Use of Intrinsic Entropy to Assess the Instantaneous Complexity of Thoracoabdominal Movement Patterns to Indicate the Effect of the Iso-Volume Maneuver Trial on the Performance of the Step Test
Abstract
:1. Introduction
2. Materials and Methods
2.1. Intrinsic Entropy (IE)
- Initialize the iterator .
- Calculate the upper envelope by interpolating the local maximum of ||.
- For all , if there exists at least one t that larger than 1, let to redo II.
- Else, output
2.2. Experiment Description
2.2.1. Step Test
2.2.2. Iso-Volume Maneuver (IVM) Trial
2.3. Statistical Analysis
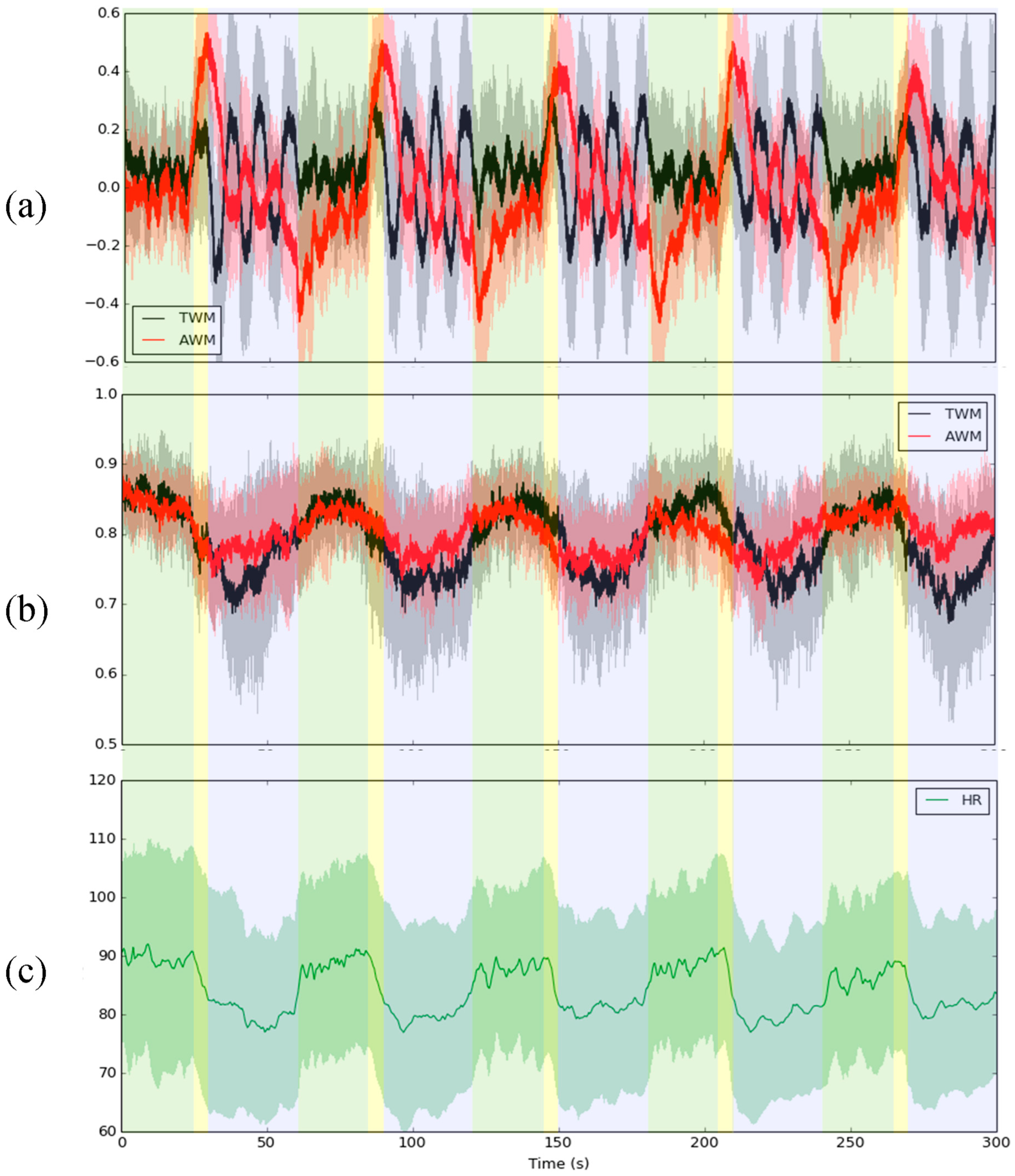
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Shannon, C.E. A mathematical theory of communication. Bell Syst. Tech. J. 1948, 27, 379–423. [Google Scholar] [CrossRef]
- Kolmogorov, A.N. A new metric invariant of transient dynamical systems and automorphisms in Lebesgue spaces. Dokl. Akad. Nauk SSSR 1958, 119, 861–864. [Google Scholar]
- Sinai, I. On the concept of entropy for a dynamic system. Dokl. Akad. Nauk. SSSR 1959, 124, 768–771. [Google Scholar]
- Ribeiro, M.; Henriques, T.; Castro, L.; Souto, A.; Antunes, L.; Costa-Santos, C.; Teixeira, A. The Entropy Universe. Entropy 2021, 23, 222. [Google Scholar] [CrossRef] [PubMed]
- Pincus, S.M. Approximate entropy as a measure of system complexity. Proc. Natl. Acad. Sci. USA 1991, 88, 2297–2301. [Google Scholar] [CrossRef]
- Richman, J.S.; Moorman, J.R. Physiological time-series analysis using approximate entropy and sample entropy. Am. J. Physiol.-Heart Circul. Physiol. 2000, 278, H2039–H2049. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of complex physiologic time series. Phys. Rev. Lett. 2002, 89, 68102. [Google Scholar] [CrossRef]
- Costa, M.; Goldberger, A.L.; Peng, C.-K. Multiscale entropy analysis of biological signals. Phys. Rev. E 2005, 71, 021906. [Google Scholar] [CrossRef]
- Hsu, C.F.; Wei, S.-Y.; Huang, H.-P.; Hsu, L.; Chi, S.; Peng, C.-K. Entropy of entropy: Measurement of dynamical complexity for biological systems. Entropy 2017, 19, 550. [Google Scholar] [CrossRef]
- Hsu, C.F.; Lin, P.Y.; Chao, H.H.; Hsu, L.; Chi, S. Average Entropy: Measurement of disorder for cardiac RR interval signals. Physica A 2019, 529, 121533. [Google Scholar] [CrossRef]
- Wang, F.; Wang, H.; Fu, R. Real-time ECG-based detection of fatigue driving using sample entropy. Entropy 2018, 20, 196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ning, X. Dynamical complexity detection in short-term physiological series using base-scale entropy. Phys. Rev. E 2006, 73, 052902. [Google Scholar] [CrossRef]
- Bian, C.; Ma, Q.; Si, J.; Wu, X.; Shao, J.; Ning, X.; Wang, D. Sign series entropy analysis of short-term heart rate variability. Chin. Sci. Bull. 2009, 54, 4610–4615. [Google Scholar] [CrossRef]
- Pham, T.D. Time-shift multiscale entropy analysis of physiological signals. Entropy 2017, 19, 257. [Google Scholar] [CrossRef]
- Pham, T.D.; Oyama-Higa, M. Nonlinear dynamics analysis of short-time photoplethysmogram in Parkinson’s disease. In Proceedings of the 2018 IEEE International Conference on Fuzzy Systems (FUZZ-IEEE), Rio de Janeiro, Brazil, 8–13 July 2018. [Google Scholar]
- Lin, G.-M.; Haryadi, B.; Yang, C.-M.; Chu, S.-C.; Yang, C.-C.; Wu, H.-T. Discrepancies between conventional multiscale entropy and modified short-time multiscale entropy of photoplethysmographic pulse signals in middle- and old- aged individuals with or without diabetes. Entropy 2017, 19, 132. [Google Scholar] [CrossRef]
- Lai, Y.-H.; Huang, P.-H.; Hsiao, T.-C. Use of sample entropy to assess sub-maximal physical load for avoiding exercise-induced cardiac fatigue. Appl. Sci. 2023, 13, 3813. [Google Scholar] [CrossRef]
- Rostaghi, M.; Azami, H. Dispersion entropy: A measure for time-series analysis. IEEE Signal Process. Lett. 2016, 23, 610–614. [Google Scholar] [CrossRef]
- Azami, H.; Rostaghi, M.; Abásolo, D.; Escudero, J. Refined composite multiscale dispersion entropy and its application to biomedical signals. IEEE Trans. Biomed. Eng. 2017, 64, 2872–2879. [Google Scholar]
- Azami, H.; Escudero, J. Amplitude- and fluctuation-based dispersion entropy. Entropy 2018, 20, 210. [Google Scholar] [CrossRef]
- Zou, S.; Qiu, T.; Huang, P.; Bai, X.; Liu, C. Constructing multi-scale entropy based on the empirical mode decomposition (EMD) and its application in recognizing driving fatigue. J. Neurosci. Methods 2020, 341, 108691. [Google Scholar] [CrossRef]
- Lau, Z.J.; Pham, T.; Chen, S.A.; Makowski, D. Brain entropy, fractal dimensions and predictability: A review of complexity measures for EEG in healthy and neuropsychiatric populations. Eur. J. Neurosci. 2022, 56, 5047–5069. [Google Scholar] [CrossRef] [PubMed]
- Chien, J.Y.; Ruan, S.Y.; Huang, Y.C.T.; Yu, C.J.; Yang, P.C. Asynchronous thoraco-abdominal motion contributes to decreased 6-minute walk test in patients with COPD. Respir. Care 2013, 58, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Reber, A.; Bobbia, S.A.; Hammer, J.; Frei, F.J. Effect of airway opening manoeuvres on thoraco-abdominal asynchrony in anaesthetized children. Eur. Resp. J. 2001, 17, 1239–1243. [Google Scholar] [CrossRef] [PubMed]
- Sivan, Y.; Newth, J.L. Thoracoabdominal asynchrony in acute upper airway obstruction in small children. Am. Rev. Respir. Dis. 1990, 142, 540–544. [Google Scholar] [CrossRef] [PubMed]
- Bedenice, D.; Mazan, M.R.; Kuehn, H.; Hoffman, A.M. Diaphragmatic paralysis due to phrenic nerve degeneration in a llama. J. Vet. Intern. Med. 2002, 16, 603–606. [Google Scholar] [CrossRef] [PubMed]
- Goldman, M.D. Interpretation of thoracoabdominal movements during breathing. Clin. Sci. 1982, 62, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Cysarz, D.; Zerm, R.; Bettermann, H.; Frühwirth, M.; Moser, M.; Kröz, M. Comparison of respiratory rates derived from heart rate variability, ECG amplitude, and nasal/oral airflow. Ann. Biomed. Eng. 2008, 36, 2085–2094. [Google Scholar] [CrossRef] [PubMed]
- Motto, A.L.; Galiana, H.L.; Brown, K.A.; Kearney, R.E. Automated estimation of the phase between thoracic and abdominal movement signals. IEEE Trans. Biomed. Eng. 2005, 52, 614–621. [Google Scholar] [CrossRef]
- Tataraidze, A.; Anishchenko, L.; Korostovtseva, L.; Kooij, B.J.; Bochkarev, M.; Sviryaev, Y. Sleep stage classification based on respiratory signal. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015. [Google Scholar]
- Fiamma, M.N.; Samara, Z.; Baconnier, P.; Similowski, T.; Straus, C. Respiratory inductive plethysmography to assess respiratory variability and complexity in humans. Respir. Physiol. Neurobiol. 2007, 156, 234–239. [Google Scholar] [CrossRef]
- Taranpal, B.; Gulam, S.H.; Harry, B.R.; Mike, I.P.; James, H.H. Exercise ventilatory irregularity can be quantified by approximate entropy to detect breathing pattern disorder. Respir. Physiol. Neurobiol. 2018, 255, 1–6. [Google Scholar]
- Huang, P.H.; Hsiao, T.C. Intrinsic entropy: A novel adaptive method for measuring the instantaneous complexity of time series. IEEE Signal Process. Lett. 2023, 30, 160–164. [Google Scholar] [CrossRef]
- Huang, N.E.; Shen, Z.; Long, S.R.; Wu, M.C.; Shih, H.H.; Zheng, Q.; Yen, N.C.; Tung, C.C.; Liu, H.H. The empirical mode decomposition and the Hilbert spectrum for nonlinear and non-stationary time series analysis. Proc. R. Soc. Lond. Ser. A-Math. Phys. Eng. Sci. 1998, 454, 903–995. [Google Scholar] [CrossRef]
- Huang, N.E.; Wu, Z.; Long, S.R.; Arnold, K.C.; Chen, X.; Blank, K. On instantaneous frequency. Adv. Data Sci. Adapt. Anal. 2009, 1, 177–229. [Google Scholar] [CrossRef]
- Physical Fitness Website of Sports Administration of Ministry of Education in Taiwan/National Society of Physical Education of the Republic of China. Available online: https://www.fitness.org.tw/measure01.php (accessed on 18 April 2023).
- Andrade, C.H.S.D.; Cianci, R.G.; Malaguti, C.; Dal Corso, S. The use of step tests for the assessment of exercise capacity in healthy subjects and in patients with chronic lung disease. J. Bras. Pneumol. 2012, 38, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.C.; Hsiao, T.C. Abdominal breathing by using an intelligent tutoring system. In Proceedings of the 6th European Conference of the International Federation for Medical and Biological Engineering: MBEC 2014, Dubrovnik, Croatia, 7–11 September 2014; pp. 419–422. [Google Scholar]
- Sackner, M.A.; Watson, H.E.; Belsito, A.S.; Feinerman, D.R.; Suarez, M.A.; Gonzalez, G.E.; Bizousky, F.R.; Krieger, B.R. Calibration of respiratory inductive plethysmograph during natural breathing. J. Appl. Physiol. 1989, 66, 410–420. [Google Scholar] [CrossRef] [PubMed]
- Konno, K.; Mead, J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J. Appl. Physiol. 1967, 22, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.Y.; Huang, P.H.; Huang, Y.H.; Hsiao, T.C. Step-test-based assessment of cardiorespiratory fitness improvement achieved through isovolume maneuver trial. Biomed. Signal Process. Control, 2023; in press. [Google Scholar]
- Huang, P.H.; Chung, W.C.; Sheu, C.C.; Tsai, J.R.; Hsiao, T.C. Is the asynchronous phase of thoracoabdominal movement a novel feature of successful extubation? A preliminary result. In Proceedings of the 2021 43rd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Guadalajara, Mexico, 1–5 November 2021; pp. 752–756. [Google Scholar]
- Huang, P.H.; Luo, Y.F.; Yeh, C.F.; Hsiao, T.C. Towards instantaneous phase difference on the COPD pre-discrimination. In Proceedings of the 2018 IEEE 23rd International Conference on Digital Signal Processing (DSP), Shanghai, China, 19–21 November 2018; pp. 1–5. [Google Scholar]
- Wysocki, M.; Fiamma, M.N.; Straus, C.; Poon, C.S.; Similowski, T. Chaotic dynamics of resting ventilatory flow in humans assessed through noise titration. Respir. Physiol. Neurobiol. 2006, 153, 54–65. [Google Scholar] [CrossRef]
- Bandt, C.; Pompe, B. Permutation entropy: A natural complexity measure for time series. Phys. Rev. Lett. 2002, 88, 174102. [Google Scholar] [CrossRef]
- Magagnin, V.; Bassani, T.; Bari, V.; Turiel, M.; Maestri, R.; Pinna, G.D.; Porta, A. Non-stationarities significantly distort short-term spectral, symbolic and entropy heart rate variability indices. Physiol Meas. 2011, 32, 1775. [Google Scholar] [CrossRef]
- Yeh, J.R.; Peng, C.K.; Huang, N.E. Scale-dependent intrinsic entropies of complex time series. Philos. Trans. R. Soc. A 2016, 374, 20150204. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zheng, D.; Li, P.; Zhao, L.; Liu, C.; Murray, A. Is cross-sample entropy a valid measure of synchronization between sequences of RR interval and pulse transit time? In Proceedings of the Computing in Cardiology 2013, Zaragoza, Spain, 22–25 September 2013; pp. 939–942. [Google Scholar]
- Zhang, T.; Yang, Z.; Coote, J.H. Cross-sample entropy statistic as a measure of complexity and regularity of renal sympathetic nerve activity in the rat. Exp. Physiol. 2007, 92, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Jamin, A.; Humeau-Heurtier, A. (Multiscale) Cross-Entropy Methods: A Review. Entropy 2020, 22, 45. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, C.; Zhang, L.; Zhao, L.; Liu, C.; Wang, H. Measuring synchronization in coupled simulation and coupled cardiovascular time series: A comparison of different cross entropy measures. Biomed. Signal Process. Control 2015, 21, 49–57. [Google Scholar] [CrossRef]
- Zheng, L.; Pan, W.; Li, Y.; Luo, D.; Wang, Q.; Liu, G. Use of mutual information and transfer entropy to assess interaction between parasympathetic and sympathetic activities of nervous system from HRV. Entropy 2017, 19, 489. [Google Scholar] [CrossRef]
- Vicente, R.; Wibral, M.; Lindner, M.; Pipa, G. Transfer entropy—A model-free measure of effective connectivity for the neurosciences. J. Comput. Neurosci. 2011, 30, 45–67. [Google Scholar] [CrossRef]
- Gelpi, F.; Bari, V.; Cairo, B.; De Maria, B.; Wells, R.; Baumert, M.; Porta, A. Evaluation of cardiovascular and cerebrovascular control mechanisms in postural orthostatic tachycardia syndrome via conditional transfer entropy: The impact of the respiratory signal type. Physiol. Meas. 2023, 44, 64001. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, Y.; Cheng, S.; Xie, P. Transfer spectral entropy and application to functional corticomuscular coupling. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 27, 1092–1102. [Google Scholar] [CrossRef]
- Mador, M.J.; Acevedo, F.A. Effect of respiratory muscle fatigue on breathing pattern during incremental exercise. Am. Rev. Respir. Dis. 1991, 143, 462–468. [Google Scholar] [CrossRef]
- Swain, D.P.; Abernathy, K.S.; Smith, C.S.; Lee, S.J.; A Bunn, S. Target heart rates for the development of cardiorespiratory fitness. Med. Sci. Sport. Exerc. 1994, 26, 112–116. [Google Scholar] [CrossRef]
- Stöhr, E.J.; González-Alonso, J.; Shave, R. Left ventricular mechanical limitations to stroke volume in healthy humans during incremental exercise. Am. J. Physiol.-Heart Circ. Physiol. 2011, 301, 478–487. [Google Scholar] [CrossRef] [PubMed]
- NBerry, N.T.; Bechke, E.; Shriver, L.H.; Calkins, S.D.; Keane, S.P.; Shanahan, L.; Wideman, L. Heart rate dynamics during acute recovery from maximal aerobic exercise in young adults. Front. Physiol. 2021, 12, 627320. [Google Scholar]






| ALL Subjects | Rest | Step | Recovery |
|---|---|---|---|
| Before IVM | −499.15 2053.6 | 1141.74 2969.58 | 1596.73 3046.28 |
| After IVM | −4475.17 6182.31 ** | 1434.31 3856.01 | −1414.40 4799.54 * |
| Rest | PFI Increase or Unchanged | PFI Decrease |
|---|---|---|
| dAUC increase | 1 | 2 |
| dAUC decrease | 8 | 8 |
| Step | PFI Increase or Unchanged | PFI Decrease |
|---|---|---|
| dAUC increase | 3 | 4 |
| dAUC decrease | 6 | 6 |
| Recovery | PFI Increase or Unchanged | PFI Decrease |
|---|---|---|
| dAUC increase | 4 | 2 |
| dAUC decrease | 5 | 8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, P.-H.; Hsiao, T.-C. Use of Intrinsic Entropy to Assess the Instantaneous Complexity of Thoracoabdominal Movement Patterns to Indicate the Effect of the Iso-Volume Maneuver Trial on the Performance of the Step Test. Entropy 2024, 26, 27. https://doi.org/10.3390/e26010027
Huang P-H, Hsiao T-C. Use of Intrinsic Entropy to Assess the Instantaneous Complexity of Thoracoabdominal Movement Patterns to Indicate the Effect of the Iso-Volume Maneuver Trial on the Performance of the Step Test. Entropy. 2024; 26(1):27. https://doi.org/10.3390/e26010027
Chicago/Turabian StyleHuang, Po-Hsun, and Tzu-Chien Hsiao. 2024. "Use of Intrinsic Entropy to Assess the Instantaneous Complexity of Thoracoabdominal Movement Patterns to Indicate the Effect of the Iso-Volume Maneuver Trial on the Performance of the Step Test" Entropy 26, no. 1: 27. https://doi.org/10.3390/e26010027






