Abstract
Quinoxaline is a privileged pharmacophore that has broad-spectrum applications in the fields of medicine, pharmacology and pharmaceutics. Similarly, the sulfonamide moiety is of considerable interest in medicinal chemistry, as it exhibits a wide range of pharmacological activities. Therefore, the therapeutic potential and biomedical applications of quinoxalines have been enhanced by incorporation of the sulfonamide group into their chemical framework. The present review surveyed the literature on the preparation, biological activities and structure-activity relationship (SAR) of quinoxaline sulfonamide derivatives due to their broad range of biomedical activities, such as diuretic, antibacterial, antifungal, neuropharmacological, antileishmanial, anti-inflammatory, anti-tumor and anticancer action. The current biological diagnostic findings in this literature review suggest that quinoxaline-linked sulfonamide hybrids are capable of being established as lead compounds; modifications on quinoxaline sulfonamide derivatives may give rise to advanced therapeutic agents against a wide variety of diseases.
1. Introduction
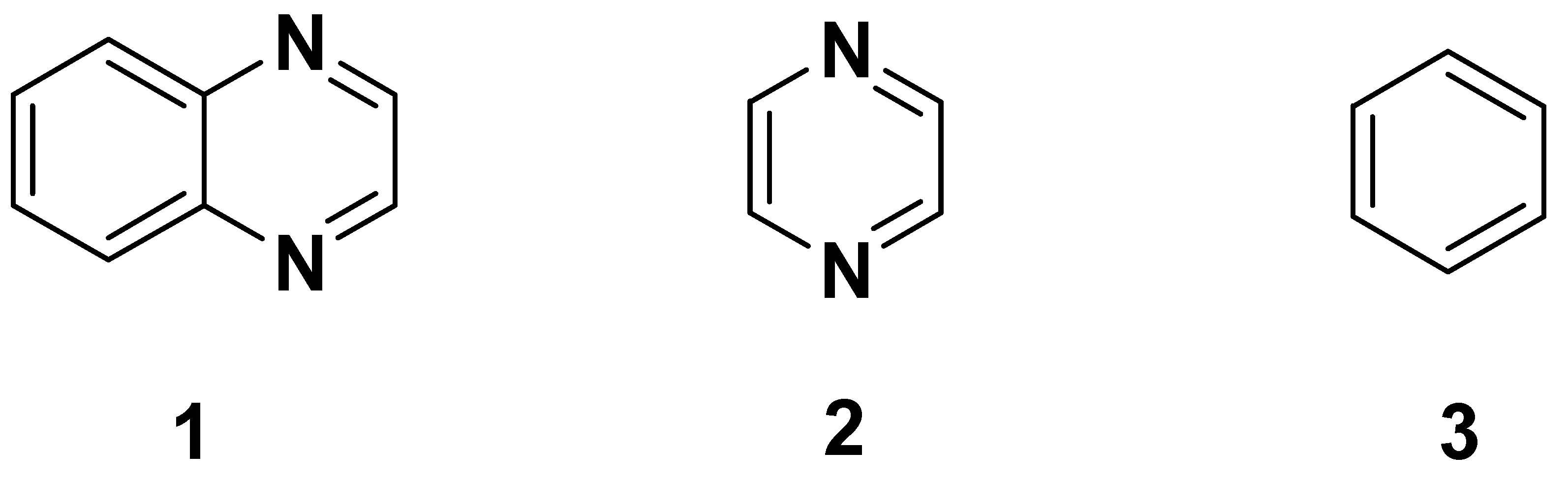
Heterocyclic scaffolds hold a pivotal place in medicinal chemistry and are one of the largest areas of research in organic chemistry due to their wide spectrum of biological activities. Therefore, continuous efforts have been undertaken to explore their therapeutic potential in the field of design and development of new drugs. Quinoxaline 1 is a privileged, ubiquitous and versatile nitrogen-containing heterocyclic motif, which exhibits a broad spectrum of medicinal, pharmacological and pharmaceutical applications. Quinoxaline, also known as benzopyrazine, was formed by the combination of pyrazine 2 and benzene 3 rings at the carbons 2 and 3 of the pyrazine ring (Figure 1) [1,2,3,4,5]. Quinoxaline derivatives are of relevant interest in medicinal chemistry because of a wide range of pharmacological and biological activities, such as antitumor, anticancer [6,7,8,9,10,11,12], antidiabetic [13,14,15,16,17], antimycobacterium tuberculosis [18,19], antimicrobial [20,21,22], antidepressant [23], anthelmintic [24], analgesic [25,26,27,28,29,30,31], anti-inflammatory [11,32,33,34,35,36], antiviral [37,38,39,40,41,42,43], antifungal [44,45,46,47,48,49], antimalarial [50,51,52,53,54,55], antibacterial [56,57,58,59,60,61,62,63], antioxidant [14,64,65], antithrombotic [66,67] and antiprotozoal [54,68].
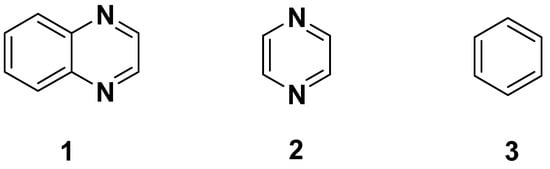
Figure 1.
Structures of quinoxaline, pyrazine and benzene.
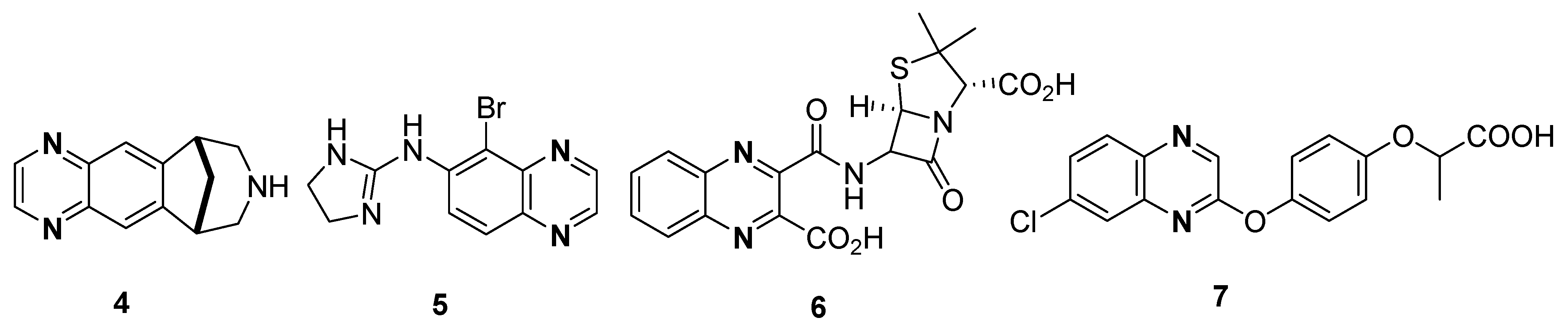
Different quinoxaline-based drugs have been playing a main role in the therapy and treatment of various diseases, such as varenicline 4 (aids in smoking cessation), brimonidine 5 (anti-glaucoma activity), quinacillin 6 (antibacterial activity) and XK469 NSC 7 (selective topoisomerase IIβ poison) (Figure 2) [69,70,71].
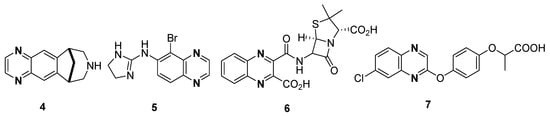
Figure 2.
Structures of quinoxaline drugs 4–7.
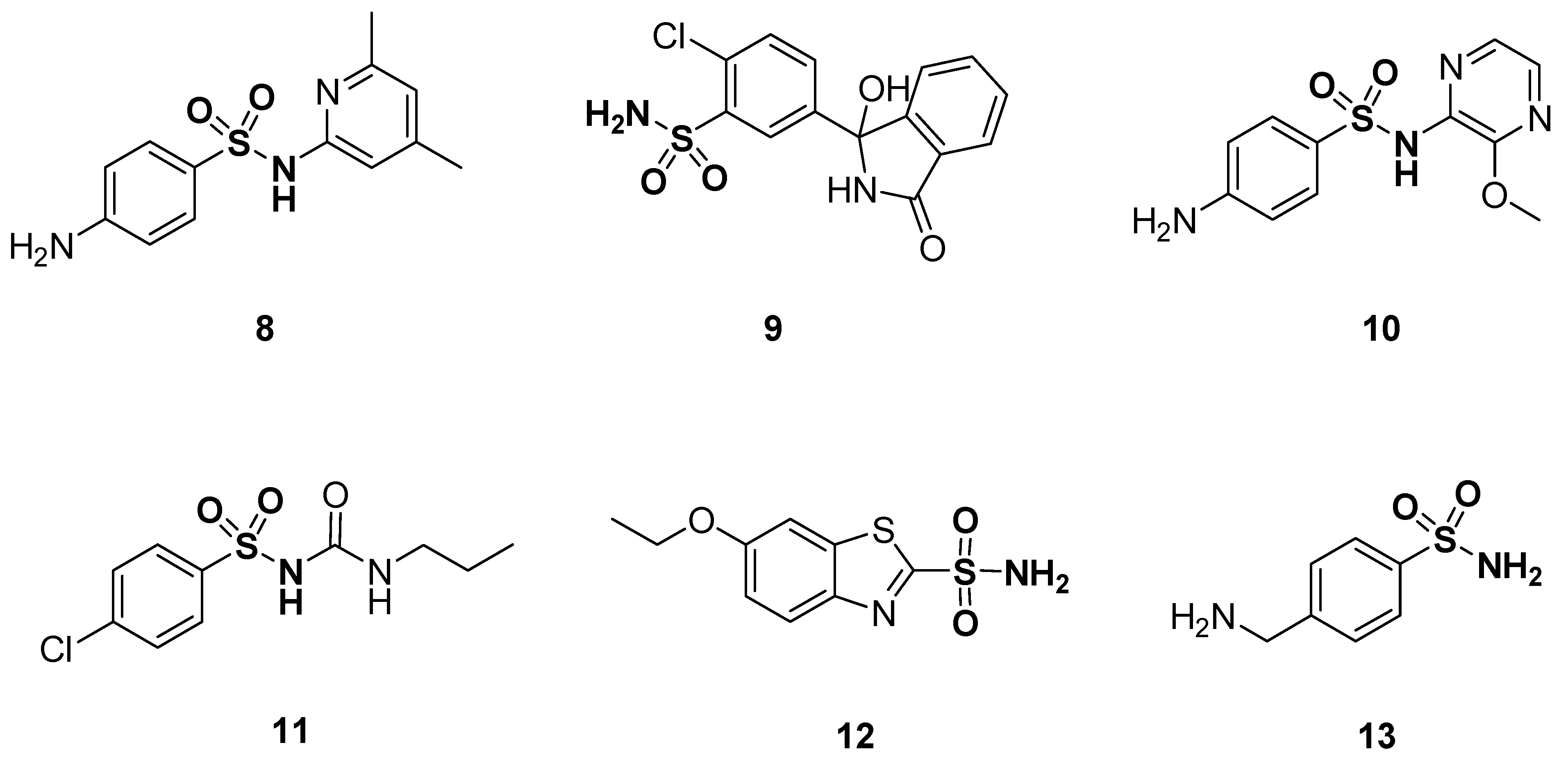
Sulfonamides were first characterized in 1932 and are organic chemical frameworks in which a sulfonyl group is linked to an amine group (-SO2-NR1R2) [72]. The sulfonamide group is a key moiety that plays a paramount role in both medicinal chemistry and organic chemistry due to their immense biological action. A series of therapeutic agents contain a sulfonamide moiety in their chemical structures [73,74,75,76,77,78,79]. Particular examples are antibiotics which are used on a large scale for many clinical purposes [80]. Heterocyclic sulfonamides exhibit different biological activities, such as antibacterial [81,82,83], anti-inflammatory [84], antibacterial [85], antimicrobial [86], antifungal [87], antiprotozoal [88], antiviral [89], antimalarial [90], antitumor [91,92,93], carbonic anhydrase inhibitors [94,95,96,97,98,99], antidiabetic [100], anticonvulsant, [101], anti-glaucoma [95,97], anti-obesity [102], diuretic [103,104,105], hypoglycemic [95,97], anti-neuropathic pain [106], matrix metalloproteinase and bacterial protease inhibitors [107,108]. The sulfonamide moiety is a basic component of drugs such as sulfamethazine 8 (antibacterial), chlortalidone 9 (diuretic), sulfametopyrazine 10 (chronic bronchitis, urinary tract infections and malaria), chlorpropamide 11 (treats diabetes mellitus type 2), ethoxzolamide 12 (carbonic anhydrase inhibitor) and mafenide 13 (antimicrobial agent used to treat severe burns) (Figure 3) [109].
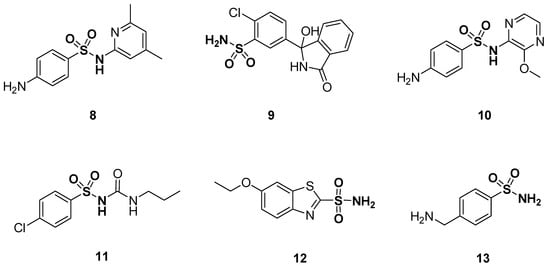
Figure 3.
Structures of sulfonamide drugs 8–13.
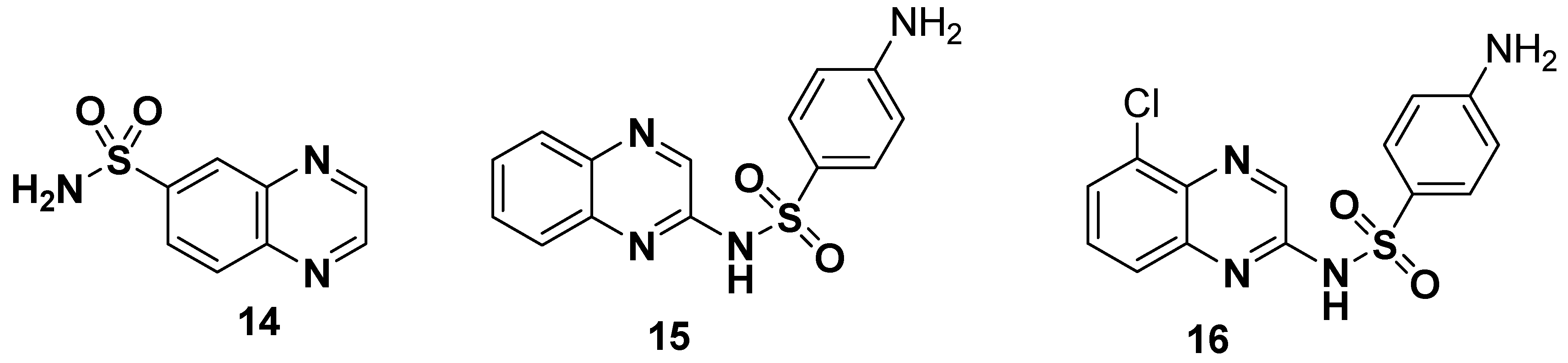
Hybrid structures formulated by the combination of quinoxaline and sulfonamide moieties display novelty and versatility and possess a consistent therapeutic potential against most diseases. The general structure of quinoxaline sulfonamide 14, sulfaquinxaline 15 (antimicrobial and a coccidiosis for veterinary use) and chloroquinoxaline sulfonamide 16 (topoisomerase-IIα and a topoisomerase-IIβ poison) are depicted in Figure 4 [110].
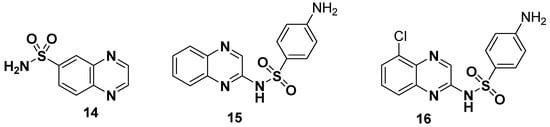
Figure 4.
Structures of quinoxaline sulfonamide drugs 14–16.
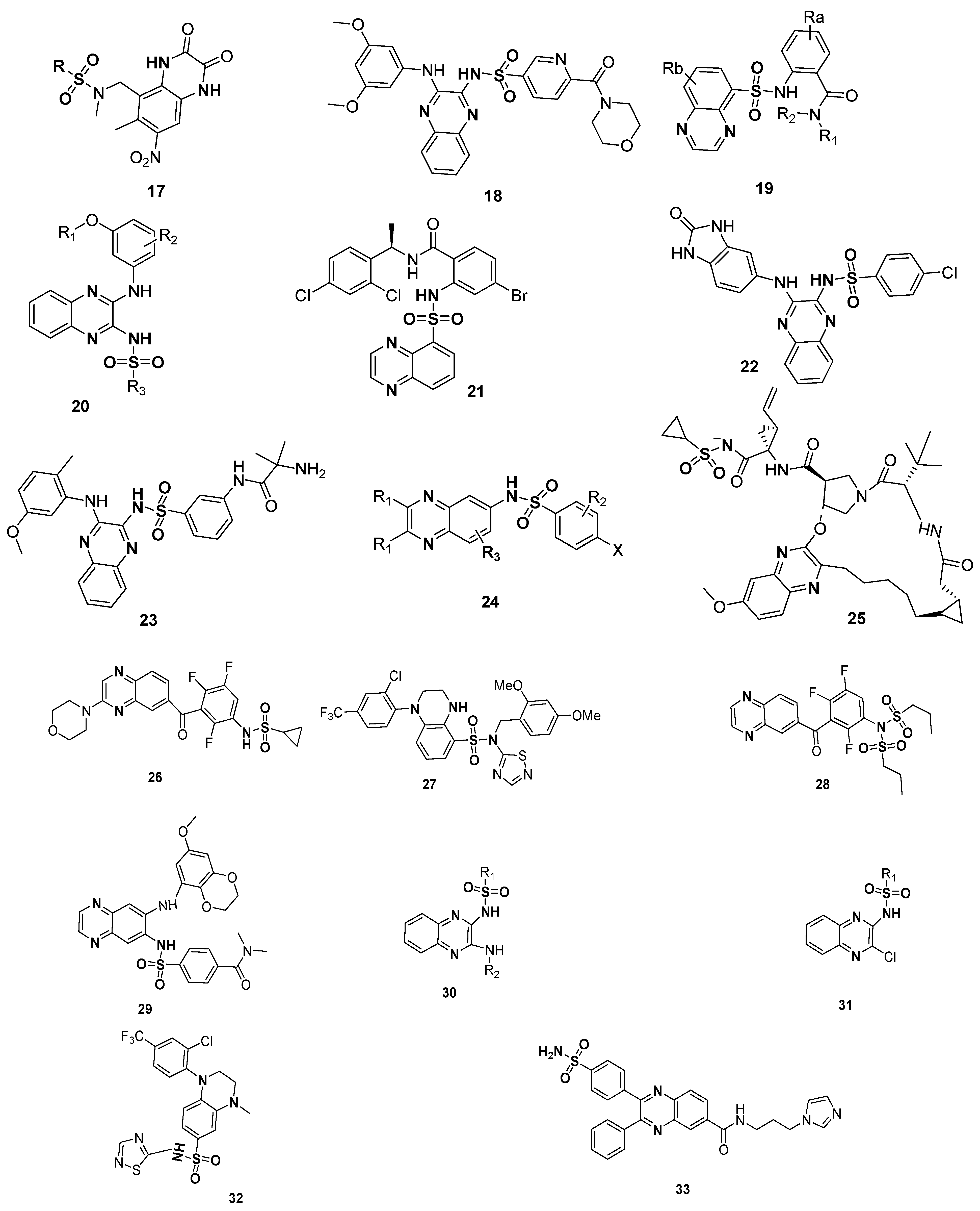
The significance of quinoxline sulfonamide derivatives in medicinal chemistry as therapeutic agents is clearly displayed by the patents. The patented sulfonamide derivatives, such as substituted quinoxaline-2,3-diones 17 (glutamate receptor antagonists) [111], quinoxaline-containing pyridine-3-sulfonamide derivative 18 (used as PI3K inhibitors) [112], amidophenyl-sulfonylamino-quinoxaline compounds 19 (CCK2 modulators useful in the treatment of CCK2 mediated diseases) [113], quinoxaline compounds 20 (for the treatment of autoimmune disorders and/or inflammatory diseases and cardiovascular diseases, etc.) [114], dichlorophenyl moiety-containing quinoxaline sulfonamide 21 (useful for the treatment of disease states mediated by CCK2 receptor activity) [115] and benzimidazole moiety-based quinoxaline benzene sulfonamide scaffold 22 (phosphatidylinositol 3-kinase inhibitors) [116], 2-chloro-5-methoxyphenylamino based quinoxaline sulfonamide derivative 23 (phosphatidylinositol 3-kinase inhibitors) [117], substituted quinoxaline compound 24 (HCV NS3 protease inhibitors) [118], macrocyclic quinoxaline compound 25 (HCV NS3 protease inhibitors) [119], benzene sulfonamide derivatives of quinoxaline 26 (anticancer agents) [120], tetrahydro quinoxaline derivative 27 (inhibitors of sodium channels/pain disorders) [121], benzene sulfonamide derivatives of quinoxaline 28 (kinase inhibitors, anticancer agents) [122], quinoxaline sulfonamides 29 (PI3K inhibitors, anti-inflammatory and autoimmune diseases) [123], substituted aminoquinoxaline sulfonamides 30 and substituted chloroquinoxaline sulfonamides 31 [124], substituted 1,2,4-thiadiazole based quinoxaline sulfonamide derivative 32 (inhibitors of sodium channels) [125] and substituted diphenyl quinoxaline sulfonamide derivative 33 (inhibitors of NAMPT) [126] are depicted in Figure 5.
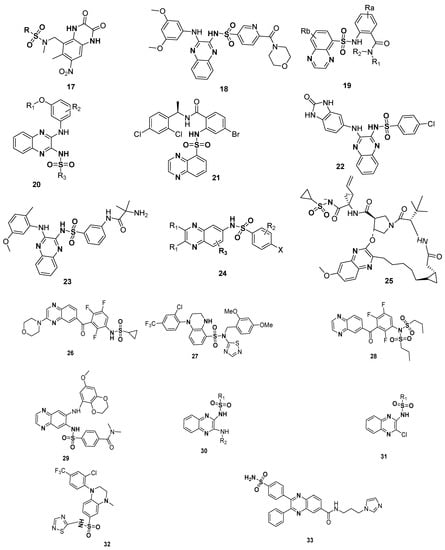
Figure 5.
Structures of patented quinoxaline sulfonamides 17–33.
2. Synthesis and Biological Activities
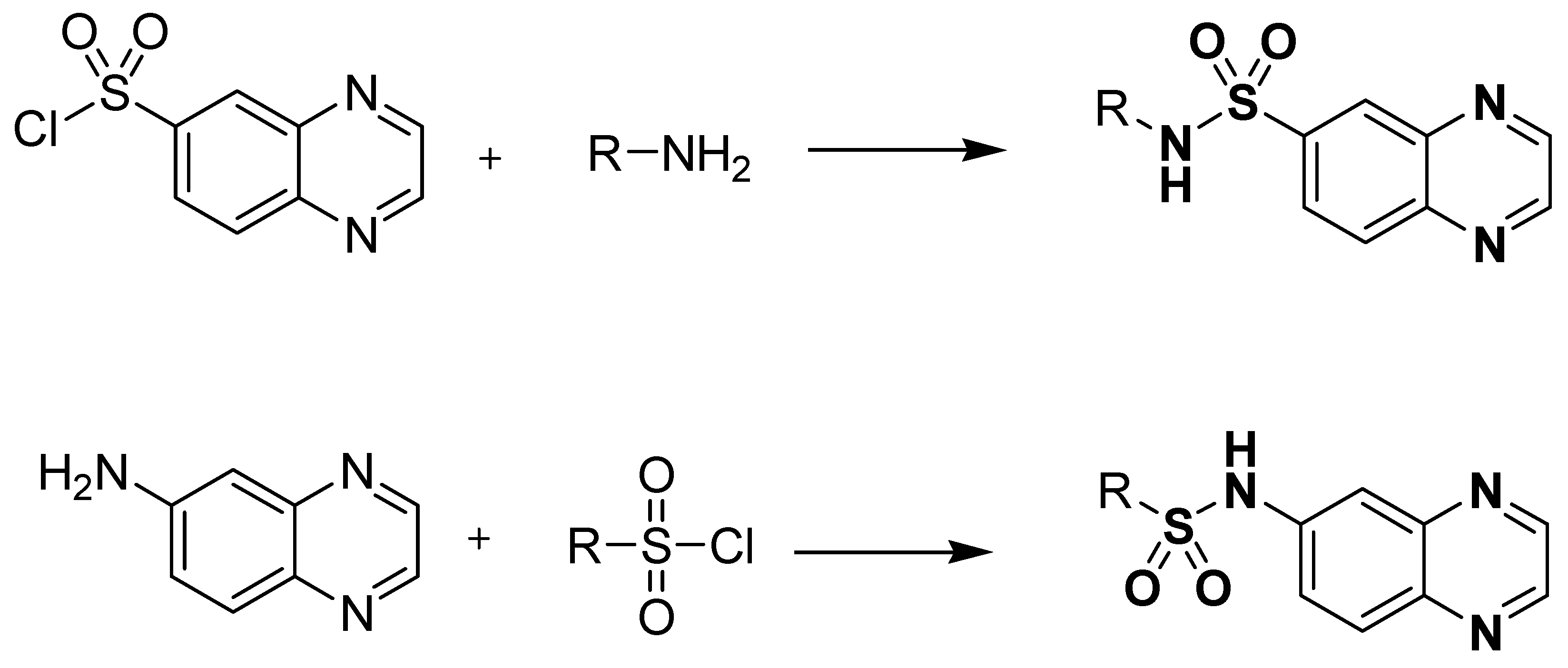
Standard reference works have shown that the orthro-phenylenediamine (OPD) can be condensed with dicarboxylic acids, diketones, α-halo-ketones and esters to give quinoxalines. The development of quinoxaline sulfonamide chemistry is linked with the presence of amino (-NH2) and sulfonyl chloride (-SO2Cl) groups in the reacting species. Commonly, quinoxaline sulfonamides can be available via general transformation of substituted amines, with the quinoxaline containing sulfonyl chloride functionality and vice versa, as depicted in Figure 6.
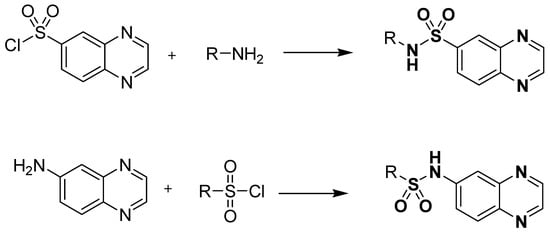
Figure 6.
General preparation of quinoxaline sulfonamides.
2.1. Benzothiazole Quinoxaline Sulfonamide Derivatives with Diuretic Activity
Medications designed to increase the excretion of water and salt in urine are termed as diuretics. Diuretics are used as therapeutics in liver cirrhosis, epilepsy, edema, hypertensions, heart failure, hypercalciuria, diabetes insipidus and in some kidney diseases. Their mechanism of action on distinct sites of nephrons involves the production of diuresis and inhibition of sodium ions re-absorption in the renal tubules of the kidney.
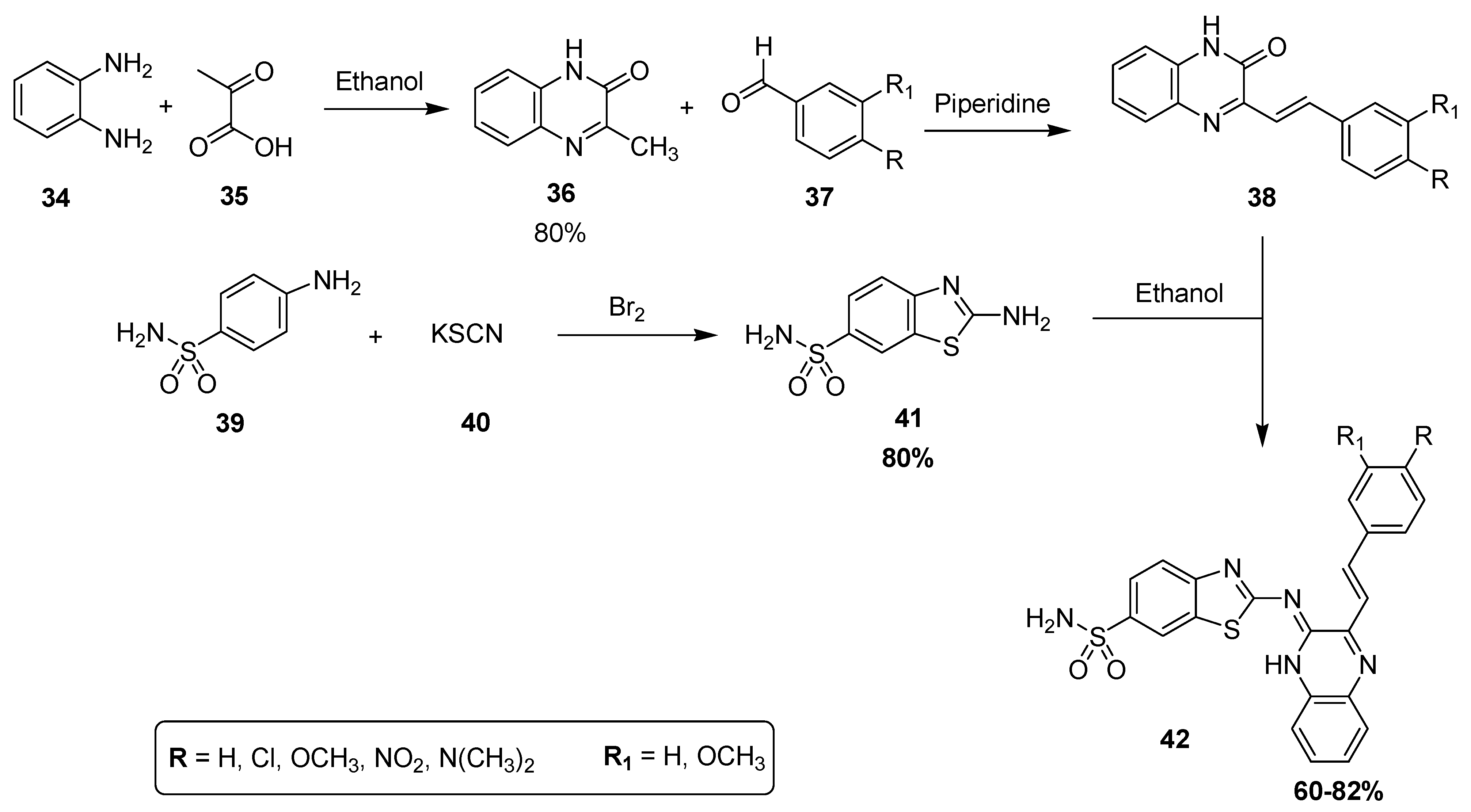
A new multistep synthetic methodology was developed for the synthesis of a novel promising class of substituted quinoxaline sulfonamides as diuretic agents, assessed in vivo by Husain and coworkers [127]. The condensation of o-phenylene diamine 34 with pyruvic acid 35 in methanol produced 3-methylquinoxaline-2H-one 36 with an 80% yield that was further reacted with substituted aldehydes 37 in the presence of piperidine to afford the substituted quinoxaline derivatives 38. The condensation of 2-amino-benzothiazole-6-sulfonamide (41) with 38 in ethanol yielded the final quinoxaline sulfonamide derivatives 42 in moderate to good yield (60–82%) (Scheme 1) [127]. The derivatives with electron-donating groups showed the maximum yield, while the electron-withdrawing groups resulted in a minimum yield.
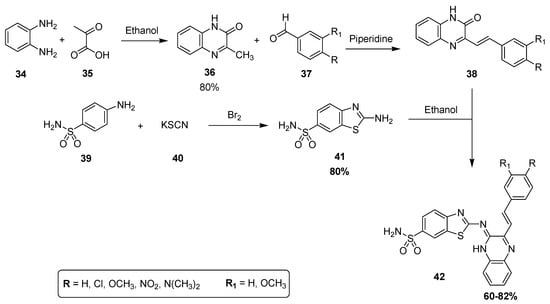
Scheme 1.
Synthesis of sulfonamide derivatives 42.
The Lipschitz method was adopted to determine the diuretic activity of the thiazole moiety-based quinoxaline sulfonamide hybrids. Fifty-four healthy adult Wistar albino rats (180–200 g) were selected and divided into nine groups, and these were acclimatized in standard environmental conditions for one week.
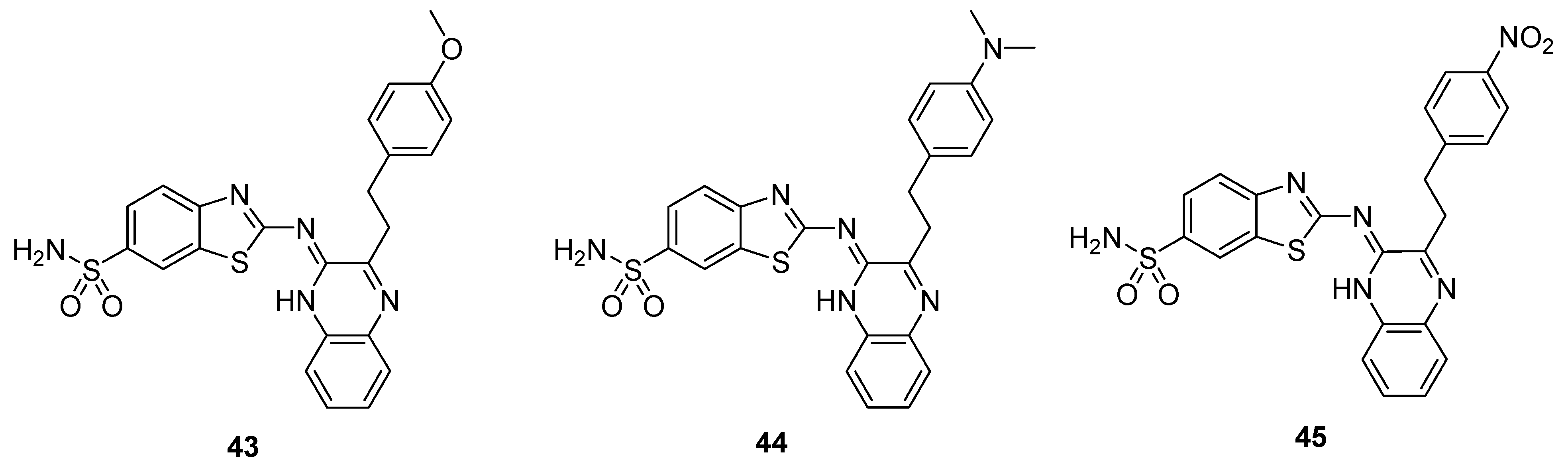
The thiazole moiety-containing quinoxaline sulfonamide derivative 43 showed a remarkably high diuretic activity and Lipschitz value (1.13 and 1.28 values, respectively) compared to the standard reference drugs, acetazolamide and urea. The derivative 44 exhibited a moderate diuretic activity (value of 0.78) and Lipstchitz value (0.88). The structural motif 45 exhibited the least diuretic action among the three compounds (value of 0.56) and a Lipschitz value of 0.63 (Table 1). These results showed that diuretic activity is directly proportional to the Lipschitz value. The SAR showed that the presence of a strong electron-donating group (EDG), such as methoxy, on the benzene ring of scaffold 43 increases the diuretic activity, while the presence of strong electron-withdrawing group (EWG), such as an -NO2 group, decreases the diuretic activity of the analog 45. The presence of an -N(CH3)2 moiety on the benzene ring of derivative 44 induced a moderate diuretic effect (Figure 7). The SAR study revealed that the diuretic activity decreased in order of –OCH3 > -N(CH3)2 > -NO2. This indicated that EDG increases the diuretic activity, while EWG decreases the diuretic activity [127].

Table 1.
Diuretic activity of benzothiazole quinoxaline sulfonamide derivatives 43–45.
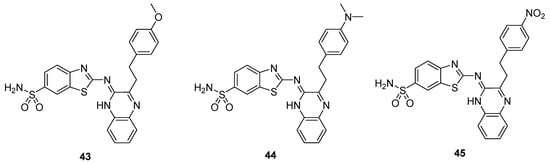
Figure 7.
Substituted quinoxaline benzothiazole sulfonamide derivative 43–45.
2.2. Quinoxaline Sulfonamides with Antibacterial Activity
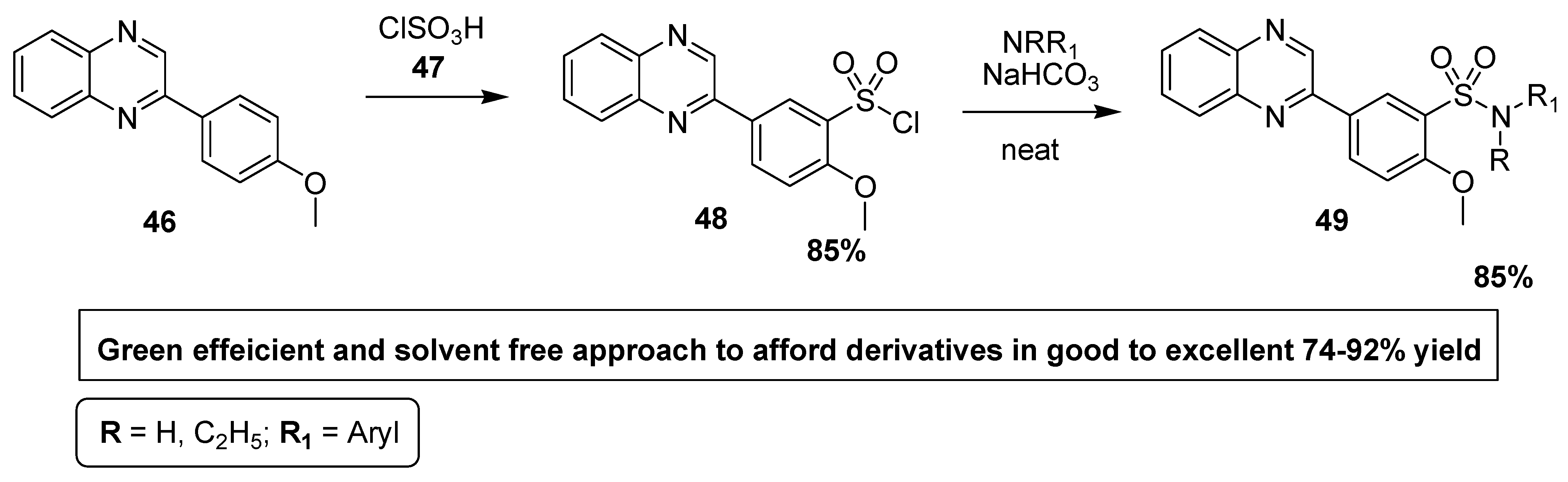
Alavi et al. reported a facile, efficient, solvent and catalyst-free green protocol for the synthesis of quinoxaline sulfonamide derivatives, which were screened for their antibacterial activity against different Gram-positive and Gram-negative bacterial strains. The quinoxaline sulfonyl chloride (QSC) 48 was synthesized in 85% yields by the treatment of methoxyphenyl quinoxaline 46 with chlorosulfonic acid 47. The QSC scaffold 48 was reacted with substituted aromatic amines in neat and ecofriendly conditions to afford substituted quinoxaline sulfonamides of the type 49 in good to excellent yield (Scheme 2). Aromatic amines with EDG, such as methyl and methoxy, reacted in 3–10 min and lead to products with a higher yield, while aromatic amines with EWG afforded products with a low yield. Aromatic amines with strongly EWG, such as nitro, do not react in the below mentioned conditions [128].
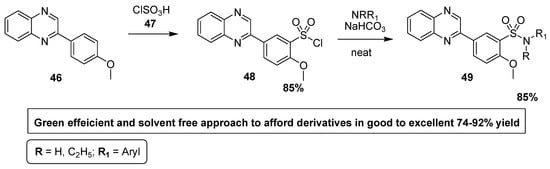
Scheme 2.
Synthesis of substituted quinoxaline sulfonamide derivatives, with structure 49.
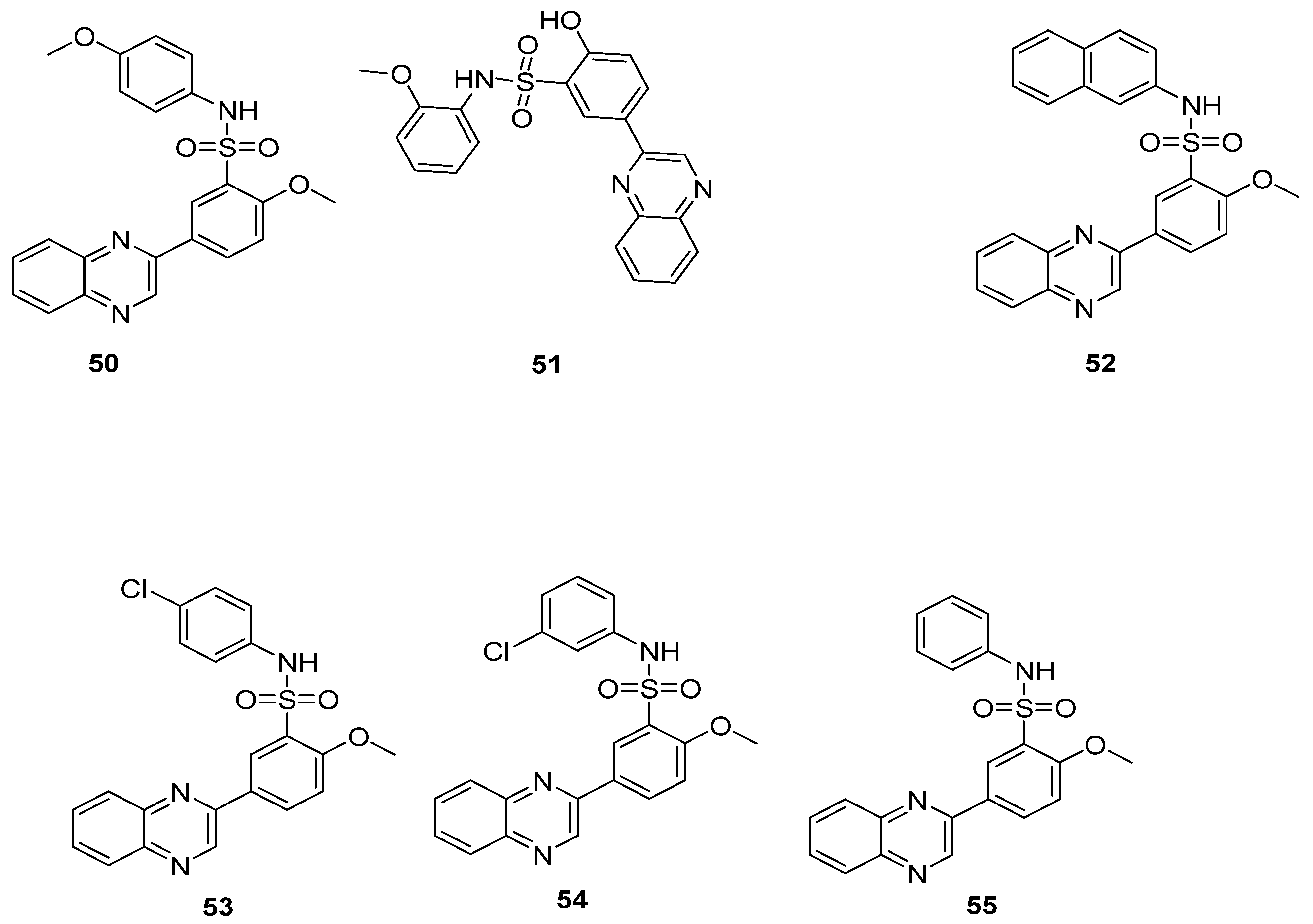
The substituted 4-methoxyphenyl quinoxaline sulfonamide 50 and substituted 2-methoxyphenyl quinoxaline sulfonamide derivative 51 showed the best antibacterial activity against S. Aureus, having a zone of inhibition (ZOI) of 20 mm and 22 mm, respectively, which is more than the standard chloramphenicol drug (ZOI 19 mm) but less than reference ampicillin drug (ZOI 28 mm). The substituted naphthalene quinoxaline sulfonamide derivatives 52 exhibited the highest antibacterial activity against E. coli, with a ZOI value of 18 mm when compared to the standard drug, ampicillin (ZOI 15 mm), but less than the reference chloramphenicol drug (ZOI 19 mm) (Table 2). A moderate antibacterial activity was observed for the substituted chlorophenyl quinoxaline sulfonamides 53 and 54 against Gram-positive S. Aureus and Gram-negative E. coli. The unsubstituted phenyl quinoxaline sulfonamide derivative 55 showed the least antibacterial activity against S. Aureus and E. coli, exhibiting a ZOI of 5 mm and 5> mm, respectively, when compared to the reference drugs, chloramphenicol and ampicillin, exhibiting ZOI values of 19 mm and 28 mm against S. Aureus and 22 mm and 15 mm against E. coli. The SAR revealed that the presence of electron-rich groups on the aminophenyl ring showed the highest antibacterial activity for derivatives 50–52, whereas the presence of the electron-withdrawing groups on the aminophenyl ring, such as a chloro atom (weak EWD group), in 53 and 54 scaffolds led to a moderate antibacterial activity, while the least antibacterial activity was observed for the unsubstituted derivative 55 (Figure 8) [128]. The results showed that electron-donating group’s substitution on the quinoxaline sulfonamide scaffolds contribute positively to increase the antibacterial activity.

Table 2.
Antibacterial activity of quinoxaline benzene sulfonamide derivatives 50–55.
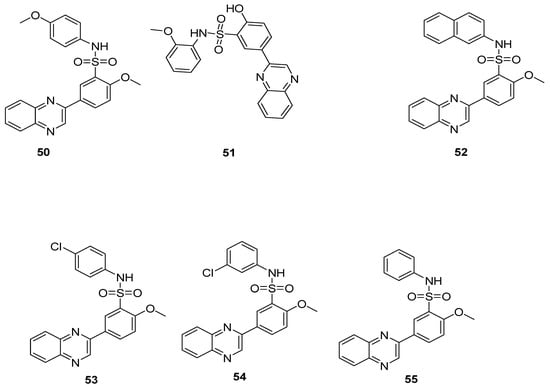
Figure 8.
Substituted quinoxaline benzene sulfonamide derivatives 50–55.
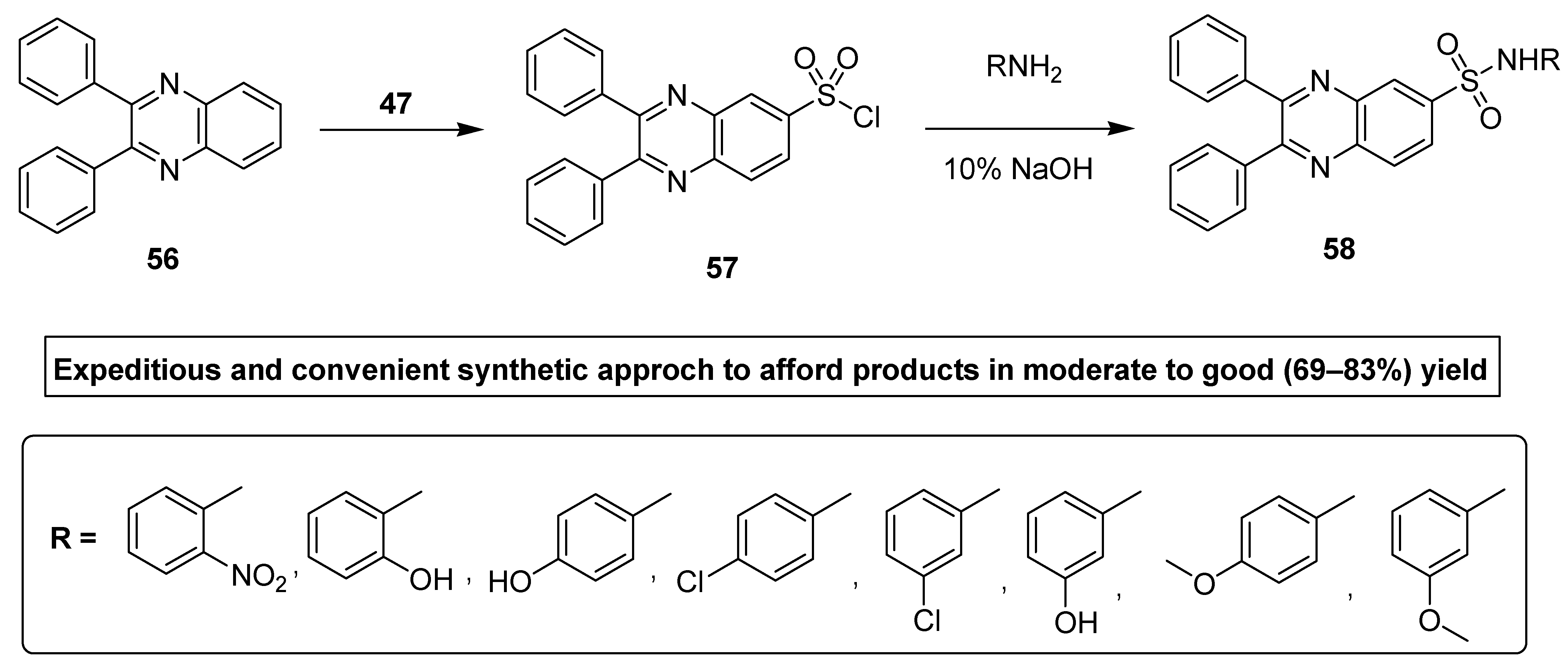
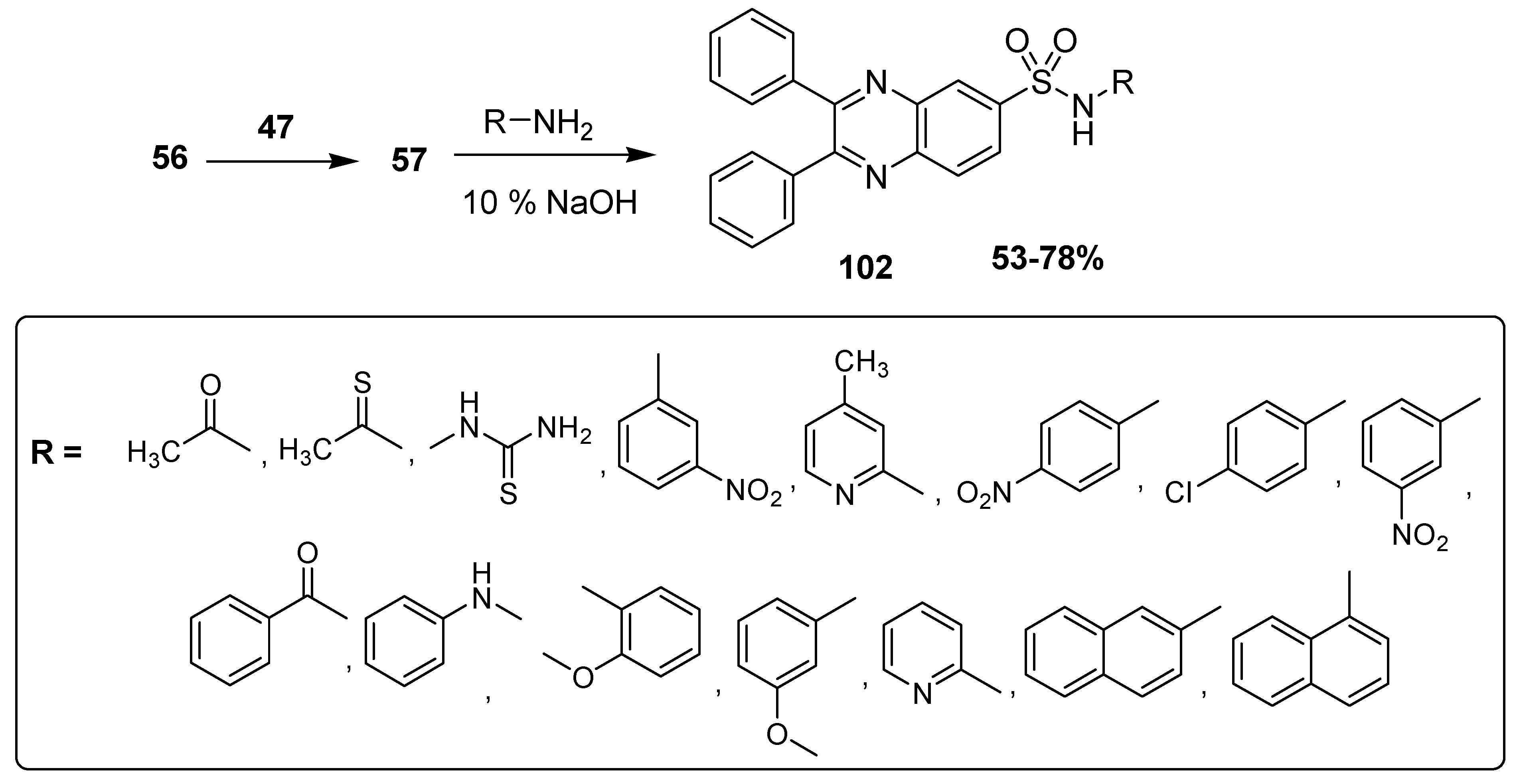
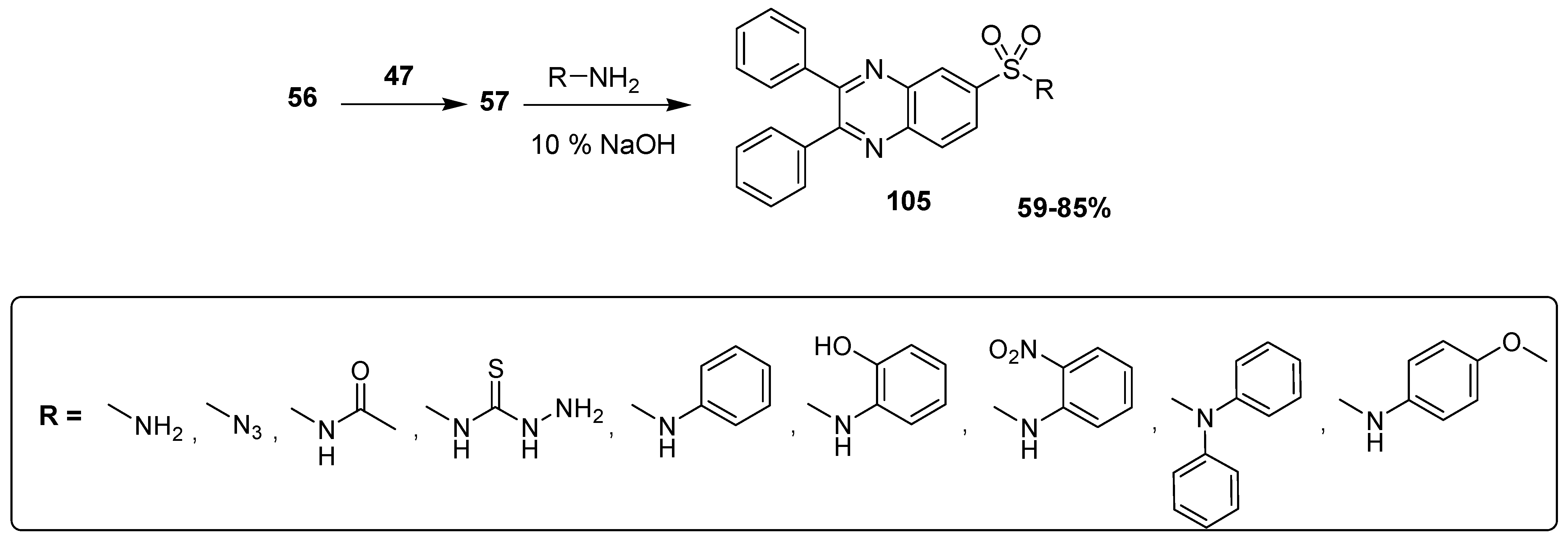
Ingle et al. synthesized antimicrobial quinoxaline sulfonamide derivatives by applying a convenient and an expeditious methodology. In this synthetic strategy, the nucleophilic substitution was performed by attacking the electrophilic center (S) of chloro sulfonic acid 47 by the benzene of diphenyl quinoxaline 56 to furnish the intermediate 2,3-diphenylquinoxaline-6-sulfonyl chloride 57, which further refluxed with the substituted primary amine under basic conditions to deliver the final product diphenyl quinoxaline sulfonamide 58 in moderate to good (69–83%) yield (Scheme 3). The aromatic amines with EWG, such as nitro, lead to the final products in a higher yield, while the aromatic amines with EDG, such as methyl and methoxy, etc, lead to final products in low yield [129].
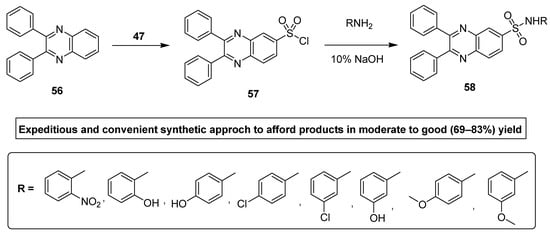
Scheme 3.
Synthesis of substituted quinoxaline sulfonamide derivatives with structure 58.
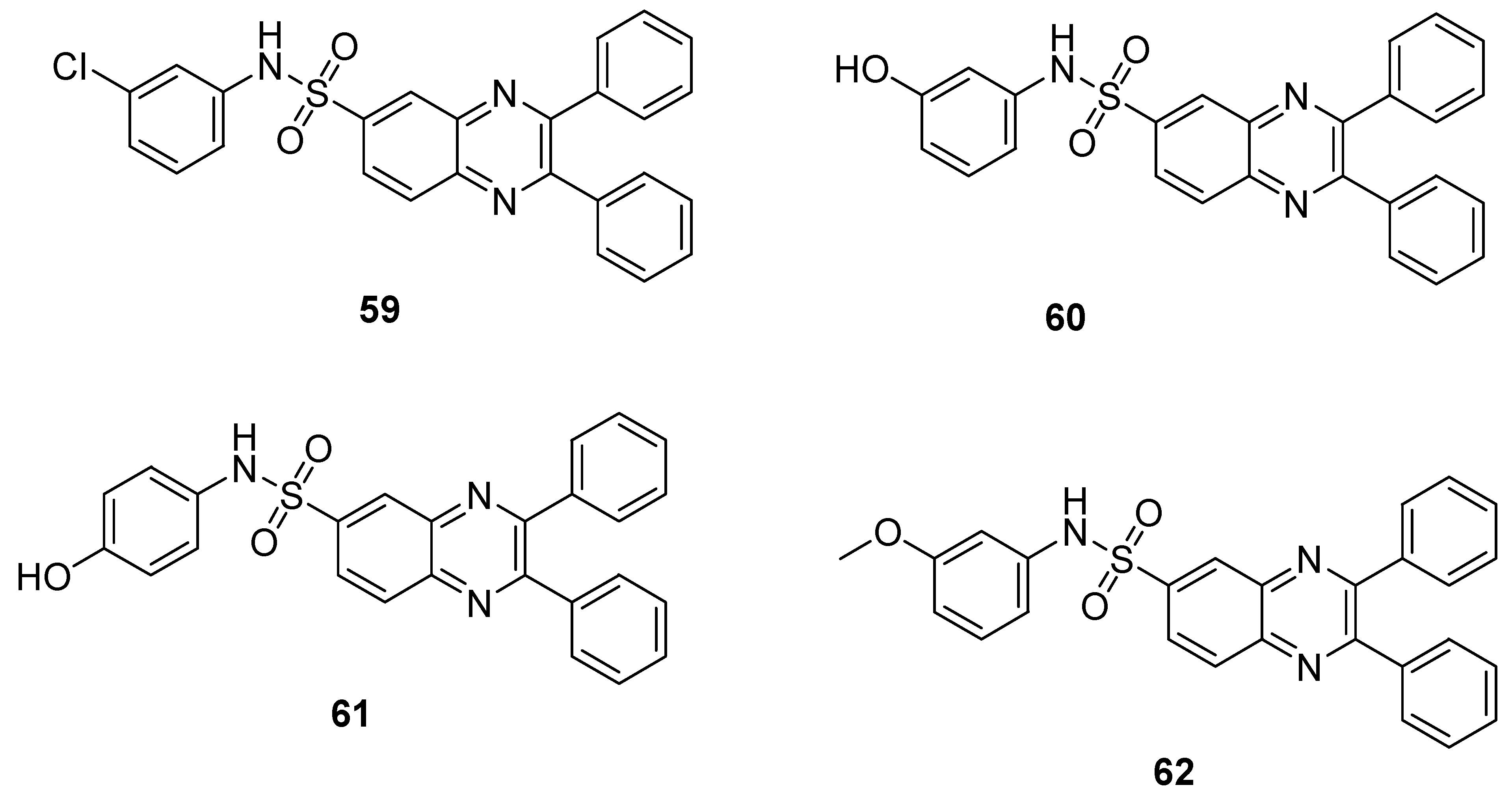
The quinoxaline sulfonamide analogs 59 displayed the highest antibacterial activity, exhibiting a ZOI value of 10 mm against S. aureus and 8 mm against E. coli at a concentration of 100 µg (microgram), while the quinoxaline sulfonamide hybrid 60 showed moderate antibacterial activity, with a ZOI of 7 mm and 6 mm at a concentration of 100 µg against S. aureus and E. coli, respectively (Table 3). The moderate antibacterial activity was observed for derivative 61 that exhibited a ZOI value of 6 mm against S. aureus at a concentration of 100 µg, and derivative 62 exhibited the least antibacterial activity by inducing a 2 mm ZOI value at the concentration of 100 µg against E. coli, when compared with the standard drug azithromycin (ZOI value of 12 mm and 10 mm against S. aureus and E. coli at the concentration of 100 µg). The SAR revealed that the maximum antibacterial activity was observed in the presence of a strong EWD group (chloro atom) on the ring of analogue 59, while the introduction of a m-OH and p-OH group on the ring produced a moderate activity for compounds 60 and 61. The derivative 62 exhibited the least antibacterial activity due to the presence of an electron-denoting methoxy group at the phenyl ring (Figure 9). The presence of EWG showed the highest antibacterial activity as compared to the presence of EDG, which displayed moderate to low antibacterial activity [129].

Table 3.
Antibacterial activity of quinoxaline-based substituted benzene sulfonamide derivatives.
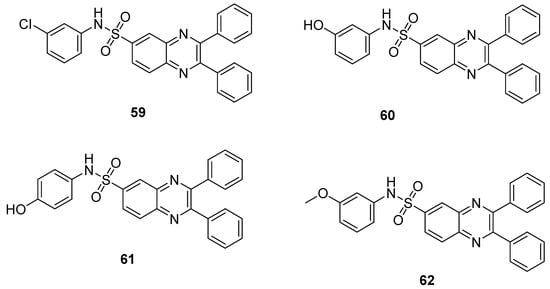
Figure 9.
Substituted quinoxaline sulfonamide derivatives 59–62.
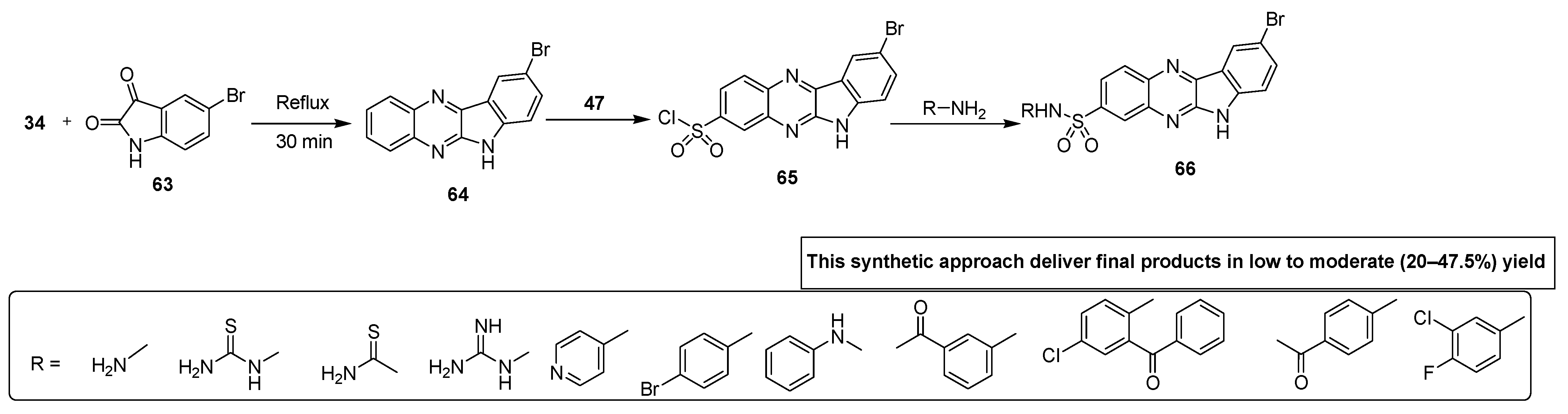
Talari and coworkers developed a novel synthetic strategy for the synthesis of indole-based quinoxaline sulfonamides. These sulfonamide derivatives were evaluated for their antimicrobial activity against different bacterial and fungal strains. Indole quinoxaline analogue 64 was afforded by the reaction of OPD 34 with 5-bromoisatin 63 heated to reflux for 30 min. The scaffold 64 was refluxed with chlorosulfonic acid 47 to obtain the quinoxaline sulfonyl chloride 65 that was further treated with substituted amines in anhydrous acetone and pyridine to furnish final quinoxaline sulfonamides 66 via dehydrohalogenation in a low to moderate (20–47.5%) yield (Scheme 4) [130].
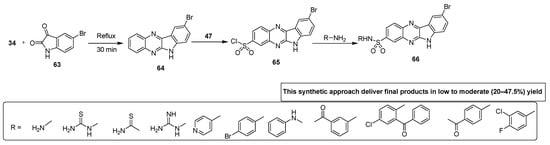
Scheme 4.
Synthesis of substituted quinoxaline sulfonamide derivatives with structure 66.
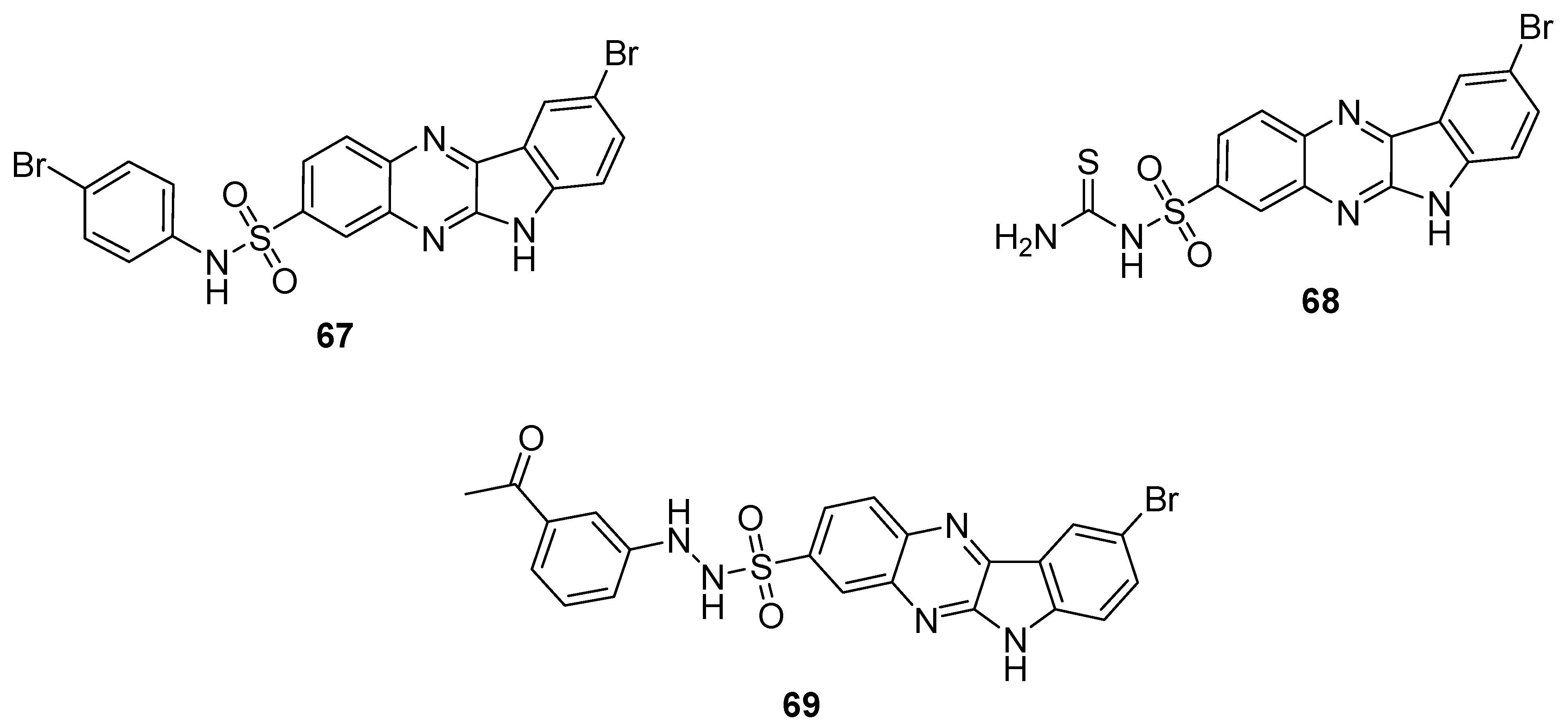
Some of the synthesized quinoxaline sulfonamide derivatives were screened for antimicrobial activities against some bacterial and fungal strains. The quinoxaline sulfonamide 67 showed the most potent antibacterial and antifungal activity, with ZOI values of 14 mm, 14 mm, 16 mm, 17 mm and 17 mm against S. aureus, B. pimilis, E. coli A. niger and P. notatum, respectively at 50 μg/mL (Table 4). The quinoxaline sulfonamide derivative 68 demonstrated a moderate activity, exhibiting ZOI values of 13 mm against S. aureus, 14 mm against B. pimilis, 15 mm against E. coli and 12 mm against A. niger at 50 μg/mL. The derivative 69 exhibited the lowest ZOI values of 10 mm against B. pimilis and 10 mm against A. niger at 50 μg/mL. The reference standard streptomycin at 50 μg/mL exhibited a ZOI of 18 mm, 20 mm and 20 mm against S. aureus, B. pimilis and E. coli strains, respectively. Miconazole nitrate exhibited, instead, 23 mm and 20 mm ZOI values at 50 μg/mL against A. niger and P. notatum strains. The SAR showed that the introduction of a bromophenyl moiety on the sulfonamide increased the antimicrobial activity of 67, while thioamide functionality was incorporated in analogue 68 to induce a moderate antimicrobial activity. The acetophenone moiety-containing derivative 69 displayed the least antimicrobial activity (Figure 10). The SAR study indicated that the antimicrobial activity decreased in the order Bromo > Thiamide > Acetyl. The results in the Table 4 showed that the structural motif 67 displayed a broad spectrum antimicrobial therapeutic potential against different bacterial and fungal strains than the reference standard drugs [130].

Table 4.
Antimicrobial activity of quinoxaline-substituted benzene sulfonamide derivatives 67–69.
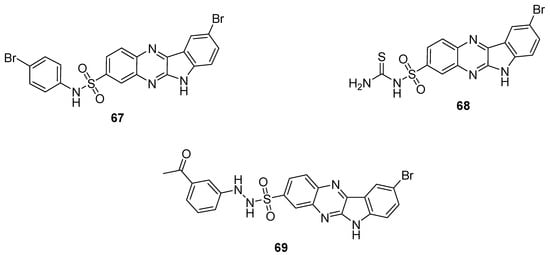
Figure 10.
Substituted quinaxoline sulfonamide derivatives 67–69.
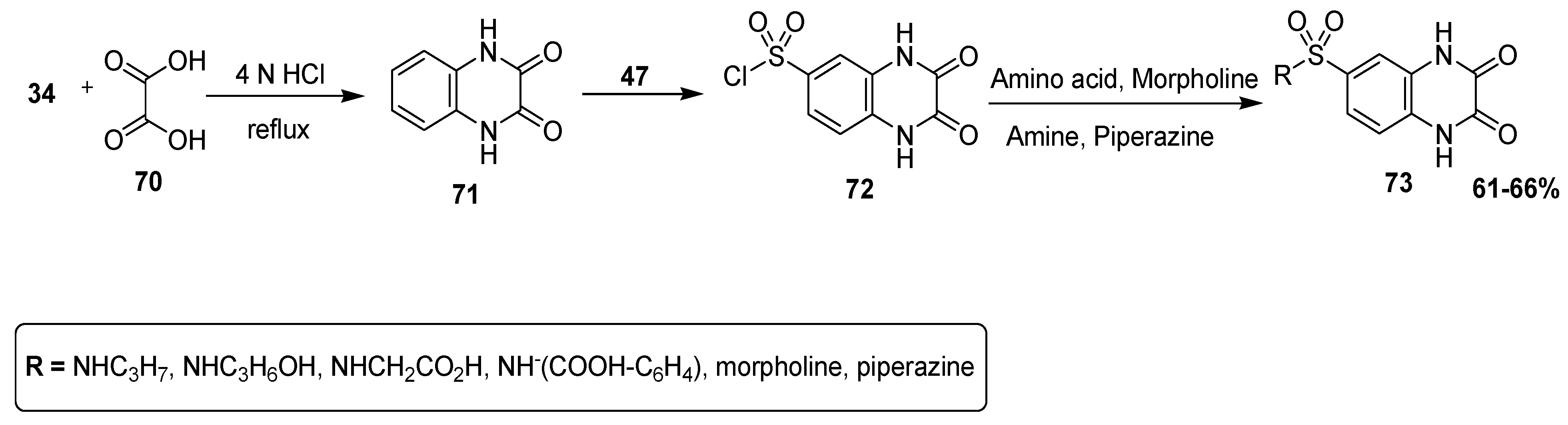
An efficient pathway was adopted by Sharaf El-Din et al. to afford substituted quinoxaline sulfonamide derivatives, and they analyzed these designed scaffolds for antibacterial therapeutic potential by the agar diffusion method. The 1,4-dihydroquinoxaline-2,3-dione (71) was prepared by condensation of 34 with oxalic acid 70. In the next step, reaction with chlorosulfonic acid (47) at 0–5 °C furnished the quinoxaline scaffold with chlorosulfonyl moiety 72, which was combined with different amino compounds, such as amines, amino acids, morpholine and piperazine, to produce final quinoxaline sulfonamide derivatives 73 in good yield (61–66%) (Scheme 5) [131].
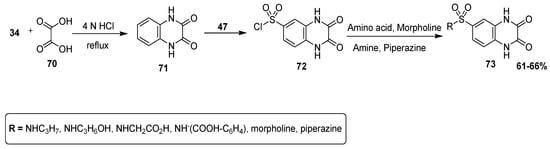
Scheme 5.
Synthesis of quinoxaline-2,3 (1H,4H) dione sulfonamide derivative with structure 73.
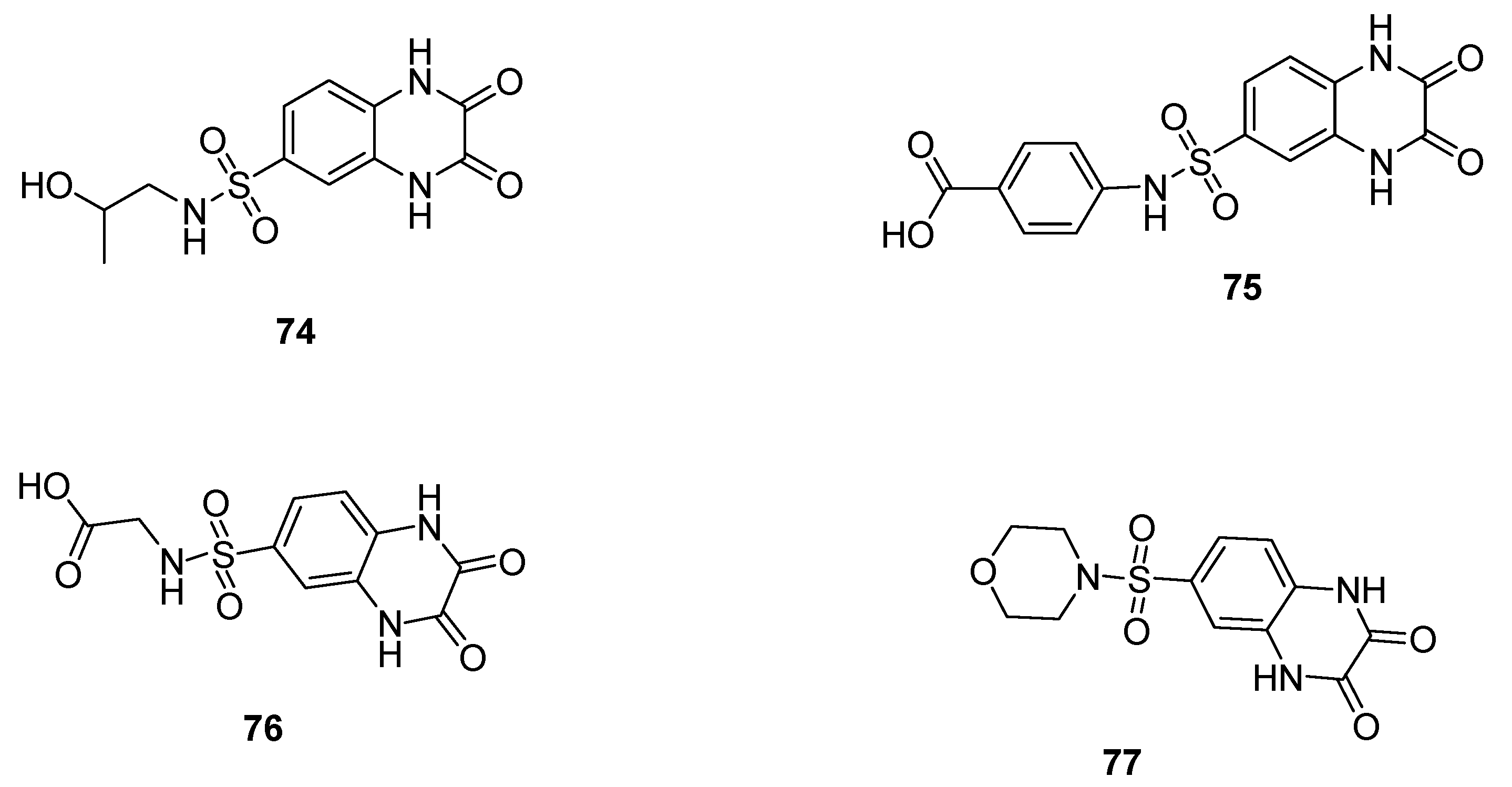
The quinoxaline sulfonamide 74 displayed the highest antibacterial activity, with a zone of inhibition (ZOI) of 15 mm and 10 mm against S. aureus and E. coli, respectively, while the reference drug, sulfadiazine, gave a ZOI of 14 mm and 13 mm zone diameter against S. aureus and E. coli (Table 5). The derivative 75 showed equipotent antibacterial activity (ZOI of 14 mm) against S. aureus in comparison with the reference drug (ZOI of 14 mm), but less antibacterial activity (ZOI of 8 mm) against E. coli than the reference drug (ZOI of 13 mm). A moderate activity was observed for the quinoxaline sulfonamide derivative 76 that led to a ZOI of 12 mm and 10 mm against S. aureus and E. coli, respectively. Compound 77 exhibited the lowest antibacterial activity, showing a ZOI of 10 mm against S. aureus with respect to the reference drug sulfadiazine, but it did not exhibit any activity against E. coli. The SAR studies demonstrated that the presence of a propanol or benzoic acid moiety on the sulfonamide analogs 74 and 75 displayed an excellent and slightly higher or equipotent antibacterial activity in comparison with the reference drug sulfadiazine, while the presence of a sulfonyl glycine moiety was responsible for the moderate activity of the derivative 76. The morpholine moiety in analog 77 was responsible for the least antibacterial activity against Gram-positive and negative bacterial strains. (Figure 11) [131].

Table 5.
Antibacterial activity of quinaxoline sulfonamide derivatives 74–77.
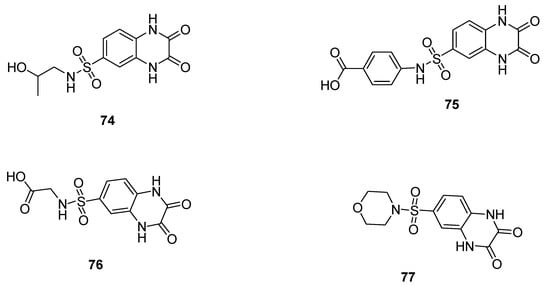
Figure 11.
Quinaxoline sulfonamide derivatives 74–77.
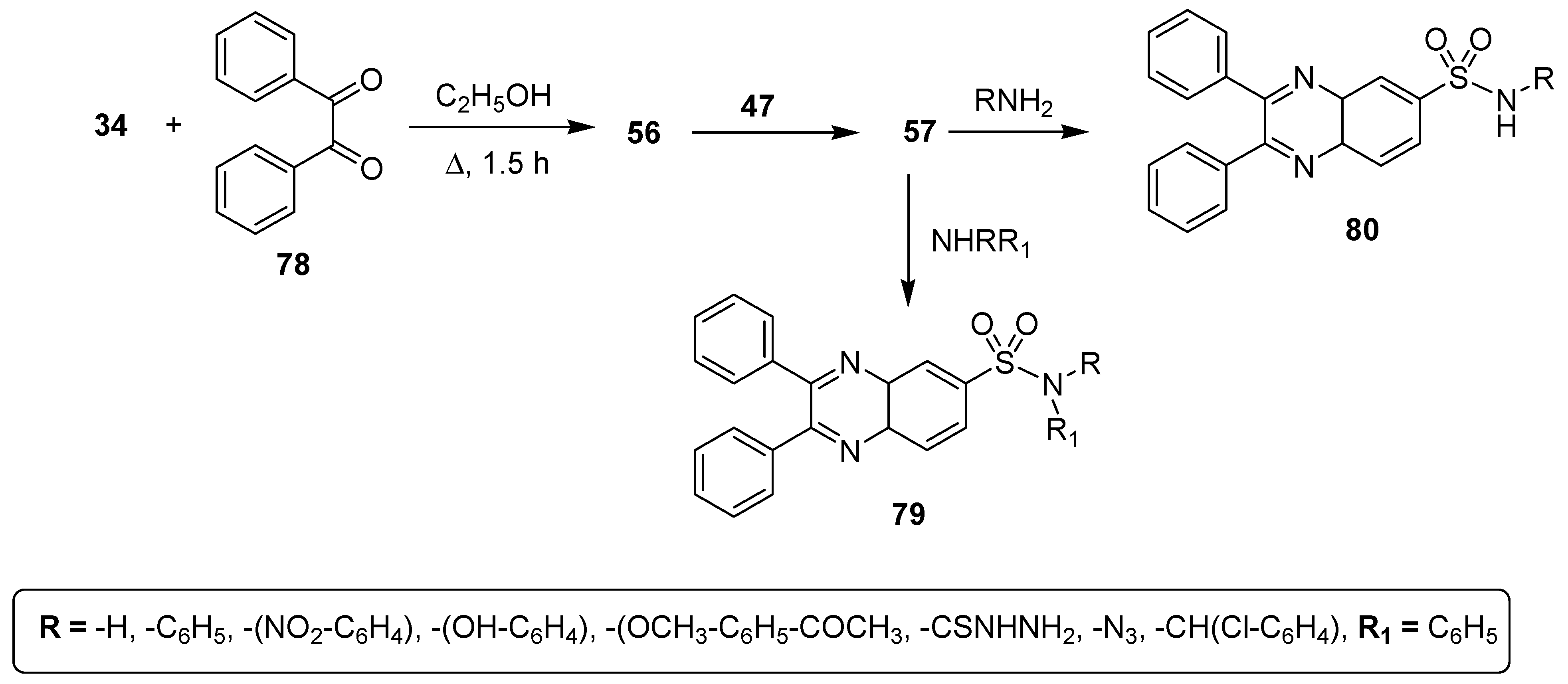
Potey et al. described a new synthetic approach for the synthesis of libraries of quinoxaline sulfonamides and studied their antimicrobial activities. The starting 2,3-diphenylquinoxaline (56) was prepared in an excellent yield by condensation of o-phenylenediamine (OPD) (34) and diketone 78. Then, quinoxaline 56 was treated with chlorosulfonic acid 47 at room temperature to afford the quinoxaline sulfonyl chloride 57 that reacted with primary and secondary amines to furnish quinoxaline sulfonamides 79 and 80 in good to excellent yield (Scheme 6) [132].
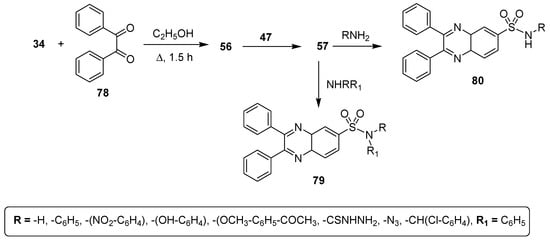
Scheme 6.
Synthesis of 2,3-diphenylquinoxaline sulfonamide derivatives 79 and 80.
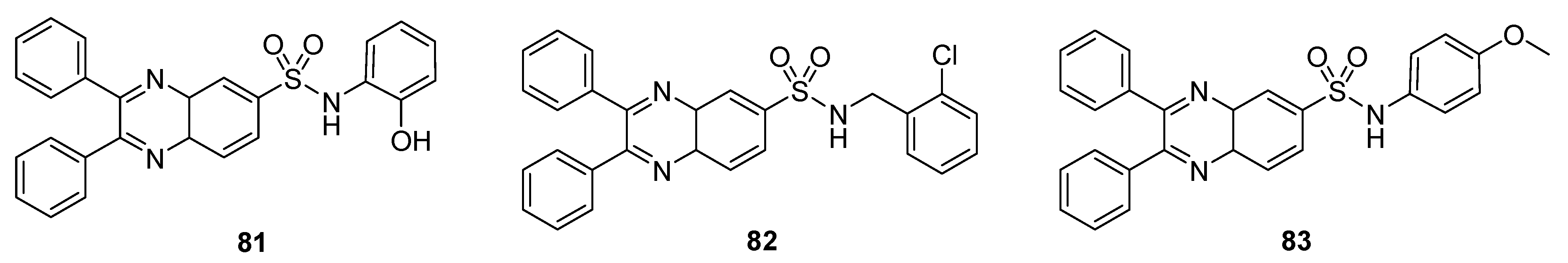
The prepared derivatives were tested against some Gram-positive and negative bacterial strains (Table 6). Among all these derivatives, the quinoxaline sulfonamide 81 exhibited an excellent antibacterial activity, with a ZOI of 30 mm and 24 mm against P. vulgaries and Enterobacteria, respectively. A moderate activity was observed for 82, with a ZOI value of 18 mm against S. aureus, 18 mm against Enterobacteria, 16 mm against V. cholorie, 16 mm against E. coli and 16 mm against P. vulgaries. Compound 83 showed the least antibacterial activity, exhibiting a ZOI of 8 mm, 8 mm, 6 mm, 6 mm and 8 mm against S. aureus, Enterobacteria, V. cholera, E. coli and P. vulgaries, respectively. The SAR showed that ortho-OH on the phenylsulfonamide moiety increased the antibacterial activity of the quinoxaline sulfonamide 81, while the presence of a 2-chlorophenyl substituent was responsible for the moderate activity of the scaffold 82. The introduction of a methoxyphenyl group into the quinoxaline sulfonamide derivative 83 decreased the antibacterial activity (Figure 12) [132].

Table 6.
Antimicrobial activity of quinoxaline derivatives 81–83.
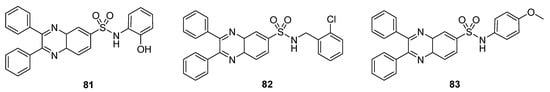
Figure 12.
Substituted quinaxoline sulfonamide derivatives 81–83.
2.3. Synthesis of Quinoxaline Sulfonamide Derivative with Neuropharmacological Activity
The activity which determines how drugs or therapeutic agents affect the cellular functions in the nervous system is called neuropharmacological activity. The neuropharmacological effects, such as analgesia, sedation, convulsion, anxiety, memory and psychosis, and the neural mechanisms through which the drugs influence behavior were studied in neuropharmacology.
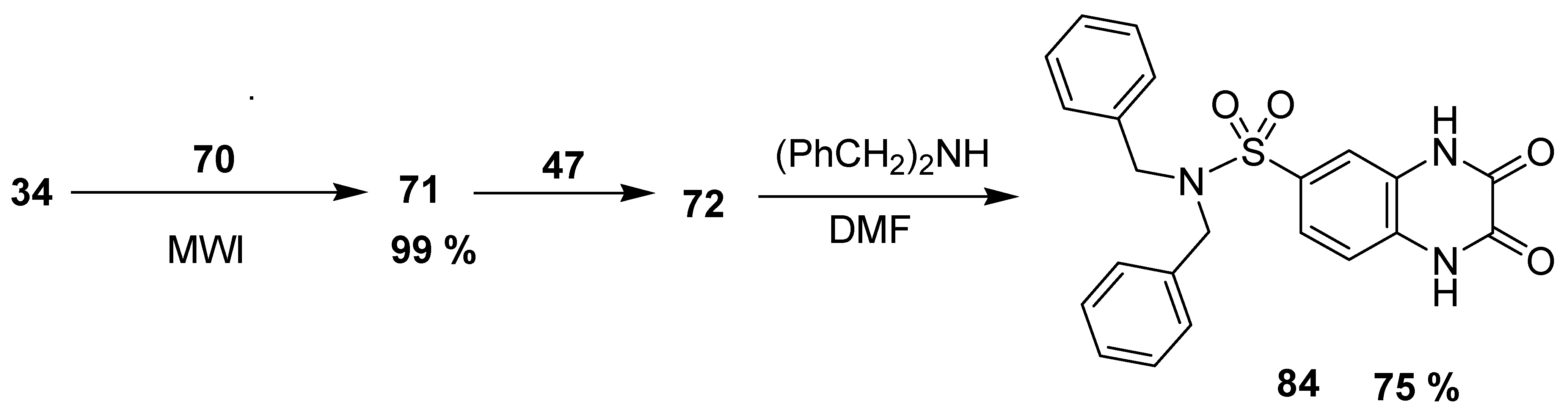
Olayiwola et al. reported the green microwave synthetic route to quinonoxaline sulfonamide with neuropharmacological activity. The 2,3-quinoxalinedione 71 was furnished in an excellent yield (99%) by the condensation of 34 and oxalic acid dihydrate (70) using microwave radiations. The scaffold 71 was treated with 47 to obtain quinoxaline-6-sulfonyl chloride 72 at 88% yield, followed by the reaction with dibenzyloamine in anhydrous dimethylformamide (DMF) to give corresponding quinoxaline sulfonamide 84 at 75% yield (Scheme 7) [133].
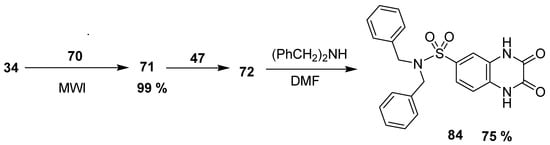
Scheme 7.
Synthesis of quinoxaline sulfonamide 84.
The obtained derivative 84 was analyzed for its neuropharmacological effects, such as anxiolytic, anticonvulsant and total locomotor activity, in mice (Table 7). It showed maximum inhibition of locomotor activity (sedative action) at 40 mg/kg. As for anxiolytic activity, 84 exhibited a maximum action at 2.5 mg/kg, having 67.3% time in open arm and 81.0% entry into open arm, and the index of open arm avoidance was 25.9 when compared with diazepam at 1 mg/kg (53.3% = time in open arm, 82.4% = entry into open arm and 32.2 = index of open arm avoidance). As for anticonvulsant activity, 84 displayed a protection effect of 100% against both leptazol at 80 mg/kg and strychnine at 2.0 mg/kg after 60 min at 25 mg/kg dose, when compared with phenobarbitone sodium, having a 90% protection effect at 20 mg/kg dose against both leptazol at 80 mg/kg and strychnine at 2.0 mg/kg after 60 min [133].

Table 7.
Neuropharmacological activity of quinoxaline-based substituted benzene sulfonamide derivatives.
2.4. Quinoxaline Sulfonamide Derivatives with Antileishmanial Activity
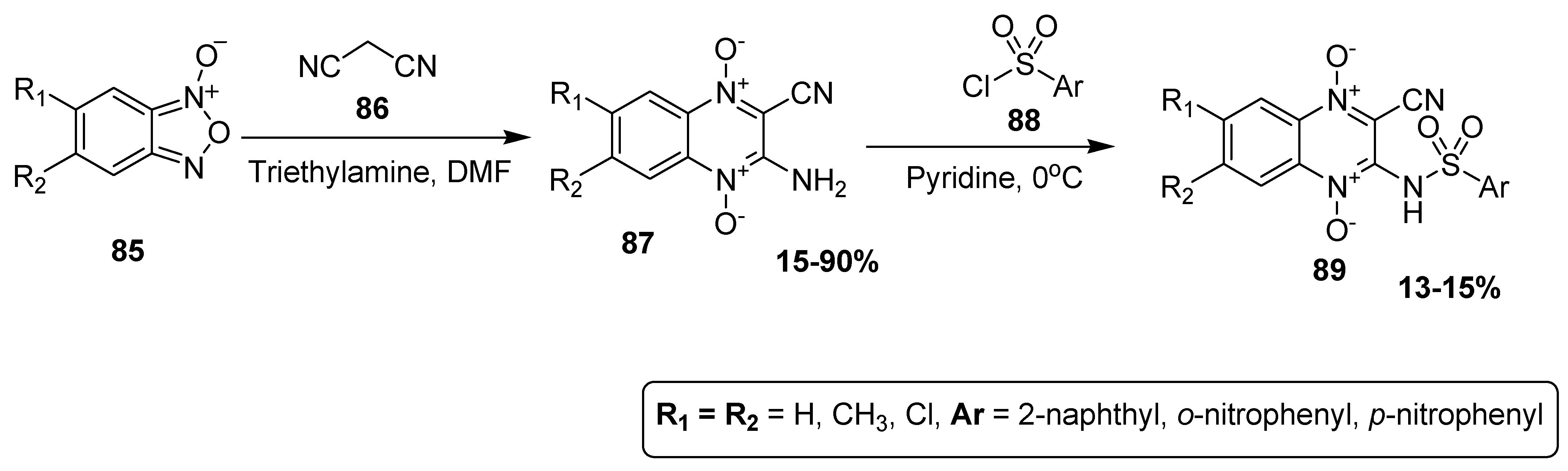
Barea et al. synthesized quinoxaline sulfonamide derivatives and studied their antileishmanial activities. The 3-amino-1,4-di-N-oxide quinoxaline-2-carbonitrile derivative 89 was synthesized in 15–90% yield by the reaction of benzofuroxane 85 and malononitrile 86, using triethylamine as catalyst and DMF as solvent. The scaffold 87 was further treated with substituted sulfonyl chlorides 88 at 0 °C to afford quinoxaline sulfonamide derivatives 89 in low yield (13–15%) (Scheme 8) [134].
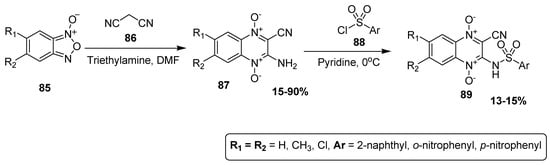
Scheme 8.
Synthesis of substituted quinoxaline sulfonamide derivatives 89.
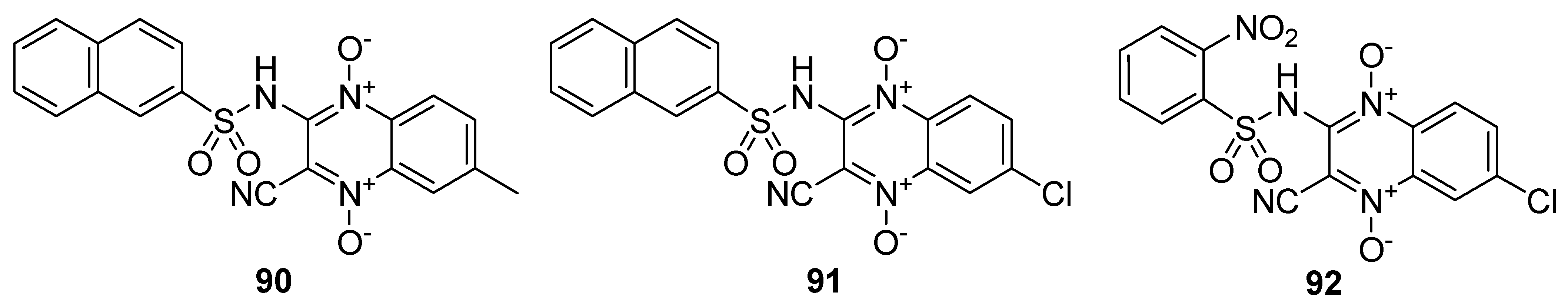
The synthesized derivatives were evaluated against Leishmania amazonensis strain MHOM/BR/76/LTB in infected macrophages (Table 8) The quinoxaline sulfonamide 92 displayed a potent antileishmanial activity (IC50 = 3.1 µM), although 15-fold less active than the standard drug amphotericin B. The quinoxaline sulfonamide analog 91 displayed a moderate antileishmanial activity (IC50 = 16.3 µM), comparable to that observed for 90 (IC50 = 20.3 µM). The SAR demonstrated that the presence of a 2-naphtyl moiety on the sulfonamide motif and a methyl group on the quinoxaline in 90 led to a potent antileishmanial activity, while a 2-naphtyl moiety and the electronegative chlorine atom was responsible for the moderate antileishmanial activity of scaffold 91. The highest antileishmanial activity was observed for 92 with the o-nitrophenyl group and chlorine atom (Figure 13) [134].

Table 8.
Antileishmanial activity of quinoxaline-1,4-dioxide sulfonamide derivatives 90–92.
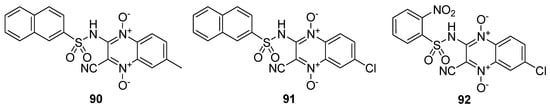
Figure 13.
Structure of quinoxaline-1,4-dioxide sulfonamide derivatives 90–92.
2.5. Synthesis of Quinoxaline Moiety-Based Benzene Sulfonamides with Antitumor Activity
A mass or lump of tissue that is formed by an accumulation of abnormal cells, resembling swelling and varying from a tiny nodule to a large mass, is specified as a tumor. Not all tumors are cancerous; some are benign (non-cancerous), premalignant (having potential to become cancerous) and some are malignant (cancerous). A chemotherapeutic agent which has the ability to prevent or inhibit the formation or growth of tumors is termed as antitumor.
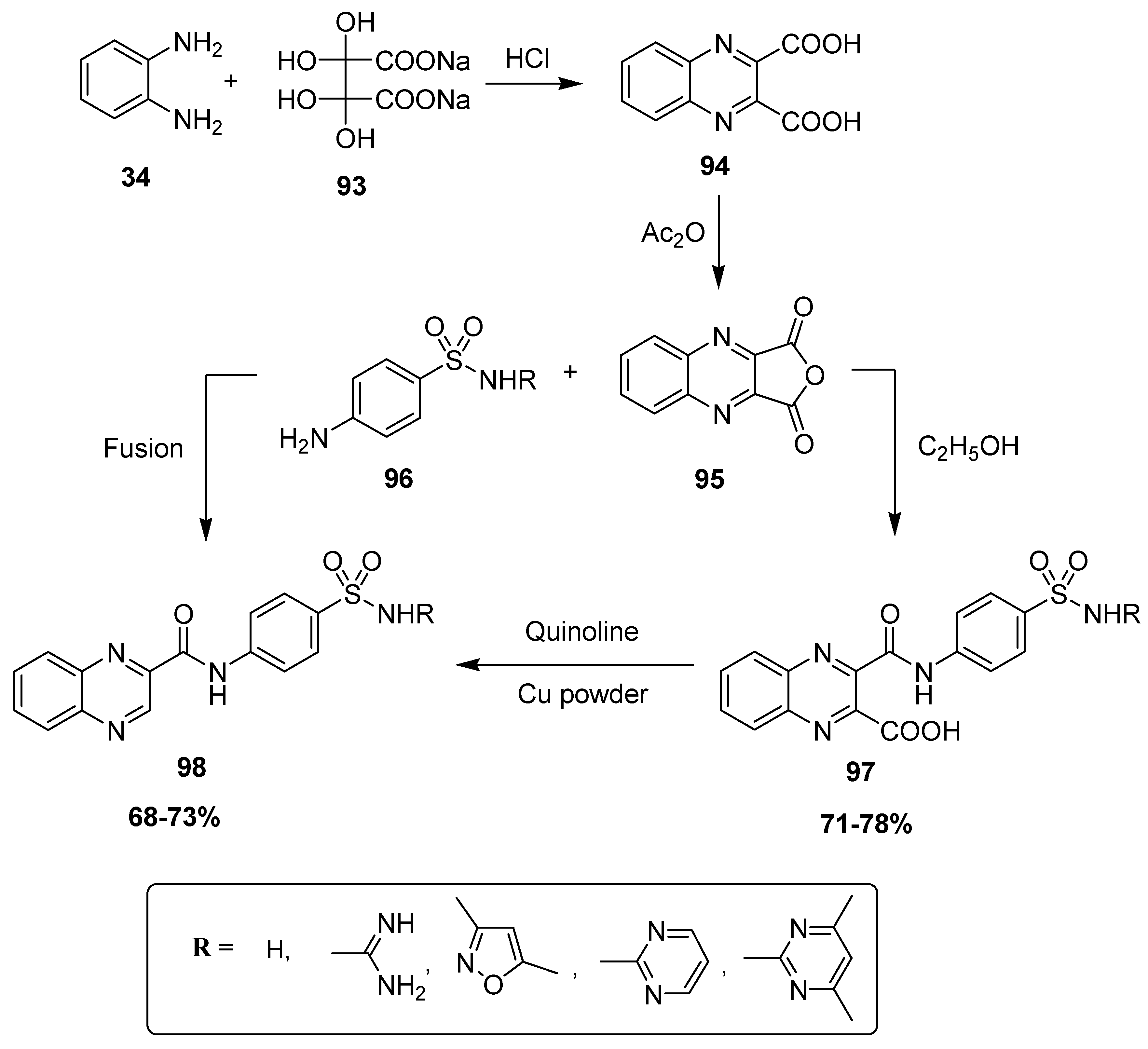
Farrag et al. developed a novel synthetic strategy to furnish novel sulfamoyl phenyl carbamoyl quinoxaline hybrid structures. In the first step, quinoxaline-2,3-dicarboxylic acid 94 was produced by the treatment of OPD 34 with disodium dihydroxytartarate 93, followed by a reaction with acetic anhydride, which resulted in the formation of quinoxaline anhydride derivative 95. The scaffold 95 was refluxed with substituted sulfonamide 96 in ethanol to afford 3-sulfamoyl phenylcarbamoyl quinoxaline-2-carboxylic acids 97 at 71–78% yield, which further reacted in quinoline-containing copper powder to obtain corresponding quinoxaline sulfonamide scaffold 98. The quinoxaline sulfonamide derivative 98 was also prepared at 68–73% yield by directly heating the anhydride compound 95 with sulfonamide compound 96 under fusing conditions (Scheme 9) [135].
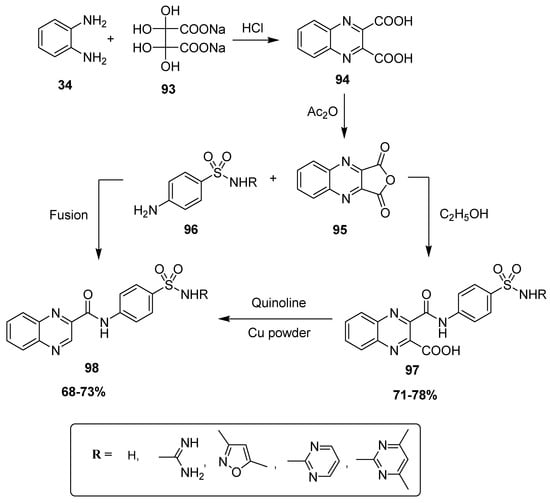
Scheme 9.
Synthesis of quinoxaline moiety-based benzene sulfonamides with structure 98.
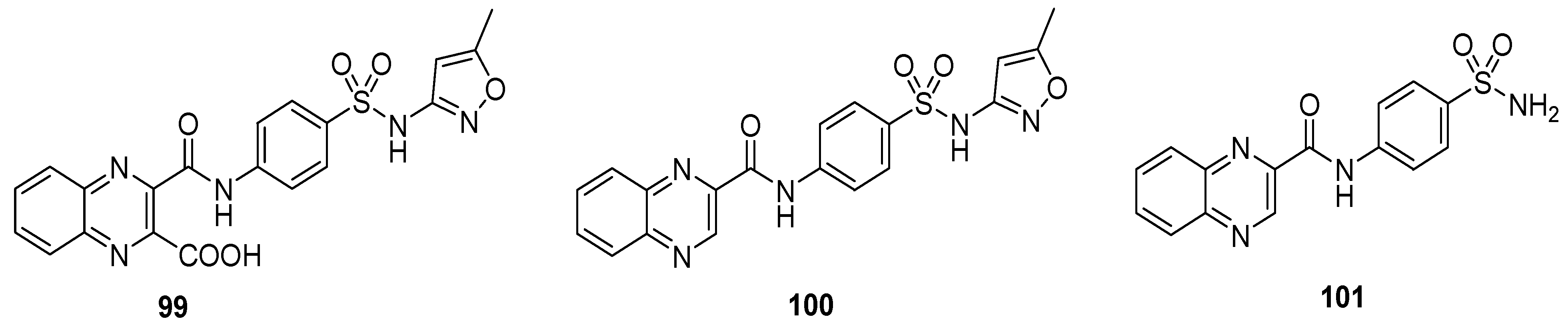
The quinoxaline sulfonamide derivative 99 displayed the most potent antitumor activity against the liver carcinoma cell line (IC50 value 0.5 μg m/L), while moderate anti-tumor activity was exerted by 100 (IC50 value 4.75 μg m/L, Table 9). The quinoxaline sulfonamide structural motif 101 demonstrated the least antitumor activity, exhibiting an IC50 value of 6.79 μg m/L. The SAR described that the presence of a carboxylic acid group on the quinoxaline was responsible for the potent antitumor activity of 99, while its absence in 100 led to a moderate activity. The absence of the isoxazole in 101 produced the worst action herein (Figure 14) [135].

Table 9.
Anti-tumor activity of quinoxaline sulfonamides 99–101.
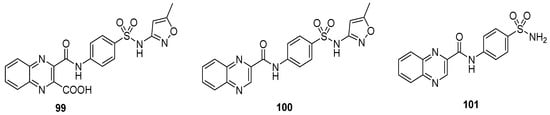
Figure 14.
Substituted quinoxaline sulfonamide derivatives 99–101.
Ingle and coworkers reported the synthesis of new anticancer quinoxaline sulfonamide derivatives. The 2,3-diphenylquinoxaline derivative 56 was treated with electrophilic compound 47 to produce 2,3-diphenyl quinoxaline-6-sulfonylchloride 57 that further refluxed with primary amines in a basic medium and resulted in the formation of the desired final product, substituted quinoxaline sulfonamides 58 at 53–78% yield (Scheme 10) [136].
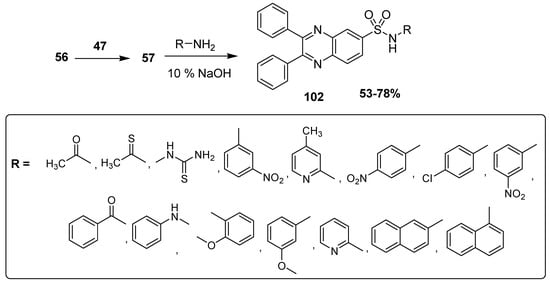
Scheme 10.
Synthesis of quinoxaline sulfonamide derivatives 102.
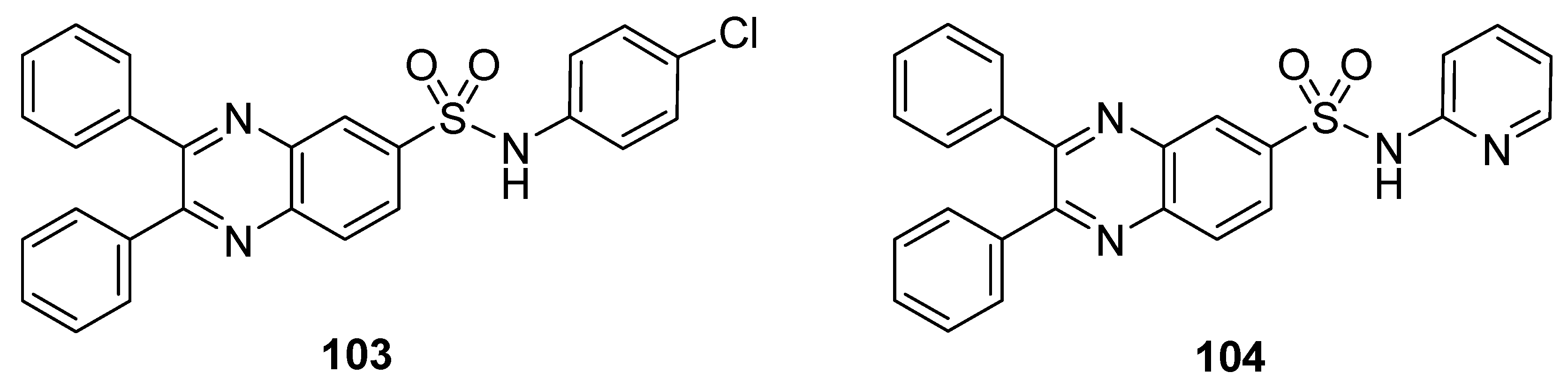
These synthesized quinoxaline sulfonamide derivatives were tested for their cytotoxicity in vitro against various cancer cell lines, such as leukemia, non-small cell lung cancer, colon cancer, CNS cancer, melanoma, ovarian cancer, renal cancer, prostate cancer and breast cancer cell lines. Among all the synthesized derivatives, the chlorophenyl-containing moiety quinoxaline sulfonamide 103 and the pyridine moiety-containing quinoxaline sulfonamide 104 (Figure 15) exhibited the greatest anticancer activities against various above mentioned cancer cell lines [136].
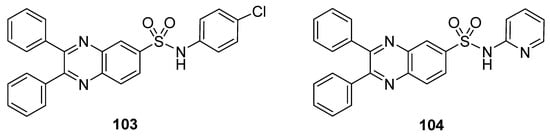
Figure 15.
Substituted quinoxaline sulfonamide derivatives 103–104.
2.6. Synthesis of Quinoxaline-Based Benzene Sulfonamide Derivatives with Anti-Inflammatory Activity
The property of a substance or drug that reduces inflammation or swelling is specified as anti-inflammatory. In other words, nonsteroidal anti-inflammatory drugs (NSAIDs) are drugs that help reduce inflammation, which often helps to relieve pain. NSAIDs can be very effective, and some of the NSAIDS are high-dose aspirin, ibuprofen (Advil, Motrin, Midol) and naproxen (Aleve, Naprosyn), etc.
Ingle et al. synthesized novel quinoxaline sulfonamide derivatives and described their anti-inflammatory activity. The sulfonation of 2,3 diphenylquinoxaline 56 was carried out by the electrophilic agent 47 to produce quinoxaline sulfonyl chloride 57 at 76% yield that was further treated with aliphatic and aromatic substituted amines to obtain the final quinoxaline sulfonamides 105 at 59–85% yield (Scheme 11) [137].
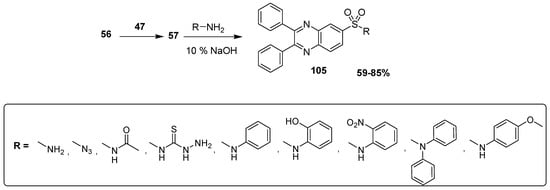
Scheme 11.
Synthesis of substituted quinoxaline sulfonamide derivative 105.
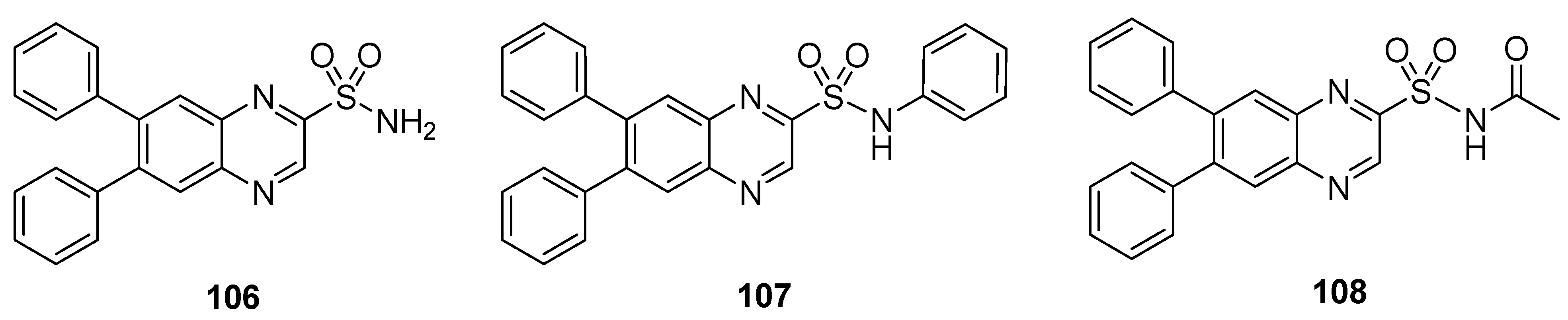
The synthesized quinaxoline sulfonamide derivatives were tested for their in vivo anti-inflammatory activity in rat paw edema. The compounds 106–108 (Figure 16) exhibited anti-inflammatory activity, showing inhibition of edema in the range 1.17–4.04% and a mean paw volume of 0.96–0.98 mL. Diclofenac sodium used as reference drug showed, instead, 15.15% inhibition of edema after 30 min with a mean paw volume of 0.85 mL (Table 10) [137].
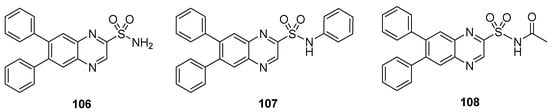
Figure 16.
Substituted quinaxoline sulfonamide derivatives 106–108.

Table 10.
Anti-inflammatory activity of quinoxaline-based substituted benzene sulfonamide derivatives 104–106.
3. Conclusions
The present review showed the escalating interest of organic and medicinal chemists in the synthesis of quinoxaline scaffolds bearing a sulfonamide moiety to target a variety of diseases. The literature survey cited in this article highlighted and revealed that quinoxaline sulfonamide derivatives show a wide array of biological activities, such as antimicrobial, anti-convulsant, anti-inflammatory, anti-leishmania, anti-tumor and anticancer. Ecofriendly and economical procedures for the synthesis of quinoxaline sulfonamide derivatives were also reported. The structure-based activity findings will be helpful for further modifications on the quinoxaline sulfonamide derivatives. In order to develop future potent therapeutic agents.
Author Contributions
Conceptualization: A.I.; resources: S.H., F.B., H.R., R.Z.; writing—original draft preparation: A.I., S.A.; writing—review and editing: K.K.-M., M.M.; All authors have read and agreed to the published version of the manuscript.
Funding
Not applicable.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seitz, L.E.; Suling, W.J.; Reynolds, R.C. Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives. J. Med. Chem. 2002, 45, 5604–5606. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.J.; Taylor, E.C.; Ellman, J.A. The Chemistry of Heterocyclic Compounds; Wiley: Hoboken, NJ, USA, 2004. [Google Scholar]
- Saifina, D.F.; Mamedov, V.A. New and modified classical methods for the synthesis of quinoxalines. Russ. Chem. Rev. 2010, 79, 351–370. [Google Scholar] [CrossRef]
- Patidar, A.K.; Jeyakanda, M.; Mobiya, A.K.; Selvam, G. Exploring potential of quinoxaline moiety. Int. J. Pharm. Tech. Res. 2011, 3, 386–392. [Google Scholar]
- Tariq, S.; Somakala, K.; Amir, M. Quinoxaline: An insight into the recent pharmacological advances. Eur. J. Med. Chem. 2017, 143, 542–557. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, C.A.; Nogueira, M.C.T.; de Souza, M.V.N. Quinoxaline Nucleus: A promising scaffold in anti-cancer drug discovery. Anticancer. Agents Med. Chem. 2016, 16, 1339–1352. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.K.; Ismail, F.M.M.; Noaman, E.; Soliman, D.H.; Ammar, Y.A. New quinoxaline 1,4-di-N-oxides. Part 1: Hypoxia-selective cytotoxins and anticancer agents derived from quinoxaline 1,4-di-N-oxides. Bioorg. Med. Chem. 2006, 14, 6917–6923. [Google Scholar] [CrossRef]
- Kotb, R.; Anwar, A.M.; Soliman, S.M.; Salama, A.M. Synthesis and reactions of some novel quinoxalines for anticancer evaluation. Phosphorus Sulfur 2007, 182, 1119–1130. [Google Scholar] [CrossRef]
- Lindsley, C.W.; Zhao, Z.; Leister, W.H.; Robinson, R.G.; Barnett, S.F.; Defeo-Jones, D.; Jones, R.E.; Hartman, G.D.; Huff, J.R.; Huber, H.E.; Duggan, M.E. Allosteric Akt (PKB) inhibitors: Discovery and SAR of isozyme selective inhibitors. Bioorg. Med. Chem. Lett. 2005, 15, 761–764. [Google Scholar] [CrossRef]
- Carta, A.; Loriga, M.; Piras, S.; Paglietti, G.; La Colla, P.; Busonera, B.; Collu, G.; Loddo, R. Synthesis of variously substituted 3-phenoxymethyl quinoxalin-2-ones and quinoxalines capable to potentiate in vitro the antiproliferative activity of anticancer drugs in multi-drug resistant cell lines. Med. Chem. 2006, 2, 113–122. [Google Scholar]
- Khan, S.A.; Mullick, P.; Pandit, S.; Kaushik, D. Synthesis of hydrazones derivatives of quinoxalinone-prospective antimicrobial and antiinflammatory agents. Acta Pol. Pharm. 2009, 66, 169–172. [Google Scholar]
- Piras, S.; Loriga, M.; Paglietti, G. Quinoxaline chemistry. Part XVII. Methyl [4-(substituted 2-quinoxalinyloxy) phenyl] acetates and ethyl N-{[4-(substituted 2-quinoxalinyloxy) phenyl] acetyl} glutamates analogues of methotrexate: Synthesis and evaluation of in vitro anticancer activity. Farmaco 2004, 59, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Bahekar, R.H.; Jain, M.R.; Gupta, A.A.; Goel, A.; Jadav, P.A.; Patel, D.N.; Prajapati, V.M.; Patel, P.R. Synthesis and antidiabetic activity of 3,6,7-trisubstituted-2-(1H-imidazol-2-ylsulfanyl)quinoxalines and quinoxalin-2-yl isothioureas. Arch. Pharm. 2007, 340, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Shintre, S.A.; Ramjugernath, D.; Islam, M.; Mopuri, R.; Mocktar, C.; Koorbanally, N.A. Synthesis, in vitro antimicrobial, antioxidant, and antidiabetic activities of thiazolidine–quinoxaline derivatives with amino acid side chains. Med. Chem. Res. 2017, 26, 2141–2151. [Google Scholar] [CrossRef]
- Kulkarni, N.V.; Revankar, V.K.; Kirasur, B.N.; Hugar, M.H. Transition metal complexes of thiosemicarbazones with quinoxaline hub: An emphasis on antidiabetic property. Med. Chem. Res. 2012, 21, 663. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, S.; Wu, B.; Ma, M.; Mingming, M.; Xin, C.; Xiangyu, Q.; Minlan, H.; Saghir, H.; Chaojun, J.; Bing, M.; Changjin, Z. An efficient synthesis of quinoxalinone derivatives as potent inhibitors of aldose reductase. Chem. Med. Chem. 2012, 7, 823–835. [Google Scholar] [CrossRef]
- Gupta, D.; Ghosh, N.N.; Chandra, R. Synthesis and pharmacological evaluation of substituted 5-[4-[2-(6, 7-dimethyl-1, 2, 3, 4-tetrahydro-2-oxo-4-quinoxalinyl) ethoxy] phenyl] methylene] thiazolidine-2, 4-dione derivatives as potent euglycemic and hypolipidemic agents. Bioorg. Med. Chem. Lett. 2005, 15, 1019–1022. [Google Scholar] [CrossRef]
- Jaso, A.; Zarranz, B.; Aldana, I.; Monge, A. Synthesis of new quinoxaline-2-carboxylate 1,4-dioxide derivatives as anti-Mycobacterium tuberculosis agents. J. Med. Chem. 2005, 48, 2019–2025. [Google Scholar] [CrossRef]
- Ancizu, S.; Moreno, E.; Solano, B.; Villar, R.; Asunción, B.; Torres, E.; Pérez-Silanes, S.; Aldana, I.; Monge, A. New 3-methylquinoxaline-2-carboxamide 1, 4-di-N-oxide derivatives as anti-Mycobacterium tuberculosis agents. Biorg. Med. Chem. 2010, 18, 2713–2719. [Google Scholar] [CrossRef]
- El-Atawy, A.M.; Ezzat, A.; Hamed, E.A.; Alhadi, M.; Omar, A.Z. Synthesis and antimicrobial activity of some new substituted quinoxalines. Molecules 2019, 24, 4198. [Google Scholar] [CrossRef]
- Dharmchand, P.S.; Sanjay, K.D.; Hashim, R.S.; Singhal, G.R. Synthesis and antimicrobial activity of some new quinoxaline derivatives. Pharmaceuticals 2010, 3, 2416–2425. [Google Scholar]
- Kim, Y.B.; Kim, Y.H.; Park, J.Y.; Kim, S.K. Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogueS. Bioorg. Med. Chem. Lett. 2004, 14, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Sarges, R.; Howard, H.R.; Browne, R.G.; Lebel, L.A.; Seymour, P.A.; Koe, B.K. 4-Amino [1, 2, 4] triazolo [4, 3-a] quinoxalines. A novel class of potent adenosine receptor antagonists and potential rapid-onset antidepressants. J. Med. Chem. 1990, 33, 2240–2254. [Google Scholar] [CrossRef] [PubMed]
- Sakata, G.; Makino, K.; Kurasawa, Y. Recent progress in the quinoxaline chemistry. Synthesis and biological activity. Heterocycles 1988, 27, 2481–2515. [Google Scholar]
- Abu-hashem, A.A.; Gouda, M.A.; Badria, F.A. Synthesis of some new pyrimido[2:2,3]thiazolo[4,5-b]quinoxaline derivatives as anti-inflammatory and analgesic agents. Eur. J. Med. Chem. 2010, 45, 1976–1981. [Google Scholar] [CrossRef]
- Wagle, S.; Adhikari, V.A.; Kumari, S.N. Synthesis of some new 2-(3-methyl-7-substituted-2-oxoquinoxalinyl)-5-(aryl)-1,3,4-oxadiazoles as potential non-steroidal anti-inflammatory and analgesic agents. Indian J. Chem. 2008, 47, 439–448. [Google Scholar]
- Ismail, M.M.; Ammar, Y.A.; Ibrahim, M.K.; El-Zahaby, H.S.A.; Mahmoud, S.S. Synthesis and pharmacological evaluation of novel quinoxalines as potential nonulcerogenic anti-inflammatory and analgesic agents. Arzneimittelforschung 2005, 55, 738–743. [Google Scholar] [CrossRef]
- Dhansay, D.; Kartik, T.N.; Vinay, S.V.; Nagori, K.; Badwaik, H.; Nair, N.; Tripathi, D.K.; Mishra, A. Synthesis and molecular docking study of novel hybrids of 1,3,4-oxadiazoles and quinoxaline as a potential analgesic and anti-inflammatory agents. J. Heterocycl. Chem. 2018, 55, 2901–2910. [Google Scholar]
- Gayathri, R.; Suresh, S.; Thirumurthy, R. Synthesis, characterization and pharmacological evaluation of some potent 2-(substituted phenylimino) quinoxaline-3-one for their analgesic activity. J. Chem. Pharm. 2015, 7, 961–966. [Google Scholar]
- Alswah, M.; Adel, G.; El-Morsy, A.; El-Gamal, K. Synthesis and biological evaluation of some [1,2,4]triazolo[4,3-a]quinoxaline derivatives as novel anticonvulsant agents. ISRN Org. Chem. 2013, 587054. [Google Scholar] [CrossRef]
- Møllerud, S.; Hansen, R.B.; Pallesen, J.; Temperini, P.; Pasini, D.; Bornholt, J.; Nielsen, B.; Mamedova, E.; Chalupnik, P.; Paternain, A.V.; et al. N-(7-(1H-imidazol-1-yl)-2,3-dioxo-6-(trifluoromethyl)-3,4- dihydroquinoxalin-1(2H)-yl)benzamide - a new kainate receptor selective antagonist and analgesic: Synthesis, X-ray crystallography, structure-affinity relationships, in vitro and in vivo pharmacology. ACS Chem. Neurosci. 2019, 10, 4685–4695. [Google Scholar]
- Burguete, A.; Pontiki, E.; Hadjipavlou-Litina, D.; Ancizu, S.; Villar, R.; Solano, B.; Moreno, E.; Torres, E.; Pérez, S.; Aldana, I.; Monge, A. Synthesis and biological evaluation of new quinoxaline derivatives as antioxidant and anti-inflammatory agents. Chem Biol. Drug Des. 2011, 77, 255–267. [Google Scholar] [CrossRef] [PubMed]
- El-Sabbagh, O.I.; El-Sadek, M.E.; Lashine, S.M.; Yassin, S.H.; El-Nabtity, S.M. Synthesis of new 2 (1H)-quinoxalinone derivatives for antimicrobial and antiinflammatory evaluation. Med. Chem. Res. 2009, 18, 782. [Google Scholar] [CrossRef]
- Qing-K, S.; Guo, H.G.; Gao, L.; Mei, J.; Li-Hua Cao, C.; Zhe-Shan, Q. Discovery and evaluation of novel synthetic 5-alkyl-4-oxo-4,5-dihydro-[1,2,4]triazolo[4,3-a]quinoxaline-1-carbox-amide derivatives as anti-inflammatory agents. J. Enzyme Inhib. Med. Chem. 2020, 35, 85–95. [Google Scholar]
- Tariq, S.; Ozair, A.; Mohd, A. Synthesis, anti-inflammatory, p38α MAP kinase inhibitory activities and molecular docking studies of quinoxaline derivatives containing triazole moiety. Bioorg. Chem. 2018, 76, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Manzano, M.T.; Guirado, A.; Martínez-Esparza, M.; Gálvez, J.; García-Peñarrubia, P.; Ruiz-Alcaraz, A.J. 1Quinoxalines potential to target pathologies. Curr. Med. Chem. 2015, 22, 3075–3108. [Google Scholar] [CrossRef]
- El-Zahabi, H.S.A. Synthesis, Characterization, and biological evaluation of some novel quinoxaline derivatives as antiviral agents. Arch. Pharm. Chem. Life Sci. 2017, 350, 1–13. [Google Scholar] [CrossRef]
- Wilhelmsson, L.M.; Kingi, N.; Bergman, J. Interactions of Antiviral Indolo[2,3-b]quinoxaline Derivatives with DNA. J. Med. Chem. 2008, 51, 7744–7750. [Google Scholar] [CrossRef]
- Rongjiao, X.; Tao, G.; Mei, C.; Shijun, S.; He, J.; Xu, T.; Jianga, S.; Xue, W. Synthesis, antiviral and antibacterial activities and action mechanism of penta-1,4-dien-3-one oxime ether derivatives containing a quinoxaline moiety. New J. Chem. 2019, 43, 16461–16467. [Google Scholar]
- Fonseca, T.; Gigante, B.; Marques, M.M.; Gilchrist, T.L.; de Clercq, E. Synthesis and antiviral evaluation of benzimidazoles, quinoxalines and indoles from dehydroabietic acid. Bioorg. Med. Chem. 2004, 12, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Ali, I.; Al-Masoudi, I.; Hassan, H.G.; Al-Masoudi, N. Synthesis and anti-HIV activity of new homo acyclic nucleosides, 1-(pent-4-enyl) quinoxalin-2-ones and 2-(pent-4-enyloxy) quinoxalines. Chem. Hetrocycl. Compd. 2007, 43, 1052–1059. [Google Scholar] [CrossRef]
- Shibinskaya, M.O.; Lyakhov, S.A.; Mazepa, A.V.; Andronati, S.A.; Turov, A.V.; Zholobak, N.M.; Spivak, N.Y. Synthesis, cytotoxicity, antiviral activity and interferon inducing ability of 6-(2-aminoethyl)-6H-indolo[2,3-b]quinoxalines. Eur. J. Med. Chem. 2010, 45, 1237–1243. [Google Scholar] [CrossRef]
- Loughran, M.H.; Ziying, H.; Wrobel, E.J.; Sarah, D.E.; Gordon, R.; Bruce, F.D.; Ronald, H.N.; Allen, R.B. Quinoxaline-based inhibitors of Ebola and Marburg VP40 egress. Bioorg Med. Chem. Lett. 2016, 26, 3429–3435. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhi-Cheng, D.; Shao-Song, Q.; Jun-Yan, L.; Yu, X.; Ai-Min, L.; Hai-Liang, Z.; Jian-Xin, W.; Yong-Hao, Y. Design, synthesis, antifungal, and antioxidant activities of (E)-6-((2 phenylhydrazono) methyl) quinoxaline derivatives. J. Agric. Food Chem. 2014, 62, 9637–9643. [Google Scholar] [CrossRef]
- Waring, M.J.; Taibi, B.H.; Kotchevar, A.T.; Ramdani, A.; Touzani, R.; Elkadiri, S.; Hakkou, A.; Bouakka, M.; Ellis, T. 2,3-Bifunctionalized quinoxalines: Synthesis, DNA interactions and evaluation of anticancer, anti-tuberculosis and antifungal activity. Molecules 2002, 7, 641–656. [Google Scholar] [CrossRef]
- El-Gazzar, M.; Nafie, N.H.; Nocentini, A.; Ghorab, M.M.; Heiba, H.I. Supuran, C.T.; Carbonic anhydrase inhibition with a series of novel benzenesulfonamide-triazole conjugates. J. Enzyme Inhib. Med. Chem. 2018, 33, 1565–1574. [Google Scholar] [CrossRef] [PubMed]
- De Lacorte Singulani, J.; Galeane, M.C.; Ramos, M.D.; Gomes, P.C.; Dos Santos, C.T.; de Souza, B.M.; Palma, M.S.; Fusco Almeida, A.M.; Mendes Giannini, M.J.S. Antifungal activity, toxicity and membranolytic action of a mastoparan analogue peptide. Front. Cell. Infect. Microbiol. 2019, 9, 1–11. [Google Scholar]
- Shaik, M.B.; Paala, K.; Shaik, T.B.; Chintha, V.; Shafi, S.S.; Naga Raju, C. Synthesis, characterization and antimicrobial activity of boron, silicon and selenium substituted quinoxaline derivatives. Pharma Innov. J. 2019, 8, 627–632. [Google Scholar]
- Soliman, D.H. Synthesis, characterization, anti-bacterial and anti-fungal activities of new quinoxaline 1,4-di-N-Oxide derivatives. IJOC 2013, 3, 65–72. [Google Scholar] [CrossRef]
- Shekhar, C.A.; Rao, S.P.; Narsaiah, B.; Allanki, A.D.; Sijwali, P.S. Emergence of pyrido quinoxalines as new family of antimalarial agents. Eur. J. Med. Chem. 2014, 77, 280–287. [Google Scholar] [CrossRef]
- Rangisetty, J.B.; Gupta, C.N.; Prasad, A.L.; Srinivas, P.; Sridhar, N.; Parimoo, P.; Veeranjaneyulu, A. Synthesis of new arylaminoquinoxalines and their antimalarial activity in mice. J. Pharm. Pharmacol. 2001, 53, 1409–1413. [Google Scholar] [CrossRef]
- Bonilla-Ramírez, L.; Galiano, S.; Quiliano, M.; Aldana, I.; Pabón, A. Primaquine–quinoxaline 1,4-di-N-oxide hybrids with action on the exo-erythrocytic forms of plasmodium induce their effect by the production of reactive oxygen species. Malar. J. 2019, 18, 201. [Google Scholar] [CrossRef]
- Guillon, J.; Cohen, A.; Gueddouda, N.M.; Das, R.N.; Moreau, S.; Ronga, L.; Savrimoutou, S.; Basmaciyan, L.; Monnier, A.; Monget, M.; Rubio, S.; Garnerin, T.; Azas, N.; Mergny, J.L.; Mullié, C.; Sonnet, P. Design, synthesis and antimalarial activity of novel bis{N- [(pyrrolo[1,2-a]quinoxalin-4-yl)benzyl]-3-aminopropyl}amine derivatives. J. Enzyme Inhib. Med. Chem. 2017, 32, 547–563. [Google Scholar] [CrossRef] [PubMed]
- Guillon, J.; Moreau, S.; Mouray, E.; Sinou, V.; Forfar, I.; Fabre, S.B.; Desplat, V.; Millet, P.; Parzy, D.; Jarry, C.; et al. New ferrocenic pyrrolo[1,2-a]quinoxaline derivatives: Synthesis and in vitro antimalarial activity. Bioorg. Med. Chem. 2008, 16, 9133–9144. [Google Scholar] [CrossRef]
- Guillon, J.; Grellier, P.; Labaied, M.; Sonnet, P.; Léger, J.M.; Déprez-Poulain, R.; Forfar-Bares, I.; Dallemagne, P.; Lemaître, N.; Péhourcq, F.; Rochette, J.; Sergheraert, C.; Jarry, C. Synthesis, antimalarial activity, and molecular modeling of new pyrrolo[1,2-a]quinoxalines, Bispyrrolo[1,2-a]quinoxalines, Bispyrido[3,2-e]pyrrolo[1,2-a]pyrazines, and Bispyrrolo[1,2-a]thieno[3,2-e]pyrazines. J. Med. Chem. 2004, 47, 1997–2009. [Google Scholar] [CrossRef] [PubMed]
- Parhi, A.K.; Zhang, Y.; Saionz, K.W.; Pradhan, P.; Kaul, M.; Trivedi, K.; Pilch, D.S.; LaVoie, E.J. Antibacterial activity of quinoxalines, quinazolines, and 1,5-naphthyridines. Bioorg. Med. Chem. Lett. 2013, 234, 4968–4974. [Google Scholar] [CrossRef] [PubMed]
- Aravind, K.; Ganesh, A.; Ashok, D. Microwave assisted synthesis, characterization and antibacterial activity of quinoxaline derivatives. J. Chem. Pharm. Res. 2013, 5, 48–52. [Google Scholar]
- Abbas, H.A.S.; Al-Marhabi, A.R.; Ammar, Y.A. Design, synthesis and biological evaluation of 2, 3-disubstituted and fused quinoxalines as potential anticancer and antimicrobial agents. Acta Pol. Pharm. 2017, 74, 445–458. [Google Scholar]
- Bayoumi, A.; Ghiaty, A.H.; Abd El-Gilil, S.M.; Husseiny, E.; Ebrahim, M.A. Exploration of quinoxaline derivatives as antimicrobial and anticancer agents. J. Heterocyclic. Chem. 2019, 4, 1–21. [Google Scholar] [CrossRef]
- Al-Marhabi, A.R.; Abbas, H.-A.S.; Ammar, Y.A. Synthesis, characterization and biological evaluation of some quinoxaline derivatives: A promising and potent new class of antitumor and antimicrobial agents. Molecules 2015, 20, 19805–19822. [Google Scholar] [CrossRef]
- Afrough, T.; Bakavoli, M.; Eshghi, H.; Beyzaei, H.; Manesh, M.-M. Synthesis, characterization and in vitro antibacterial evaluation of novel 4-(1-(Pyrimidin-4-yl)Ethyl)-12H-Pyrimido[4′,5′:5,6] [1,4]Thiazino[2,3-b] quinoxaline derivatives. Polycycl. Aromat. Comp. 2019, 1–11. [Google Scholar] [CrossRef]
- Vieira, M.; Pinheiro, C.; Fernandes, R.; Noronha, J.P.; Prudencio, C. Antimicrobial activity of quinoxaline 1,4-dioxide with 2- and 3-substituted derivatives. Microbiol. Res. 2014, 169, 287–293. [Google Scholar] [CrossRef] [PubMed]
- Nicola, O.; O’Neill, A.J. Revisiting unexploited antibiotics in search of new antibacterial drug candidates: The case of MSD-819 (6-chloro-2-quinoxalinecarboxylic acid 1,4-dioxide). J. Antibiot. 2017, 7, 317–319. [Google Scholar]
- Settypalli, T.; Chunduri, V.R.; Maddineni, A.K.; Begari, N.; Allagadda, R.; Kotha, P.; Chippada, A.R. Design, synthesis, in silico docking studies and biological evaluation of novel quinoxaline-hydrazide hydrazone-1,2,3-triazole hybrids as α-glucosidase inhibitors and antioxidants. New. J. Chem. 2019, 43, 15435–15452. [Google Scholar] [CrossRef]
- Umesh, T.P.; Bhautik, T.B.; Dipak, R.K. A green protocol for the synthesis of quinoxaline derivatives catalyzed by polymer supported sulphanilic acid. Arab. J. Chem. 2017, 10, S2902–S2907. [Google Scholar]
- Vijay, K.; Karakavalasa, P.; Vasanthi, R. Synthesis, characterization and pharmacological evaluation of some novel quinoxaline derived chalcones. Der. Pharma. Chemica. 2013, 5, 301–307. [Google Scholar]
- Elkaeed, E.; Ghiaty, A.; El-Morsy, A.; El-Gamal, K.; Sakr, H. Synthesis and Biological Evaluation of Some quinoxaline-2-one Derivatives as Novel Anticonvulsant Agents. Chem. Sci. Rev. Lett. 2014, 3, 1375–1387. [Google Scholar]
- Hui, X.; Desrivot, J.; Bories, C.; Loiseau, P.M.; Franck, X.; Hocquemiller, R.; Figadere, B. Synthesis and antiprotozoal activity of some new synthetic substituted quinoxalines. Bioorg. Med. Chem. Lett. 2006, 16, 815–820. [Google Scholar] [CrossRef]
- E-Chavez, J.J.; Merino, V.; Cervantes, M.-L.; Cruz, I.-R.; Guerrero, D.-Q.; Quintanar, A.-G. The use of iontophoresis in the administration of nicotine and new non-nicotine drugs through the skin for smoking cessation. Curr. Drug Discov. Technol. 2009, 6, 171–185. [Google Scholar] [CrossRef]
- Rohde, B.H.; McLaughlin, M.A.; Chiou, L.Y. Existence and role of endogenous ocular Melatonin. J. Ocul. Pharmacol. Th. 1985, 1, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Hugo, W.B.; Stretton, R.G. Action of quinacillin on staphylococcus aureus. Nature 1964, 202, 1217. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Scozzafava, A.; Supuran, C.T. Sulfonamides: A patent review (2008–2012). Expert. Opin. Ther. Pat. 2012, 22, 747–758. [Google Scholar] [CrossRef]
- Actor, P.; Chow, A.W.; Dutko, F.; McKinlay, M.A. "Chemotherapeutics", Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2005; p. 8. [Google Scholar]
- Supuran, C.T. Advances in structure-based drug discovery of carbonic anhydrase inhibitors. Expert. Opin. Drug. Discov. 2017, 12, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Carta, F.; Supuran, C.T.; Scozzafava, A. Sulfonamides and their isosters as carbonic anhydrase inhibitors. Future Med. Chem. 2014, 6, 1149–1165. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Owa, T.; Mastrolorenzo, A.; Supuran, C.T. Anticancer and antiviral sulfonamides. Curr. Med. Chem. 2003, 10, 925–953. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. Sulfa and trimethoprim-like drugs–antimetabolites acting as carbonic anhydrase, dihydropteroate synthase and dihydrofolate reductase inhibitors. J. Enzym. Inhib. Med. Chem. 2014, 29, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Pareek, A.; Rani, P.; Kishore, D. A Short Review On: Sulphonamides. Int. J. Pharma. BioSci. 2013, 4, 812–820. [Google Scholar]
- Khan, H.U.N.; Zaib, S.; Sultana, K.; Khan, I.; Soume, B.-M.; Nadeem, H.; Hassan, M.; Iqbal, J. Metal complexes of tosyl sulfonamides: Design, x-ray structure, biological activities and molecular docking studies. RSC. Adv. 2015, 5, 30125–30132. [Google Scholar] [CrossRef]
- Hager, T. The Demon Under The Microscope: From Battlefield Hospitals To Nazi Labs, One Doctor’s Heroic Search For. The World’s First Miracle Drug; Broadway Books: New York, NY, USA, 2006; ISBN 1-4000-8214-5. [Google Scholar]
- Al-Mohammed, N.N.; Alias, Y.; Abdullah, Z.; Shakir, R.M.; Taha, E.M.; Hamid, A.A. Synthesis and antibacterial evaluation of some novel imidazole and benzimidazole sulfonamides. Molecules 2013, 18, 11978–11995. [Google Scholar] [CrossRef]
- Alsughayer, A.; Elassar, A.A.Z.; Mustafa, S.; Sagheer, F. Synthesis, structure analysis and antibacterial activity of new potent sulfonamide derivatives. J. Biomater. Nanobiotechnol. 2011, 2, 143–148. [Google Scholar] [CrossRef]
- Gorantla, V.; Gundlac, R.; Jadavc, S.S.; Anugu, S.R.; Chimakurthy, J.; Nidasanametla, S.K.; Korupolu, R. Molecular hybrid design, synthesis and biological evaluation of nphenyl sulfonamide linked n-acyl hydrazone derivatives functioning as cox-2 inhibitors: New anti-inflammatory, anti-oxidant and anti-bacterial agents. New J. Chem. 2017, 41, 13516–13532. [Google Scholar] [CrossRef]
- Abdel-Aziz, A.A.M.; Angeli, A.; El-Azab, A.S.; Hammouda, M.E.A.; El-Sherbeny, M.A.; Supuran, C.T. Synthesis and anti-inflammatory activity of sulfonamides and carboxylates incorporating trimellitimides: Dual cyclooxygenase/carbonic anhydrase inhibitory actions. Bioorg. Chem. 2019, 84, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Capasso, C.; Supuran, C.T. An overview of the alpha-, beta-and gamma-carbonic anhydrases from Bacteria: Can bacterial carbonic anhydrases shed new light on evolution of bacteria. J. Enzym Inhib. Med. Chem. 2015, 30, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Isik, K.; Kocak, O.F. Antimicrobial activity screening of some sulfonamide derivatives on some nocardia species and isolates. Microbiol. Res. 2009, 164, 49–58. [Google Scholar] [CrossRef]
- Chohan, Z.H.; Rauf, A.; Naseer, M.M.; Somra, M.A.; Supuran, C.T. Antibacterial, antifungal and cytotoxic properties of some sulfonamide-derived chromones. J. Enzyme Inhib. Med. Chem. 2006, 21, 173–177. [Google Scholar] [CrossRef] [PubMed]
- Chibale, K.; Haupt, H.; Kendrick, H.; Yardley, V.; Saravanamuthu, A.; Fairlamb, A.H.; Croft, S.L. Antiprotozoal and cytotoxicity evaluation of sulfonamide and urea analogues of quinacrine. Bioorg. Med. Chem. Lett. 2001, 11, 2655–2657. [Google Scholar] [CrossRef]
- Supuran, C.T.; Casini, A.; Scozzafava, A. Protease inhibitors of the sulfonamide type: Anticancer, antiinflammatory, and antiviral agents. Med. Res. Rev. 2003, 23, 535–558. [Google Scholar] [CrossRef]
- Andrews, K.T.; Fisher, G.M.; Sumanadasa, S.D.M.; Adams, T.-S.; Moeker, J.; Lopez, M.; S-Poulsen, A. Antimalarial activity of compounds comprising a primary benzene sulfonamide fragment. Bioorg. Med. Chem. Lett. 2013, 23, 6114–6117. [Google Scholar] [CrossRef]
- Puccetti, L.; Fasolis, G.; Vullo, D.; Chohan, Z.H.; Scozzafava, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors. Inhibition of Cytosolic/Tumor-Associated Carbonic Anhy-drase Isozymes I, II, IX, and XII with Schiff Bases Incorpo-rating Chromone and Aromatic Sulfonamide Moieties, and Their Zinc Complexes. Bioorg. Med. Chem. Lett. 2005, 15, 3096–3101. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic Anhydrase Inhibition and the Management of Hypoxic Tumors. Metabolites 2017, 7, 48. [Google Scholar] [CrossRef]
- Abbate, F.; Winum, J.Y.; Potter, B.V.; Casini, A.; Montero, L.-J.; Scozzafava, A.; Supuran, C.T. Carbonic anhydrase inhibitors: X-ray crystallographic structure of the adduct of human isozyme II with EMATE, a dual inhibitor of carbonic anhydrases and steroid sulfatase. Bioorg. Med. Chem. Lett. 2004, 14, 231–234. [Google Scholar] [CrossRef]
- Garaj, V.; Puccetti, L.; Fasolis, G.; Winum, Y.J.; Montero, L.J.; Scozzafava, A.; Vullo, D.; Innocenti, A.; Supuran, C.T. Carbonic Anhydrase Inhibitors: Synthesis and In-hibition of Cytosolic/Tumor-Associated Carbonic Anhydrase Isozymes I, Ii, and Ix with sulfonamides incorporating 1,2,4-Triazine Moieties. Bioorg. Med. Chem. Lett. 2004, 14, 5427–5433. [Google Scholar] [CrossRef]
- Supuran, C.T. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat. Rev. Drug Discov. 2008, 7, 168–181. [Google Scholar] [CrossRef]
- Neri, D.; Supuran, C.T. Interfering with pH regulation in tumours as a therapeutic strategy. Nat. Rev. Drug Discov. 2011, 10, 767–777. [Google Scholar] [CrossRef]
- Masini, E.; Carta, F.; Scozzafava, A.; Supuran, C.T. Antiglaucoma carbonic anhydrase inhibitors: A patent review. Expert Opin. Ther. Pat. 2013, 23, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Monti, S.M.; Supuran, C.T.; Simone, G.D. Anticancer carbonic anhydrase inhibitors: A patent review (2008–2013). Expert Opin. Ther. Pat. 2013, 23, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Supuran, C.T. Structure-based drug discovery of carbonic anhydrase inhibitors. J. Enzym Inhib. Med. Chem. 2012, 27, 759–772. [Google Scholar] [CrossRef] [PubMed]
- Berredjem, H.; Reggami, Y.; Benlaifa, M.; Berredjem, M.; Bouzerna, N. Antidiabetic and hypolipidemic potential of 3, 4-dihydroisoquinolin-2(1H)- sulfonamide in alloxan induced diabetic rats. Int. J. Pharmacol. 2015, 11, 226–235. [Google Scholar] [CrossRef]
- Masereel, B.; Thiry, A.; Dogne, J.M.; Supuran, C.T. Anticonvulsant sulfonamides/sulfamates/sulfamides with carbonic anhydrase inhibitory activity: Drug design and mechanism of action. Curr. Pharm. Des. 2008, 14, 661–671. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T.; Carta, F. Antiobesity carbonic anhydrase inhibitors: A literature and patent review. Expert Opin. Ther. Pat. 2013, 23, 725–735. [Google Scholar] [CrossRef]
- Supuran, C.T. How many carbonic anhydrase inhibition mechanisms exist. J. Enzym Inhib. Med. Chem. 2016, 31, 345–360. [Google Scholar] [CrossRef]
- Carta, F.; Supuran, C.T. Diuretics with carbonic anhydrase inhibitory action: A patent and literature review (2005–2013). Expert Opin. Ther. Pat. 2013, 23, 681–691. [Google Scholar] [CrossRef]
- Boyd, A.E. Sulfonylurea receptors, ion channels and fruit flies. Diabetes 1988, 37, 847–850. [Google Scholar] [CrossRef]
- Carta, F.; Mannelli, L.D.C.; Pinard, M.; Ghelardini, C.; Scozzafava, A.; McKenna, R.; Supuran, C.T. A class of sulfonamide carbonic anhydrase inhibitors with neuropathic pain modulating effects. Bioorg. Med. Chem. 2015, 23, 1828–1840. [Google Scholar] [CrossRef]
- Scozzafava, A.; Supuran, C.T. Carbonic anhydrase and matrix metalloproteinase inhibitors: sulfonylated amino acid hydroxamates with mmp inhibitory properties act as efficient inhibitors of ca isozymes i, ii, and iv, and N-hydroxysulfonamides, Inhibit Both These Zinc. Enzymes J. Med. Chem. 2000, 43, 3677–3687. [Google Scholar] [CrossRef] [PubMed]
- Scozzafava, A.; Supuran, C.T. Protease Inhibitors: Synthesis of potent bacterial collagenase and matrix metalloproteinase inhibitors incorporating n-4-nitrobenzylsulfonylglycine hydroxamate moieties. J. Med. Chem. 2000, 43, 1858–1865. [Google Scholar] [CrossRef] [PubMed]
- Ajeet, A.; Mishra, K.; Kumar, A. Recent advances in development of sulfonamide derivatives and their pharmacological effects- A Review. Am. J. Pharmacol. Sci. 2015, 3, 18–24. [Google Scholar]
- Gao, H.; Yamasaki, E.F.; Chan, K.K.; Shen, L.L.; Snapka, R.M. Chloroquinoxaline Sulfonamide (NSC 339004) is a Topoisomerase IIα/β Poison. Cancer Res. 2000, 60, 5937–5940. [Google Scholar] [PubMed]
- Kornberg, B.E.; Nikam, S.S. Sulfonamide Derivatives of Substituted Quinoxaline 2,3-Diones as Glutamate Receptor Antagonsts. U.S. Patent 6,096,744, 1 August 2000. [Google Scholar]
- Gaillard, P.; Quattropani, A.; Pomel, V.; Rueckle, T.; Klicic, J.; Church, D. Pyrazine Derivatives and Use as pi3k Inhibitors. U.S. Patent 8,877,757, 4 November 2014. [Google Scholar]
- Allison, B.D.; Hack, M.D.; Rabinowitz, M.H.; Rosen, M.D. Quinoxaline Compounds. U.S. Patent 7,304,051 B2, 4 December 2007. [Google Scholar]
- Pascale, G.; Vincent, P.; J-Isabelle, E.; Jerome, D.; Jasna, K.; Cyril, M. Quinoxaline Compounds and Use Thereof. WO 2008/101979 Al, 28 August 2008. [Google Scholar]
- Deng, X.; Liang, J.T.; Mani, N.; Pandit, C.R. Preparation of Quinoxaline Compounds. U.S. Patent 2008/0103300 A, 1 May 2008. [Google Scholar]
- Bajjalieh, W.; Bannen, L.C.; Brown, S.D.; Mac, M.B.; Marlowe, C.K.; Nuss, J.M.; Tesfai, Z.; Wang, Y.; Xu, W. Phosphatidylinositol, 3-Kinase Inhibitors and Methods of Their Use. U.S. Patent 7,989,622 B2011, 2 August 2011. [Google Scholar]
- Aftab, D.T.; Decillis, A. Phosphatidylinositol 3-Kinase Inhibitors and Methods of Their Use. WO 2012/065057 A3, 18 May 2012. [Google Scholar]
- Natarajan, A.; Chen, Q.; Bryant, V.C.; Radhakrishnan, P.; Rajule, R.; Chaturvedi, N. Quinoxaline Compounds and Uses Thereof. WO 2012/071414 A2, 31 May 2012. [Google Scholar]
- Harper, S.; Summa, V.; Liverton, N.J.; McCauley, J.A. Macrocyclic Quinoxaline Compounds as HCV NS3 Protease Inhibitors. U.S. Patent 7,973,040 B2, 5 July 2011. [Google Scholar]
- Zhu, Y.-L.; Qian, X. Benzenesulfonamide Derivatives of Quinoxaline, Pharmaceutical Compositions Thereof, and Their Use in Methods for Treating Cancer. U.S. Patent 9.295,671 B2, 29 March 2016. [Google Scholar]
- Boezio, C.; Boezio, A.; Bregman, H.; Coats, J.R.; Copeland, K.W.; Dimauro, E.F.; Dineen, T.; Gao, H.; La, D.; Marx, I.E.; Nguyen, H.N.; Peterson, E.A.; Weiss, M. Dhydrobenzoxazine and Tetrahydroquinoxaline Sodium Channel Inhibitors. U.S. Patent 9,346,798 B2016, 28 August 2013. [Google Scholar]
- Zhu, L.-Y.; Qian, X. Benzene Sulfonamide Derivatives of Quinoxaline, Pharmaceutical Compositions Thereof, and Their Use in Methods for Treating Cancer. U.S. Patent 9,572,808 B2, 21 February 2017. [Google Scholar]
- Klein, M.; Emde, U.; Buchstaller, H.P.; Esdar, C.; Poeschke, O. Quinoxaline Derivatives. U.S. Patent 2013/0217670 A1, 22 August 2013. [Google Scholar]
- Danig, P.; Dominique, S. Method for Preparing Substituted n-(3-Amino-Quinoxalin-2-yl)-Sulfonamides and Their Intermediates n-(3-Chloro-Quinoxalin-2-yl)Sulfonamides. WO 2012 /052420 Al, 26 April 2012. [Google Scholar]
- Boezio, C.; Boezio, A.; Bregman, H.; Chakka, N.; Coats, J.R.; Copeland, K.W.; Dimauro, E.F.; Dineen, T.; Gao, H.; La, D.; Marx, I.E.; Nguyen, H.N.; Peterson, E.A.; Weiss, M. Tetrahydroquinoxaline Dihydrobenzoxazine and Sodium Channel Inhibitors. WO 2013/122897 Al, 22 August 2013. [Google Scholar]
- Gigstad, K.M.; Cardin, D.P.; Hirayama, T.; Hirose, M.; Hu, Y.; Kakei, H.; Lee, H.M.; Motoyaji, T.; NII, N.; Shi, Z.; Vyskocil, S.; Watanabe, H. Quinoxaline Compounds and Uses Thereof. U.S. Patent 10,144,742 B2018, 4 December 2018. [Google Scholar]
- Husain, A.; Madhesia, D.; Rashid, M.; Ahmad, A.; Khan, S.A. Synthesis and in vivo diuretic activity of some new benzothiazole sulfonamides containing quinoxaline ring system. J. Enzyme Inhib. Med. Chem. 2016, 31, 1682–1689. [Google Scholar] [CrossRef]
- Alavi, S.; Mosslemin, M.H.; Mohebat, R.; Massah, A.R. Green synthesis of novel quinoxaline sulfonamides with antibacterial activity. Res. Chem. Intermed. 2017, 43, 4549–4559. [Google Scholar] [CrossRef]
- Ingle, R.; Wadher, S. Synthetic development of antimicrobial novel quinoxaline derivatives. Int. J. Pharm. Chem. 2013, 3, 34–38. [Google Scholar] [CrossRef]
- Talari, S.; Karunakaram, D.R.G.; Jupudi, S.; Udhayavani, S. Synthesis of 9-bromo-n-substituted- 6h- indolo [2, 3-b] quinoxaline-3-sulfonamide derivatives containing quinoxaline moiety as prospective antimicrobial agents. Int. J. Pharm. 2013, 3, 145–151. [Google Scholar]
- Sharaf El-Din, N. Synthesis of some sulfonamide derivatives with potential antibacterial activity. Chem. Heterocycl. Compd. 2000, 36, 523–528. [Google Scholar]
- Potey, C.L.; Kosalge, B.S.; Hadke, A.M. Synthesis and Antimicrobial Activity of Quinoxaline Sulfonamide. IJOART 2013, 2, 126–134. [Google Scholar]
- Olayiwola, G.; Obafemi, C.A.; Taiwo, F.O. Synthesis and neuropharmacological activity of some quinoxalinone derivatives. Afr. J. Biotechnol. 2007, 6, 777–786. [Google Scholar]
- Barea, C.; Pabon, A.; Castillo, D.; Zimic, M.; Quiliano, M.; Galiano, S.; Pérez-Silanes, S.; Monge, A.; Deharo, E.; Aldana, I. New Salicylamide And Sulfonamide Derivatives of Quinoxaline 1,4-Di-N-Oxide with Antileishmanial And Antimalarial Activities. Bioorg Med. Chem Lett. 2011, 21, 4498–4502. [Google Scholar] [CrossRef] [PubMed]
- Farrag, A.A.; Ammar, A.Y.; El-Sehemi, G.A.; Thabet, H.K.; Hassan, N.A.-A.; Samy, A.H. Synthesis And Pharmacological Screening of Novel Sulfamoylphenyl Carbamoyl Quinoxaline Derivatives As Anti-Inflammatory, Analgesic And Antitumour Agents. J. Chem. Res. 2011, 35, 163–166. [Google Scholar] [CrossRef]
- Ingle, R.; Marathe, R.; Magar, D.; Patel, H.M.; Surana, S.J. Sulphonamido-quinoxalines: Search for anticancer agent. Eur. J. Med. Chem. 2013, 65, 168–186. [Google Scholar] [CrossRef]
- Ingle, G.R.; Marathe, P.R. Sulfonamido Quinoxalines—Search for Anti-Inflammatory Agents. Int. J. Pharm. Res. Allied Sci. 2012, 4, 46–51. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).