Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa
Abstract
:1. Introduction
2. Results and Discussion
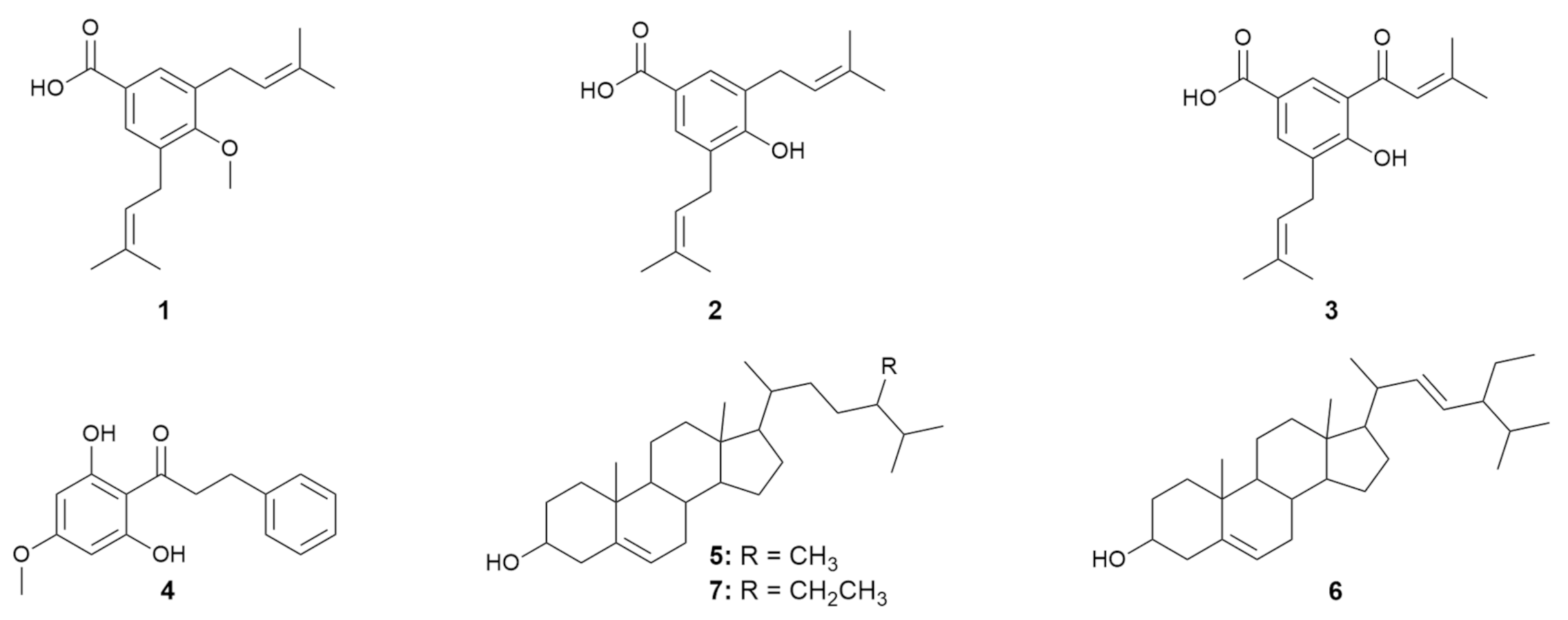
2.1. Phytochemical Study
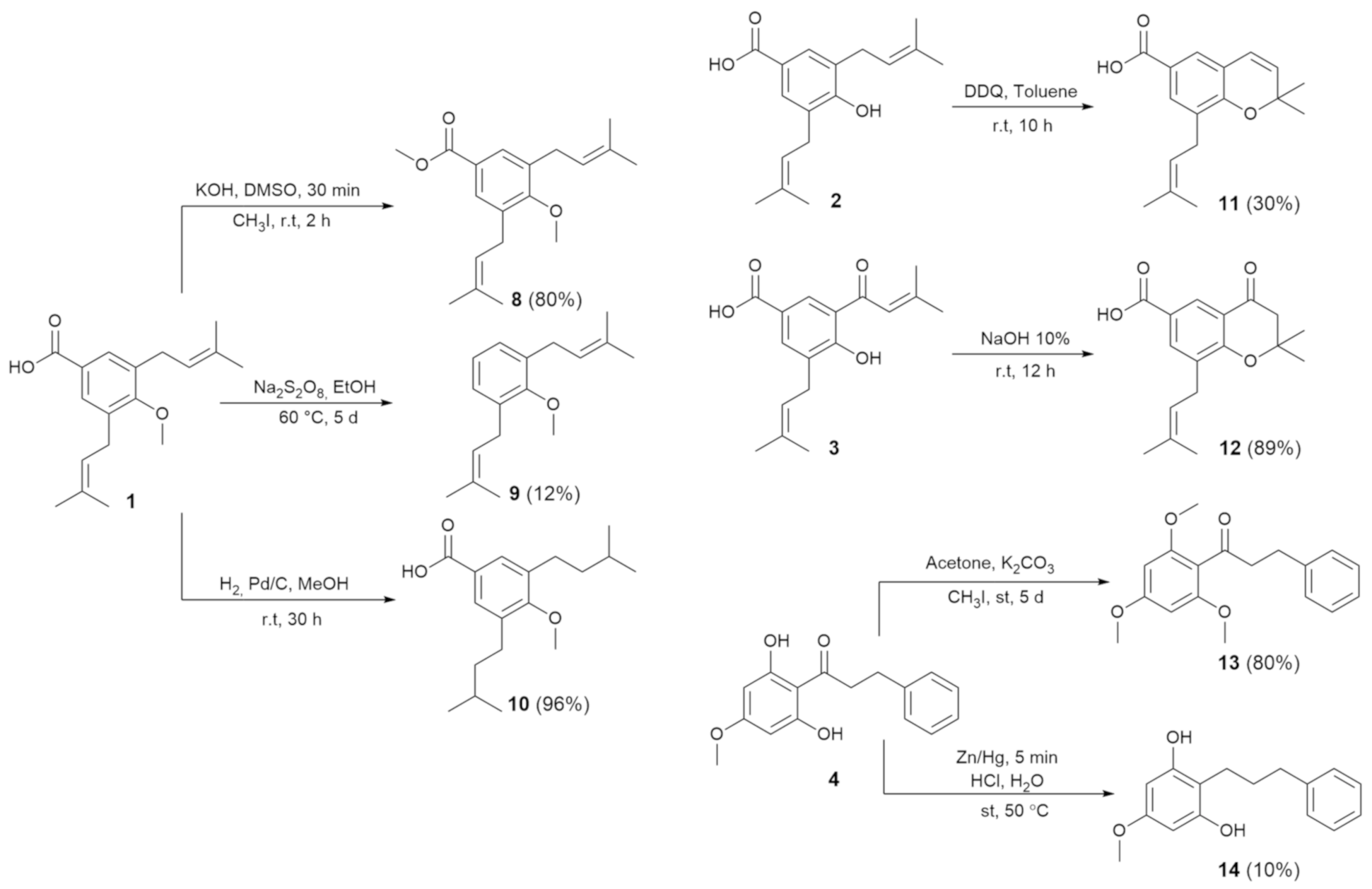
2.2. Synthesis of Derivates
2.3. Antifungal Activity and Qualitative Structure–Activity Relationship Analysis
3. Materials and Methods
3.1. General Experimental Procedures
3.2. Plant Material
3.3. Extraction and Isolation of Compounds
3.4. Preparation of Derivatives
3.5. Fungal Strains
3.6. Mycelial Growth Inhibition Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Beg, M.S.; Ahmad, S.; Jan, K.; Bashir, K. Status, supply chain and processing of cocoa—A review. Trends Food Sci. Technol. 2017, 66, 108–116. [Google Scholar] [CrossRef]
- Vásquez, Z.S.; De Calvalho Neto, D.P.; Pereira, G.V.; Vandenberghe, L.P.; De Oliveira, P.Z.; Tiburcio, P.B.; Rogez, H.L.; Neto, A.G.; Soccol, C.R. Biotechnological approaches for cocoa waste management: A review. Waste Manag. 2019, 90, 72–83. [Google Scholar] [CrossRef] [PubMed]
- Magrone, T.; Russo, M.A.; Jirillo, E. Cocoa and Dark Chocolate Polyphenols: From Biology to Clinical Applications. Front. Immunol. 2017, 8, 677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arunkumar, K.; Jegadeeswari, V. Evaluating the processed beans of different cocoa (Theobroma cacao L.) accessions for quality parameters. J. Phytol. 2019, 11, 1–4. [Google Scholar]
- Noble, M.D. Chocolate and the consumption of forests: A cross-national examination of ecologically unequal exchange in cocoa exports. J. World-Syst. Res. 2017, 23, 236–268. [Google Scholar] [CrossRef] [Green Version]
- Voora, V.; Bermúdez, S.; Larrea, C. Global Market Report: Cocoa; International Institute for Sustainable Development: Winnipeg, Manitoba, Canada, 2019; pp. 2–5. [Google Scholar]
- ICCO: International Cocoa Organization. 2021. Available online: https://www.icco.org/ (accessed on 20 March 2021).
- Cilas, C.; Bastide, P. Challenges to Cocoa Production in the Face of Climate Change and the Spread of Pests and Diseases. Agronomy 1232, 10, 1232. [Google Scholar] [CrossRef]
- Cubillos, G. Frosty pod rot, disease that affects the cocoa (Theobroma cacao) crops in Colombia. Crop Prot. 2017, 96, 77–82. [Google Scholar] [CrossRef]
- Artero, A.S.; Silva, J.Q.; Albuquerque, P.S.B.; Bressan, E.A.; Leal Jr, G.A.; Sebbenn, A.M.; Griffith, G.W.; Figueira, A. Spatial genetic structure and dispersal of the cacao pathogen Moniliophthora perniciosa in the Brazilian Amazon. Plant Pathol. 2016, 66, 912–923. [Google Scholar] [CrossRef] [Green Version]
- Pakora, G.A.; Mpika, J.; Kone, D.; Ducamp, M.; Kebe, I.; Nay, B.; Buisson, D. Inhibition of Phytophthora species, agents of cocoa black pod disease, by secondary metabolites of Trichoderma species. Env. Sci. Pollut. Res. Int. 2018, 25, 29901–29909. [Google Scholar] [CrossRef]
- Antonio, G.L.; Donato, R.D.L.; Lossi, M.R.; Firmino, A.C. Action of resistance inducers in cocoa plants infected with Ceratocystis cacaofunesta. Summa Phytopathol. 2019, 45, 104–106. [Google Scholar] [CrossRef] [Green Version]
- Adu-Acheampong, R.; Archer, S.; Leather, S. Resistance to dieback disease caused by Fusarium and Lasiodiplodia species in cacao (Theobroma cacao L.) genotypes. Exp. Agric. 2012, 48, 85–98. [Google Scholar] [CrossRef]
- Rosmana, A.; Papalangi, I.; Kannapadang, S.; Rahim Danial, M.; Asman, N. Cultural and pathogenic characterization of Fusarium fungi isolated from dieback branches of cacao. Int. J. Curr. Res. Acad. Rev. 2014, 2, 1–6. [Google Scholar]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bolanle, O.O. In vitro antifungal effects of medicinal plants extracts on the mycelia growth of Phytophthora megakarya causal agent of cocoa blackpod disease. RJLBPCS 2017, 29, 1–17. [Google Scholar]
- Prieto, J.A.; Patiño, O.J.; Plazas, E.A.; Pabón, L.C.; Ávila, M.C.; Guzmán, J.D.; Delgado, W.A.; Cuca, L.E. Natural Products from Plants as Potential Source Agents for Controlling Fusarium. In Fungicides-Showcases of Integrated Plant Disease Management from Around the World; IntechOpen: London, UK, 2013; pp. 233–278. [Google Scholar]
- Ramirez-Mares, M.V.; Hernandez-Carlos, B. Plant-derived natural products from the American continent for the control of phytopathogenic fungi: A review. J. Glob. Innov. Agric. Soc. Sci. 2015, 3, 2312–5225. [Google Scholar] [CrossRef]
- Abad, M.J.; Ansuategui, M.; Bermejo, P. Active antifungal substances from natural sources. Arkivoc. 2007, 7, 116–145. [Google Scholar] [CrossRef]
- TPL: The Plant List. 2021. Available online: http://www.theplantlist.org/ (accessed on 15 March 2021).
- Ladino, C. Potencialidad del género Piper como fuente de sustancias para el control de hongos fitopatógenos. Master Thesis, Universidad Nacional de Colombia, Bogotá, Columbia, 2019. Available online: https://repositorio.unal.edu.co/handle/unal/64107 (accessed on 20 March 2021).
- Win, N.K.K.; Jitareerat, P.; Kanlayanarat, S.; Sangchote, S. Effects of cinnamon extract, chitosan coating, hot water treatment and their combinations on crown rot disease and quality of banana fruit. Postharvest Biol. Technol. 2007, 45, 333–340. [Google Scholar] [CrossRef]
- Xu, W.H.; Li, X.C. Antifungal compounds from Piper Species. Curr. Bioact. Compd. 2011, 7, 262–267. [Google Scholar] [CrossRef] [Green Version]
- WFO: World Flora Online. 2021. Available online: http://www.worldfloraonline.org/ (accessed on 20 March 2021).
- Aricapa, G. Estudio fitoquímico de Piper pesaresanum y Piper crassinervium (Piperaceae). Undergraduate Thesis, Universidad Tecnológica de Pereira, Pereira, Risaralda, Columbia, 2012. Available online: http://repositorio.utp.edu.co/dspace/handle/11059/2705 (accessed on 10 March 2021).
- Nitola, L.Y.; Muñoz, D.R.; Patiño, O.J.; Prieto, J.A. Caracterización fitoquímica y evaluación de actividad inhibitoria sobre acetilcolinesterasa de hojas de Piper pesaresanum C. DC. Rev. Cuba. Plantas Med. 2016, 21, 1–10. [Google Scholar]
- López, S.N.; Lopes, A.A.; Batista, J.M.; Flausino, O.; Bolzani, V.D.; Kato, M.J.; Furlan, M. Geranylation of benzoic acid derivatives by enzymatic extracts from Piper crassinervium (Piperaceae). Bioresour. Technol. 2010, 101, 4251–4260. [Google Scholar] [CrossRef]
- Malami, I. Prenylated benzoic acid derivatives from Piper species as source of anti-infective agents. Int. J. Pharm. Sci. Res. 2012, 3, 1554–1559. [Google Scholar]
- Parmar, V.S.; Jain, S.C.; Bisht, K.S.; Jain, R.; Taneja, P.; Jha, A.; Tyagi, O.D.; Prasad, A.K.; Wengel, J.; Olsen, C.E. Phytochemistry of the genus Piper. Phytochem. 1997, 46, 597–673. [Google Scholar] [CrossRef]
- Casuga, F.P.; Castillo, A.L.; Corpuz, M.J. GC–MS analysis of bioactive compounds present in different extracts of an endemic plant Broussonetia luzonica (Blanco) (Moraceae) leaves. Asian Pac. J. Trop. Biomed. 2016, 6, 957–961. [Google Scholar] [CrossRef] [Green Version]
- Rojas, M.; Rincón, J.; Cárdenas, G. Estudio fitoquímico y evaluación de la actividad antimalárica de Piper cumanense y Piper holtonii. Doctoral Thesis, Universidad Nacional de Colombia, Bogotá, Columbia, 2012. Available online: https://repositorio.unal.edu.co/handle/unal/75097 (accessed on 5 March 2021).
- Andrade, M. Alcaloides de Rutaceae: Química e Atividade Biológica. Ph.D. Thesis, Universidade Federal de Sao Carlos, São Carlos, Brazil, 2003. Available online: https://repositorio.ufscar.br/handle/ufscar/6368 (accessed on 5 March 2021).
- Orjala, J.; Erdelmeier, C.A.; Wright, A.D.; Rali, T.; Sticher, O. Five new prenylated p-hydroxybenzoic acid derivatives with antimicrobial and molluscicidal activity from Piper aduncum leaves. Planta Med. 1993, 59, 546–551. [Google Scholar] [CrossRef] [PubMed]
- Wagner, H.; Bladt, S. Plant Drug Analysis: A Thin Layer Chromatography Atlas, 2nd ed.; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 2001; pp. 55–57. [Google Scholar]
- Hermoso, A.; Jiménez, I.A.; Mamani, Z.A.; Bazzocchi, I.L.; Piñero, J.E.; Ravelo, A.G.; Valladares, B. Antileishmanial activities of dihydrochalcones from Piper elongatum and synthetic related compounds. Structural requirements for activity. Bioorg. Med. Chem. 2003, 11, 3975–3980. [Google Scholar] [CrossRef]
- Avila-Zárraga, J.G.; Martínez, R. Efficient methylation of carboxylic acids with potassium hydroxide/methyl sulfoxide and iodomethane. Synth. Commun. 2001, 31, 2177–2183. [Google Scholar] [CrossRef]
- Fang, J.; Wang, D.; Deng, G.J.; Gong, H. Transition metal-free protodecarboxylation of electron rich aromatic acids under mild conditions. Tetrahedron Lett. 2017, 58, 4503–4506. [Google Scholar] [CrossRef]
- Zheng, G.-Q.; Kenney, P.M.; Lam, L.K.T. Myristicin: A Potential Cancer Chemopreventive Agent from Parsley Leaf Oil. J. Agric. Food Chem. 1992, 40, 107–110. [Google Scholar] [CrossRef]
- Keßberg, A.; Lübken, T.; Metz, P. Enantioselective Total Synthesis of Natural Isoflavans: Asymmetric Transfer Hydrogenation/Deoxygenation of Isoflavanones with Dynamic Kinetic Resolution. Org. Lett. 2018, 20, 3006–3009. [Google Scholar] [CrossRef] [PubMed]
- Iguchi, D.; Erra-Balsells, R.; Bonesi, S.M. Expeditious photochemical reaction toward the preparation of substituted chroman-4-ones. Tetrahedron Lett. 2014, 55, 4653–4656. [Google Scholar] [CrossRef]
- Rao, P.S.; Reddy, P.P.; Seshadri, T.R. Methylation of hydroxy flavonols using methyl iodide and potassium carbonate. Proc. Natl. Acad. Sci. India A. 1940, 12, 495–497. [Google Scholar] [CrossRef]
- Vogel, A. Practical Organic Chemistry, 3rd ed.; Longman Group Ltd.: London, UK, 1957; pp. 199–200. [Google Scholar]
- Cabanillas, B.J.; Le Lamer, A.-C.; Castillo, D.; Arevalo, J.; Estevez, Y.; Rojas, R.; Valadeau, C.; Bourdy, G.; Sauvain, M.; Fabre, N. Dihydrochalcones and benzoic acid derivatives from Piper dennisii. Planta Med. 2012, 78, 914–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lago, J.H.G.; Young, M.C.M.; Reigada, J.B.; Soares, M.G.; Roesler, B.P.; Kato, M.J. Antifungal derivatives from Piper mollicomum and P. lhotzkyanum (Piperaceae). Quim. Nova 2007, 30, 1222–1224. [Google Scholar] [CrossRef]
- Plazas, E.; Cuca, L.E.; Delgado, W.A. Flavonoides aislados de las inflorescencias de Piper hispidum kunth (Piperaceae) y derivados acetilados. Rev. Colomb. Quim. 2008, 37, 135–144. [Google Scholar]
- Orjala, J.; Erdelmeier, C.A.J.; Wright, A.D.; Rali, T.; Sticher, O. Two chromenes and a prenylated benzoic acid derivate from Piper aduncum. Phytochemistry 1993, 34, 813–818. [Google Scholar] [CrossRef]
- Burmaoglu, S.; Algul, O.; Anil, D.A.; Gobek, A.; Duran, G.G.; Ersan, R.H.; Duran, N. Synthesis and anti-proliferative activity of fluoro-substituted chalcones. Bioorg. Med. Chem. Lett. 2016, 26, 3172–3176. [Google Scholar] [CrossRef] [PubMed]
- Cosoveanu, A.; Da Silva, E.D.; Gimenez Marino, C.; Nuñez Trujillo, G.; González Coloma, A.; Frias Viera, I.; Cabrera, R. Artemisia thuscula Cav.: Antibacterial, Antifungal Activity of the Plant Extracts and Associated Endophytes. J. Hortic. For. Biotechnol. 2012, 16, 87–90. [Google Scholar]
- Deressa, T.; Lemessa, F.; Wakjira, M. Antifungal activity of some invasive alien plant leaf extracts against mango (Mangifera indica) anthracnose caused by Colletotrichum gloeosporioides. Int. J. Pest. Manag. 2015, 61, 99–105. [Google Scholar] [CrossRef]
| Compounds | M. roreri | F. solani | Phytophthora sp. | p-Value | |||
|---|---|---|---|---|---|---|---|
| IC50 (µg/mL) | IC50 (µM) | IC50 (µg/mL) | IC50 (µM) | IC50 (µg/mL) | IC50 (µM) | ||
| 1 | 16.2 ± 1.5 | 56.1 ± 5.2 | 27.2 ± 0.2 | 94.3 ± 0.7 | 12.4 ± 2.5 | 43.1 ± 8.6 | ≤0.05 |
| 2 | 26.9 ± 0.7 | 97.4 ± 2.6 | 14.7 ± 0.7 | 50.9 ± 2.5 | 12.0 ± 0.5 | 31.6 ± 1.9 | ≤0.05 |
| 3 | 12.8 ± 2.3 | 44.4 ± 8.0 | 9.4 ± 0.9 | 32.5 ± 3.3 | 24.0 ± 1.0 | 83.3 ± 3.4 | ≤0.05 |
| 4 | 17.7 ± 0.8 | 63.0 ± 2.8 | 14.5 ± 0.2 | 34.3 ± 0.7 | 8.7 ± 1.5 | 26.7 ± 5.3 | ≤0.05 |
| 8 | 2.0 ± 0.2 | 6.7 ± 0.7 | 13.8 ± 0.4 | 45.5 ± 1.4 | 13.7 ± 0.4 | 45.3 ± 1.4 | ≤0.05 |
| 9 | 53.2 ± 1.4 | 216.6 ± 5.9 | 30.9 ± 0.5 | 124.6 ± 2.2 | 121.7 ± 0.5 | 496.0 ± 2.2 | ≤0.001 |
| 10 | 13.0 ± 1.0 | 40.1 ± 3.3 | 24.0 ± 0.7 | 81.0 ± 2.5 | 12.5 ± 1.9 | 38.8 ± 6.5 | ≤0.05 |
| 11 | 1.8 ± 0.5 | 3.0 ± 0.8 | 9.5 ± 0.7 | 35.0 ± 2.7 | 10.9 ± 1.7 | 39.1 ± 6.4 | ≤0.05 |
| 12 | 12.4 ± 0.5 | 43.1 ± 1.9 | 10.4 ± 0.5 | 36.0 ± 1.7 | 10.4 ± 0.5 | 35.9 ± 1.8 | =0.70 * |
| 13 | 19.1 ± 0.9 | 98.0 ± 3.0 | 20.5 ± 0.2 | 68.0 ± 0.8 | 10.2 ± 0.9 | 32.2 ± 2.9 | ≤0.05 |
| 14 | 24.1 ± 0.7 | 243.1 ± 2.7 | 24.0 ± 0.2 | 101.8 ± 0.8 | 13.4 ± 1.2 | 29.0 ± 4.8 | ≤0.05 |
| 15 | >200 | >2000 | >200 | >2000 | >200 | >2000 | nd |
| 16 | >200 | >1600 | >200 | >1600 | >200 | >1600 | nd |
| 17 | >200 | >1400 | >200 | >1400 | >200 | >1400 | nd |
| Mancozeb® | 2.8 ± 0.2 | 4.9 ± 0.4 | 5.6 ± 0.7 | 10.2 ± 1.3 | 5.3 ± 0.7 | 9.9 ± 1.4 | nd |
| p-value | ≤0.001 | ≤0.001 | ≤0.001 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chitiva-Chitiva, L.C.; Ladino-Vargas, C.; Cuca-Suárez, L.E.; Prieto-Rodríguez, J.A.; Patiño-Ladino, O.J. Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa. Molecules 2021, 26, 3256. https://doi.org/10.3390/molecules26113256
Chitiva-Chitiva LC, Ladino-Vargas C, Cuca-Suárez LE, Prieto-Rodríguez JA, Patiño-Ladino OJ. Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa. Molecules. 2021; 26(11):3256. https://doi.org/10.3390/molecules26113256
Chicago/Turabian StyleChitiva-Chitiva, Luis C., Cristóbal Ladino-Vargas, Luis E. Cuca-Suárez, Juliet A. Prieto-Rodríguez, and Oscar J. Patiño-Ladino. 2021. "Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa" Molecules 26, no. 11: 3256. https://doi.org/10.3390/molecules26113256
APA StyleChitiva-Chitiva, L. C., Ladino-Vargas, C., Cuca-Suárez, L. E., Prieto-Rodríguez, J. A., & Patiño-Ladino, O. J. (2021). Antifungal Activity of Chemical Constituents from Piper pesaresanum C. DC. and Derivatives against Phytopathogen Fungi of Cocoa. Molecules, 26(11), 3256. https://doi.org/10.3390/molecules26113256








