Self-Improvising Memory: A Perspective on Memories as Agential, Dynamically Reinterpreting Cognitive Glue
Abstract
:1. Introduction and Overview
“To live is to be other. It’s not even possible to feel, if one feels today what he felt yesterday. To feel today what one felt yesterday is not to feel—it’s to remember today what was felt yesterday, to be today’s living corpse of what yesterday was lived and lost. To erase everything from the slate from one day to the next, to be new with each new morning, in a perpetual revival of our emotional virginity—this, and only this, is worth being or having, to be or have what we imperfectly are.”Fernando Pessoa
2. Background: The Shifting Sands of Selves and Memories
“The material present in the form of memory traces being subjected from time to time to a rearrangement in accordance with fresh circumstances—to a re-transcription.”Sigmund Freud writing to Wilhelm Fliess on 2 November 1896
3. Remapping Memories: Beyond Storage and Simple Modification
“No man ever steps in the same river twice. For it’s not the same river and he’s not the same man.”Heraclitus
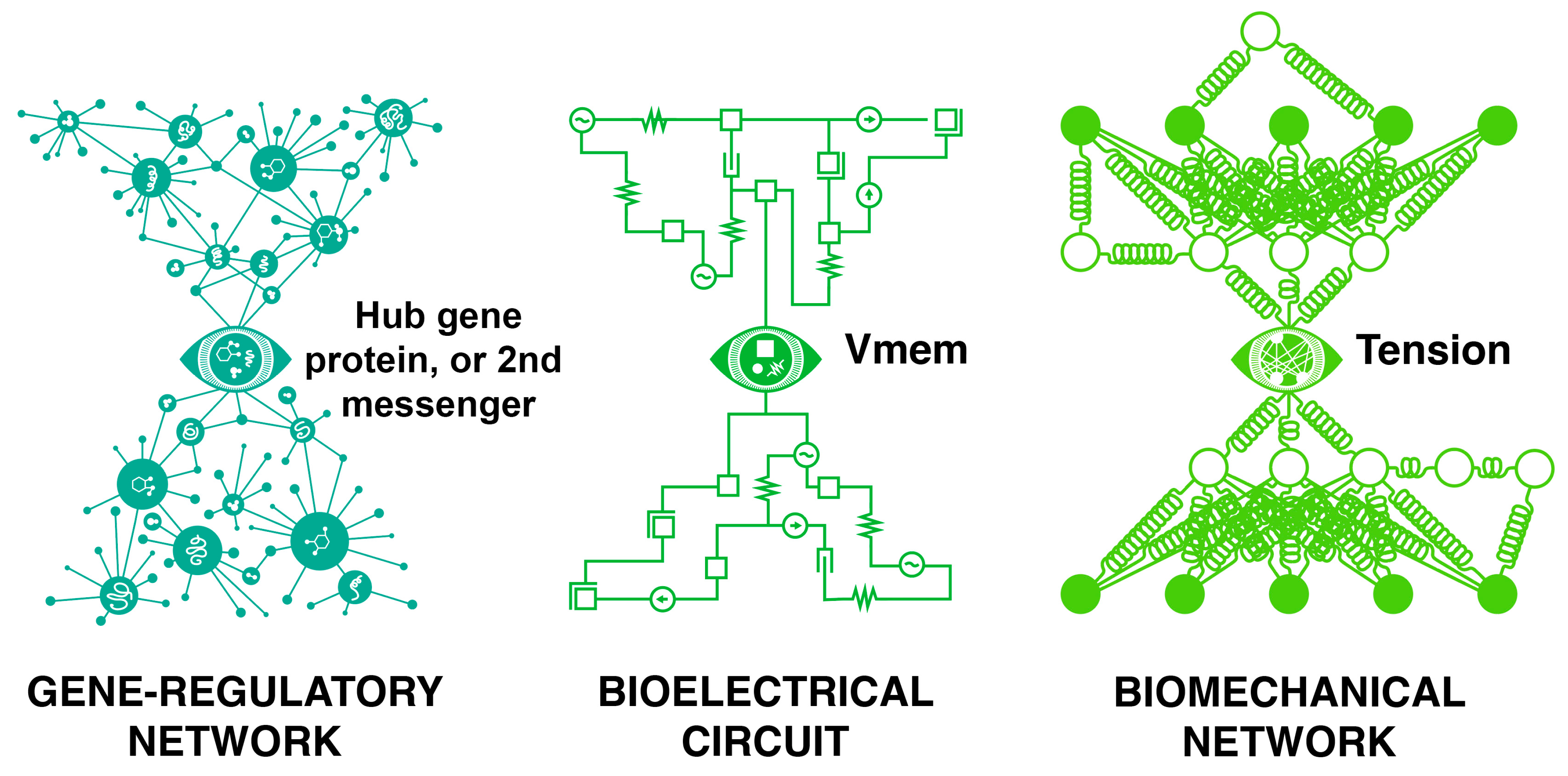
4. Beyond the Brain: Bowties Everywhere
“The past is a foreign country; they do things differently there.”L.P. Hartley
5. Beyond Biology
| Scenario/Scale | Bowtie Hub Node | Remapping Process |
|---|---|---|
| Developmental lineage | Egg | Morphogenetic problem-solving competencies |
| Stress | Integrated stress response | Multiple physiological systems performing credit assignment to adaptively adjust to general stress indicators |
| Hyper-embryo groups [167] | Calcium/ATP signal through the medium between embryos | Increased morphogenetic problem-solving competencies |
| Hologram43 | Holographic film, storing a compressed complex 3D pattern in a 2D substrate | Laser interrogation |
| Regeneration | Bioelectric pattern | Planaria remapping Vmem map from whole to fragment |
| Single cognitive Self across time | Memory media (engrams) | Neural interpretation of engrams |
| Transplants between cognitive Selves | Extracts (RNA) or tissue implants | Neural interpretation of engrams |
| Communication | Language [185] | Neural interpretation of spoken/written messages |
| Psychoanalysis | Dreams, speech acts | Creative, intuitive, skillful interpretation for a therapeutic goal |
| Song | Written musical scores | Replaying the same song on a totally different instrument |
| Science, in the short term | Talks/manuscripts | Interpretation of data by scientists in the same/other fields |
| Science, in the long term | Ideas and paradigms, explanations | Interpretation and use by the scientific community: some ideas become immortalized as engineering tech; others are revised, or forgotten. |
| Art | Poetry, paintings, etc. | Personal interpretation and finding personal meaning |
| Society | Religious frameworks | Adapting (or not) as technology and science advance |
6. What It Means, and What Next: A Research Program for Further Development
“A story has no beginning or end: arbitrarily one chooses that moment of experience from which to look back or from which to look ahead.”Graham Greene
7. A Continuum: From Thoughts to Thinkers
“We are pleased to have helped you. Goodbye.”a hallucinatory voice which correctly diagnosed a patient’s unrecognized brain tumor [242]
8. Conclusions
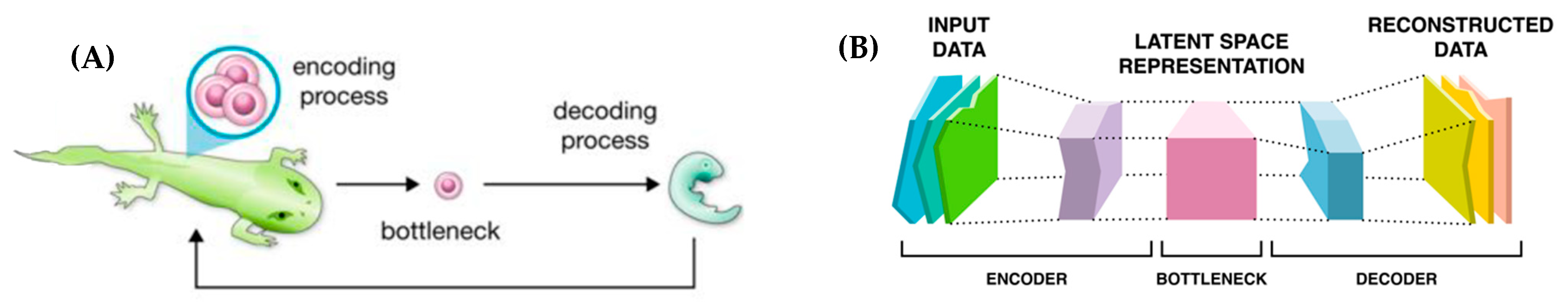
“Now I do not know whether I was then a man dreaming I was a butterfly, or whether I am now a butterfly dreaming I am a man. Between a man and a butterfly there is necessarily a barrier.”Chuang Tzu
Funding
Acknowledgments
Conflicts of Interest
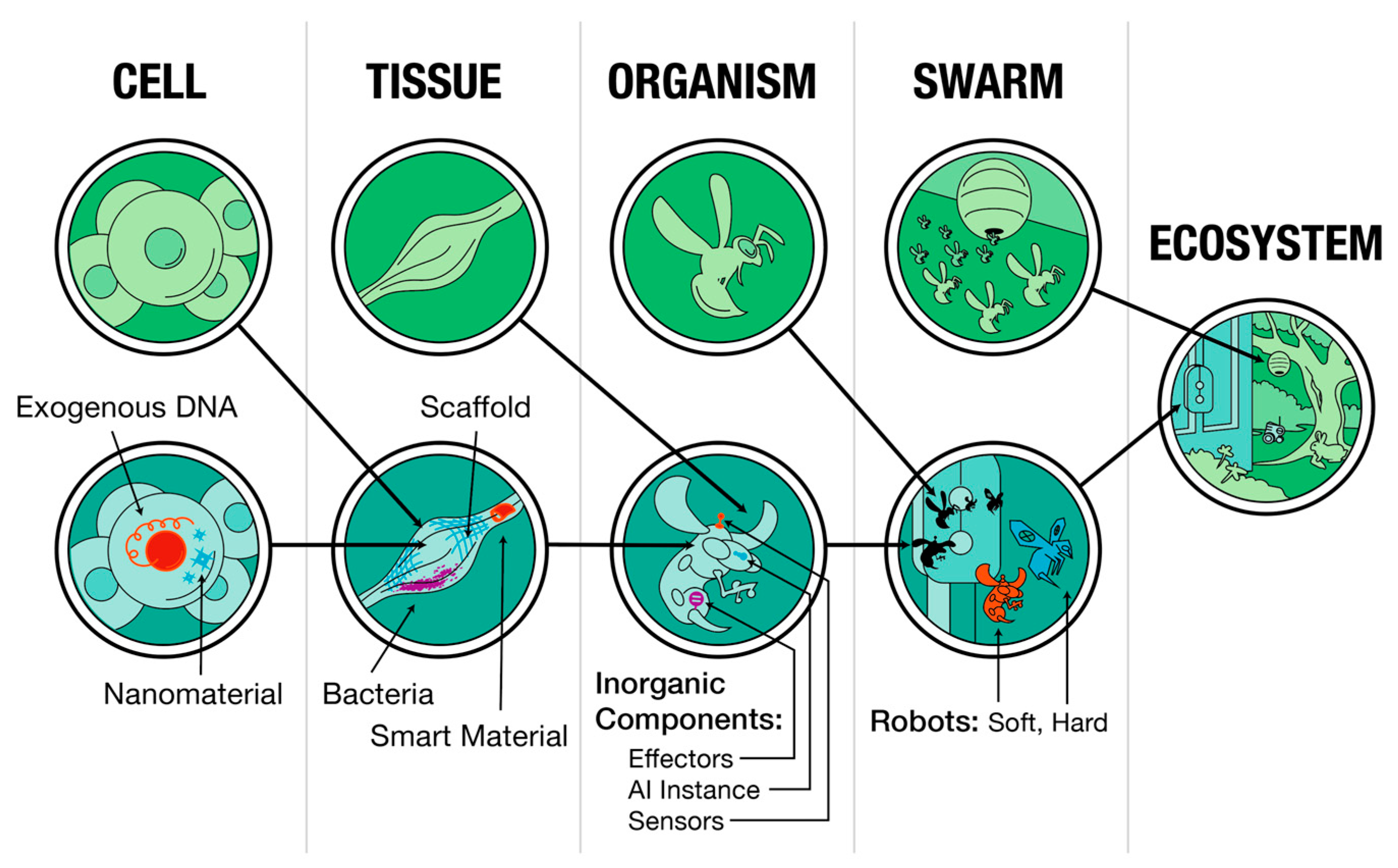
| 1 | The below analysis of this problem is performed in the context of the emerging field of Diverse Intelligence, which seeks symmetries among agents regardless of their composition, spatiotemporal scale, or origin (evolved vs. engineered). This field also seeks to understand the relationship between life and cognition; to understand the deep nature of embodied minds beyond contingent facts of evolutionary lineage on Earth [1,2,3,4,5,6,7,8,9]. |
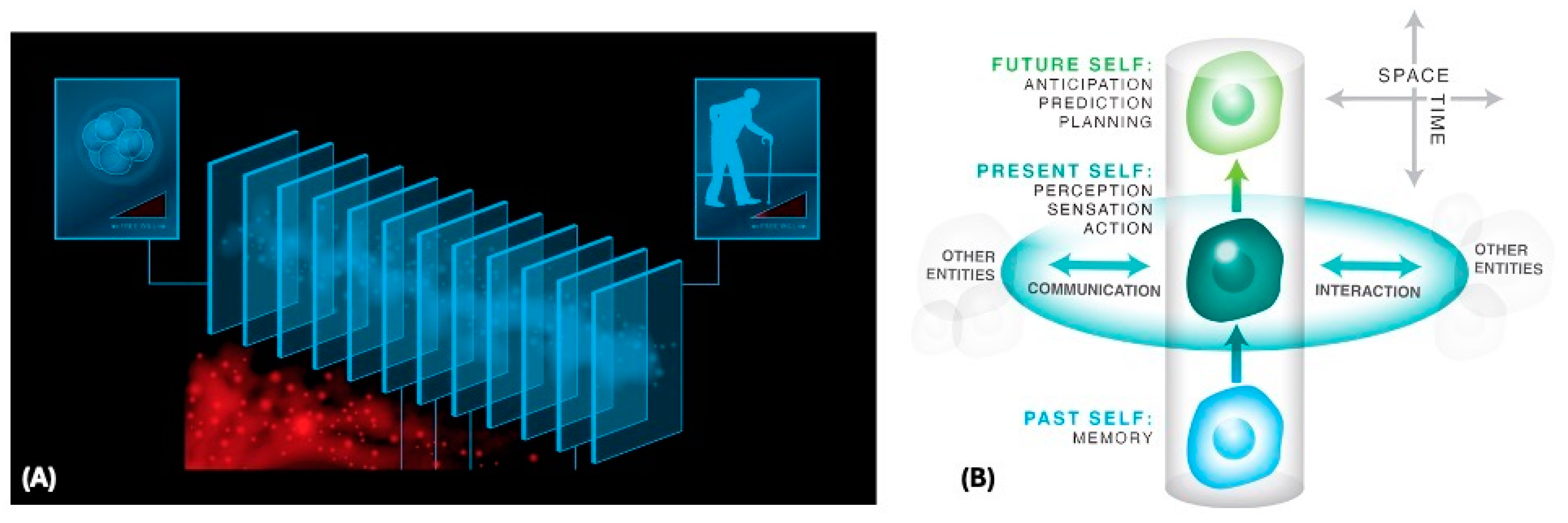
| 2 | Definitions, for the purpose of this paper: Self = a process by which an emergent perspective on the world persists and embodies, but feels (from the inside, from a first-person perspective) as a persistent structure. Intelligence = a publicly observable (third-person perspective) degree of competency at reaching the same goal by different means as necessary [14], with respect to a problem space and goals identified by some observer (which can also be the system itself). |
| 3 | Iain McGilchrist points out that snapshot selves are particularly a “left hemisphere” invention—a framing used because it does not understand flow. |
| 4 | This is related to the concept of “precariousness” in the enactive cognition field [18] and to ideas on the relationship between the different kinds of disorder and death in living systems and the need for freedom from fixed interpretations of the past [19]. Note that this is a matter of timescales, with respect to how quickly various components degrade relative to the role they perform within the agent. See [20,21,22] for more on the relationship between vulnerability, agency, and plasticity. |
| 5 | |
| 6 | This maintenance requires constant adjustment of the estimated boundary between the Self and the world, and of the sensors and effectors an agent thinks it has. The plasticity of this process, and its lack of allegiance to evolutionary priors, is revealed for example when humans, having spent millions of years of their history as a tetrapod, adopt a new limb as their own within minutes of experience in the “rubber hand illusion” experiments and other cases of sensorimotor augmentation [48,49,50,51], or in syndromes where patients deny that their conventional bodyparts belong to them [52]. The ongoing maintenance of the cognitive Self is highly analogous to the ongoing maintenance required of the somatic self, as the Ship of Theseus of the body resists aging and degenerative processes while remodeling as needed to fit environmental demands. |
| 7 | Polycomputing [58] is a kind of observer-first way to see living systems, in which each subsystem can and must interpret the actions and outputs of their neighbors and their own parts. In this framework, computations do not have a single, objective, correct meaning: the same set of physical events can be seen as performing different computations by different observers. By focusing on different ways to interpret the same processes, biological subsystems can adaptively make use of each other; within a body, this allows evolution great freedom to innovate—adding new functionality by adding new interpretations of mechanisms, rather than having to change those mechanisms and risk ruining all of the other systems that depend on them (thus avoiding catastrophic forgetting—a problem that plagues machine learning—and preserving hard-won gains). This freedom from how others in the past intended something to be used or understood is an essential component of the notion of hacking, which is ubiquitous in biology. |
| 8 | This can be further unpacked into a multi-scale vision of endless stacks of virtualization, where we—as patterns—have thoughts, and thus thoughts can have thoughts of their own. |
| 9 | |
| 10 | This applies both laterally, and across time (because of saccades). |
| 11 | These begin at the very front of the perception cycle, during active processing by the retina. |
| 12 | The corresponding memory medium for ant colonies—another collective intelligence—is the ground, on which they leave chemical messages that mediate the colony-level decision-making. |
| 13 | Memory is communication, and the mechanisms used to assemble those communications into an emergent Self-model that seems to persist over time is analogous to the mechanisms that bind individual agents (like cells) into an emergent Body-model, because in both cases a continuous stream of signals (passing messages) underlies a temporally and spatially extended whole (as perceived by itself and by external observers). An interesting direction for future research then is to ask what role, within a persistent cognitive Self, is played by the competitive and cooperative dynamics studied in evolutionary game theory within communication between agents [37,78,79,80]. |
| 14 | “The Mind is Flat” [81] is an excellent discussion of how this happens in real time. |
| 15 | The model of intelligence implemented as the analogy-making Copycat system [85] is a great example of this: learning that “abc” maps to “123” will later have to be used to guess what “def” (or even “deg” or “456”) maps to, and it is not the original stimulus but rather the extracted mapping that has to be stored and applied as best as one can to a future situation. |
| 16 | This is because our memories are the reference point for everything else we think we know about ourselves—if our memories were changed, in a consistent way, we would never know. |
| 17 | They can even lead to existential crises—“a thought that breaks the thinker”—for some. |
| 18 | There may be a useful definition of “life” that could be developed with respect to this. We certainly do not call unchanging informational media (e.g., a magnetic disk) “living”, and change is not enough to signal the presence of life, but change in a way that preserves saliency for some observer across transformative steps may be a signature of life in unfamiliar embodiments. |
| 19 | This may be related to the limits of formal systems with respect to polysemy, and raises the question of how to go beyond these limits [86] in creating Artificial Intelligence systems that are both reliable (consistent) and creative (inconsistent) [87]. For example, GPUs allow for high performance at the cost of nondeterministic results. |
| 20 | Von Foerster [17,94,95] talks about the role of inductive inference, and suggests that living organisms’ and human organizations’ efforts to use past experiences to predict the future, and memory, are fundamentally a form of generalization and not inherently temporal. This emphasis on sense-making over time, as a uniquely privileged aspect, is consistent with previous suggestions that evolution readily pivoted the competencies of bioelectric networks’ control of spatial pattern during morphogenesis into nervous systems’ control of temporal patterns during behavior in a 3D space [96,97]. |
| 21 | In other words, unlike error correction (de-noising), which aims to reconstruct the low-level details of the message, the information is instead reinterpreted as needed to extract useful meaning, with allegiance not to the original sender and their intent, but to the current recipient and their perspective. This is related to the notion of universal hacking of biological parts exploiting neighbors and subcomponents [58], in the sense of a lack of commitment to others’ interpretations, and even your own past interpretation (an essential ingredient of a growth in wisdom). |
| 22 | Such patients cannot remember a doctor they just met but will refuse to shake hands with him if he poked them with a concealed pin the first time they shook hands, rapidly concocting stories like “I don’t like to shake hands” [63]—an alternate form of memory that also requires confabulation to fit it into their sense of Self. |
| 23 | From this perspective, memories can be seen as prompts—just extremely rich versions of trigger stimuli—that push the cognitive system into specific movements in linguistic or behavioral spaces. Similar phenomena have been studied in anatomical morphospace, where simple bioelectric states likewise trigger memories of organogenesis [99,100]. |
| 24 | |
| 25 | However, the same issue comes up for human practices, leading to self-transcendence. |
| 26 | Perceiving pattern memories in morphogenesis (the anatomical collective intelligence) is also not a pure “read” operation, as many biochemical signals are modified/destroyed by receptors that bind to them during cell–cell instructions in the navigation of anatomical space [96]. |
| 27 | One thing that this model does not yet deal with, however, is exceptional cases of eidetic memory, as well as failures of generalization [107]. |
| 28 | The injection of odorant molecules into an egg, which changes the behavior of an adult animal [122], is an example of a small information structure taking over the functionality of a much larger whole, with a past history and its own information structures in place. Another example is the induction of ectopic organs by a small region of bioelectrically-modified cells which disagree with the established fate of the much larger tissue environment and induces neighboring cells to change course and participate in the ectopic organ formation [100]. Why does this work? One possibility is that it triggers a built-in drive meant to overcome the dark-room problem of active inference: if nothing new happens in a long time, it is possible that the lack of surprise is due to a maladaptive failure of infotaxis; thus, there may be selection for a module that counteracts boredom with excessive attention paid to any new information that disagrees with the too-successful world model. The ability of tissues to change on a dime with small trigger inputs makes systems hackable (and susceptible to exploitation) but also enables cognition and evolution (mutations, like ideas, can make huge differences). One formalism that might be useful is that of tiling the plane (because of the relevance of alignment as cognitive glue), where one abnormal shape nucleates changes and geometric frustration that can spread through the whole system. |
| 29 | |
| 30 | In this scheme, we can think of the left and right sides of the bowtie architecture (Figure 3) as two agents, having to communicate via a narrow in-between interface, like language that helps complex beings cooperate through a low-bandwidth interface. Being agential/intelligent means that you do not track microstates—the communication channel does not preserve the lowest-level bits, but rather it preserves meaning or saliency. In biology, this is essential because the parts are never guaranteed to preserve low-level details: engineers make cables where the slightest change of pinout can destroy the functionality; biology assumes there will be “pins” missing, reversed, hacked, etc., and that it will have to spend effort to decode and extract meaning not dependent on the microstates of the message. This is why one can inject brain homogenates blindly into a recipient in a memory transfer experiment, and perhaps why you can randomize the pixel elements of a photo of a dog and an ANN can still recognize the dog-ness of it. |
| 31 | As Douglas Brash points out (personal communication), re-writable memory could be viewed as the art of building a new triggerable module. |
| 32 | The process of planarian regeneration, in which tiny pieces are able to restore an entire animal, is especially reminiscent of holographic properties [43], but all reproduction can be seen this way—restoring the entire body from one egg cell. |
| 33 | |
| 34 | |
| 35 | Indeed, one recent paper shows DNA damage and repair in the context of memory formation [161]. |
| 36 | This enormous compression is achieved because of the intelligence of both sides of the bowtie, and their incentive for mutual understanding. In general, there is a fascinating dynamic here between forces that incentivize clear communication (encodings that are understandable to future instances of oneself) and cryptographic resistance to hacking from future instances of others [165,166]. |
| 37 | It is possible that large groups work better than small ones for the Cross-Embryo Morphogenetic Assistance (CEMA, [167]) effect because bigger groups provide a more compelling, more fault-tolerant context for interpretation of the simple signals. With increased distance between the individuals, the latency makes it hard for the collective to maintain the bowtie architecture. |
| 38 | This is well recognized, for example, by SETI (Search for Extraterrestrial Intelligence) workers, who must grapple with the fact that messages from advanced beings will very likely look very random to us. |
| 39 | Pattern completion—filling in gaps (physical and informational) and out-painting—is a key aspect of machine learning and evolution of cognition [174]. |
| 40 | It is actually not despite them, but because of them. Asexual planaria accumulate mutations due to their somatic inheritance reproductive mode, and largely ignore them—evolution put all of its effort into an algorithm that can reliably make and remake a planarian body despite the extremely variable (mixoploid!) and unpredictable hardware. I have hypothesized that this is due to a competency ratchet in which the remapping process hides information from selection, putting all of the pressure onto the competency mechanisms, not the structural genome [176,177]. Because of their chaotic hardware, planaria commit to a large-scale body plan, not the details of the structural genome—an extreme type of morphogenetic intelligence in the sense of “same goal by different means”. They epitomize top-down control and sense-making in the form of extracting high-level wisdom from the low-level details provided by their genomic data. |
| 41 | As Chris Fields points out, “this kind of boundary/Markov blanket thinking says that a shared semantics can never be inferred from observations. It turns Wittgenstein’s private language argument on its head—languages are useless if they are only private, but mechanistically they are private. “Shared semantics” is a constantly evolving negotiation between language users” (personal communication). |
| 42 | This connects to ongoing debates about the status of an author’s intended meaning vs. that assigned by consumers of their work. We can visualize how semantic autonomy and deconstructive interpretation extends beyond written works to their antecedents—the dynamic patterns inside minds which progressively broaden out and change the more they interact with other agents, and even their author’s future Self, in a kind of (non-quantum, but perhaps related) decoherence. |
| 43 | |
| 44 | |
| 45 | In the same way, reservoir computing needs materials with high degrees of freedom. |
| 46 | Much like the eggshell and the egg’s maternal resources protect an embryo from the environment and harsh selection until its maturity, competency protects a fragile genome from selection too (and assimilation may “mature” it). |
| 47 | Some of this dynamic is captured by the emphasis of the extended evolutionary synthesis approach [190,191,192,193,194]. This is especially consistent with the concepts of constructive neutral evolution and the paradox of robustness developed by Susan Lindquist and Steve Frank [195,196,197,198,199,200,201,202,203,204,205,206,207,208]. In the evolutionary context, robust traits enable degeneracy and drift to accumulate underneath, providing a ratchet which stabilizes the trait (since it is now required to tame the noise underneath). This could work similarly with memories: once there are degeneracies in the generalization/mapping of events->engram, the agent becomes complex to be able to actively interpret them, and can no longer easily go backwards—a ratchet for minds as well as for DNA/phenotypes. |
| 48 | |
| 49 | |
| 50 | |
| 51 | |
| 52 | |
| 53 | Perhaps small implants are sometimes able to override the patterns of a much larger body [247] because they work harder—being at risk of extinction, maybe they exert more effort to hack their host (their niche) to persist. There are data showing that cells which are in their correct positions with their anatomical setpoints satisfied complacently ignore global signals that are actively processed by cells that are in a precarious or uncertain state [125,126,248]. |
| 54 | Moreover, the more robust, remappable, deep thoughts are the most agentially potent ones (it is not the physical agent that is robust; it is the behavioral, physiological, and anatomical memories that are). This view is the opposite of the superficially similar Dawkensian memes [249] because, I think, like in other aspects of biology, limiting oneself to a view in which propagating patterns are pawns of a purely mechanical process leads to many missed opportunities afforded by an agential lens [244,250]. |
| 55 | The shelf which helps Huygens clocks achieve synchrony is a very basal example of a low-bandwidth hub node that helps active agents transfer information. This leads to a further question of whether wave patterns need a medium that is waving, or whether, like with electromagnetic waves and their back-and-forth action between the electric and magnetic components, it can be a self-reinforcing pattern that needs no “cogniferous aether” in the form of a brain in which to persist. While resonance is one way to understand the interaction of the elements of thought, Von Foerster suggests another, more geometric formalism for the chemistry of thought: tiling, in which “cognitive tiles” tesselate into larger patterns [82]. |
| 56 | Niche construction is basically like stigmergy (memory), when we look at the evolutionary timescale. The actions and perceptions of an agent leave information in its environment, which then alters how it acts in the future—the environment is like a memory medium, external from the perspective of individual organisms but internal from the perspective of the lineage mind. |
| 57 | This is also a possible framework for understanding addictions to processes that remodel the mind to facilitate their own continuation. Another potential risk of systems that enable data to manipulate them is that this makes these systems susceptible to permanent changes [255]. |
| 58 | It has already been proposed [259], in the case of an evolutionary-scale thinker, that the fundamental units of evolution are metabolic and developmental interaction patterns. A relevant science-fiction story concerns some extremely dense beings emerging from the center of the Earth, to whom we surface-dwellers are part of the gas phase—in this story, these beings cannot see us. One of them claims that he has been studying ripples in this gas that almost look like they have a degree of agency, despite the fact that they only persist for ~100 years and are easily disrupted by his swirling, but the others do not believe him as patterns in a gas cannot be a “thing”, much less an agential being, and certainly not ones that are formed and disappear in what to them is the blink of an eye. Thus, whether one sees patterns, or the physical medium in which they exist, as the agent, is dependent on the cognitive properties of that observer. Extending this idea, one might imagine alien beings wanting to communicate with life on Earth—would they try talking to people, or to our cells, or to our financial system, or to our ecosystems? Every cognitive being will have a preferred vantage point from which to recognize other minds, and it is essential to note that such vantage points are just evolutionary user interfaces [70,71,72], not privileged/correct ways to pick the right level of organization and cognition. |
| 59 | Perhaps the dichotomy between goals and goal-havers could be made into a continuum in the same way. |
| 60 | Perhaps all of the “stable objects” (including organisms) that we see are just the low-dimensional hub nodes—the medium bearing the pattern—and it is the persistent, deep pattern that is the true agent. This links to ideas we are developing on the active Platonic space concept, which focuses attention on the patterns and their interactions, not the media that they temporarily animate. On this view, we are just slightly coarser variants of our memories, all essentially denizens of Platonic space, projected temporarily into low-dimensional media. |
| 61 | Regarding how differently and for how long, that is a matter of degree, determined by (and determining) the system’s cognitive light cone—the spatiotemporal distance of events, from the here and now, that are needed to have a powerful understanding of a system. |
| 62 | Polycomputing emphasizes both the read side of the interaction (where agents perceive and interpret others’ activity in whichever way they want) and the write side of the interaction (where agents use this information to optimally direct the behavior of other systems in ways that bring adaptive benefits to them). |
References
- Baluska, F.; Reber, A.S.; Miller, W.B., Jr. Cellular sentience as the primary source of biological order and evolution. Biosystems 2022, 218, 104694. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Miller, W.B.; Reber, A.S. Cellular and evolutionary perspectives on organismal cognition: From unicellular to multicellular organisms. Biol. J. Linn. Soc. 2023, 139, 503–513. [Google Scholar] [CrossRef]
- Reber, A.S.; Baluska, F. Cognition in some surprising places. Biochem. Biophys. Res. Commun. 2021, 564, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Baluška, F.; Levin, M. On Having No Head: Cognition throughout Biological Systems. Front. Psychol. 2016, 7, 902. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P.; Keijzer, F.; Arendt, D.; Levin, M. Reframing cognition: Getting down to biological basics. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20190750. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Keijzer, F.; Lyon, P.; Arendt, D. Uncovering cognitive similarities and differences, conservation and innovation. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2021, 376, 20200458. [Google Scholar] [CrossRef] [PubMed]
- Lyon, P. Of what is “minimal cognition” the half-baked version? Adapt. Behav. 2019, 28, 407–424. [Google Scholar] [CrossRef]
- Lyon, P. The cognitive cell: Bacterial behavior reconsidered. Front. Microbiol. 2015, 6, 264. [Google Scholar] [CrossRef]
- Lyon, P. The biogenic approach to cognition. Cogn. Process 2006, 7, 11–29. [Google Scholar] [CrossRef]
- Goodwin, B.C. A cognitive view of biological process. J. Social. Biol. Struct. 1978, 1, 117–125. [Google Scholar] [CrossRef]
- Solch, D. Wheeler and Whitehead: Process Biology and Process Philosophy in the Early Twentieth Century. J. Hist. Ideas 2016, 77, 489–507. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, A. Process and Reality: An Essay in Cosmology; Free Press: New York, NY, USA, 1978. [Google Scholar]
- Kapstein, M. Self, Non-self, and Personal Identity. In Oxford Bibliography; Oxford University Press: Oxford, UK, 2021. [Google Scholar] [CrossRef]
- James, W. The Principles of Psychology; H. Holt and Company: New York, NY, USA, 1890. [Google Scholar]
- Vervaeke, J.; Lillicrap, T.P.; Richards, B.A. Relevance Realization and the Emerging Framework in Cognitive Science. J. Log. Comput. 2009, 22, 79–99. [Google Scholar] [CrossRef]
- Fields, C.; Levin, M. How Do Living Systems Create Meaning? Philosophies 2020, 5, 36. [Google Scholar] [CrossRef]
- von Foerster, H. Logical Principles of Information Storage and Retrieval. In Electronic Handling of Information, Testing, and Evaluation; Academic Press: London, UK, 1967; Volume BCL Publication 150, pp. 123–147. [Google Scholar]
- Beer, R.D.; Di Paolo, E.A. The theoretical foundations of enaction: Precariousness. Biosystems 2023, 223, 104823. [Google Scholar] [CrossRef]
- Miller, W.B., Jr.; Baluska, F.; Reber, A.S.; Slijepcevic, P. Why death and aging ? All memories are imperfect. Prog. Biophys. Mol. Biol. 2024, 187, 21–35. [Google Scholar] [CrossRef] [PubMed]
- Man, K.; Damasio, A. Homeostasis and soft robotics in the design of feeling machines. Nat. Mach. Intell. 2019, 1, 446–452. [Google Scholar] [CrossRef]
- Jablonka, E.; Ginsburg, S. Learning and the Evolution of Conscious Agents. Biosemiotics 2022, 15, 401–437. [Google Scholar] [CrossRef]
- Froese, T. Life is Precious Because it is Precarious: Individuality, Mortality and the Problem of Meaning. In Representation and Reality in Humans, Other Living Organisms and Intelligent Machines; Dodig-Crnkovic, G., Giovagnoli, R., Eds.; Studies in Applied Philosophy, Epistemology and Rational Ethics; Springer International Publishing: Cham, Switzerland, 2017; pp. 33–50. [Google Scholar]
- Rosen, R. Anticipatory Systems in Retrospect and Prospect. Gen. Syst. 1979, 24, 11–23. [Google Scholar]
- Rosen, R. Anticipatory Systems: Philosophical, Mathematical, and Methodological Foundations, 1st ed.; Pergamon Press: Oxford, UK; New York, NY, USA, 1985; pp. 867–871. [Google Scholar]
- Gershenson, C. On the Scales of Selves: Information, Life, and Buddhist Philosophy. In Alife 2021: The 2021 Conference on Artificial Life; Čejková, J., Soros, S.H.L., Witkowski, O., Eds.; MIT Press: Cambridge, MA, USA, 2021. [Google Scholar]
- Blackiston, D.J.; Shomrat, T.; Levin, M. The stability of memories during brain remodeling: A perspective. Commun. Integr. Biol. 2015, 8, e1073424. [Google Scholar] [CrossRef]
- Blackiston, D.J.; Silva Casey, E.; Weiss, M.R. Retention of memory through metamorphosis: Can a moth remember what it learned as a caterpillar? PLoS ONE 2008, 3, e1736. [Google Scholar] [CrossRef]
- Gershman, S.J.; Monfils, M.H.; Norman, K.A.; Niv, Y. The computational nature of memory modification. Elife 2017, 6, e23763. [Google Scholar] [CrossRef] [PubMed]
- Dennett, D.C. Real Patterns. J. Philos. 1991, 88, 27–51. [Google Scholar] [CrossRef]
- Heylighen, F. Relational agency: A new ontology for co-evolving systems. In Evolution ‘On Purpose’: Teleonomy in Living Systems; Corning, P., Kauffman, S.A., Noble, D., Shapi, J.A., Vane-Wright, R.I., Pross, A., Eds.; MIT Press: Cambridge, MA, USA, 2023; pp. 79–104. [Google Scholar]
- Chemero, A. Radical Embodied Cognitive Science; MIT Press: Cambridge, MA, USA, 2009; pp. 145–150. [Google Scholar]
- Lebois, L.A.M.; Kaplan, C.S.; Palermo, C.A.; Pan, X.; Kaufman, M.L. A Grounded Theory of Dissociative Identity Disorder: Placing DID in Mind, Brain, and Body. In Dissociation and the Dissociative Disorders; Routledge: London, UK, 2022. [Google Scholar]
- Godfrey-Smith, P. Gradualism and the Evolution of Experience. Philos. Top. 2020, 48, 201–220. [Google Scholar] [CrossRef]
- Robins, S.K. Stable Engrams and Neural Dynamics. Philos. Sci. 2020, 87, 1130–1139. [Google Scholar] [CrossRef]
- Robins, S. The 21st century engram. Wiley Interdiscip. Rev. Cogn. Sci. 2023, 14, e1653. [Google Scholar] [CrossRef] [PubMed]
- Heylighen, F. The meaning and origin of goal-directedness: A dynamical systems perspective. Biol. J. Linn. Soc. 2023, 139, 370–387. [Google Scholar] [CrossRef]
- Artiga, M.; Birch, J.; Martinez, M. The meaning of biological signals. Stud. Hist. Philos. Biol. Biomed. Sci. 2020, 84, 101348. [Google Scholar] [CrossRef] [PubMed]
- Neuman, Y. “Meaning-making” in language and biology. Perspect. Biol. Med. 2005, 48, 317–327. [Google Scholar] [CrossRef]
- Barbieri, M. Biology with information and meaning. Hist. Philos. Life Sci. 2003, 25, 243–254. [Google Scholar] [CrossRef]
- Atlan, H. Self Creation of Meaning. Phys. Scr. 1987, 36, 563–576. [Google Scholar] [CrossRef]
- Kull, K. Semiosis stems from logical incompatibility in organic nature: Why biophysics does not see meaning, while biosemiotics does. Prog. Biophys. Mol. Biol. 2015, 119, 616–621. [Google Scholar] [CrossRef]
- Barbieri, M. Biosemiotics: A new understanding of life. Naturwissenschaften 2008, 95, 577–599. [Google Scholar] [CrossRef]
- Pietsch, P. Shufflebrain; Houghton Mifflin: Boston, MA, USA, 1981; 273p. [Google Scholar]
- Torday, J.S.; Miller, W.B., Jr. The resolution of ambiguity as the basis for life: A cellular bridge between Western reductionism and Eastern holism. Prog. Biophys. Mol. Biol. 2017, 131, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. Is the free-energy principle neurocentric? Nat. Rev. Neurosci. 2010, 11, 605. [Google Scholar] [CrossRef]
- Constant, A.; Ramstead, M.J.D.; Veissiere, S.P.L.; Campbell, J.O.; Friston, K.J. A variational approach to niche construction. J. R. Soc. Interface 2018, 15. [Google Scholar] [CrossRef]
- Allen, M.; Friston, K.J. From cognitivism to autopoiesis: Towards a computational framework for the embodied mind. Synthese 2018, 195, 2459–2482. [Google Scholar] [CrossRef]
- Niizato, T.; Nishiyama, Y.; Sakamoto, K.; Kazama, T.; Okabayashi, T.; Yamaguchi, T. The ambiguous feeling between “mine” and “not-mine” measured by integrated information theory during rubber hand illusion. Sci. Rep. 2022, 12, 18002. [Google Scholar] [CrossRef]
- Ramakonar, H.; Franz, E.A.; Lind, C.R. The rubber hand illusion and its application to clinical neuroscience. J. Clin. Neurosci. 2011, 18, 1596–1601. [Google Scholar] [CrossRef] [PubMed]
- Kieliba, P.; Clode, D.; Maimon-Mor, R.O.; Makin, T.R. Robotic hand augmentation drives changes in neural body representation. Sci. Robot. 2021, 6, eabd7935. [Google Scholar] [CrossRef] [PubMed]
- Shull, P.B.; Damian, D.D. Haptic wearables as sensory replacement, sensory augmentation and trainer—A review. J. Neuroeng. Rehabil. 2015, 12, 59. [Google Scholar] [CrossRef]
- Hassan, A.; Josephs, K.A. Alien Hand Syndrome. Curr. Neurol. Neurosci. Rep. 2016, 16, 73. [Google Scholar] [CrossRef]
- Miller, W.B., Jr.; Torday, J.S.; Baluška, F. Biological evolution as defense of ‘self’. Prog. Biophys. Mol. Biol. 2019, 142, 54–74. [Google Scholar] [CrossRef]
- Hofstadter, D.R. I Am a Strange Loop; Basic Books: New York, NY, USA, 2007; 436p. [Google Scholar]
- McGilchrist, I. The Master and His Emissary: The Divided Brain and the Making of the Western World; Yale University Press: New Haven, CT, USA, 2019; p. 597. [Google Scholar]
- Varela, F.J.; Thompson, E.; Rosch, E. The Embodied Mind: Cognitive Science and Human Experience; MIT Press: Cambridge, MA, USA, 1991; 392p. [Google Scholar]
- Maturana, H.R.; Varela, F.J. Autopoiesis and Cognition: The Realization of the Living; Reidel, D., Ed.; Pub. Co.: Dordrecht, The Netherlands; Boston, MA, USA, 1980; 141p. [Google Scholar]
- Bongard, J.; Levin, M. There’s Plenty of Room Right Here: Biological Systems as Evolved, Overloaded, Multi-Scale Machines. Biomimetics 2023, 8, 110. [Google Scholar] [CrossRef]
- Fried, I.; Wilson, C.L.; MacDonald, K.A.; Behnke, E.J. Electric current stimulates laughter. Nature 1998, 391, 650. [Google Scholar] [CrossRef]
- Desmurget, M.; Reilly, K.T.; Richard, N.; Szathmari, A.; Mottolese, C.; Sirigu, A. Movement intention after parietal cortex stimulation in humans. Science 2009, 324, 811–813. [Google Scholar] [CrossRef]
- Serino, A.; Bockbrader, M.; Bertoni, T.; Colachis Iv, S.; Solcà, M.; Dunlap, C.; Eipel, K.; Ganzer, P.; Annetta, N.; Sharma, G.; et al. Sense of agency for intracortical brain-machine interfaces. Nat. Hum. Behav. 2022, 6, 565–578. [Google Scholar] [CrossRef]
- Keeling, S. Confabulation and rational obligations for self-knowledge. Philos. Psychol. 2018, 31, 1215–1238. [Google Scholar] [CrossRef]
- Sacks, O.W. The Man Who Mistook His Wife for a Hat and Other Clinical Tales; Simon & Schuster: New York, NY, USA, 1998. [Google Scholar]
- Dennett, D.C. Consciousness Explained; Brown, L., Ed.; Penguin Co.: Boston, MA, USA, 1991. [Google Scholar]
- Seth, A.K. Being You: A New Science of Consciousness; Dutton, an imprint of Penguin Random House LLC: New York, NY, USA, 2021. [Google Scholar]
- Jahn, C.I.; Markov, N.T.; Morea, B.; Daw, N.D.; Ebitz, R.B.; Buschman, T.J. Learning attentional templates for value-based decision-making. Cell 2024, 187, 1476–1489.e1421. [Google Scholar] [CrossRef]
- Solms, M. The statistical mechanics of felt uncertainty under active inference. Behav. Brain Sci. 2023, 46, e108. [Google Scholar] [CrossRef]
- Friston, K. The free-energy principle: A unified brain theory? Nat. Rev. Neurosci. 2010, 11, 127–138. [Google Scholar] [CrossRef]
- Osorio-Gomez, D.; Miranda, M.I.; Guzman-Ramos, K.; Bermudez-Rattoni, F. Transforming experiences: Neurobiology of memory updating/editing. Front. Syst. Neurosci. 2023, 17, 1103770. [Google Scholar] [CrossRef]
- Hoffman, D.D. The Case Against Reality: Why Evolution Hid the Truth from Our Eyes; WW Norton & Company: New York, NY, USA, 2019. [Google Scholar]
- Hoffman, D.D. The Interface Theory of Perception. In Stevens’ Handbook of Experimental Psychology and Cognitive Neuroscience; Stevens, Ed.; Wiley Online Library: Hoboken, NJ, USA, 2017. [Google Scholar]
- Prakash, C.; Fields, C.; Hoffman, D.D.; Prentner, R.; Singh, M. Fact, Fiction, and Fitness. Entropy 2020, 22, 514. [Google Scholar] [CrossRef]
- Friston, K.J.; Stephan, K.E.; Montague, R.; Dolan, R.J. Computational psychiatry: The brain as a phantastic organ. Lancet Psychiatry 2014, 1, 148–158. [Google Scholar] [CrossRef]
- Seth, A.K. Interoceptive inference, emotion, and the embodied self. Trends Cogn. Sci. 2013, 17, 565–573. [Google Scholar] [CrossRef]
- Semon, R.W.; Simon, L. The Mneme; The Macmillan Company: London, UK; New York, NY, USA, 1921; 304p. [Google Scholar]
- Heylighen, F. Stigmergy as a universal coordination mechanism II: Varieties and evolution. Cogn. Syst. Res. 2016, 38, 50–59. [Google Scholar] [CrossRef]
- Heylighen, F. Stigmergy as a universal coordination mechanism I: Definition and components. Cogn. Syst. Res. 2016, 38, 4–13. [Google Scholar] [CrossRef]
- Skyrms, B. Evolution of signalling systems with multiple senders and receivers. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009, 364, 771–779. [Google Scholar] [CrossRef] [PubMed]
- Martinez, M. Deception as cooperation. Stud. Hist. Philos. Biol. Biomed. Sci. 2019, 77, 101184. [Google Scholar] [CrossRef]
- Planer, R.J.; Godfrey-Smith, P. Communication and representation understood as sender–receiver coordination. Mind Lang. 2020, 36, 750–770. [Google Scholar] [CrossRef]
- Chater, N. The Mind Is Flat: The Illusion of Mental Depth and the Improvised Mind; Allen Lane: London, UK, 2018; 251p. [Google Scholar]
- von Foerster, H. What is memory that it may have hindsight and foresight as well? In The Future of the Brain Sciences; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1969; pp. 19–65. [Google Scholar]
- Nagel, T. What is it like to be a bat? Philos. Rev. 1974, 83, 435–450. [Google Scholar] [CrossRef]
- Dennett, D.C. Who’s on First? Heterophenomenology Explained. J. Conscious. Stud. 2003, 10, 19–30. [Google Scholar]
- Hofstadter, D.R.; Mitchell, M. The Copycat project: A model of mental fluidity and analogy-making. In Analogical Connections; Advances in connectionist and neural computation theory; Ablex Publishing: Westport, CT, USA, 1994; Volume 2, pp. 31–112. [Google Scholar]
- Gershenson, C. Complexity, Artificial Life, and Artificial Intelligence. Preprints 2024, 2024041826. [Google Scholar] [CrossRef]
- Hofstadter, D.R. Godel, Escher, Bach: An Eternal Golden Braid; Basic Books: New York, NY, USA, 1979. [Google Scholar]
- Alloway, T.M. Retention of Learning through Metamorphosis in Grain Beetle (Tenebrio-Molitor). Am. Zool. 1972, 12, 471–472. [Google Scholar] [CrossRef]
- Sheiman, I.M.; Tiras, K.L. Memory and morphogenesis in planaria and beetle. In Russian Contributions to Invertebrate Behavior, Abramson, C.I., Shuranova, Z.P., Burmistrov, Y.M., Eds.; Praeger: Westport, CT, USA, 1996; pp. 43–76. [Google Scholar]
- Ray, S. Survival of olfactory memory through metamorphosis in the fly. Neurosci. Lett. 1999, 259, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Punzo, F.; Malatesta, R.J. Brain-Rna Synthesis and the Retention of Learning through Metamorphosis in Tenebrio-Obscurus (Insecta, Coleoptera). Comp. Biochem. Physiol. A-Mol. Integr. Physiol. 1988, 91, 675–678. [Google Scholar] [CrossRef] [PubMed]
- Goldsmith, C.M.; Hepburn, H.R.; Mitchell, D. Retention of an Associative Learning-Task after Metamorphosis in Locusta-Migratoria-Migratorioides. J. Insect Physiol. 1978, 24, 737–741. [Google Scholar] [CrossRef]
- Miller, R.R.; Berk, A.M. Retention over metamorphosis in the African claw-toed frog. J. Exp. Psychol. Anim. Behav. Process. 1977, 3, 343–356. [Google Scholar] [CrossRef]
- von Foerster, H.; Inselberg, A.; Weston, P. Memory and Inductive Inference. In Bionics Symposium 1966: Cybernetic Problems in Bionics; New York, NY, USA; Dayton, OH, USA, 1966; pp. 31–68. [Google Scholar]
- von Foerster, H. Memory without Record. In Learning, Remembering, and Forgetting: Volume I; Kimble, D.P., Ed.; Science and Behavior Books: Palo Alto, CA, USA, 1965; Volume BCL Publication 120, pp. 388–433. [Google Scholar]
- Fields, C.; Levin, M. Competency in Navigating Arbitrary Spaces as an Invariant for Analyzing Cognition in Diverse Embodiments. Entropy 2022, 24, 819. [Google Scholar] [CrossRef]
- Fields, C.; Bischof, J.; Levin, M. Morphological Coordination: A Common Ancestral Function Unifying Neural and Non-Neural Signaling. Physiology 2020, 35, 16–30. [Google Scholar] [CrossRef]
- Sole, R.; Moses, M.; Forrest, S. Liquid brains, solid brains. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20190040. [Google Scholar] [CrossRef]
- Levin, M. Bioelectric signaling: Reprogrammable circuits underlying embryogenesis, regeneration, and cancer. Cell 2021, 184, 1971–1989. [Google Scholar] [CrossRef]
- Pai, V.P.; Aw, S.; Shomrat, T.; Lemire, J.M.; Levin, M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development 2012, 139, 313–323. [Google Scholar] [CrossRef] [PubMed]
- Bedard-Gilligan, M.; Zoellner, L.A.; Feeny, N.C. Is Trauma Memory Special? Trauma Narrative Fragmentation in PTSD: Effects of Treatment and Response. Clin. Psychol. Sci. 2017, 5, 212–225. [Google Scholar] [CrossRef] [PubMed]
- van der Hart, O.; Bolt, H.; van der Kolk, B.A. Memory fragmentation in dissociative identity disorder. J. Trauma. Dissociation 2005, 6, 55–70. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, B.A.; Fisler, R. Dissociation and the fragmentary nature of traumatic memories: Overview and exploratory study. J. Trauma. Stress 1995, 8, 505–525. [Google Scholar] [CrossRef] [PubMed]
- van der Kolk, B.A. The body keeps the score: Memory and the evolving psychobiology of posttraumatic stress. Harv. Rev. Psychiatry 1994, 1, 253–265. [Google Scholar] [CrossRef] [PubMed]
- Bridge, D.J.; Paller, K.A. Neural correlates of reactivation and retrieval-induced distortion. J. Neurosci. 2012, 32, 12144–12151. [Google Scholar] [CrossRef] [PubMed]
- Thakral, P.P.; Barberio, N.M.; Devitt, A.L.; Schacter, D.L. Constructive episodic retrieval processes underlying memory distortion contribute to creative thinking and everyday problem solving. Mem. Cogn. 2023, 51, 1125–1144. [Google Scholar] [CrossRef] [PubMed]
- Luriia, A.R. The Mind of a Mnemonist: A Little Book About a Vast Memory; Harvard University Press: Cambridge, MA, USA, 1987. [Google Scholar]
- Trivers, R. The Folly of Fools: The Logic of Deceit and Self-Deception in Human Life; Basic Books: New York, NY, USA, 2011. [Google Scholar]
- Nisbett, R.E.; Wilson, T.D. Telling More Than We Can Know—Verbal Reports on Mental Processes. Psychol. Rev. 1977, 84, 231–259. [Google Scholar] [CrossRef]
- Stammers, S.; Bortolotti, L. Introduction: Philosophical Perspectives on Confabulation. Topoi-Int. Rev. Philos. 2020, 39, 115–119. [Google Scholar] [CrossRef]
- Byrne, W.L.; Samuel, D.; Bennett, E.L.; Rosenzweig, M.R.; Wasserman, E. Memory transfer. Science 1966, 153, 658–659. [Google Scholar] [CrossRef] [PubMed]
- Mcgaugh, J.L. Analysis of Memory Transfer and Enhancement. Proc. Am. Philos. Soc. 1967, 111, 347–351. [Google Scholar]
- Bedecarrats, A.; Chen, S.; Pearce, K.; Cai, D.; Glanzman, D.L. RNA from Trained Aplysia Can Induce an Epigenetic Engram for Long-Term Sensitization in Untrained Aplysia. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Reinis, S. Analysis of “memory transfer” by puromycin. Act. Nerv. Super. 1970, 12, 289–294. [Google Scholar]
- Frank, B.; Stein, D.G.; Rosen, J. Interanimal “memory” transfer: Results from brain and liver homogenates. Science 1970, 169, 399–402. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, H.; Tablante, A. Behavioral transfer in praying mantis by injection of brain homogenate. Physiol. Behav. 1976, 16, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Miller, B.E.; Holt, G.L. Memory transfer in rats by injection of brain and liver RNA. J. Biol. Psychol. 1977, 19, 4–9. [Google Scholar]
- Holt, G.L.; Bentz, G. Interanimal memory transfer of a barpress response through brain and liver RNA injections. Bull. Psychon. Soc. 1983, 21, 51–53. [Google Scholar] [CrossRef]
- Ungar, G.; Irwin, L.N. Transfer of acquired information by brain extracts. Nature 1967, 214, 453–455. [Google Scholar] [CrossRef]
- Liester, M.B. Personality changes following heart transplantation: The role of cellular memory. Med. Hypotheses 2020, 135, 109468. [Google Scholar] [CrossRef]
- Pearsall, P.; Schwartz, G.E.; Russek, L.G. Changes in heart transplant recipients that parallel the personalities of their donors. Integr. Med. 2000, 2, 65–72. [Google Scholar] [CrossRef]
- Hepper, P.G.; Waldman, B. Embryonic olfactory learning in frogs. Q. J. Exp. Psychol. B 1992, 44, 179–197. [Google Scholar] [CrossRef]
- Pezzulo, G.; Levin, M. Re-membering the body: Applications of computational neuroscience to the top-down control of regeneration of limbs and other complex organs. Integr. Biol. 2015, 7, 1487–1517. [Google Scholar] [CrossRef]
- Levin, M. Technological Approach to Mind Everywhere: An Experimentally-Grounded Framework for Understanding Diverse Bodies and Minds. Front. Syst. Neurosci. 2022, 16, 768201. [Google Scholar] [CrossRef]
- Tseng, A.S.; Beane, W.S.; Lemire, J.M.; Masi, A.; Levin, M. Induction of vertebrate regeneration by a transient sodium current. J. Neurosci. 2010, 30, 13192–13200. [Google Scholar] [CrossRef]
- Tseng, A.; Levin, M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun. Integr. Biol. 2013, 6, e22595. [Google Scholar] [CrossRef] [PubMed]
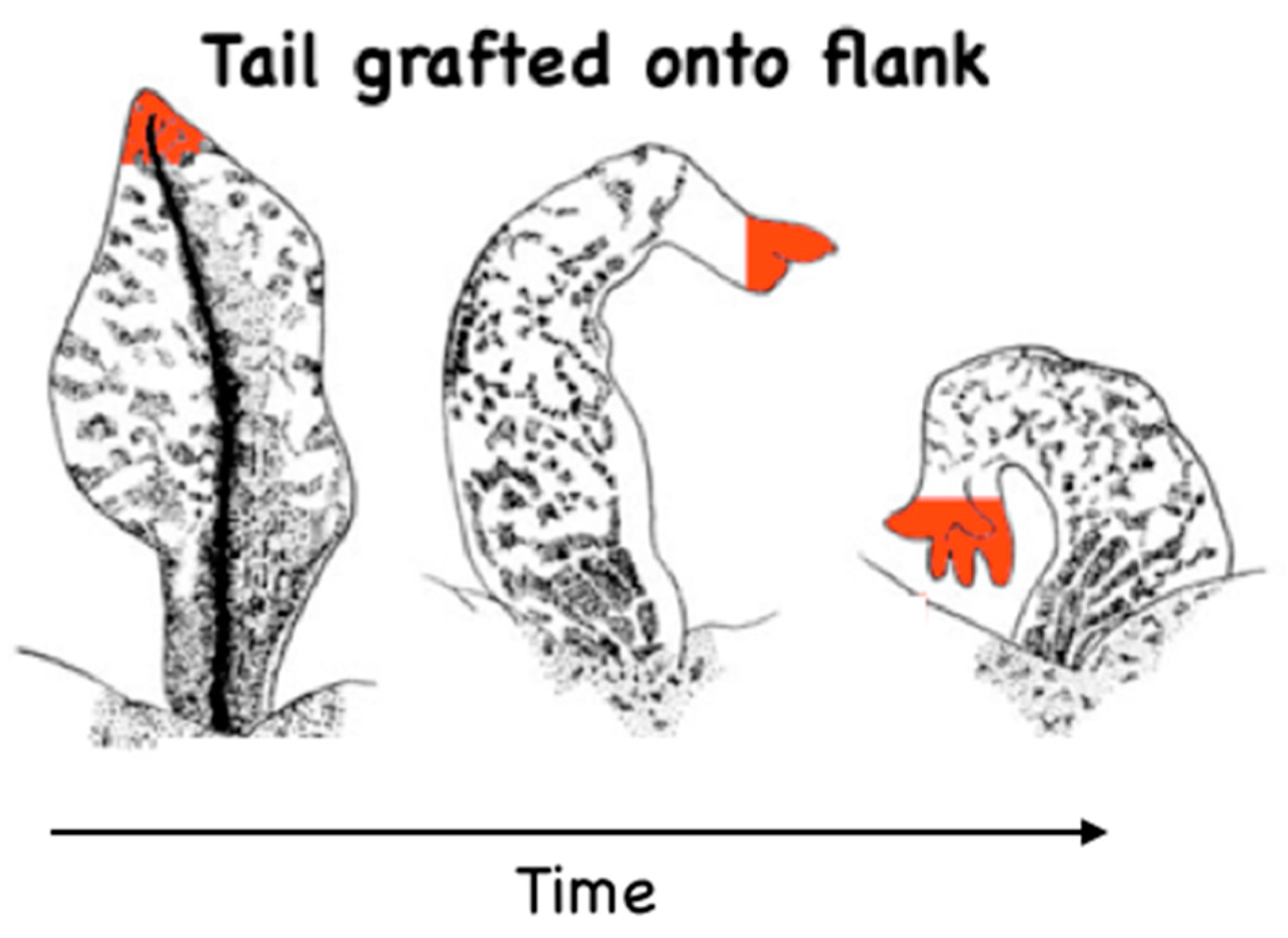
- Farinella-Ferruzza, N. The transformation of a tail into a limb after xenoplastic transformation. Experientia 1956, 15, 304–305. [Google Scholar] [CrossRef]
- Farinella-Ferruzza, N. Risultati di trapianti di bottone codale di urodeli su anuri e vice versa. Riv. Biol. 1953, 45, 523–527. [Google Scholar]
- Holtfreter, J. Transformation of a Tail into a Limb or Gill-Like Structures. J. Exp. Zool. 1955, 129, 623–648. [Google Scholar] [CrossRef]
- Jablonka, E. The evolutionary implications of epigenetic inheritance. Interface Focus 2017, 7, 20160135. [Google Scholar] [CrossRef] [PubMed]
- Bourrat, P.; Lu, Q.; Jablonka, E. Why the missing heritability might not be in the DNA. BioEssays 2017, 39, 1700067. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. The inheritance of acquired epigenetic variations. Int. J. Epidemiol. 2015, 44, 1094–1103. [Google Scholar] [CrossRef] [PubMed]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef] [PubMed]
- Pakos-Zebrucka, K.; Koryga, I.; Mnich, K.; Ljujic, M.; Samali, A.; Gorman, A.M. The integrated stress response. EMBO Rep. 2016, 17, 1374–1395. [Google Scholar] [CrossRef] [PubMed]
- Costa-Mattioli, M.; Walter, P. The integrated stress response: From mechanism to disease. Science 2020, 368, eaat5314. [Google Scholar] [CrossRef] [PubMed]
- Boone, M.; Zappa, F. Signaling plasticity in the integrated stress response. Front. Cell Dev. Biol. 2023, 11, 1271141. [Google Scholar] [CrossRef] [PubMed]
- Ryoo, H.D. The integrated stress response in metabolic adaptation. J. Biol. Chem. 2024, 300, 107151. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Levin, M. Ectopic eyes outside the head in Xenopus tadpoles provide sensory data for light-mediated learning. J. Exp. Biol. 2013, 216, 1031–1040. [Google Scholar] [CrossRef] [PubMed]
- Lovas, J.R.; Yuste, R. Dissociation and reaggregation of Hydra vulgaris for studies of self-organization. STAR Protoc. 2022, 3, 101504. [Google Scholar] [CrossRef]
- Martín-Durán, J.M. General Principles of Planarian Embryogenesis and Its Analysis by In Situ Hybridization and Immunohistochemistry Methods. In Planarian Regeneration: Methods and Protocols; Rink, J.C., Ed.; Springer New York: New York, NY, USA, 2018; pp. 405–421. [Google Scholar]
- Blackiston, D.; Lederer, E.; Kriegman, S.; Garnier, S.; Bongard, J.; Levin, M. A cellular platform for the development of synthetic living machines. Sci. Robot. 2021, 6, eabf1571. [Google Scholar] [CrossRef]
- Fankhauser, G. Maintenance of normal structure in heteroploid salamander larvae, through compensation of changes in cell size by adjustment of cell number and cell shape. J. Exp. Zool. 1945, 100, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Fankhauser, G. The Effects of Changes in Chromosome Number on Amphibian Development. Q. Rev. Biol. 1945, 20, 20–78. [Google Scholar] [CrossRef]
- Thornton, C. Predictive processing simplified: The infotropic machine. Brain Cogn. 2017, 112, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Falandays, J.B.; Nguyen, B.; Spivey, M.J. Is prediction nothing more than multi-scale pattern completion of the future? Brain Res. 2021, 1768, 147578. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J.; Webber, J.A. Dynamics of the Control of Body Pattern in the Development of Xenopus-Laevis.1. Timing and Pattern in the Development of Dorsoanterior and Posterior Blastomere Pairs, Isolated at the 4-Cell Stage. J. Embryol. Exp. Morphol. 1985, 88, 85–112. [Google Scholar] [CrossRef] [PubMed]
- Cooke, J. Scale of body pattern adjusts to available cell number in amphibian embryos. Nature 1981, 290, 775–778. [Google Scholar] [PubMed]
- Cooke, J. Cell number in relation to primary pattern formation in the embryo of Xenopus laevis. I: The cell cycle during new pattern formation in response to implanted organisers. J. Embryol. Exp. Morphol. 1979, 51, 165–182. [Google Scholar]
- Blackiston, D.; Kriegman, S.; Bongard, J.; Levin, M. Biological Robots: Perspectives on an Emerging Interdisciplinary Field. Soft Robot. 2023, 10, 674–686. [Google Scholar] [CrossRef] [PubMed]
- Kriegman, S.; Blackiston, D.; Levin, M.; Bongard, J. Kinematic self-replication in reconfigurable organisms. Proc. Natl. Acad. Sci. USA 2021, 118, e2112672118. [Google Scholar] [CrossRef]
- Kriegman, S.; Blackiston, D.; Levin, M.; Bongard, J. A scalable pipeline for designing reconfigurable organisms. Proc. Natl. Acad. Sci. USA 2020, 117, 1853–1859. [Google Scholar] [CrossRef]
- Gumuskaya, G.; Srivastava, P.; Cooper, B.G.; Lesser, H.; Semegran, B.; Garnier, S.; Levin, M. Motile Living Biobots Self-Construct from Adult Human Somatic Progenitor Seed Cells. Adv. Sci. 2024, 11, e2303575. [Google Scholar] [CrossRef]
- Mani, M.S. Ecology of plant galls. Monogr. Biol. 1964, 1. online resource. [Google Scholar] [CrossRef]
- Nanos, V.; Levin, M. Multi-scale Chimerism: An experimental window on the algorithms of anatomical control. Cells Dev. 2022, 169, 203764. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.G.; Huang, M.S.; Li, T.L.; Feig, V.R.; Jiang, Y.; Cui, B.; Greely, H.T.; Bao, Z.; Pasca, S.P.; Heilshorn, S.C. Advancing models of neural development with biomaterials. Nat. Rev. Neurosci. 2021, 22, 593–615. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Wan, X.; Wang, Z.L.; Li, L. Electroactive Biomaterials and Systems for Cell Fate Determination and Tissue Regeneration: Design and Applications. Adv. Mater. 2021, 33, e2007429. [Google Scholar] [CrossRef]
- Bernheim-Groswasser, A.; Gov, N.S.; Safran, S.A.; Tzlil, S. Living Matter: Mesoscopic Active Materials. Adv. Mater. 2018, 30, e1707028. [Google Scholar] [CrossRef]
- Gil, E.S.; Panilaitis, B.; Bellas, E.; Kaplan, D.L. Functionalized silk biomaterials for wound healing. Adv. Healthc. Mater. 2013, 2, 206–217. [Google Scholar] [CrossRef] [PubMed]
- Muskovich, M.; Bettinger, C.J. Biomaterials-based electronics: Polymers and interfaces for biology and medicine. Adv. Healthc. Mater. 2012, 1, 248–266. [Google Scholar] [CrossRef]
- Cohen-Karni, T.; Langer, R.; Kohane, D.S. The smartest materials: The future of nanoelectronics in medicine. ACS Nano 2012, 6, 6541–6545. [Google Scholar] [CrossRef]
- Jovasevic, V.; Wood, E.M.; Cicvaric, A.; Zhang, H.; Petrovic, Z.; Carboncino, A.; Parker, K.K.; Bassett, T.E.; Moltesen, M.; Yamawaki, N.; et al. Formation of memory assemblies through the DNA-sensing TLR9 pathway. Nature 2024, 628, 145–153. [Google Scholar] [CrossRef]
- Doyle, J.C.; Csete, M. Architecture, constraints, and behavior. Proc. Natl. Acad. Sci. USA 2011, 108 (Suppl. 3), 15624–15630. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.; Doyle, J. Bow ties, metabolism and disease. Trends Biotechnol. 2004, 22, 446–450. [Google Scholar] [CrossRef] [PubMed]
- Csete, M.E.; Doyle, J.C. Reverse engineering of biological complexity. Science 2002, 295, 1664–1669. [Google Scholar] [CrossRef] [PubMed]
- Krakauer, D. Cryptographic Nature. arXiv 2015, arXiv:1505.01744. [Google Scholar] [CrossRef]
- Levin, M. The evolution of understanding: A genetic algorithm model of the evolution of communication. Biosystems 1995, 36, 167–178. [Google Scholar] [CrossRef] [PubMed]
- Tung, A.; Sperry, M.M.; Clawson, W.; Pavuluri, A.; Bulatao, S.; Yue, M.; Flores, R.M.; Pai, V.P.; McMillen, P.; Kuchling, F.; et al. Embryos assist morphogenesis of others through calcium and ATP signaling mechanisms in collective teratogen resistance. Nat. Commun. 2024, 15, 535. [Google Scholar] [CrossRef] [PubMed]
- Derrida, J. Of Grammatology, 1st ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1976; 560p. [Google Scholar]
- Truby, J. The Anatomy of Story: 22 Steps to Becoming a Master Storyteller, 1st ed.; Faber and Faber: New York, NY, USA, 2008; 445p. [Google Scholar]
- Kuchling, F.; Friston, K.; Georgiev, G.; Levin, M. Integrating variational approaches to pattern formation into a deeper physics: Reply to comments on “Morphogenesis as Bayesian inference: A variational approach to pattern formation and manipulation in complex biological systems”. Phys. Life Rev. 2020, 33, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kuchling, F.; Friston, K.; Georgiev, G.; Levin, M. Morphogenesis as Bayesian inference: A variational approach to pattern formation and control in complex biological systems. Phys. Life Rev. 2020, 33, 88–108. [Google Scholar] [CrossRef]
- Annila, A. The art of abstraction: Comment on “Morphogenesis as Bayesian inference: A variational approach to pattern formation and control in complex biological systems” by F. Kuchling, K. Friston, G. Georgiev, M. Levin. Phys. Life Rev. 2020, 33, 119–120. [Google Scholar] [CrossRef]
- Friston, K.; Levin, M.; Sengupta, B.; Pezzulo, G. Knowing one’s place: A free-energy approach to pattern regulation. J. R. Soc. Interface 2015, 12, 20141383. [Google Scholar] [CrossRef]
- Levin, M.; Yuste, R. Modular Cognition. Aeon Essays. 2022. Available online: https://aeon.co/essays/how-evolution-hacked-its-way-to-intelligence-from-the-bottom-up (accessed on 2 May 2024).
- Pianka, E.R. On r- and K-Selection. Am. Nat. 1970, 104, 592–597. [Google Scholar] [CrossRef]
- Shreesha, L.; Levin, M. Cellular Competency during Development Alters Evolutionary Dynamics in an Artificial Embryogeny Model. Entropy 2023, 25, 131. [Google Scholar] [CrossRef] [PubMed]
- Levin, M.; Pietak, A.M.; Bischof, J. Planarian regeneration as a model of anatomical homeostasis: Recent progress in biophysical and computational approaches. Semin. Cell Dev. Biol. 2019, 87, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Dor, D. The Instruction of Imagination: Language as a Social Communication Technology; Oxford University Press: Oxford, UK; New York, NY, USA, 2015. [Google Scholar]
- Veissière, S.P.L.; Constant, A.; Ramstead, M.J.D.; Friston, K.J.; Kirmayer, L.J. Thinking through other minds: A variational approach to cognition and culture. Behav. Brain Sci. 2019, 43, e90. [Google Scholar] [CrossRef] [PubMed]
- Vasil, J.; Badcock, P.B.; Constant, A.; Friston, K.; Ramstead, M.J.D. A World Unto Itself: Human Communication as Active Inference. Front. Psychol. 2020, 11, 417. [Google Scholar] [CrossRef] [PubMed]
- Wess, O.; Roder, U. A holographic model for associative memory chains. Biol. Cybern. 1977, 27, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Poggio, T. On holographic models of memory. Kybernetik 1973, 12, 237–238. [Google Scholar] [CrossRef] [PubMed]
- Gabor, D. Improved holographic model of temporal recall. Nature 1968, 217, 1288–1289. [Google Scholar] [CrossRef]
- Chopping, P.T. Holographic model of temporal recall. Nature 1968, 217, 781–782. [Google Scholar] [CrossRef]
- Yngve, V.H. A Model and an Hypothesis for Language Structure. Proc. Am. Philos. Soc. 1960, 104, 444–466. [Google Scholar]
- Feduccia, A.A.; Mithoefer, M.C. MDMA-assisted psychotherapy for PTSD: Are memory reconsolidation and fear extinction underlying mechanisms? Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 84, 221–228. [Google Scholar] [CrossRef]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Raut, S.B.; Marathe, P.A.; van Eijk, L.; Eri, R.; Ravindran, M.; Benedek, D.M.; Ursano, R.J.; Canales, J.J.; Johnson, L.R. Diverse therapeutic developments for post-traumatic stress disorder (PTSD) indicate common mechanisms of memory modulation. Pharmacol. Ther. 2022, 239, 108195. [Google Scholar] [CrossRef]
- Sarmanlu, M.; Kuypers, K.P.C.; Vizeli, P.; Kvamme, T.L. MDMA-assisted psychotherapy for PTSD: Growing evidence for memory effects mediating treatment efficacy. Prog. Neuropsychopharmacol. Biol. Psychiatry 2024, 128, 110843. [Google Scholar] [CrossRef]
- Muller, G.B. Why an extended evolutionary synthesis is necessary. Interface Focus. 2017, 7, 20170015. [Google Scholar] [CrossRef] [PubMed]
- Futuyma, D.J. Evolutionary biology today and the call for an extended synthesis. Interface Focus. 2017, 7, 20160145. [Google Scholar] [CrossRef] [PubMed]
- Calvey, T. The extended evolutionary synthesis and addiction: The price we pay for adaptability. Prog. Brain Res. 2017, 235, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Lindholm, M. DNA Dispose, but Subjects Decide. Learning and the Extended Synthesis. Biosemiotics 2015, 8, 443–461. [Google Scholar] [CrossRef]
- Laland, K.N.; Uller, T.; Feldman, M.W.; Sterelny, K.; Müller, G.B.; Moczek, A.; Jablonka, E.; Odling-Smee, J. The extended evolutionary synthesis: Its structure, assumptions and predictions. Proc. Biol. Sci. 2015, 282, 20151019. [Google Scholar] [CrossRef]
- Shorter, J.; Lindquist, S. Prions as adaptive conduits of memory and inheritance. Nat. Rev. Genet. 2005, 6, 435–450. [Google Scholar] [CrossRef]
- Jarosz, D.F.; Taipale, M.; Lindquist, S. Protein homeostasis and the phenotypic manifestation of genetic diversity: Principles and mechanisms. Annu. Rev. Genet. 2010, 44, 189–216. [Google Scholar] [CrossRef] [PubMed]
- Taipale, M.; Jarosz, D.F.; Lindquist, S. HSP90 at the hub of protein homeostasis: Emerging mechanistic insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.L.; Lindquist, S. Hsp90 as a capacitor for morphological evolution. Nature 1998, 396, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Queitsch, C.; Sangster, T.A.; Lindquist, S. Hsp90 as a capacitor of phenotypic variation. Nature 2002, 417, 618–624. [Google Scholar] [CrossRef] [PubMed]
- Sollars, V.; Lu, X.; Xiao, L.; Wang, X.; Garfinkel, M.D.; Ruden, D.M. Evidence for an epigenetic mechanism by which Hsp90 acts as a capacitor for morphological evolution. Nat. Genet. 2003, 33, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, S.; Hirate, Y.; Swalla, B.J. The Hsp90 capacitor, developmental remodeling, and evolution: The robustness of gene networks and the curious evolvability of metamorphosis. Crit. Rev. Biochem. Mol. Biol. 2007, 42, 355–372. [Google Scholar] [CrossRef] [PubMed]
- Yeyati, P.L.; van Heyningen, V. Incapacitating the evolutionary capacitor: Hsp90 modulation of disease. Curr. Opin. Genet. Dev. 2008, 18, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A.; Bascompte, J. Invariance in ecological pattern. F1000Res 2019, 8, 2093. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A. Evolutionary design of regulatory control. I. A robust control theory analysis of tradeoffs. J. Theor. Biol. 2019, 463, 121–137. [Google Scholar] [CrossRef]
- Frank, S.A. Evolutionary design of regulatory control. II. Robust error-correcting feedback increases genetic and phenotypic variability. J. Theor. Biol. 2019, 468, 72–81. [Google Scholar] [CrossRef]
- Frank, S.A. Measurement invariance explains the universal law of generalization for psychological perception. Proc. Natl. Acad. Sci. USA 2018, 115, 9803–9806. [Google Scholar] [CrossRef]
- Frank, S.A. Evolution of robustness and cellular stochasticity of gene expression. PLoS Biol. 2013, 11, e1001578. [Google Scholar] [CrossRef] [PubMed]
- Frank, S.A. Natural selection. III. Selection versus transmission and the levels of selection. J. Evol. Biol. 2012, 25, 227–243. [Google Scholar] [CrossRef]
- Haig, D. From Darwin to Derrida: Selfish Genes, Social Selves, and the Meanings of Life; The MIT Press: Cambridge, MA, USA; London, UK, 2020. [Google Scholar]
- Anderson, M.L.; Richardson, M.J.; Chemero, A. Eroding the boundaries of cognition: Implications of embodiment. Top. Cogn. Sci. 2012, 4, 717–730. [Google Scholar] [CrossRef] [PubMed]
- Stepp, N.; Chemero, A.; Turvey, M.T. Philosophy for the rest of cognitive science. Top. Cogn. Sci. 2011, 3, 425–437. [Google Scholar] [CrossRef]
- Cisek, P. Beyond the computer metaphor: Behavior as interaction. J. Conscious. Stud. 1999, 6, 125–142. [Google Scholar]
- Cisek, P.; Pastor-Bernier, A. On the challenges and mechanisms of embodied decisions. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130479. [Google Scholar] [CrossRef] [PubMed]
- Powers, W.T. Behavior: The Control of Perception; Aldine Pub. Co.: Chicago, IL, USA, 1973. [Google Scholar]
- Buss, L.W. The Evolution of Individuality; Princeton University Press: Princeton, NJ, USA, 1987. [Google Scholar]
- Brash, D.E. Cellular proofreading. Nat. Med. 1996, 2, 525–526. [Google Scholar] [CrossRef] [PubMed]
- Gershman, S.J. The molecular memory code and synaptic plasticity: A synthesis. Biosystems 2023, 224, 104825. [Google Scholar] [CrossRef]
- Fields, C.; Levin, M. Scale-Free Biology: Integrating Evolutionary and Developmental Thinking. BioEssays 2020, 42, e1900228. [Google Scholar] [CrossRef]
- Shapiro, J.A. Evolution: A View from the 21st Century; FT Press Science: Upper Saddle River, NJ, USA, 2011. [Google Scholar]
- Froese, T.; Izquierdo, E.J. A Dynamical Approach to the Phenomenology of Body Memory Past Interactions Can Shape Present Capacities Without Neuroplasticity. J. Conscious. Stud. 2018, 25, 20–46. [Google Scholar]
- Biswas, S.; Clawson, W.; Levin, M. Learning in Transcriptional Network Models: Computational Discovery of Pathway-Level Memory and Effective Interventions. Int. J. Mol. Sci. 2022, 24, 285. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Manicka, S.; Hoel, E.; Levin, M. Gene Regulatory Networks Exhibit Several Kinds of Memory: Quantification of Memory in Biological and Random Transcriptional Networks. iScience 2021, 24, 102131. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, K. Physical reservoir computing-an introductory perspective. Jpn. J. Appl. Phys. 2020, 59, 060501. [Google Scholar] [CrossRef]
- Tanaka, G.; Yamane, T.; Heroux, J.B.; Nakane, R.; Kanazawa, N.; Takeda, S.; Numata, H.; Nakano, D.; Hirose, A. Recent advances in physical reservoir computing: A review. Neural Netw. 2019, 115, 100–123. [Google Scholar] [CrossRef]
- Seoane, L.F. Evolutionary aspects of reservoir computing. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180377. [Google Scholar] [CrossRef]
- Niraula, D.; El Naqa, I.; Tuszynski, J.A.; Gatenby, R.A. Modeling non-genetic information dynamics in cells using reservoir computing. iScience 2024, 27, 109614. [Google Scholar] [CrossRef]
- Miller, W.B., Jr.; Baluška, F.; Reber, A.S. A revised central dogma for the 21st century: All biology is cognitive information processing. Prog. Biophys. Mol. Biol. 2023, 182, 34–48. [Google Scholar] [CrossRef]
- Bird, J.; Layzell, P. The evolved radio and its implications for modelling the evolution of novel sensors. In Proceedings of the 2002 Congress on Evolutionary Computation. CEC’02 (Cat. No.02TH8600), Honolulu, HI, USA, 12–17 May 2002; Volume 1832, pp. 1836–1841. [Google Scholar]
- Lehman, J.; Clune, J.; Misevic, D.; Adami, C.; Altenberg, L.; Beaulieu, J.; Bentley, P.J.; Bernard, S.; Beslon, G.; Bryson, D.M.; et al. The Surprising Creativity of Digital Evolution: A Collection of Anecdotes from the Evolutionary Computation and Artificial Life Research Communities. Artif. Life 2020, 26, 274–306. [Google Scholar] [CrossRef]
- Friston, K. Life as we know it. J. R. Soc. Interface R. Soc. 2013, 10, 20130475. [Google Scholar] [CrossRef]
- Gabalda-Sagarra, M.; Carey, L.B.; Garcia-Ojalvo, J. Recurrence-based information processing in gene regulatory networks. Chaos 2018, 28, 106313. [Google Scholar] [CrossRef] [PubMed]
- Goertzel, B. The General Theory of General Intelligence: A Pragmatic Patternist Perspective. arXiv 2021, arXiv:2103.15100. [Google Scholar] [CrossRef]
- Lagasse, E.; Levin, M. Future medicine: From molecular pathways to the collective intelligence of the body. Trends Mol. Med. 2023, 29, 687–710. [Google Scholar] [CrossRef] [PubMed]
- Mathews, J.; Chang, A.J.; Devlin, L.; Levin, M. Cellular signaling pathways as plastic, proto-cognitive systems: Implications for biomedicine. Patterns 2023, 4, 100737. [Google Scholar] [CrossRef] [PubMed]
- Bongard, J.; Zykov, V.; Lipson, H. Resilient machines through continuous self-modeling. Science 2006, 314, 1118–1121. [Google Scholar] [CrossRef] [PubMed]
- Izquierdo-Torres, E.; Bührmann, T. Analysis of a Dynamical Recurrent Neural Network Evolved for Two Qualitatively Different Tasks: Walking and Chemotaxis. In Proceedings of the IEEE Symposium on Artificial Life, Winchester, UK, 8–10 September 2008. [Google Scholar]
- Cully, A.; Clune, J.; Tarapore, D.; Mouret, J.B. Robots that can adapt like animals. Nature 2015, 521, 503–507. [Google Scholar] [CrossRef]
- Saniova, B.; Drobny, M.; Sulaj, M. Delirium and postoperative cognitive dysfunction after general anesthesia. Med. Sci. Monit. 2009, 15, CS81–CS87. [Google Scholar] [PubMed]
- Veyckemans, F. Excitation and delirium during sevoflurane anesthesia in pediatric patients. Minerva Anestesiol. 2002, 68, 402–405. [Google Scholar] [PubMed]
- Wentlandt, K.; Samoilova, M.; Carlen, P.L.; El Beheiry, H. General anesthetics inhibit gap junction communication in cultured organotypic hippocampal slices. Anesth. Analg. 2006, 102, 1692–1698. [Google Scholar] [CrossRef]
- Peracchia, C. Effects of the anesthetics heptanol, halothane and isoflurane on gap junction conductance in crayfish septate axons: A calcium- and hydrogen-independent phenomenon potentiated by caffeine and theophylline, and inhibited by 4-aminopyridine. J. Membr. Biol. 1991, 121, 67–78. [Google Scholar] [CrossRef]
- Azuonye, I.O. Diagnosis made by hallucinatory voices. Br. Med. J. 1997, 315, 1685–1686. [Google Scholar] [CrossRef] [PubMed]
- Dewan, E.M.; Globus, G. Consciousness as an Emergent Causal Agent in the Context of Control System Theory; Globus, G., Maxwell, G., Savodnik, I., Eds.; Plenum Press: New York, NY, USA, 1976; pp. 179–198. [Google Scholar]
- Pezzulo, G.; Levin, M. Top-down models in biology: Explanation and control of complex living systems above the molecular level. J. R. Soc. Interface 2016, 13, 20160555. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A. Algorithms Are the Matter. 2021. Available online: https://www.adamjuliangoldstein.com/blog/algorithms-are-the-matter/ (accessed on 10 April 2024).
- Fields, C.; Friston, K.; Glazebrook, J.F.; Levin, M. A free energy principle for generic quantum systems. Prog. Biophys. Mol. Biol. 2022, 173, 36–59. [Google Scholar] [CrossRef] [PubMed]
- Pai, V.P.; Cervera, J.; Mafé, S.; Willocq, V.; Lederer, E.K.; Levin, M. HCN2 Channel-Induced Rescue of Brain Teratogenesis via Local and Long-Range Bioelectric Repair. Front. Cell Neurosci. 2020, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Blackiston, D.J.; Vien, K.; Levin, M. Serotonergic stimulation induces nerve growth and promotes visual learning via posterior eye grafts in a vertebrate model of induced sensory plasticity. NPJ Regen. Med. 2017, 2, 8. [Google Scholar] [CrossRef] [PubMed]
- Dawkins, R. The Selfish Gene; Oxford University Press: Oxford, UK, 1989. [Google Scholar]
- Manicka, S.; Levin, M. The Cognitive Lens: A primer on conceptual tools for analysing information processing in developmental and regenerative morphogenesis. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2019, 374, 20180369. [Google Scholar] [CrossRef] [PubMed]
- Friston, K.J.; Parr, T.; Heins, C.; Constant, A.; Friedman, D.; Isomura, T.; Fields, C.; Verbelen, T.; Ramstead, M.; Clippinger, J.; et al. Federated inference and belief sharing. Neurosci. Biobehav. Rev. 2024, 156, 105500. [Google Scholar] [CrossRef] [PubMed]
- Vernon, D.; Lowe, R.; Thill, S.; Ziemke, T. Embodied cognition and circular causality: On the role of constitutive autonomy in the reciprocal coupling of perception and action. Front. Psychol. 2015, 6, 1660. [Google Scholar] [CrossRef] [PubMed]
- Tasaki, K.M. Circular causality in integrative multi-scale systems biology and its interaction with traditional medicine. Prog. Biophys. Mol. Biol. 2013, 111, 144–146. [Google Scholar] [CrossRef]
- Thomas, R. Circular causality. Syst. Biol. 2006, 153, 140–153. [Google Scholar] [CrossRef]
- Jones, P.D.; Holding, D.H. Extremely long-term persistence of the McCollough effect. J. Exp. Psychol. Hum. Percept. Perform. 1975, 1, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Palmer-Cooper, E.; McGuire, N.; Wright, A. Unusual experiences and their association with metacognition: Investigating ASMR and Tulpamancy. Cogn. Neuropsychiatry 2022, 27, 86–104. [Google Scholar] [CrossRef] [PubMed]
- Luhrmann, T.M.; Alderson-Day, B.; Chen, A.; Corlett, P.; Deeley, Q.; Dupuis, D.; Lifshitz, M.; Moseley, P.; Peters, E.; Powell, A.; et al. Learning to Discern the Voices of Gods, Spirits, Tulpas, and the Dead. Schizophr. Bull. 2023, 49, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Braude, S.E. First Person Plural: Multiple Personality and the Philosophy of Mind; Rowman & Littlefield Publishers: Lanham, MD, USA, 1995. [Google Scholar]
- Doolittle, W.F.; Booth, A. It’s the song, not the singer: An exploration of holobiosis and evolutionary theory. Biol. Philos. 2017, 32, 5–24. [Google Scholar] [CrossRef]
- Heylighen, F.; Beigi, S.; Busseniers, E. The role of self-maintaining resilient reaction networks in the origin and evolution of life. Biosystems 2022, 219, 104720. [Google Scholar] [CrossRef] [PubMed]
- Busseniers, E.; Veloz, T.; Heylighen, F. Goal Directedness, Chemical Organizations, and Cybernetic Mechanisms. Entropy 2021, 23, 1039. [Google Scholar] [CrossRef] [PubMed]
- Egbert, M.; Gagnon, J.S.; Perez-Mercader, J. From chemical soup to computing circuit: Transforming a contiguous chemical medium into a logic gate network by modulating its external conditions. J. R. Soc. Interface 2019, 16, 20190190. [Google Scholar] [CrossRef] [PubMed]
- Egbert, M.; Gagnon, J.S.; Perez-Mercader, J. Dynamic modulation of external conditions can transform chemistry into logic gates. J. R. Soc. Interface 2018, 15, 20180169. [Google Scholar] [CrossRef] [PubMed]
- Letelier, J.C.; Soto-Andrade, J.; Guinez Abarzua, F.; Cornish-Bowden, A.; Luz Cardenas, M. Organizational invariance and metabolic closure: Analysis in terms of (M,R) systems. J. Theor. Biol. 2006, 238, 949–961. [Google Scholar] [CrossRef]
- Kauffman, S.; Clayton, P. On emergence, agency, and organization. Biol. Philos. 2006, 21, 501–521. [Google Scholar] [CrossRef]
- Harisch, K. How the mind comes to life: The physics and metaphysics of dissipative structures. OSF Prepr. 2022. [Google Scholar]
- Goldbeter, A. Dissipative structures in biological systems: Bistability, oscillations, spatial patterns and waves. Philos. Trans. A Math. Phys. Eng. Sci. 2018, 376, 20170376. [Google Scholar] [CrossRef] [PubMed]
- Deloof, A.; Broeck, J.V. The Key to Defining Life, Death and the Force Driving Evolution—Organic Chemistry-Based-Life Versus Artificial Life—Communication. Belg. J. Zool. 1995, 125, 5–28. [Google Scholar]
- Prigogine, I.; Stengers, I. Order out of Chaos: Man’s New Dialogue with Nature, 1st ed.; New Science Library; Distributed by Random House: Boulder, CO, USA, 1984. [Google Scholar]
- Prigogine, I. From Being to Becoming: Time and Complexity in the Physical Sciences; W. H. Freeman: San Francisco, CA, USA, 1980. [Google Scholar]
- Rosen, R. Self-Organization in Non-Equilibrium Systems—Nicolis, G, Prigogine, I. Int. J. Gen. Syst. 1978, 4, 266–269. [Google Scholar] [CrossRef]
- Solms, M. The Hard Problem of Consciousness and the Free Energy Principle. Front. Psychol. 2018, 9, 2714. [Google Scholar] [CrossRef]
- Maturana, H.R.; Varela, F.J. The Tree of Knowledge: The Biological Roots of Human Understanding; Distributed in the U.S. by Random House: Boston, MA, USA, 1992; 269p. [Google Scholar]
- Varela, F.G.; Maturana, H.R.; Uribe, R. Autopoiesis: The organization of living systems, its characterization and a model. Curr. Mod. Biol. 1974, 5, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Bateson, G. Mind and Nature: A Necessary Unity, 1st ed.; Dutton: New York, NY, USA, 1979. [Google Scholar]
- Piaget, J. Behaviour and Evolution; Routledge: London, UK, 1976. [Google Scholar]
- Walsh, D. Piaget’s Paradox: Adaptation, Evolution, and Agency. Hum. Dev. 2023, 67, 273–287. [Google Scholar] [CrossRef]
- Kolchinsky, A.; Wolpert, D.H. Semantic information, autonomous agency and non-equilibrium statistical physics. Interface Focus 2018, 8, 20180041. [Google Scholar] [CrossRef]
- Ororbia, A.; Friston, K. Mortal Computation: A Foundation for Biomimetic Intelligence. arXiv 2023, arXiv:2311.09589. [Google Scholar] [CrossRef]
- Ramstead, M.J.; Sakthivadivel, D.A.; Heins, C.; Koudahl, M.; Millidge, B.; Da Costa, L.; Klein, B.; Friston, K.J. On Bayesian Mechanics: A Physics of and by Beliefs. arXiv 2022, arXiv:2205.11543. [Google Scholar] [CrossRef]
- Ramstead, M.J.D.; Constant, A.; Badcock, P.B.; Friston, K.J. Variational ecology and the physics of sentient systems. Phys. Life Rev. 2019, 31, 188–205. [Google Scholar] [CrossRef] [PubMed]
- Friston, K. A free energy principle for a particular physics. arXiv 2019, arXiv:1906.10184. [Google Scholar]
- Fields, C.; Glazebrook, J.F.; Levin, M. Minimal physicalism as a scale-free substrate for cognition and consciousness. Neurosci. Conscious. 2021, 2021, niab013. [Google Scholar] [CrossRef] [PubMed]
- Goff, P. The Case for Panpsychism. In Consciousness Studies in Sciences and Humanities: Eastern and Western Perspectives; Satsangi, P.S., Horatschek, A.M., Srivastav, A., Eds.; Springer International Publishing: Cham, Switzerland, 2024; pp. 55–61. [Google Scholar]
- Wigner, E.P. Remarks on the mind-body question. In The Scientist Speculates; Good, I.J., Ed.; Heineman: Portsmouth, NH, USA, 1961. [Google Scholar]
- Dietrich, E.; Fields, C.; Hoffman, D.D.; Prentner, R. Editorial: Epistemic Feelings: Phenomenology, Implementation, and Role in Cognition. Front. Psychol. 2020, 11, 606046. [Google Scholar] [CrossRef] [PubMed]
- Paulson, S.; Hustvedt, S.; Solms, M.; Shamdasani, S. The deeper self: An expanded view of consciousness. Ann. N. Y Acad. Sci. 2017, 1406, 46–63. [Google Scholar] [CrossRef]
- Friston, K.J.; Wiese, W.; Hobson, J.A. Sentience and the Origins of Consciousness: From Cartesian Duality to Markovian Monism. Entropy 2020, 22, 516. [Google Scholar] [CrossRef] [PubMed]
- Clawson, W.P.; Levin, M. Endless forms most beautiful 2.0: Teleonomy and the bioengineering of chimaeric and synthetic organisms. Biol. J. Linn. Soc. 2023, 139, 457–486. [Google Scholar] [CrossRef]
- Pio-Lopez, L. The rise of the biocyborg: Synthetic biology, artificial chimerism and human enhancement. New Genet. Soc. 2021, 40, 599–619. [Google Scholar] [CrossRef]
- Orive, G.; Taebnia, N.; Dolatshahi-Pirouz, A. A New Era for Cyborg Science Is Emerging: The Promise of Cyborganic Beings. Adv. Healthc. Mater. 2020, 9, e1901023. [Google Scholar] [CrossRef]
- McGilchrist, I. The Matter with Things; Perspectiva: London, UK, 2021. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Levin, M. Self-Improvising Memory: A Perspective on Memories as Agential, Dynamically Reinterpreting Cognitive Glue. Entropy 2024, 26, 481. https://doi.org/10.3390/e26060481
Levin M. Self-Improvising Memory: A Perspective on Memories as Agential, Dynamically Reinterpreting Cognitive Glue. Entropy. 2024; 26(6):481. https://doi.org/10.3390/e26060481
Chicago/Turabian StyleLevin, Michael. 2024. "Self-Improvising Memory: A Perspective on Memories as Agential, Dynamically Reinterpreting Cognitive Glue" Entropy 26, no. 6: 481. https://doi.org/10.3390/e26060481
APA StyleLevin, M. (2024). Self-Improvising Memory: A Perspective on Memories as Agential, Dynamically Reinterpreting Cognitive Glue. Entropy, 26(6), 481. https://doi.org/10.3390/e26060481






