Accuracy Analysis of a Next-Generation Tissue Microarray on Various Soft Tissue Samples of Wistar Rats
Abstract
:1. Introduction
2. Materials and Methods
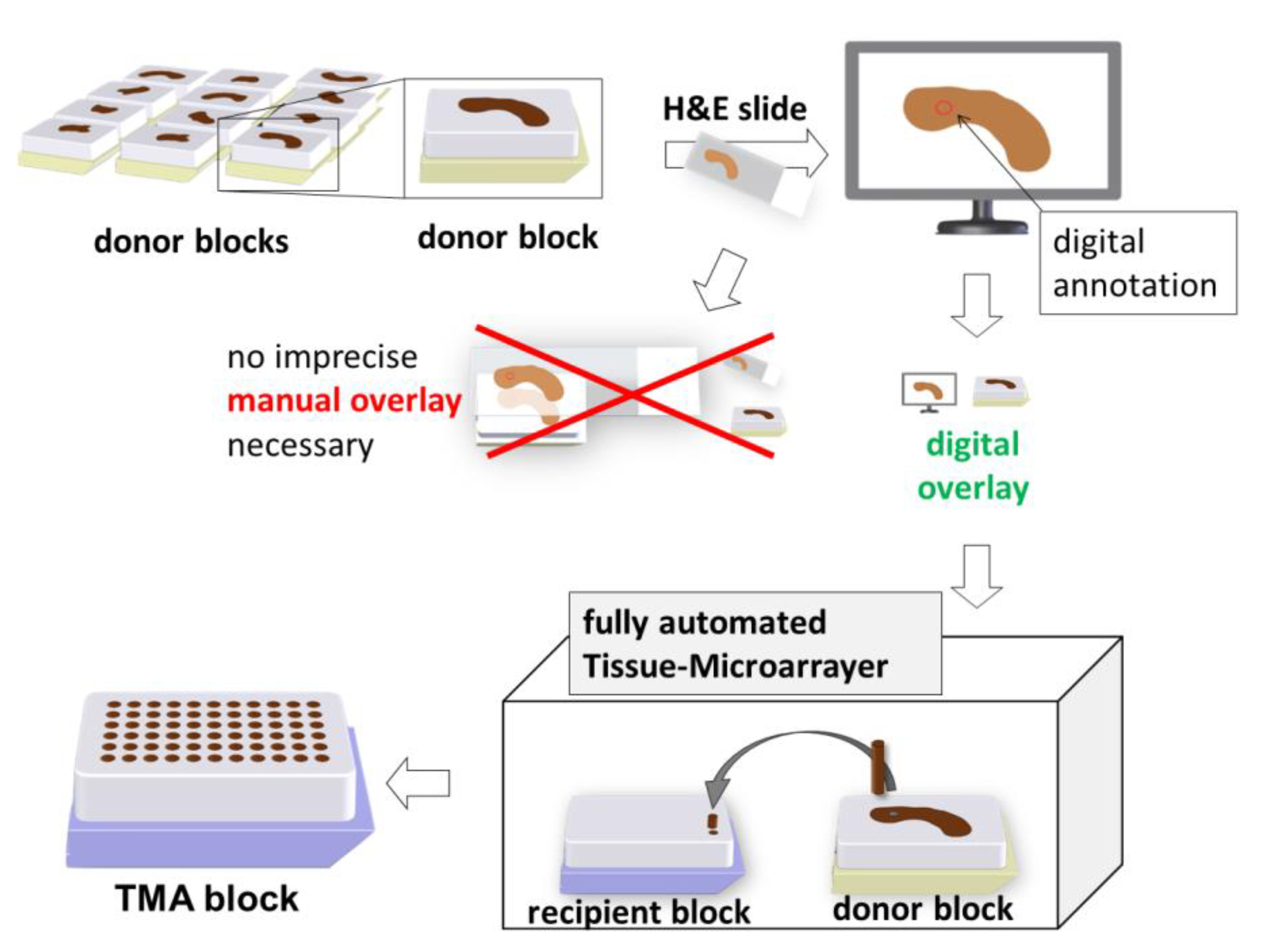
2.1. Construction of ngTMA®
2.1.1. Inspection and Selection of the Tissue Samples
2.1.2. Punching Diameter
2.1.3. Sectioning and Staining of the H&E Slides
2.1.4. Digitalisation and Determination of Annotations
2.1.5. Execution Using the Tissue Microarrayer
2.2. Methods for Analysing Accuracy
2.2.1. Core Loss
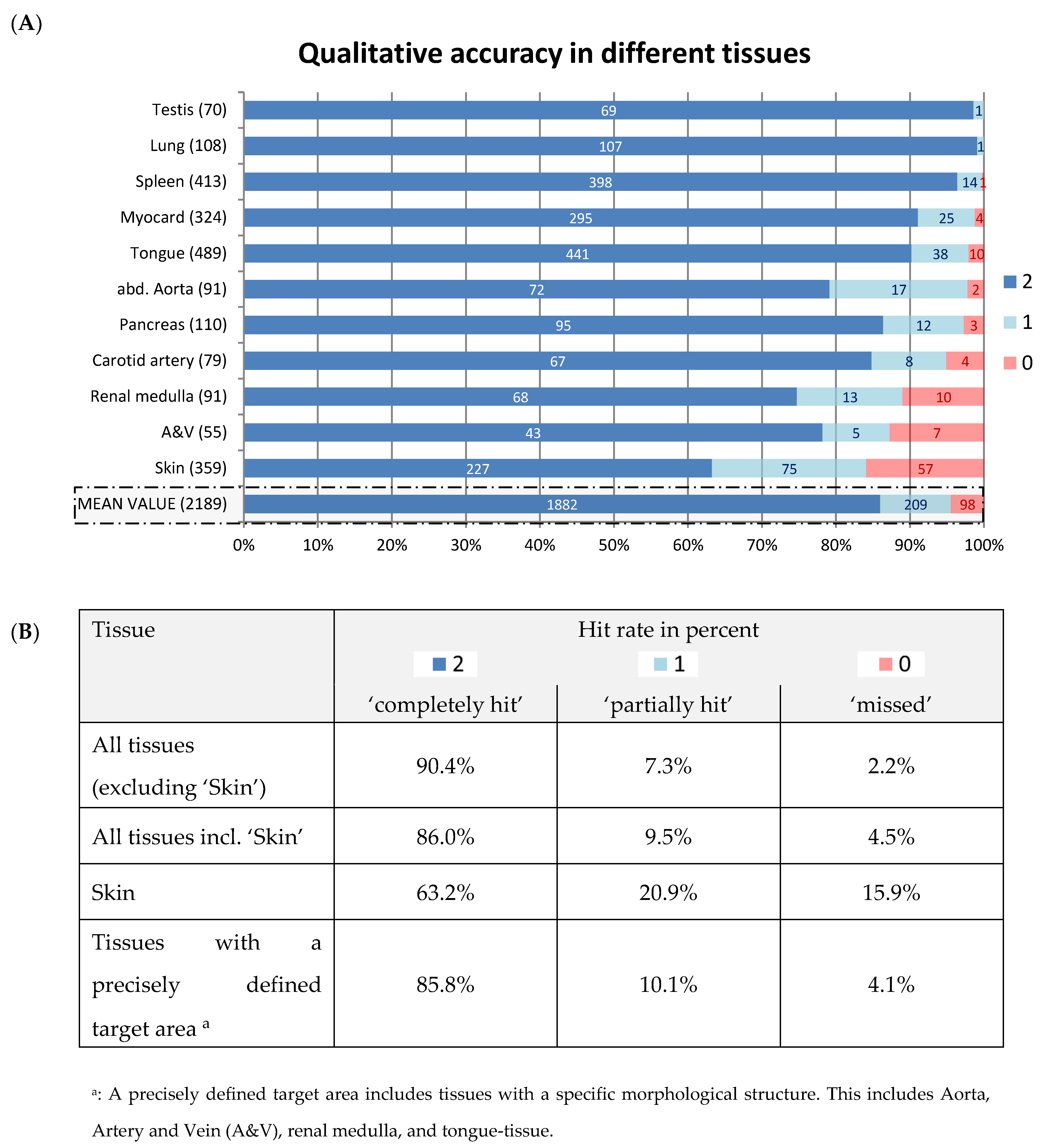
2.2.2. Qualitative Accuracy
2.2.3. Quantitative Accuracy
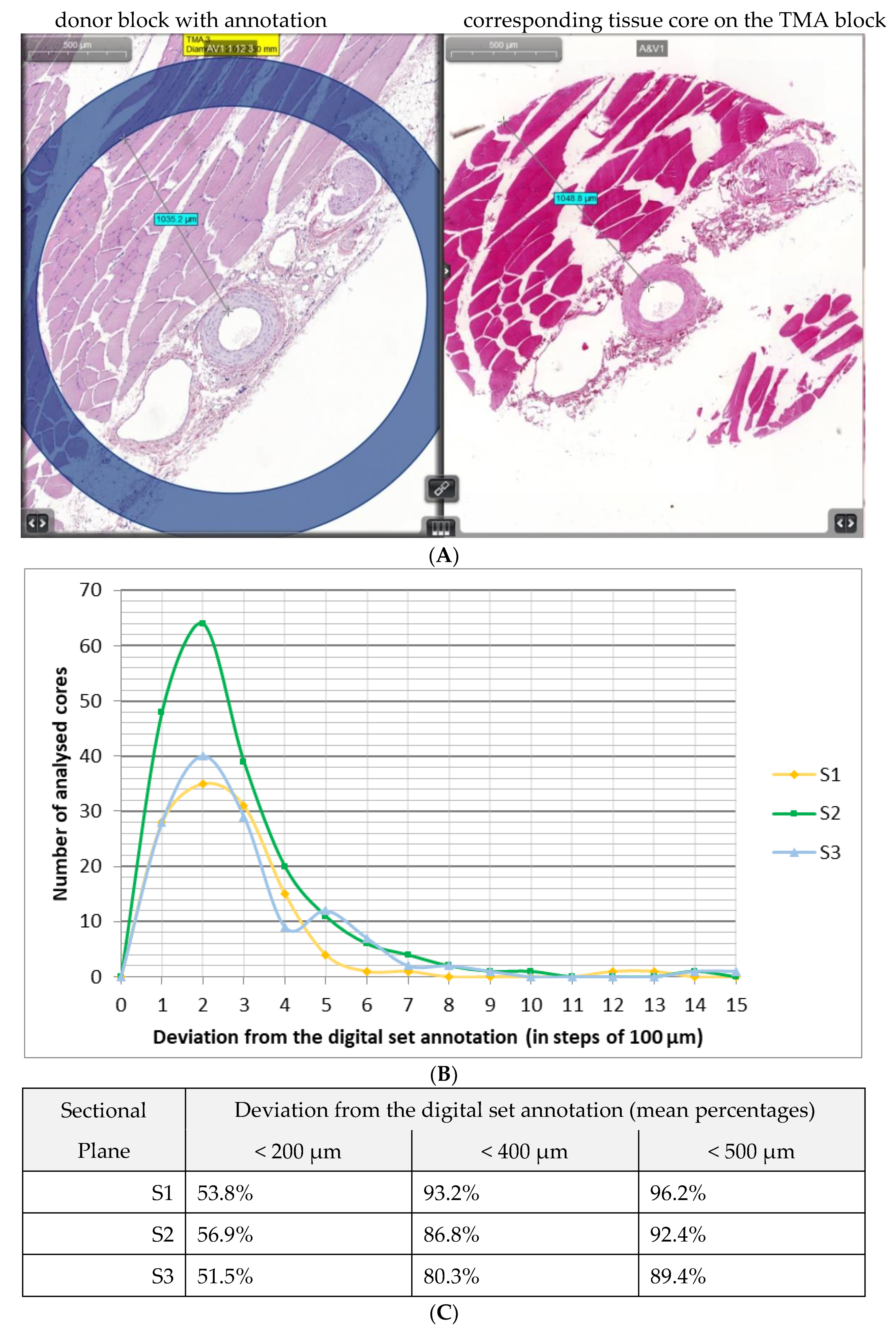
- The distance between a distinct structure and the annotation;
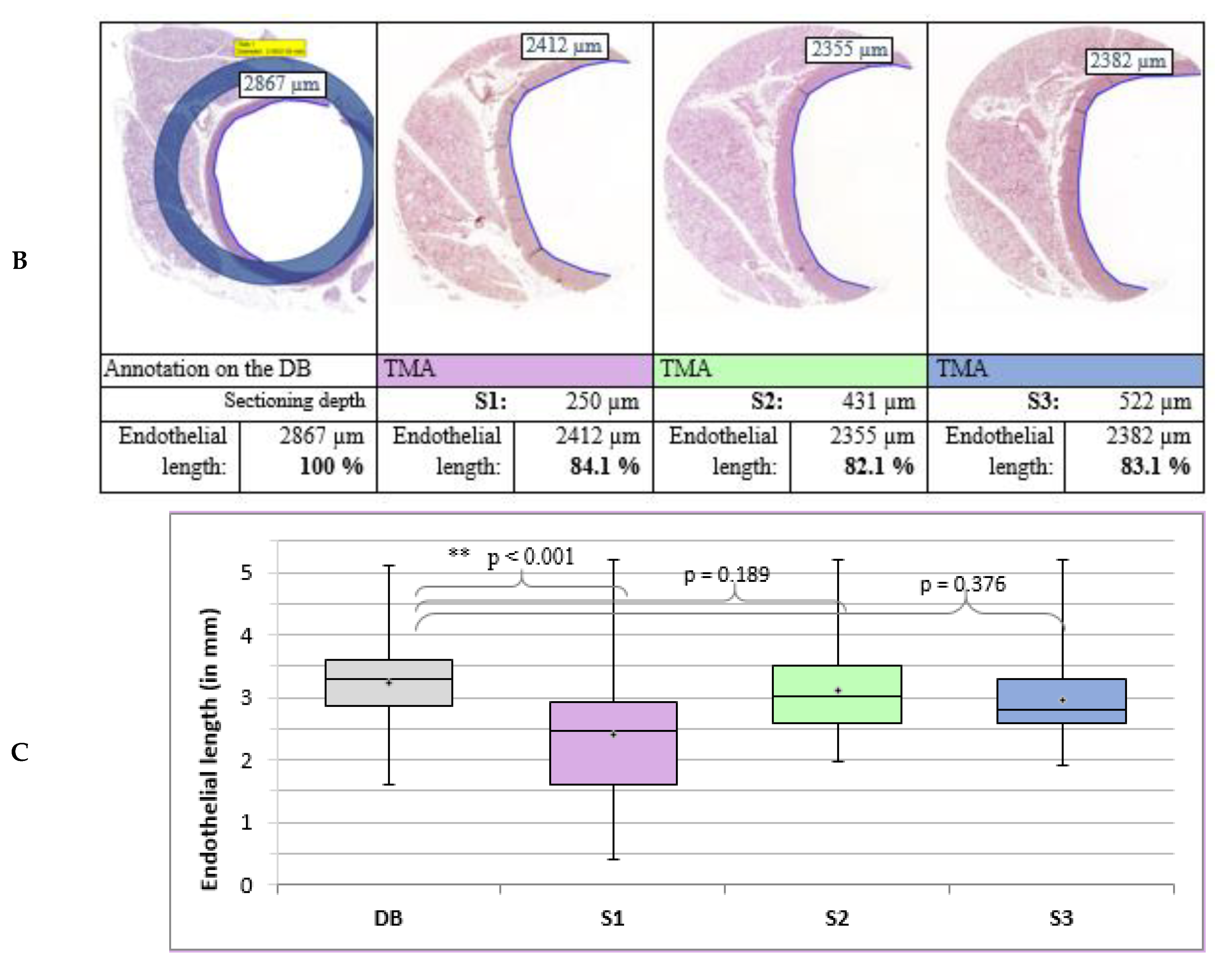
- The length of the endothelium inside the aorta.
3. Results
3.1. Overall Number of Core Losses
3.2. Correlation of Core Loss with the Punched Tissue Type
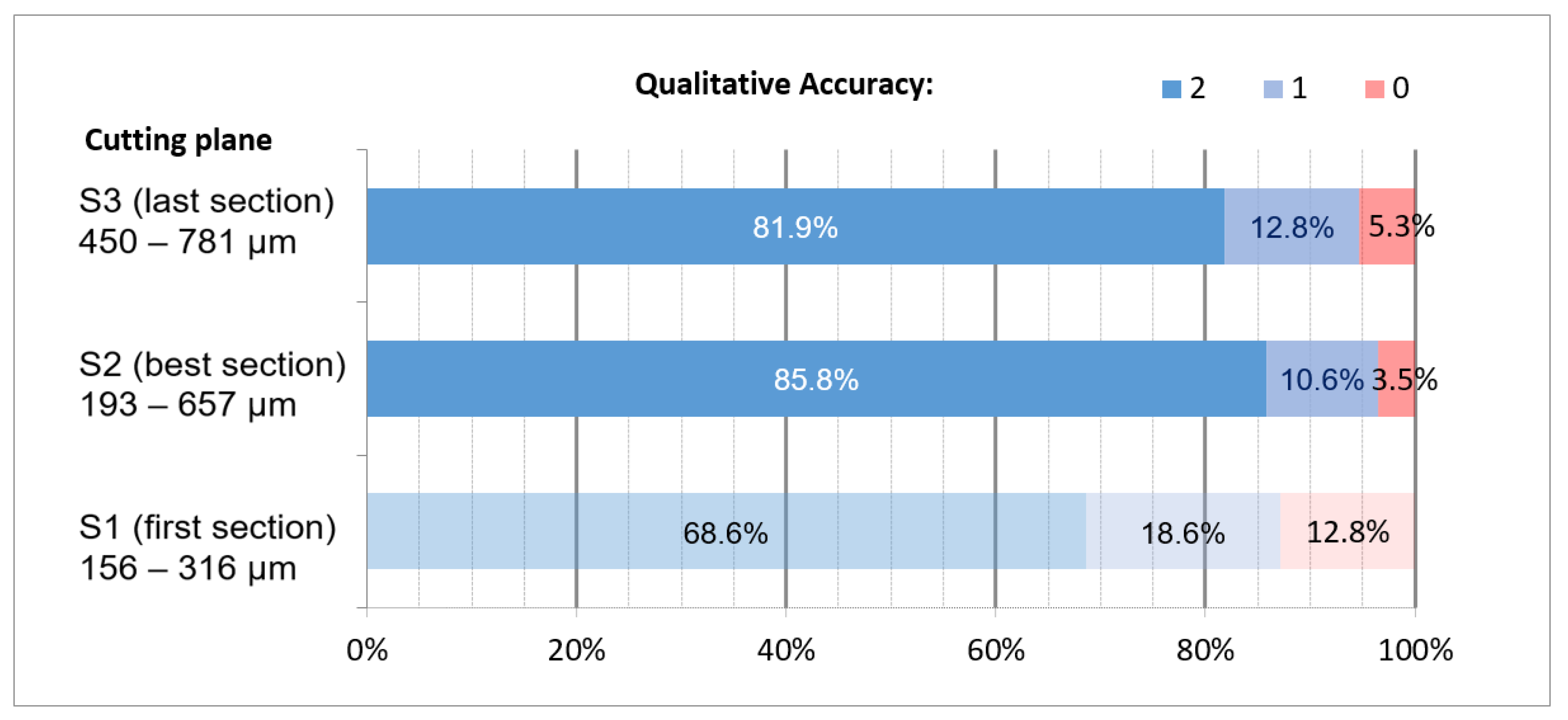
3.3. Qualitative Accuracy
3.4. Quantitative Accuracy
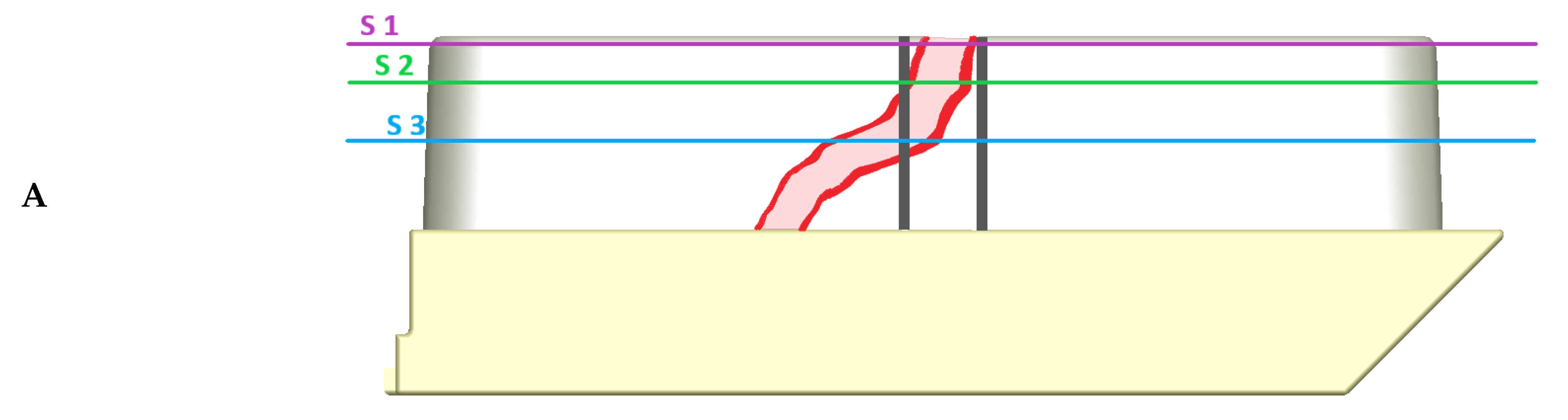
3.4.1. Distance between Target Structure and Inner Circumference
3.4.2. Length of the Endothelium in the Aorta
4. Discussion
4.1. Determination of Punching Diameter
4.2. Selection of the H&E Slides
4.3. Core Loss
4.4. Qualitative Accuracy
4.5. Quantitative Accuracy
4.6. Technical Recommendations and Limitations
- In this study, animal organs were resected and prepared especially for the execution of a TMA procedure. Hence, idealised conditions were created to embed organs and tissues into the paraffin in a good angulation and with a proper block thickness. If donor blocks are acquired from patient tissue samples and previous histopathological examinations were performed, it is important to ensure that the remaining tissue thickness is sufficient. The result of a decreased block thickness may be a higher amount of core loss due to reduced friction between tissue block and the used punching tool. Furthermore, proper block thickness ensures a good yield of TMA slides for further immunohistochemistry staining.
- The organs used in this study were from healthy animals. No tumour tissue or inflammatory tissue was included. Therefore, a higher core loss when using samples from squamous cell carcinoma of the mouth or other oral malignant or inflammatory lesions is likely.
- When a sample is collected, immediate and thorough fixation is paramount to reduce any degradation process and optimise tissue preservation and conservation.
- In the case of skin tissue, we recommend using samples which are not too thick to shift the ratio of tissue to the surrounding paraffin. This may decrease the tissue-specific effect of skin, leading to a lower amount of core loss.
- Different specific melting temperatures of the punched tissue and the surrounding paraffin may lead to deformation of tissue cores due to expansion and contraction. Paraffin blocks enriched with polymers are recommended [10].
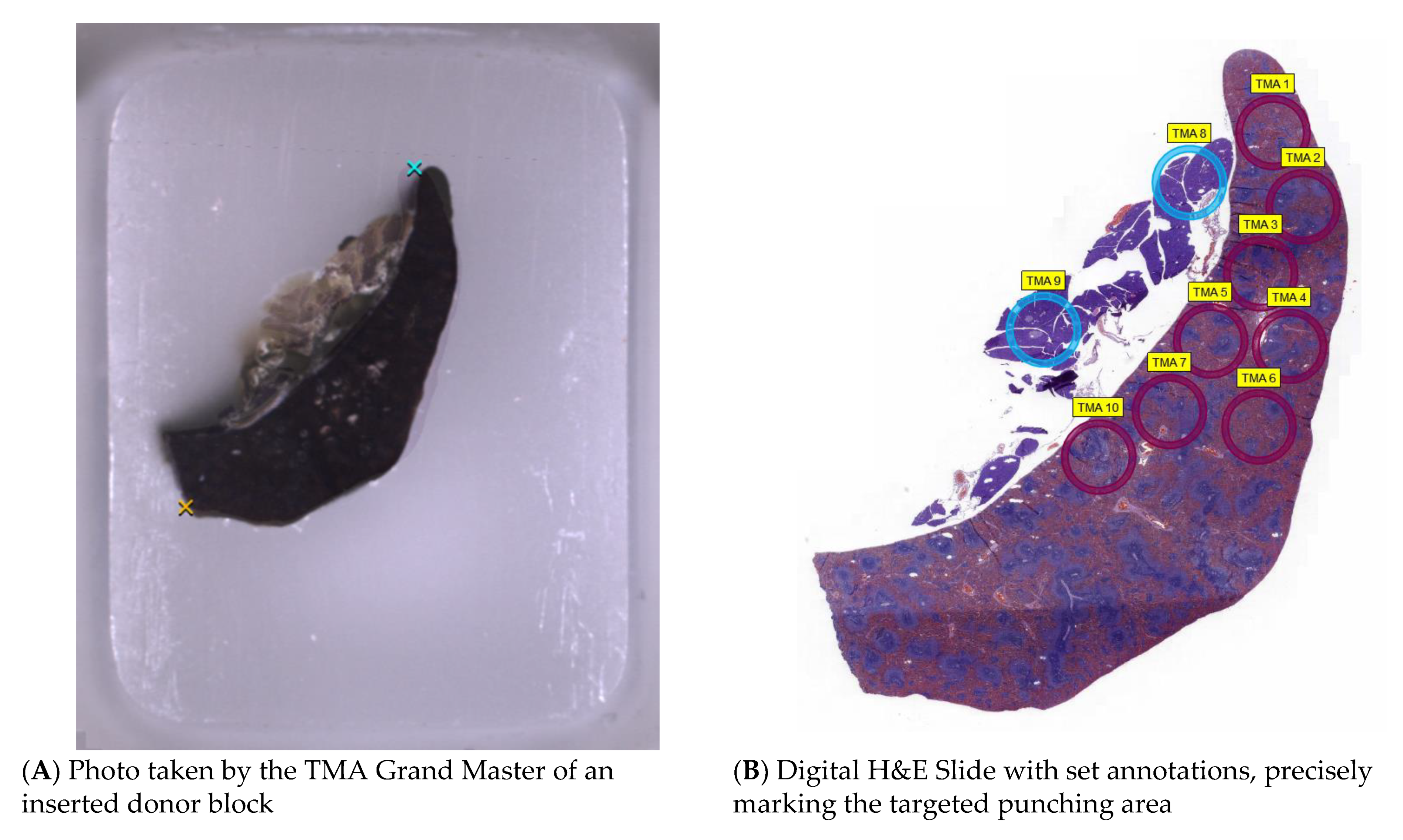
- Mismatching of the H&E slide and the picture taken from the donor block can lead to inaccurate digital aligning (Figure 2). This may happen due to shrinkage in the water bath and distortion during the slicing and processing of the H&E slide. If possible, the last acquired slide from the donor block should be used, due to it having highest concordance to the remaining tissue.
- Difficulties may arise during the slicing of the multi-tissue TMA blocks. Due to differing adjacent types of tissues, they may behave in an inhomogeneous way, if cut with the microtome. Therefore, it is advisable to use only one tissue type on each TMA if possible.
- Unilateral contraction due to temperature differences in the sectioning process may result in a shifted ratio of the different areas of the punched core.
- There should be empty paraffin on the sides of the TMA block to ensure enough space for the microtome to gain momentum to provide a clean cut when reaching embedded tissue cores. We recommend at least 2 mm.
- Coated slides should be used to increase friction and minimise distortion effects.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Battifora, H. The multitumor (sausage) tissue block: Novel method for immunohistochemical antibody testing. Lab. Investig. 1986, 55, 244–248. [Google Scholar]
- Kononen, J.; Bubendorf, L.; Kallioniemi, O.; Bärlund, M.; Schraml, P.; Leighton, S.; Torhorst, J.; Mihatsch, M.J.; Sauter, G.; Kallionimeni, O.-P. Tissue microarrays for high-throughput molecular profiling of tumor specimens. Nat. Med. 1998, 4, 844–847. [Google Scholar] [CrossRef]
- Behling, F.; Schittenhelm, J. Tissue microarrays—Translational biomarker research in the fast lane. Expert Rev. Mol. Diagn. 2018, 18, 833–835. [Google Scholar] [CrossRef]
- Voduc, D.; Kenney, C.; Nielsen, T.O. Tissue Microarrays in Clinical Oncology. Semin. Radiat. Oncol. 2008, 18, 89–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eskaros, A.R.; Egloff, S.A.A.; Boyd, K.L.; E Richardson, J.; Hyndman, M.E.; Zijlstra, A. Larger core size has superior technical and analytical accuracy in bladder tissue microarray. Lab. Investig. 2017, 97, 335–342. [Google Scholar] [CrossRef]
- Barrette, K.; Oord, J.J.V.D.; Garmyn, M. Tissue Microarray. J. Investig. Dermatol. 2014, 134, 1–4. [Google Scholar] [CrossRef] [Green Version]
- Ilyas, M.; Grabsch, H.; O Ellis, I.; Womack, C.; Brown, R.; Berney, D.; Fennell, D.; Salto-Tellez, M.; Jenkins, M.; Landberg, G.; et al. Guidelines and considerations for conducting experiments using tissue microarrays. Histopathology 2013, 62, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Merseburger, A.S. Die Tissue Microarray-Technik als neues “high throughput-tool” für den Nachweis differentieller Proteinexpression. J. Urol. Urogynaekol. 2013, 10, 7–11. [Google Scholar]
- Bubendorf, L. Tissue microarray (TMA) technology: Miniaturised pathology archives for high-throughput in situ studies. J. Pathol. 2001, 195, 72–79. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.; Robeldo, T.; Castañeda, E.; Pagliarone, A.; Pinto, K.; Borra, R. Tissue microarray: Physical and chemical parameters involved in the construction of recipient blocks. J. Bras. Patol. Med. Lab. 2017, 53, 261–269. [Google Scholar] [CrossRef]
- Zlobec, I.; Koelzer, V.H.; Dawson, H.; Perren, A.; Lugli, A. Next-generation tissue microarray (ngTMA) increases the quality of biomarker studies: An example using CD3, CD8, and CD45RO in the tumor microenvironment of six different solid tumor types. J. Transl. Med. 2013, 11, 104. [Google Scholar] [CrossRef] [Green Version]
- Fowler, C.B.; Man, Y.-G.; Zhang, S.; O’Leary, T.; Mason, J.T.; Cunningham, R.E. Tissue Microarrays: Construction and Uses. Methods Mol. Biol. 2011, 724, 23–35. [Google Scholar] [CrossRef]
- Nolte, S.; Zlobec, I.; Lugli, A.; Hohenberger, W.; Croner, R.; Merkel, S.; Hartmann, A.; I Geppert, C.; Rau, T.T. Construction and analysis of tissue microarrays in the era of digital pathology: A pilot study targeting CDX1 and CDX2 in a colon cancer cohort of 612 patients. J. Pathol. Clin. Res. 2017, 3, 58–70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zlobec, I.; Suter, G.; Perren, A.; Lugli, A. A Next-generation Tissue Microarray (ngTMA) Protocol for Biomarker Studies. J. Vis. Exp. 2014, 2014, e51893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thunnissen, E.; for the European Thoracic Oncology Platform Lungscape Consortium; Kerr, K.M.; Dafni, U.; Bubendorf, L.; Finn, S.P.; Soltermann, A.; Biernat, W.; Cheney, R.; Verbeken, E.; et al. Programmed death-ligand 1 expression influenced by tissue sample size. Scoring based on tissue microarrays’ and cross-validation with resections, in patients with, stage I–III, non-small cell lung carcinoma of the European Thoracic Oncology Platform Lungscape cohort. Mod. Pathol. 2019, 33, 792–801. [Google Scholar] [CrossRef] [PubMed]
- Camp, R.L.; Neumeister, V.; Rimm, D.L. A Decade of Tissue Microarrays: Progress in the Discovery and Validation of Cancer Biomarkers. J. Clin. Oncol. 2008, 26, 5630–5637. [Google Scholar] [CrossRef] [PubMed]
- Andeen, N.K.; Bowman, R.; Baullinger, T.; Brooks, J.M.; Tretiakova, M.S. Epitope Preservation Methods for Tissue Microarrays: Longitudinal Prospective Study. Am. J. Clin. Pathol. 2017, 148, 380–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- E Pinder, S.; Brown, J.P.; Gillett, C.; A Purdie, C.; Speirs, V.; Thompson, A.M.; Shaaban, A.M. The manufacture and assessment of tissue microarrays: Suggestions and criteria for analysis, with breast cancer as an example. J. Clin. Pathol. 2012, 66, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Visser, N.C.M.; Van Der Wurff, A.A.M.; Pijnenborg, J.M.A.; Massuger, L.F.A.G.; Bulten, J.; Nagtegaal, I. Tissue microarray is suitable for scientific biomarkers studies in endometrial cancer. Virchows Archiv. 2018, 472, 407–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chavan, S.S.; Ravindra, S.; Prasad, M. Breast Biomarkers-Comparison on Whole Section and Tissue Microarray Section. J. Clin. Diagn. Res. 2017, 11, EC40–EC44. [Google Scholar] [CrossRef] [PubMed]
- Neves-Silva, R.; Fonseca, F.P.; de Jesus, A.S.; Pontes, H.A.R.; Rocha, A.C.; Brandão, T.B.; Vargas, P.A.; Lopes, M.A.; Almeida, O.P.; Santos-Silva, A.R. Tissue microarray use for immunohistochemical study of ameloblastoma. J. Oral Pathol. Med. 2016, 45, 704–711. [Google Scholar] [CrossRef]
- Jones, S.; Prasad, M.L. Comparative Evaluation of High-Throughput Small-Core (0.6-mm) and Large-Core (2-mm) Thyroid Tissue Microarray: Is Larger Better? Arch. Pathol. Lab. Med. 2012, 136, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Tramm, T.; Kyndi, M.; Sørensen, F.B.; Overgaard, J.; Alsner, J. Influence of intra-tumoral heterogeneity on the evaluation of BCL2, E-cadherin, EGFR, EMMPRIN, and Ki-67 expression in tissue microarrays from breast cancer. Acta Oncol. 2017, 57, 102–106. [Google Scholar] [CrossRef] [Green Version]
- Böhm, J.; Muenzner, J.K.; Caliskan, A.; Ndreshkjana, B.; Erlenbach-Wünsch, K.; Merkel, S.; Croner, R.; Rau, T.T.; Geppert, C.I.; Hartmann, A.; et al. Loss of enhancer of zeste homologue 2 (EZH2) at tumor invasion front is correlated with higher aggressiveness in colorectal cancer cells. J. Cancer Res. Clin. Oncol. 2019, 145, 2227–2240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoos, A.; Cordon-Cardo, C. Tissue Microarray Profiling of Cancer Specimens and Cell Lines: Opportunities and Limitations. Lab. Investig. 2001, 81, 1331–1338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shergill, I.S.; Rao, A.R.; Anjum, F.H.; Arya, M.; Patel, H.R.; Mundy, A.R. Tissue Microarrays and Their Relevance to the Urologist. J. Urol. 2006, 175, 19–26. [Google Scholar] [CrossRef]
- Vesterinen, T.; Mononen, S.; Salmenkivi, K.; Mustonen, H.; Räsänen, J.; Salo, J.A.; Ilonen, I.; Knuuttila, A.; Haglund, C.; Arola, J. Clinicopathological indicators of survival among patients with pulmonary carcinoid tumor. Acta Oncol. 2018, 57, 1109–1116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edin, S.; Kaprio, T.; Hagström, J.; Larsson, P.; Mustonen, H.; Böckelman, C.; Strigård, K.; Gunnarsson, U.; Haglund, C.; Palmqvist, R. The Prognostic Importance of CD20+ B lymphocytes in Colorectal Cancer and the Relation to Other Immune Cell subsets. Sci. Rep. 2019, 9, 19997–19999. [Google Scholar] [CrossRef]
- Laedrach, C.; Salhia, B.; Cihoric, N.; Zlobec, I.; Tapia, C. Immunophenotypic profile of tumor buds in breast cancer. Pathol. Res. Pract. 2018, 214, 25–29. [Google Scholar] [CrossRef]
- Hadgu, E.; Seifu, D.; Tigneh, W.; Bokretsion, Y.; Bekele, A.; Abebe, M.; Sollie, T.; Merajver, S.D.; Karlsson, C.; Karlsson, M.G. Breast cancer in Ethiopia: Evidence for geographic difference in the distribution of molecular subtypes in Africa. BMC Women’s Health 2018, 18, 40. [Google Scholar] [CrossRef] [Green Version]
- Montaser-Kouhsari, L.; Knoblauch, N.W.; Oh, E.-Y.; Baker, G.; Christensen, S.; Hazra, A.; Tamimi, R.M.; Beck, A.H. Image-guided Coring for Large-scale Studies in Molecular Pathology. Appl. Immunohistochem. Mol. Morphol. 2016, 24, 431–435. [Google Scholar] [CrossRef] [Green Version]
- Fedor, H.L.; De Marzo, A.M. Practical methods for tissue microarray construction. Methods Mol. Med. 2005, 103, 89–101. [Google Scholar] [PubMed] [Green Version]
- Kampf, C.; Olsson, I.; Ryberg, U.; Sjöstedt, E.; Pontén, F. Production of tissue microarrays, immunohistochemistry staining and digitalisation within the human protein atlas. J. Vis. Exp. 2012, 63, 3620. [Google Scholar]
- Kwon, M.J.; Nam, E.S.; Cho, S.J.; Park, H.R.; Shin, H.S.; Park, J.H.; Park, C.H.; Lee, W.J. Comparison of tissue microarray and full section in immunohistochemistry of gastrointestinal stromal tumors. Pathol. Int. 2009, 59, 851–856. [Google Scholar] [CrossRef]
- Fonseca, F.P.; De Andrade, B.A.B.; Rangel, A.L.C.A.; Della Coletta, R.; Lopes, M.A.; D Almeida, O.P.; Vargas, P.A. Tissue microarray is a reliable method for immunohistochemical analysis of pleomorphic adenoma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 81–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Targeted Punching Areas | Number Tissue Samples/Donor Blocks | Number of Punches | Punching Diameter in mm |
|---|---|---|---|
| Abdominal Aorta | 92 | 92 | 2 |
| Femoral artery, nerve and vein | 97 | 97 | 2 |
| Carotid artery | 82 | 82 | 1.5 |
| Myocardial muscle tissue | 108 | 324 | 1.5 |
| Lung parenchyma | 105 | 315 | 1.5 |
| Spleen parenchyma (red and white pulp) | 92 | 550 | 1.5 |
| Pancreas | 92 | 112 | 1.5 |
| Renal medulla or cortex | 114 | 636 | 1.5 |
| Basal lamina of the tongue | 107 | 492 | 2 |
| Skin tissue | 104 | 541 | 2/1.5/1 |
| Testis (control-tissue-sample) | 8 | 80 | 2/1.5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Werry, J.-E.; Müller, S.; Wehrhan, F.; Geppert, C.; Frohwitter, G.; Ries, J.; Kämmerer, P.W.; Moest, T.; Lutz, R.; Homm, A.; et al. Accuracy Analysis of a Next-Generation Tissue Microarray on Various Soft Tissue Samples of Wistar Rats. Appl. Sci. 2021, 11, 5589. https://doi.org/10.3390/app11125589
Werry J-E, Müller S, Wehrhan F, Geppert C, Frohwitter G, Ries J, Kämmerer PW, Moest T, Lutz R, Homm A, et al. Accuracy Analysis of a Next-Generation Tissue Microarray on Various Soft Tissue Samples of Wistar Rats. Applied Sciences. 2021; 11(12):5589. https://doi.org/10.3390/app11125589
Chicago/Turabian StyleWerry, Jan-Erik, Stefan Müller, Falk Wehrhan, Carol Geppert, Gesche Frohwitter, Jutta Ries, Peer W. Kämmerer, Tobias Moest, Rainer Lutz, Andi Homm, and et al. 2021. "Accuracy Analysis of a Next-Generation Tissue Microarray on Various Soft Tissue Samples of Wistar Rats" Applied Sciences 11, no. 12: 5589. https://doi.org/10.3390/app11125589







