In Silico Analysis of Functionalized Hydrocarbon Production Using Ehrlich Pathway and Fatty Acid Derivatives in an Endophytic Fungus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Construction of Fungal Endophyte Metabolic Model
2.2. Stoichiometric Analyses
3. Results
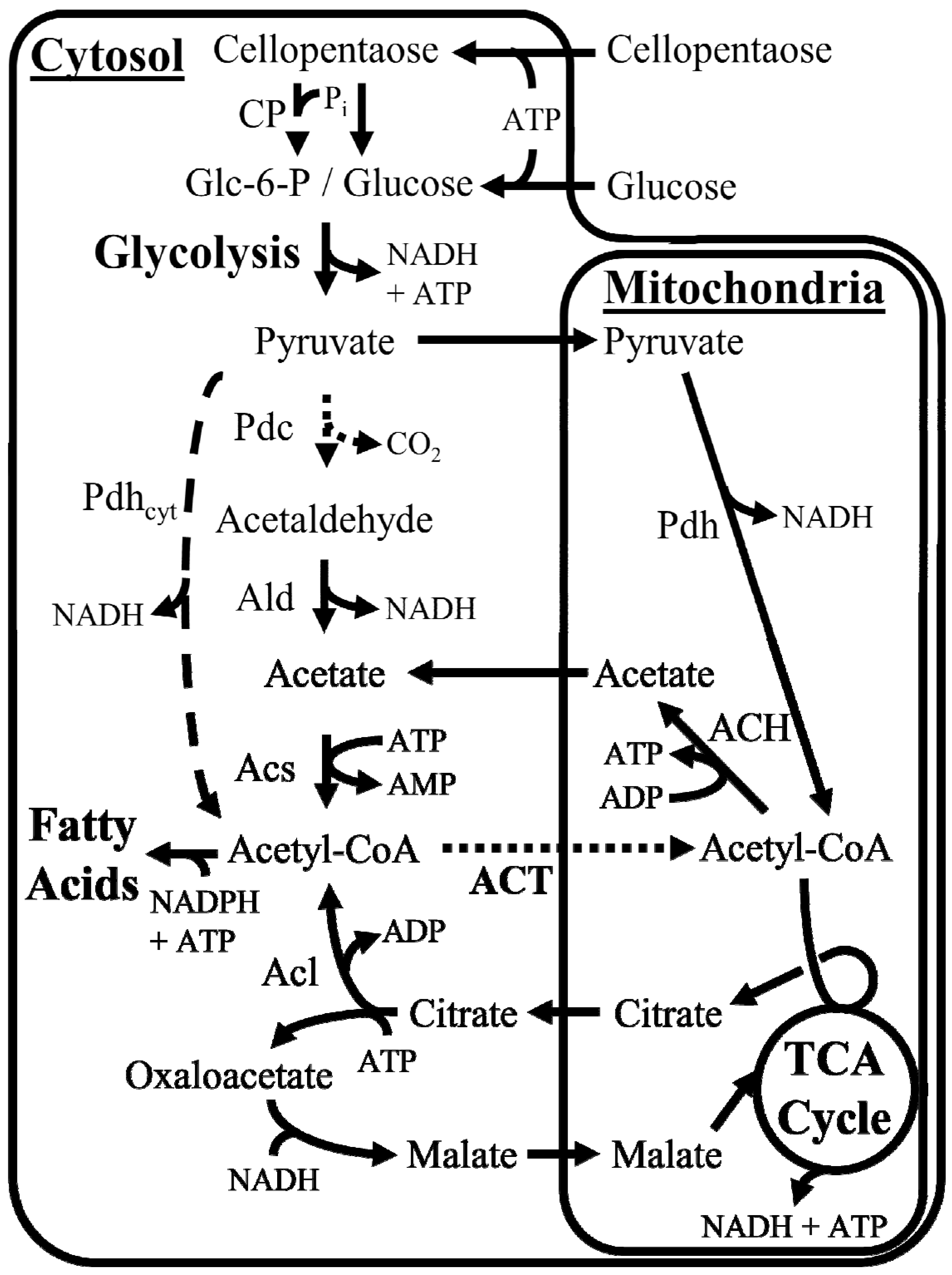
3.1. In Silico Reconstruction of Carbon Uptake, Compartmentalization, and Functionalized Hydrocarbon Production
3.2. In Silico Analysis of Cellular Energy Production
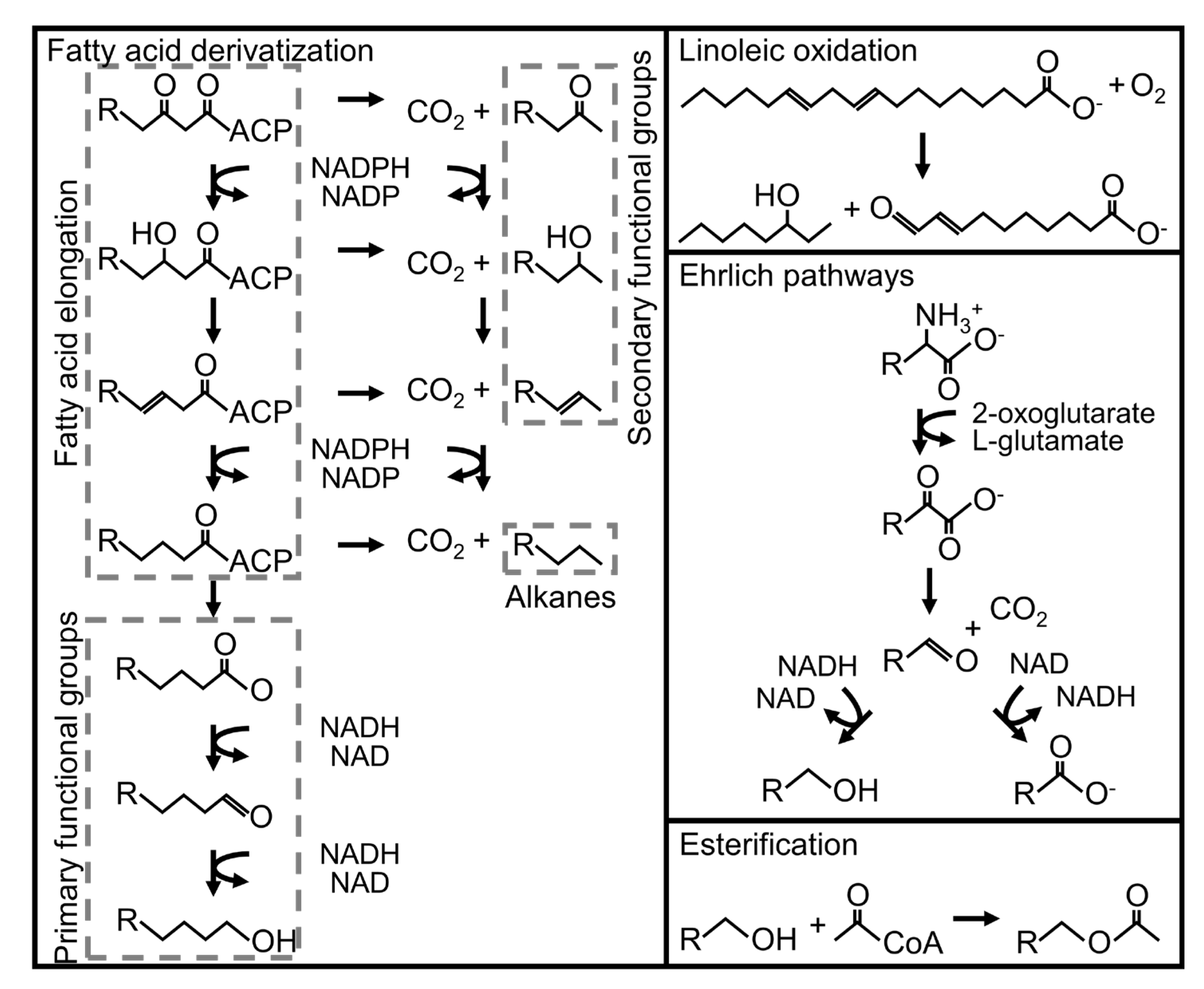
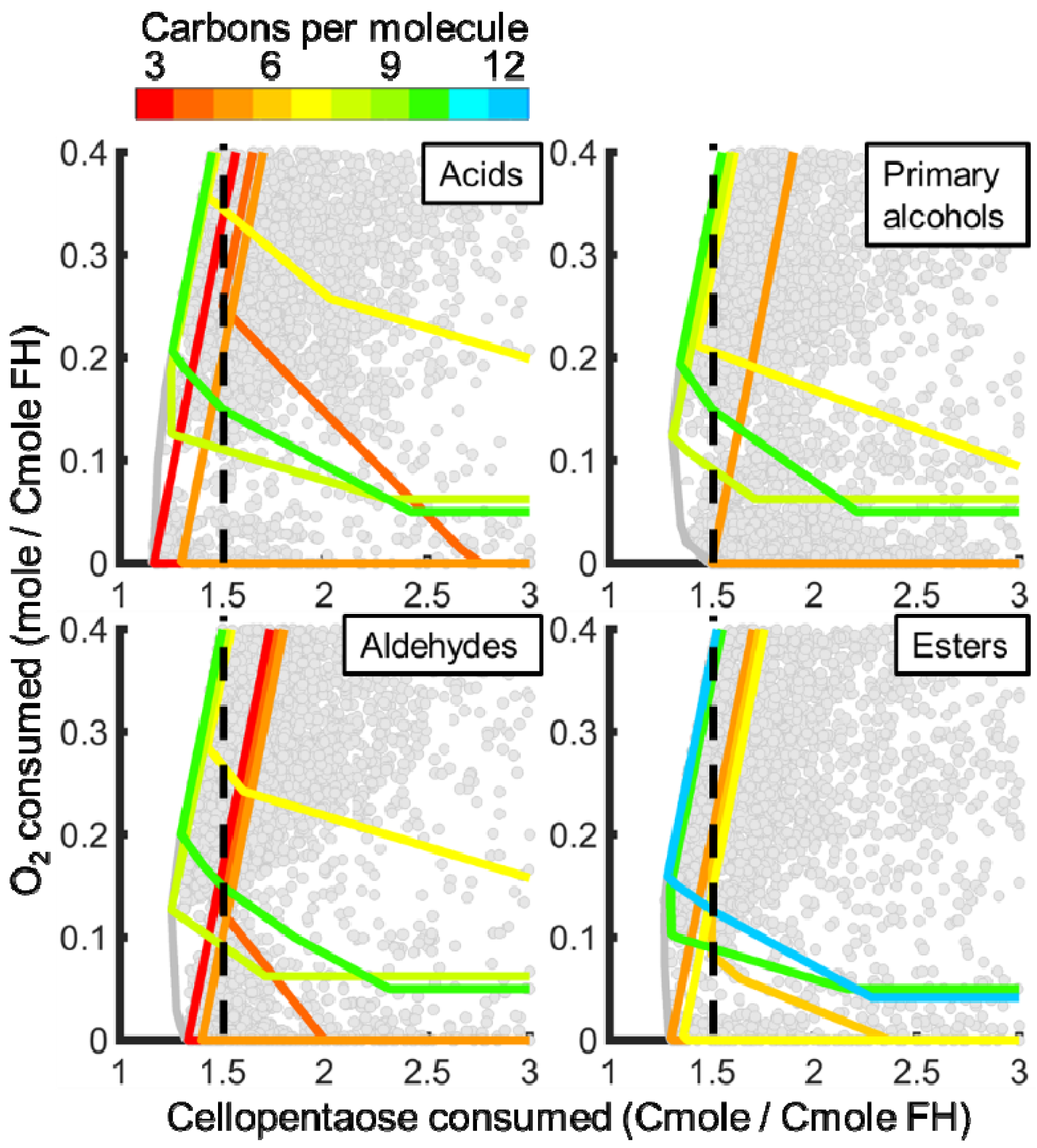
3.3. In Silico Analysis of Functionalized Hydrocarbon Production
3.3.1. Ehrlich Pathway Derivatives
3.3.2. Fatty Acid Derivatives
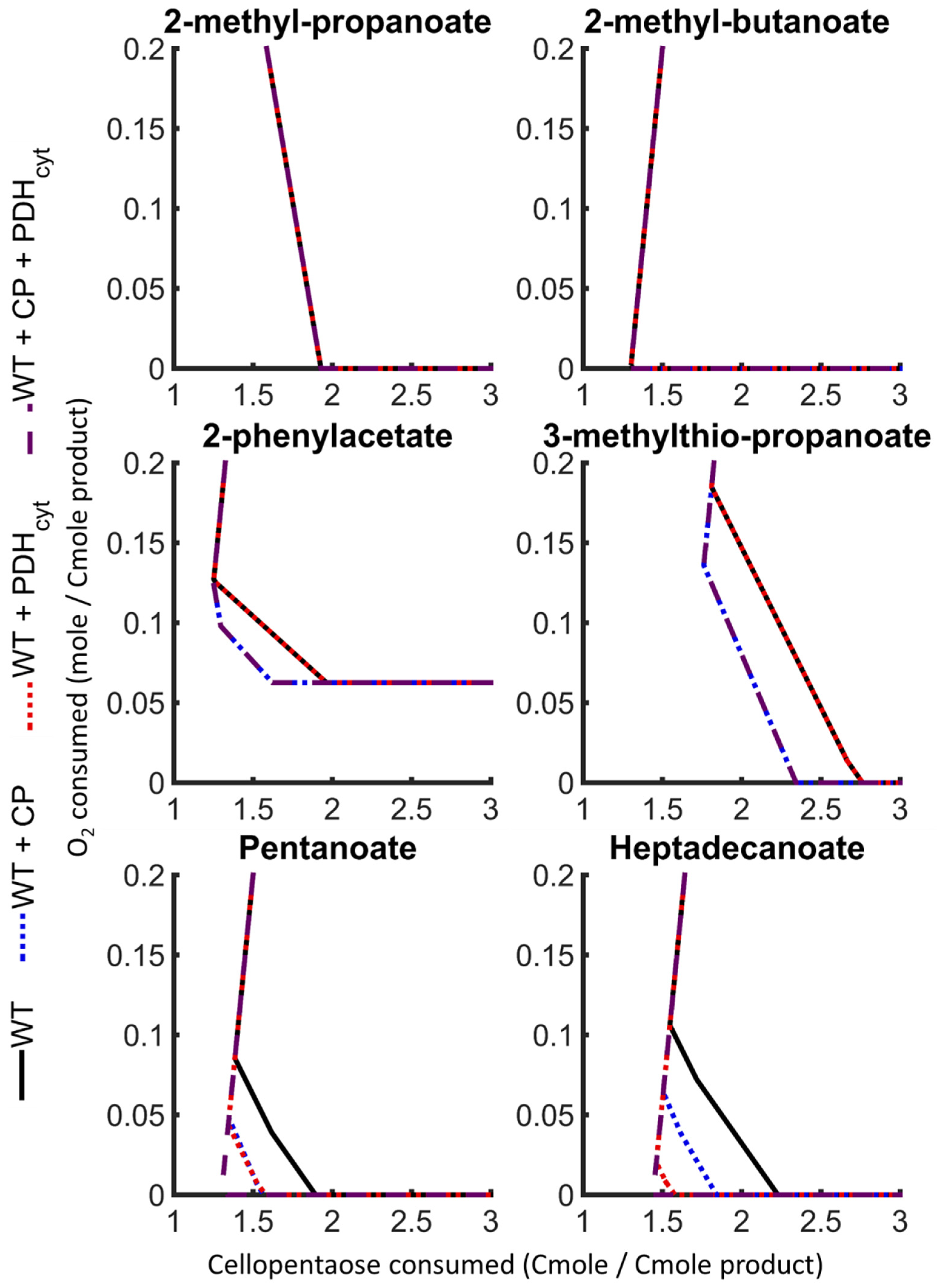
3.4. In Silico Metabolic Engineering Targets
3.4.1. Cellobiose Phosphorylase
3.4.2. Cytosolic Pyruvate Dehydrogenase
3.4.3. Concurrent Cellobiose Phosphorylase and Cytosolic Pyruvate Dehydrogenase Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strobel, G.A.; Knighton, B.; Kluck, K.; Ren, Y.; Livinghouse, T.; Griffin, M.A.; Spakowicz, D.J.; Sears, J. The production of myco-diesel hydrocarbons and their derivatives by the endophytic fungus Gliocladium roseum (NRRL 50072). Microbiology 2008, 154, 3319–3328. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Griffin, M.A.; Spakowicz, D.J.; Gianoulis, T.A.; Strobel, S.A. Volatile organic compound production by organisms in the genus Ascocoryne and a re-evaluation of myco-diesel production by NRRL 50072. Microbiology 2010, 156, 3814–3829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gianoulis, T.A.; Griffin, M.A.; Spakowicz, D.J.; Dunican, B.F.; Alpha, C.J.; Sboner, A.; Michael Sismour, A.; Kodira, C.; Egholm, M.; Church, G.M.; et al. Genomic analysis of the hydrocarbon-producing, cellulolytic, endophytic fungus Ascocoryne sarcoides. PLoS Genet. 2012, 8, e1002558. [Google Scholar] [CrossRef] [Green Version]
- Mallette, N.D.; Knighton, B.; Strobel, G.A.; Carlson, R.P.; Peyton, B.M. Resolution of volatile fuel compound profiles from Ascocoryne sarcoides: A comparison by proton transfer reaction-mass spectrometry and solid phase microextraction gas chromatography-mass spectrometry. AMB Express 2012, 2, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mallette, N.; Pankratz, E.M.; Parker, A.E.; Strobel, G.A.; Busse, S.C.; Carlson, R.P.; Peyton, B.M. Evaluation of cellulose as a substrate for hydrocarbon fuel production by Ascocoryne sarcoides (NRRL 50072). J. Sustain. Bioenergy Syst. 2014, 04, 33–49. [Google Scholar] [CrossRef] [Green Version]
- Stinson, M.; Ezra, D.; Hess, W.M.; Sears, J.; Strobel, G. An endophytic Gliocladium sp. of Eucryphia cordifolia producing selective volatile antimicrobial compounds. Plant Sci. 2003, 165, 913–922. [Google Scholar] [CrossRef]
- Hussa, E.a.; Goodrich-Blair, H. It takes a village: Ecological and fitness impacts of multipartite mutualism. Annu. Rev. Microbiol. 2013, 67, 161–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strobel, G.A.; Daisy, B.; Castillo, U.F.; Harper, J. Natural products from endophytic microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef]
- Verma, V.C.; Kharwar, R.N.; Strobel, G.A. Chemical and functional diversity of natural products from plant associated endophytic fungi. Nat. Prod. Commun. 2009, 4, 1511–1532. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Pfleger, B.F. Revisiting metabolic engineering strategies for microbial synthesis of oleochemicals. Metab. Eng. 2020, 58, 35–46. [Google Scholar] [CrossRef]
- Zhou, Y.J.; Hu, Y.; Zhu, Z.; Siewers, V.; Nielsen, J. Engineering 1-Alkene Biosynthesis and Secretion by Dynamic Regulation in Yeast. ACS Synth. Biol. 2018, 7, 584–590. [Google Scholar] [CrossRef] [PubMed]
- Yan, Q.; Simmons, T.R.; Cordell, W.T.; Hernández Lozada, N.J.; Breckner, C.J.; Chen, X.; Jindra, M.A.; Pfleger, B.F. Metabolic engineering of β-oxidation to leverage thioesterases for production of 2-heptanone, 2-nonanone and 2-undecanone. Metab. Eng. 2020, 61, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Flores, A.; Wang, X.; Nielsen, D.R. Recent trends in integrated bioprocesses: Aiding and expanding microbial biofuel/biochemical production. Curr. Opin. Biotechnol. 2019, 57, 82–87. [Google Scholar] [CrossRef]
- Schulz, S.; Dickschat, J.S. Bacterial volatiles: The smell of small organisms. Nat. Prod. Rep. 2007, 24, 814–842. [Google Scholar] [CrossRef]
- Hazelwood, L.A.; Daran, J.-M.; van Maris, A.J.A.; Pronk, J.T.; Dickinson, J.R. The Ehrlich pathway for fusel alcohol production: A century of research on Saccharomyces cerevisiae metabolism. Appl. Environ. Microbiol. 2008, 74, 2259–2266. [Google Scholar] [CrossRef] [Green Version]
- Tejero Rioseras, A.; Garcia Gomez, D.; Ebert, B.E.; Blank, L.M.; Ibáñez, A.J.; Sinues, P.M.-L. Comprehensive Real-Time Analysis of the Yeast Volatilome. Sci. Rep. 2017, 7, 14236. [Google Scholar] [CrossRef]
- Lee, J.-W.; Trinh, C.T. Towards renewable flavors, fragrances, and beyond. Curr. Opin. Biotechnol. 2020, 61, 168–180. [Google Scholar] [CrossRef]
- Xu, P.; Qiao, K.; Ahn, W.S.; Stephanopoulos, G. Engineering Yarrowia lipolytica as a platform for synthesis of drop-in transportation fuels and oleochemicals. Proc. Natl. Acad. Sci. USA 2016, 113, 10848–10853. [Google Scholar] [CrossRef] [Green Version]
- Tehlivets, O.; Scheuringer, K.; Kohlwein, S.D. Fatty acid synthesis and elongation in yeast. Biochim. Biophys. Acta 2007, 1771, 255–270. [Google Scholar] [CrossRef]
- Carlson, R.; Fell, D.; Srienc, F. Metabolic pathway analysis of a recombinant yeast for rational strain development. Biotechnol. Bioeng. 2002, 79, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Beopoulos, A.; Cescut, J.; Haddouche, R.; Uribelarrea, J.-L.; Molina-Jouve, C.; Nicaud, J.-M. Yarrowia lipolytica as a model for bio-oil production. Prog. Lipid Res. 2009, 48, 375–387. [Google Scholar] [CrossRef] [PubMed]
- Blazeck, J.; Hill, A.; Liu, L.; Knight, R.; Miller, J.; Pan, A.; Otoupal, P.; Alper, H.S. Harnessing Yarrowia lipolytica lipogenesis to create a platform for lipid and biofuel production. Nat. Commun. 2014, 5, 3131. [Google Scholar] [CrossRef] [Green Version]
- Hynes, M.J.; Murray, S.L. ATP-citrate lyase is required for production of cytosolic acetyl coenzyme A and development in Aspergillus nidulans. Eukaryot. Cell 2010, 9, 1039–1048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, C.; Kim, G.B.; Kim, W.J.; Kim, H.U.; Lee, S.Y. Current status and applications of genome-scale metabolic models. Genome Biol. 2019, 20, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poolman, M.G.; Miguet, L.; Sweetlove, L.J.; Fell, D.A. A Genome-Scale Metabolic Model of Arabidopsis and Some of Its Properties. Plant Physiol. 2009, 151, 1570–1581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, C.Y.M.; Williams, T.C.R.; Poolman, M.G.; Fell, D.A.; Ratcliffe, R.G.; Sweetlove, L.J. A method for accounting for maintenance costs in flux balance analysis improves the prediction of plant cell metabolic phenotypes under stress conditions. Plant J. 2013, 75, 1050–1061. [Google Scholar] [CrossRef]
- Moejes, F.W.; Matuszyńska, A.; Adhikari, K.; Bassi, R.; Cariti, F.; Cogne, G.; Dikaios, I.; Falciatore, A.; Finazzi, G.; Flori, S.; et al. A systems-wide understanding of photosynthetic acclimation in algae and higher plants. J. Exp. Bot. 2017, 68, 2667–2681. [Google Scholar] [CrossRef]
- Klamt, S.; Stelling, J. Two approaches for metabolic pathway analysis? Trends Biotechnol. 2003, 21, 64–69. [Google Scholar] [CrossRef]
- Orth, J.D.; Thiele, I.; Palsson, B.Ø. What is flux balance analysis? Nat. Biotechnol. 2010, 28, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Reed, J.L.; Palsson, B.O. Thirteen years of building constraint-based in silico models of Escherichia coli. J. Bacteriol. 2003, 185, 2692–2699. [Google Scholar] [CrossRef] [Green Version]
- Trinh, C.T.; Wlaschin, A.; Srienc, F. Elementary mode analysis: A useful metabolic pathway analysis tool for characterizing cellular metabolism. Appl. Microbiol. Biotechnol. 2009, 81, 813–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varma, A.; Boesch, B.W.; Palsson, B.O. Stoichiometric interpretation of Escherichia coli glucose catabolism under various oxygenation rates. Appl. Environ. Microbiol. 1993, 59, 2465–2473. [Google Scholar] [CrossRef] [Green Version]
- Llaneras, F.; Picó, J. Which metabolic pathways generate and characterize the flux space? A comparison among elementary modes, extreme pathways and minimal generators. J. Biomed. Biotechnol. 2010, 2010, 753904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schilling, C.H.; Letscher, D.; Palsson, B.Ø. Theory for the systemic definition of metabolic pathways and their use in interpreting metabolic function from a pathway-oriented perspective. J. Theor. Biol. 2000, 203, 229–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Ye, C.; Che, J.; Xu, X.; Shao, D.; Jiang, C.; Liu, Y.; Shi, J. Genomic sequencing, genome-scale metabolic network reconstruction, and in silico flux analysis of the grape endophytic fungus Alternaria sp. MG1. Microb. Cell Fact. 2019, 18. [Google Scholar] [CrossRef] [Green Version]
- Emanuelsson, O.; Nielsen, H.; Brunak, S.; von Heijne, G. Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J. Mol. Biol. 2000, 300, 1005–1016. [Google Scholar] [CrossRef] [Green Version]
- Grube, M.; Zagreba, E.; Gromozova, E.; Fomina, M. Comparative investigation of the macromolecular composition of mycelia forms Thielavia terrestris by infrared spectroscopy. Vib. Spectrosc. 1999, 19, 301–306. [Google Scholar] [CrossRef]
- Klamt, S.; Saez-Rodriguez, J.; Gilles, E.D. Structural and functional analysis of cellular networks with CellNetAnalyzer. BMC Syst. Biol. 2007, 129, 329–351. [Google Scholar] [CrossRef] [Green Version]
- Klamt, S.; von Kamp, A. An application programming interface for CellNetAnalyzer. Biosystems 2011, 105, 162–168. [Google Scholar] [CrossRef] [PubMed]
- Jungreuthmayer, C.; Ruckerbauer, D.E.; Zanghellini, J. regEfmtool: Speeding up elementary flux mode calculation using transcriptional regulatory rules in the form of three-state logic. Biosystems 2013, 113, 37–39. [Google Scholar] [CrossRef]
- Schellenberger, J.; Que, R.; Fleming, R.M.T.; Thiele, I.; Orth, J.D.; Feist, A.M.; Zielinski, D.C.; Bordbar, A.; Lewis, N.E.; Rahmanian, S.; et al. Quantitative prediction of cellular metabolism with constraint-based models: The COBRA Toolbox v2.0. Nat. Protoc. 2011, 6, 1290–1307. [Google Scholar] [CrossRef] [Green Version]
- Minty, J.J.; Singer, M.E.; Scholz, S.a.; Bae, C.-H.H.; Ahn, J.-H.H.; Foster, C.E.; Liao, J.C.; Lin, X.N. Design and characterization of synthetic fungal-bacterial consortia for direct production of isobutanol from cellulosic biomass. Proc. Natl. Acad. Sci. USA 2013, 110, 14592–14597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schirmer, A.; Rude, M.A.; Li, X.; Popova, E.; del Cardayre, S.B. Microbial biosynthesis of alkanes. Science 2010, 329, 559–562. [Google Scholar] [CrossRef]
- Ladygina, N.; Dedyukhina, E.G.; Vainshtein, M.B. A review on microbial synthesis of hydrocarbons. Process Biochem. 2006, 41, 1001–1014. [Google Scholar] [CrossRef]
- Carlson, R.; Srienc, F. Fundamental Escherichia coli biochemical pathways for biomass and energy production: Creation of overall flux states. Biotechnol. Bioeng. 2004, 86, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Runguphan, W.; Keasling, J.D. Metabolic engineering of Saccharomyces cerevisiae for production of fatty acid-derived biofuels and chemicals. Metab. Eng. 2014, 21, 103–113. [Google Scholar] [CrossRef]
- Schoen, H.R.; Hunt, K.A.; Strobel, G.A.; Peyton, B.M.; Carlson, R.P. Carbon chain length of biofuel- and flavor-relevant volatile organic compounds produced by lignocellulolytic fungal endophytes changes with culture temperature. Mycoscience 2017, 58. [Google Scholar] [CrossRef] [Green Version]
- Schoen, H.R.; Knighton, W.B.; Peyton, B.M. Endophytic fungal production rates of volatile organic compounds are highest under microaerophilic conditions. Microbiology 2017, 163, 1767–1777. [Google Scholar] [CrossRef]
- Gupta, K.J.; Zabalza, A.; van Dongen, J.T. Regulation of respiration when the oxygen availability changes. Physiol. Plant. 2009, 137, 383–391. [Google Scholar] [CrossRef]



| Fatty Acid Derivatives | Ehrlich Pathway Derivatives | Linoleic Acid Derivatives |
|---|---|---|
| 1-butanol | 2-methyl-1-propyl acid, alcohol, and esters | 3-octanone |
| 1-hexanol | 1-octen-3-ol | |
| Hexanoate | 3-methyl-1-butyl acid, alcohol, and esters | |
| 1-heptanol | ||
| Octane | 2-phenylethyl alcohol and esters | Miscellaneous |
| 2-nonanone | Propyl-cyclopropane b | |
| C2, C5–C10 alkyl acetates | Phenyl methanol | 2-pentyl-furan b |
| Wild-type (WT) | WT + CP a | WT + PDHcyt a | WT + CP + PDHcyt a | |||||
|---|---|---|---|---|---|---|---|---|
| Functional group b | Carbon c | O2 c | Carbon c | O2 c | Carbon c | O2 c | Carbon c | O2 c |
| Acids | 1.17 (1.2) | 0.00 | 1.15 (1.2) | 0.03 | 1.16 (1.2) | 0.003 | 1.15 (1.2) | 0.03 |
| Aldehydes | 1.25 (1.3) | 0.13 | 1.23 (1.3) | 0.08 | 1.25 (1.3) | 0.13 | 1.23 (1.3) | 0.08 |
| Esters | 1.29 | 0 | 1.29 | 0 | 1.29 | 0 | 1.29 | 0 |
| Primary alcohols | 1.31 (1.5) | 0.12 | 1.28 (1.5) | 0.09 | 1.31 (1.5) | 0.12 | 1.28 (1.5) | 0.09 |
| Ketones | 1.5 (2.7) | 0.12 | 1.5 (2.2) | 0.08 | 1.5 (2.0) | 0.08 | 1.5 (1.7) | 0.08 |
| Alkenes | 1.62 (2.4) | 0.12 | 1.57 (2.0) | 0.07 | 1.52 (1.7) | 0.02 | 1.5 | 0 |
| Secondary alcohols | 1.62 (2.4) | 0.12 | 1.57 (2.0) | 0.07 | 1.52 (1.7) | 0.02 | 1.5 | 0 |
| Alkanes | 1.64 (2.4) | 0.11 | 1.60 (2.0) | 0.07 | 1.54 (1.6) | 0.01 | 1.53 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hunt, K.A.; Mallette, N.D.; Peyton, B.M.; Carlson, R.P. In Silico Analysis of Functionalized Hydrocarbon Production Using Ehrlich Pathway and Fatty Acid Derivatives in an Endophytic Fungus. J. Fungi 2021, 7, 435. https://doi.org/10.3390/jof7060435
Hunt KA, Mallette ND, Peyton BM, Carlson RP. In Silico Analysis of Functionalized Hydrocarbon Production Using Ehrlich Pathway and Fatty Acid Derivatives in an Endophytic Fungus. Journal of Fungi. 2021; 7(6):435. https://doi.org/10.3390/jof7060435
Chicago/Turabian StyleHunt, Kristopher A., Natasha D. Mallette, Brent M. Peyton, and Ross P. Carlson. 2021. "In Silico Analysis of Functionalized Hydrocarbon Production Using Ehrlich Pathway and Fatty Acid Derivatives in an Endophytic Fungus" Journal of Fungi 7, no. 6: 435. https://doi.org/10.3390/jof7060435







