Metastable Oscillatory Modes as a Signature of Entropy Management in the Brain
Abstract
:1. Introduction
1.1. Broad Formulation of the Free Energy Principle
1.2. Synchronization Drives Metastable Oscillatory Modes
2. Materials and Methods
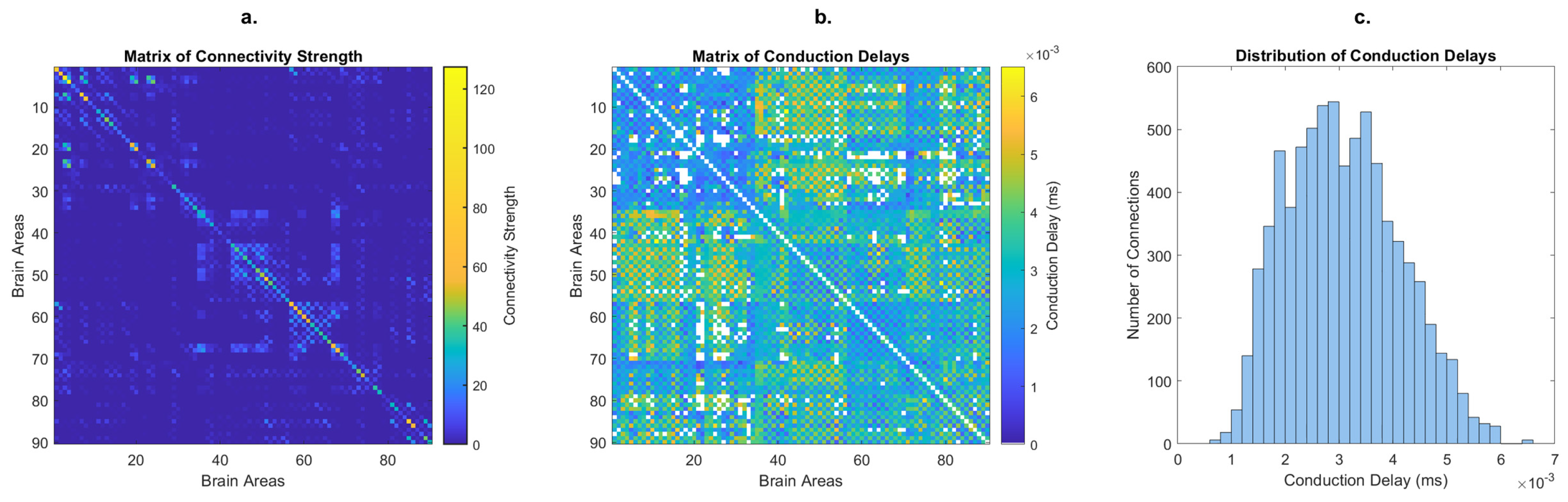
2.1. Computational Model of Coupled Oscillators
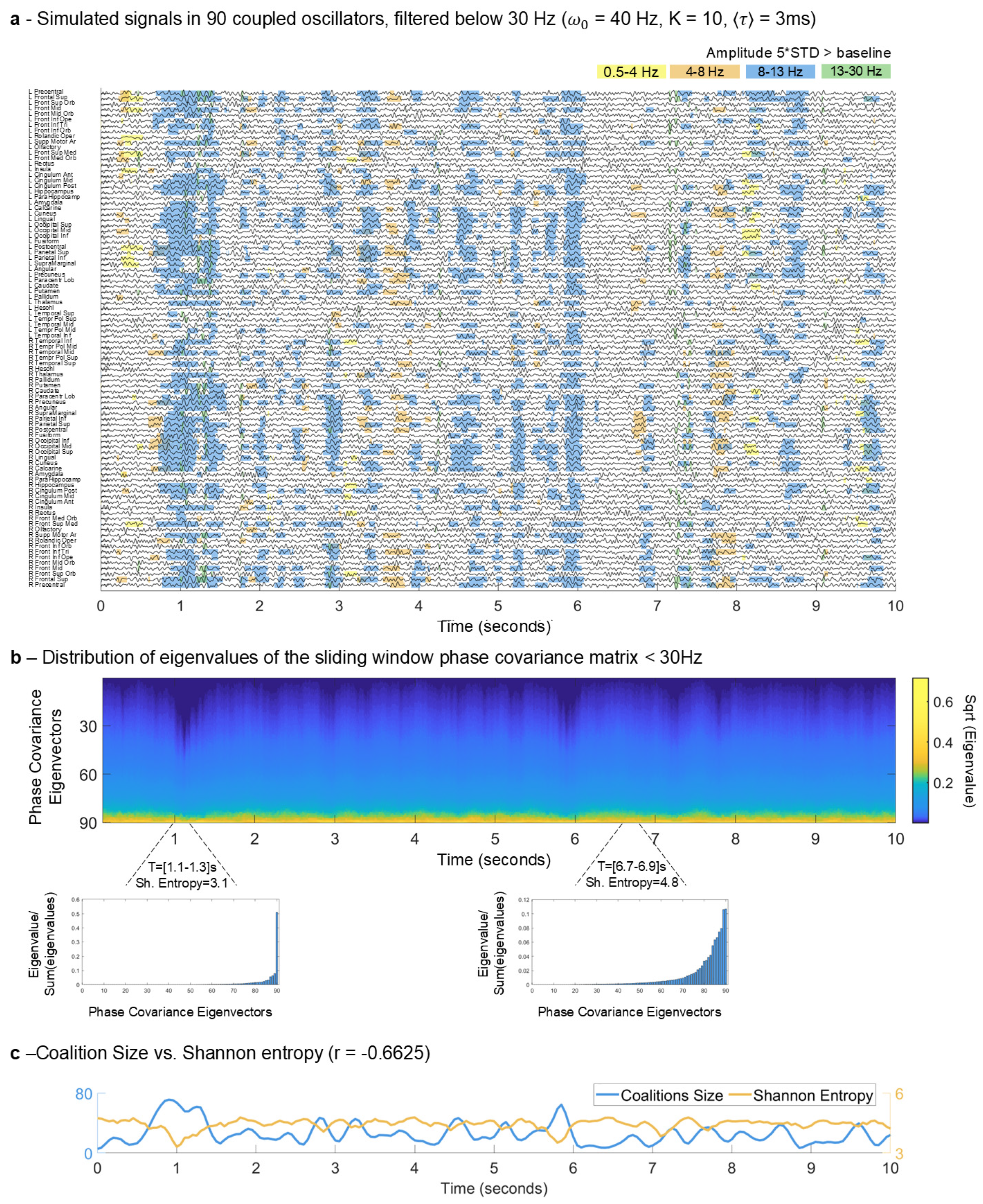
2.2. Detection and Characterization of MOMs
2.3. Entropy of the System over Time
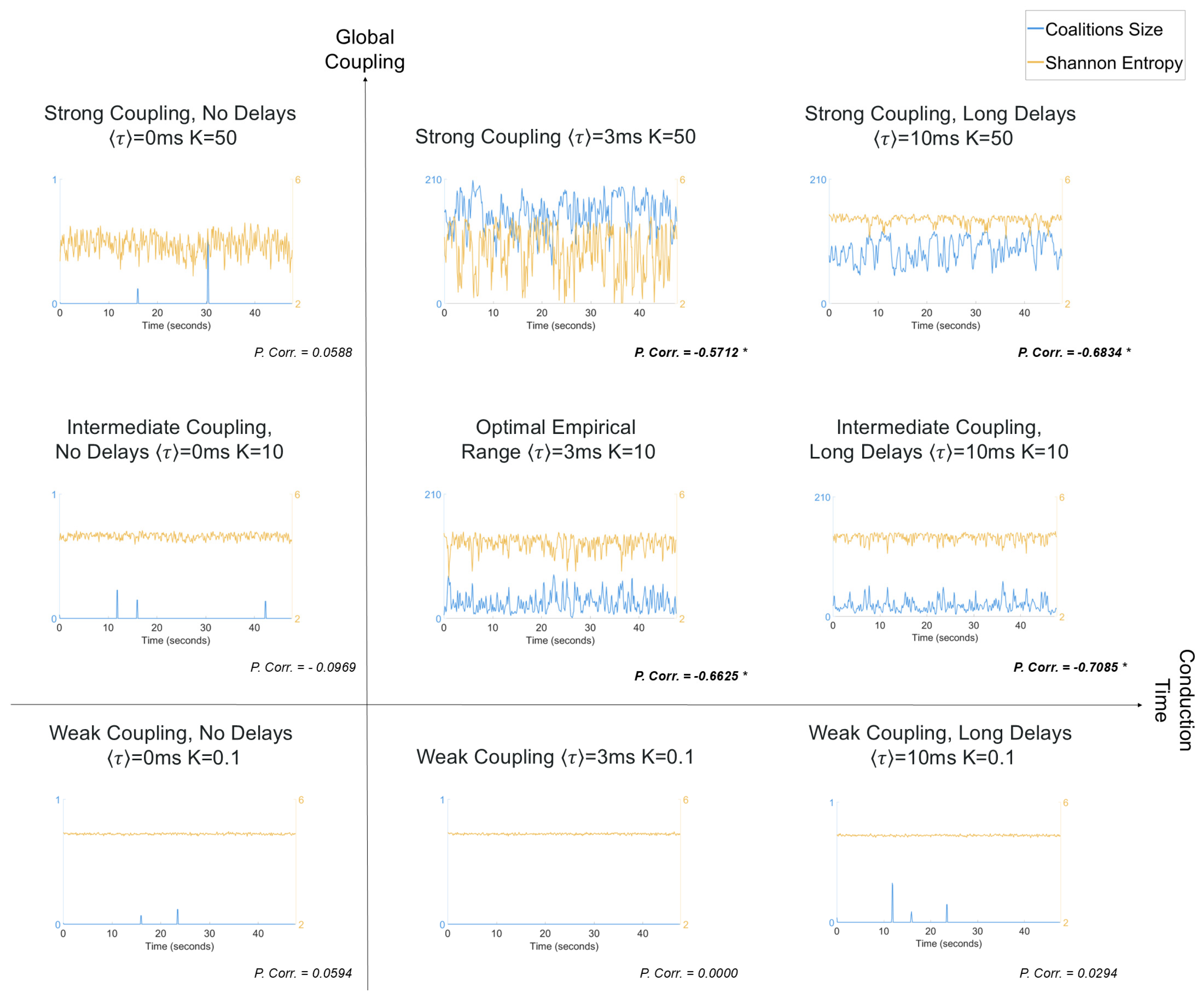
2.4. MOMs and Entropy Analyses
3. Results
4. Discussion
4.1. MOMs as a Signature of Free Energy Management
4.2. Self-Organized Itinerant Dynamics
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Friston, K.J. A Free Energy Principle for the Brain. J. Physiol. 2006, 100, 70–87. [Google Scholar] [CrossRef] [PubMed]
- Cabral, J.; Castaldo, F.; Vohryzek, J.; Litvak, V.; Bick, C.; Lambiotte, R.; Friston, K.; Kringelbach, M.L.; Deco, G. Metastable Oscillatory Modes Emerge from Synchronization in the Brain Spacetime Connectome. Commun. Phys. 2022, 5, 184. [Google Scholar] [CrossRef] [PubMed]
- Hancock, F.; Rosas, F.E.; Zhang, M.; Mediano, P.A.M.; Luppi, A.; Cabral, J.; Deco, G.; Kringelbach, M.; Breakspear, M.; Scott Kelso, J.A.; et al. Metastability Demystified—the Foundational Past, the Pragmatic Present, and the Potential Future. Preprint 2023. [Google Scholar] [CrossRef]
- Cocchi, L.; Gollo, L.L.; Zalesky, A.; Breakspear, M. Criticality in the Brain: A Synthesis of Neurobiology, Models and Cognition. Prog. Neurobiol. 2017, 158, 132–152. [Google Scholar] [CrossRef]
- Shanahan, M. Metastable Chimera States in Community-Structured Oscillator Networks. Chaos 2010, 20, 013108. [Google Scholar] [CrossRef]
- Lee, W.S.; Ott, E.; Antonsen, T.M. Large Coupled Oscillator Systems with Heterogeneous Interaction Delays. Phys. Rev. Lett. 2009, 103, 044101. [Google Scholar] [CrossRef]
- Niebur, E.; Schuster, H.G.; Kammen, D.M. Collective Frequencies and Metastability in Networks of Limit-Cycle Oscillators with Time Delay. Phys. Rev. Lett. 1991, 67, 2753. [Google Scholar] [CrossRef]
- Dos Santos, F.P.; Timms, R.C.; Cabral, J.; Vohryzek, J.; Deco, G.; Woolrich, M.; Friston, K.; Verschure, P.; Litvak, V. Multi-Modal and Multi-Model Interrogation of Large-Scale Functional Brain Networks. Neuroimage 2023, 277, 120236. [Google Scholar] [CrossRef]
- Cabral, J.; Luckhoo, H.; Woolrich, M.; Joensson, M.; Mohseni, H.; Baker, A.; Kringelbach, M.L.; Deco, G. Exploring Mechanisms of Spontaneous Functional Connectivity in MEG: How Delayed Network Interactions Lead to Structured Amplitude Envelopes of Band-Pass Filtered Oscillations. Neuroimage 2014, 90, 423–435. [Google Scholar] [CrossRef]
- Buzsaki, G. Rhythms of the Brain; Oxford University Press: Oxford, UK, 2006. [Google Scholar] [CrossRef]
- Kauffman, S.A. The Origins of Order: Self-Organization and Selection in Evolution; Oxford University Press: Oxford, UK, 1993; ISBN 9780195079517. [Google Scholar]
- Friston, K.J. The Free-Energy Principle: A Unified Brain Theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Berger, H. Über das Elektrenkephalogramm des Menschen. Arch. Für Psychiatr. Nervenkrankh. 1929, 87, 527–570. [Google Scholar] [CrossRef]
- Friston, K.J. Transients, Metastability, and Neuronal Dynamics. Neuroimage 1997, 5, 164–171. [Google Scholar] [CrossRef] [PubMed]
- Vidaurre, D.; Hunt, L.T.; Quinn, A.J.; Hunt, B.A.E.; Brookes, M.J.; Nobre, A.C.; Woolrich, M.W. Spontaneous Cortical Activity Transiently Organizes into Frequency Specific Phase-Coupling Networks. Nat. Commun. 2018, 9, 2987. [Google Scholar] [CrossRef] [PubMed]
- Strogatz, S. Sync: The Emerging Science of Spontaneous Order; Penguin: London, UK, 2004; ISBN 9780141933184. [Google Scholar]
- Strogatz, S.H. Sync: How Order Emerges from Chaos in the Universe, Nature, and Daily Life; Hachette UK: London, UK, 2012; ISBN 9781401304461. [Google Scholar]
- O’Keeffe, K.P.; Hong, H.; Strogatz, S.H. Oscillators That Sync and Swarm. Nat. Commun. 2017, 8, 1504. [Google Scholar] [CrossRef]
- Campbell, S.A. Time delays in neural systems. In Handbook of Brain Connectivity; Springer: Berlin/Heidelberg, Germany, 2007; pp. 65–90. [Google Scholar]
- Lakshmanan, M.; Senthilkumar, D. Dynamics of Nonlinear Time-Delay Systems; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar] [CrossRef]
- Atay, F.M. Complex Time-Delay Systems: Theory and Applications, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Biswas, D.; Banerjee, T. Time-Delayed Chaotic Dynamical Systems: From Theory to Electronic Experiment; Springer: Berlin/Heidelberg, Germany, 2017. [Google Scholar] [CrossRef]
- Otto, A.; Just, W.; Radons, G. Nonlinear dynamics of delay systems: An overview. Philos. Trans. A Math. Phys. Eng. Sci. 2019, 377, 20180389. [Google Scholar] [CrossRef]
- Tzourio-Mazoyer, N.; Landeau, B.; Papathanassiou, D.; Crivello, F.; Etard, O.; Delcroix, N.; Mazoyer, B.; Joliot, M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. Neuroimage 2002, 15, 273–289. [Google Scholar] [CrossRef]
- Atay, F.M.; Jost, J.; Wende, A. Delays, connection topology, and synchronization of coupled chaotic maps. Phys. Rev. Lett. 2004, 92, 144101. [Google Scholar] [CrossRef]
- Jolliffe, I.T. Principal Component Analysis for Special Types of Data. In Principal Component Analysis; Springer: New York, NY, USA, 2002; pp. 338–372. [Google Scholar] [CrossRef]
- Inouye, T.; Shinosaki, K.; Sakamoto, H.; Toi, S.; Ukai, S.; Iyama, A.; Katsuda, Y.; Hirano, M. Quantification of EEG irregularity by use of the entropy of the power spectrum. Electroencephalogr. Clin. Neurophysiol. 1991, 79, 204–210. [Google Scholar] [CrossRef]
- Bengtsson, I.; Zyczkowski, K. Geometry of Quantum States: An Introduction to Quantum Entanglement; Cambridge University Press (CUP): Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Stephen Yeung, M.K.; Strogatz, S.H. Time Delay in the Kuramoto Model of Coupled Oscillators. Phys. Rev. Lett. 1999, 82, 648. [Google Scholar] [CrossRef]
- Wildie, M.; Shanahan, M. Metastability and chimera states in modular delay and pulse-coupled oscillator networks. Chaos 2012, 22, 043131. [Google Scholar] [CrossRef]
- Caprioglio, E.; Berthouze, L. Emergence of metastability in frustrated oscillatory networks: The key role of hierarchical modularity. Front. Netw. Physiol. 2024, 4, 1436046. [Google Scholar] [CrossRef] [PubMed]
- Hizanidis, J.; Kouvaris, N.E.; Zamora-López, G.; Díaz-Guilera, A.; Antonopoulos, C.G. Chimera-like States in Modular Neural Networks. Sci. Rep. 2016, 6, 19845. [Google Scholar] [CrossRef] [PubMed]
- Van Essen, D.C.; Smith, S.M.; Barch, D.M.; Behrens, T.E.J.; Yacoub, E.; Ugurbil, K. The WU-Minn Human Connectome Project: An overview. Neuroimage 2013, 80, 62–79. [Google Scholar] [CrossRef] [PubMed]
- Horn, A.; Li, N.; Dembek, T.A.; Kappel, A.; Boulay, C.; Ewert, S.; Tietze, A.; Husch, A.; Perera, T.; Neumann, W.-J.; et al. Lead-DBS v2: Towards a comprehensive pipeline for deep brain stimulation imaging. Neuroimage 2019, 184, 293–316. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xavier, M.; Figueiredo, P.; Deco, G.; Luppi, A.I.; Cabral, J. Metastable Oscillatory Modes as a Signature of Entropy Management in the Brain. Entropy 2024, 26, 1048. https://doi.org/10.3390/e26121048
Xavier M, Figueiredo P, Deco G, Luppi AI, Cabral J. Metastable Oscillatory Modes as a Signature of Entropy Management in the Brain. Entropy. 2024; 26(12):1048. https://doi.org/10.3390/e26121048
Chicago/Turabian StyleXavier, Marta, Patrícia Figueiredo, Gustavo Deco, Andrea I. Luppi, and Joana Cabral. 2024. "Metastable Oscillatory Modes as a Signature of Entropy Management in the Brain" Entropy 26, no. 12: 1048. https://doi.org/10.3390/e26121048
APA StyleXavier, M., Figueiredo, P., Deco, G., Luppi, A. I., & Cabral, J. (2024). Metastable Oscillatory Modes as a Signature of Entropy Management in the Brain. Entropy, 26(12), 1048. https://doi.org/10.3390/e26121048





