Google Scholar] [CrossRef]
- Claassen, J.A.; Zhang, R.; Fu, Q.; Witkowski, S.; Levine, B.D. Transcranial Doppler estimation of cerebral blood flow and cerebrovascular conductance during modified rebreathing. J. Appl. Physiol. 2007, 102, 870–877. [Google Scholar] [CrossRef]
- Hetzel, A.; Braune, S.; Guschlbauer, B.; Dohms, K. CO2 reactivity testing without blood pressure monitoring? Stroke 1999, 30, 398–401. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Panerai, R.; Deverson, S.; Mahony, P.; Hayes, P.; Evans, D. Effect of CO2 on dynamic cerebral autoregulation measurement. Physiol. Meas. 1999, 20, 265. [Google Scholar] [CrossRef] [PubMed]
- Mitsis, G.D.; Poulin, M.J.; Robbins, P.A.; Marmarelis, V.Z. Nonlinear modeling of the dynamic effects of arterial pressure and CO2 variations on cerebral blood flow in healthy humans. IEEE Trans. Biomed. Eng. 2004, 51, 1932–1943. [Google Scholar] [CrossRef]
- Porta, A.; Bari, V.; De Maria, B.; Takahashi, A.C.; Guzzetti, S.; Colombo, R.; Catai, A.M.; Raimondi, F.; Faes, L. Quantifying net synergy/redundancy of spontaneous variability regulation via predictability and transfer entropy decomposition frameworks. IEEE Trans. Biomed. Eng. 2017, 64, 2628–2638. [Google Scholar]
| Brain | Discretisation | Respiratory Exercise Phase | ||
|---|---|---|---|---|
| Side | Method | Rest | Hyperventilation | Recovery |
| Right | FBD | |||
| EFD | ||||
| Left | FBD | |||
| EFD | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Muñoz, J.; Haunton, V.J.; Panerai, R.B.; Jara, J.L. Detection of Blood CO2 Influences on Cerebral Hemodynamics Using Transfer Entropy. Entropy 2024, 26, 23. https://doi.org/10.3390/e26010023
Fernández-Muñoz J, Haunton VJ, Panerai RB, Jara JL. Detection of Blood CO2 Influences on Cerebral Hemodynamics Using Transfer Entropy. Entropy. 2024; 26(1):23. https://doi.org/10.3390/e26010023
Chicago/Turabian StyleFernández-Muñoz, Juan, Victoria J. Haunton, Ronney B. Panerai, and José Luis Jara. 2024. "Detection of Blood CO2 Influences on Cerebral Hemodynamics Using Transfer Entropy" Entropy 26, no. 1: 23. https://doi.org/10.3390/e26010023
APA StyleFernández-Muñoz, J., Haunton, V. J., Panerai, R. B., & Jara, J. L. (2024). Detection of Blood CO2 Influences on Cerebral Hemodynamics Using Transfer Entropy. Entropy, 26(1), 23. https://doi.org/10.3390/e26010023