Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review
Abstract
:1. Introduction
2. Magnetic Materials
3. Synthesis
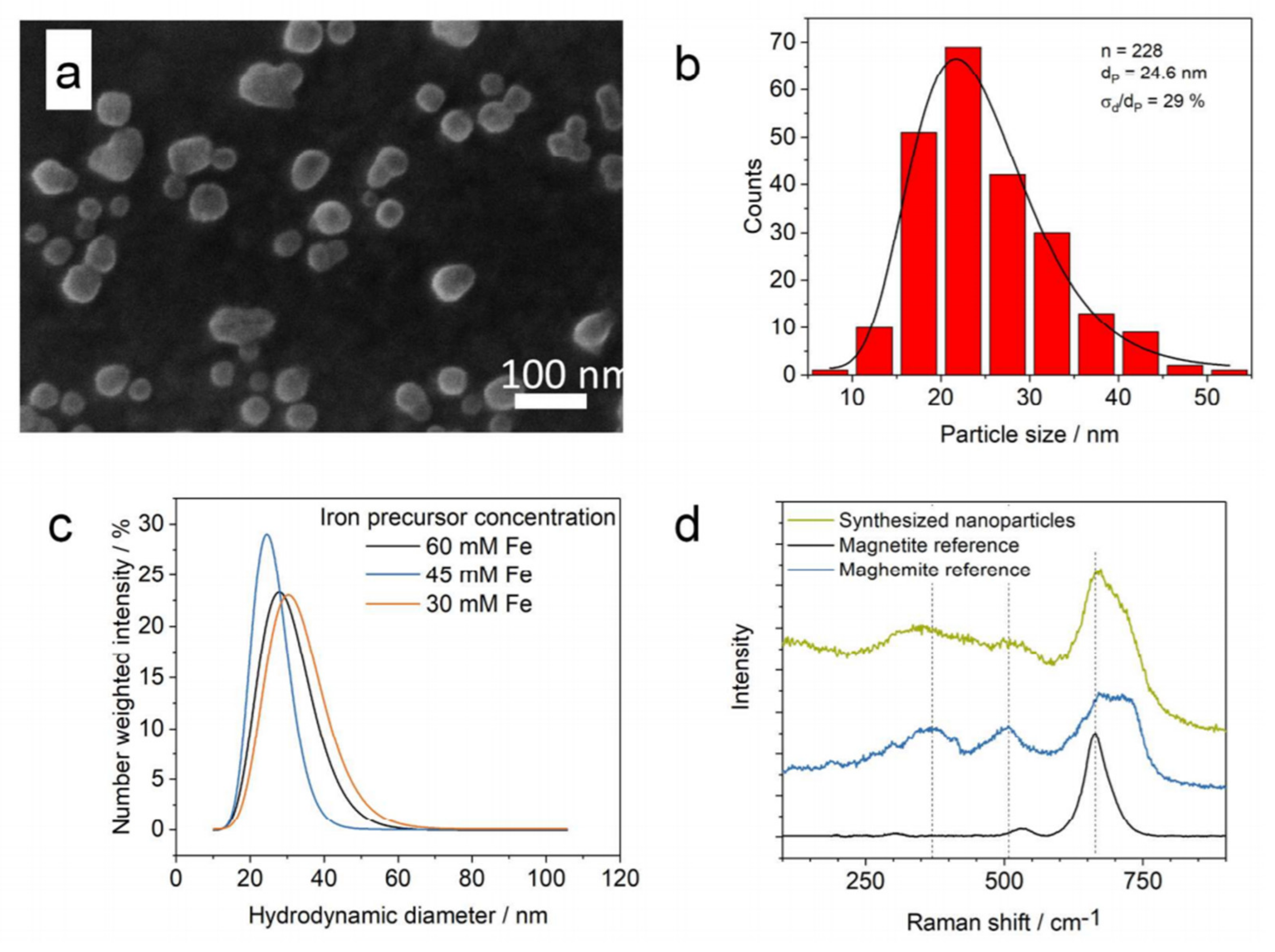
3.1. Continuous-Flow Microreactors
3.2. Droplet-Based Microreactors
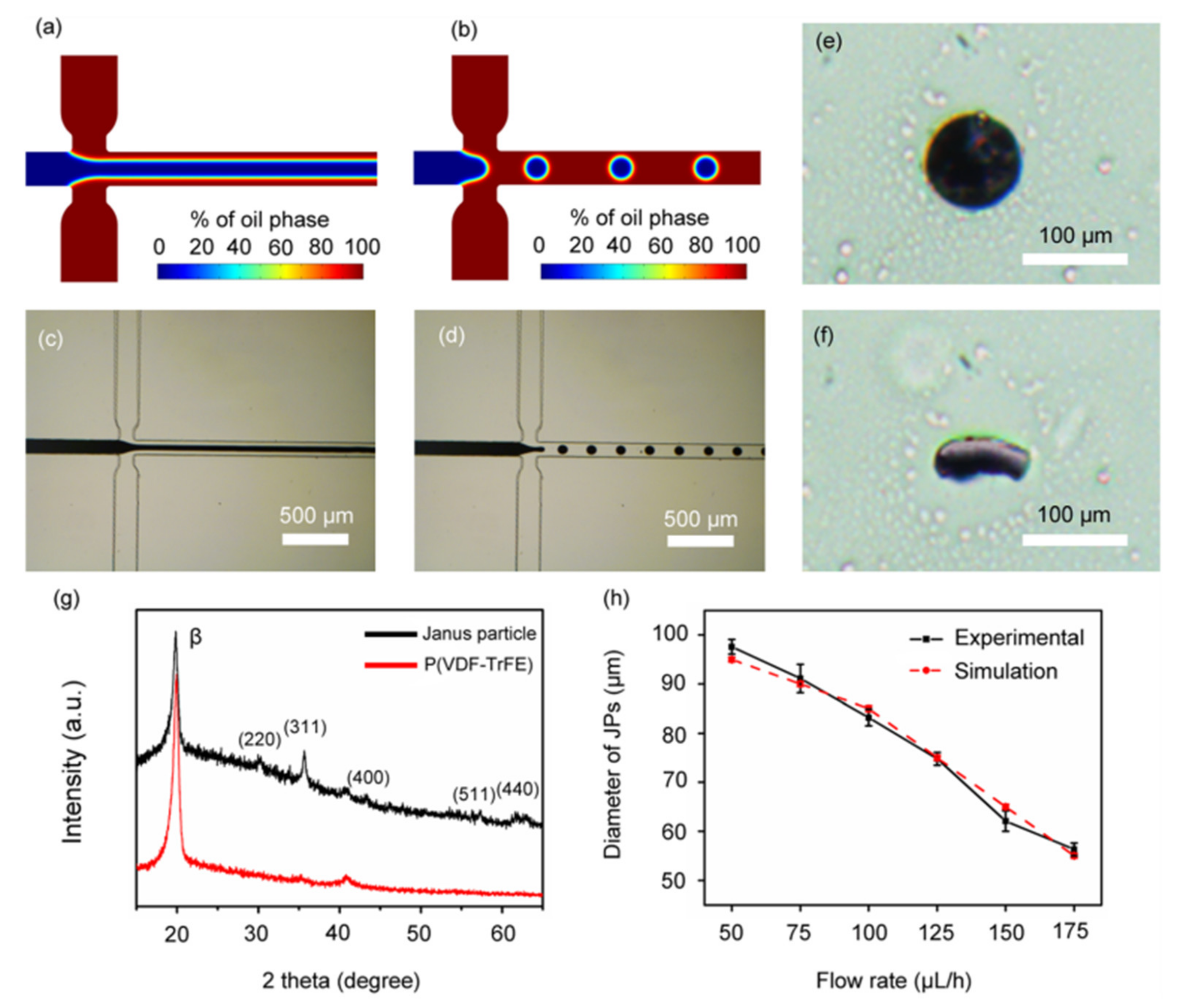
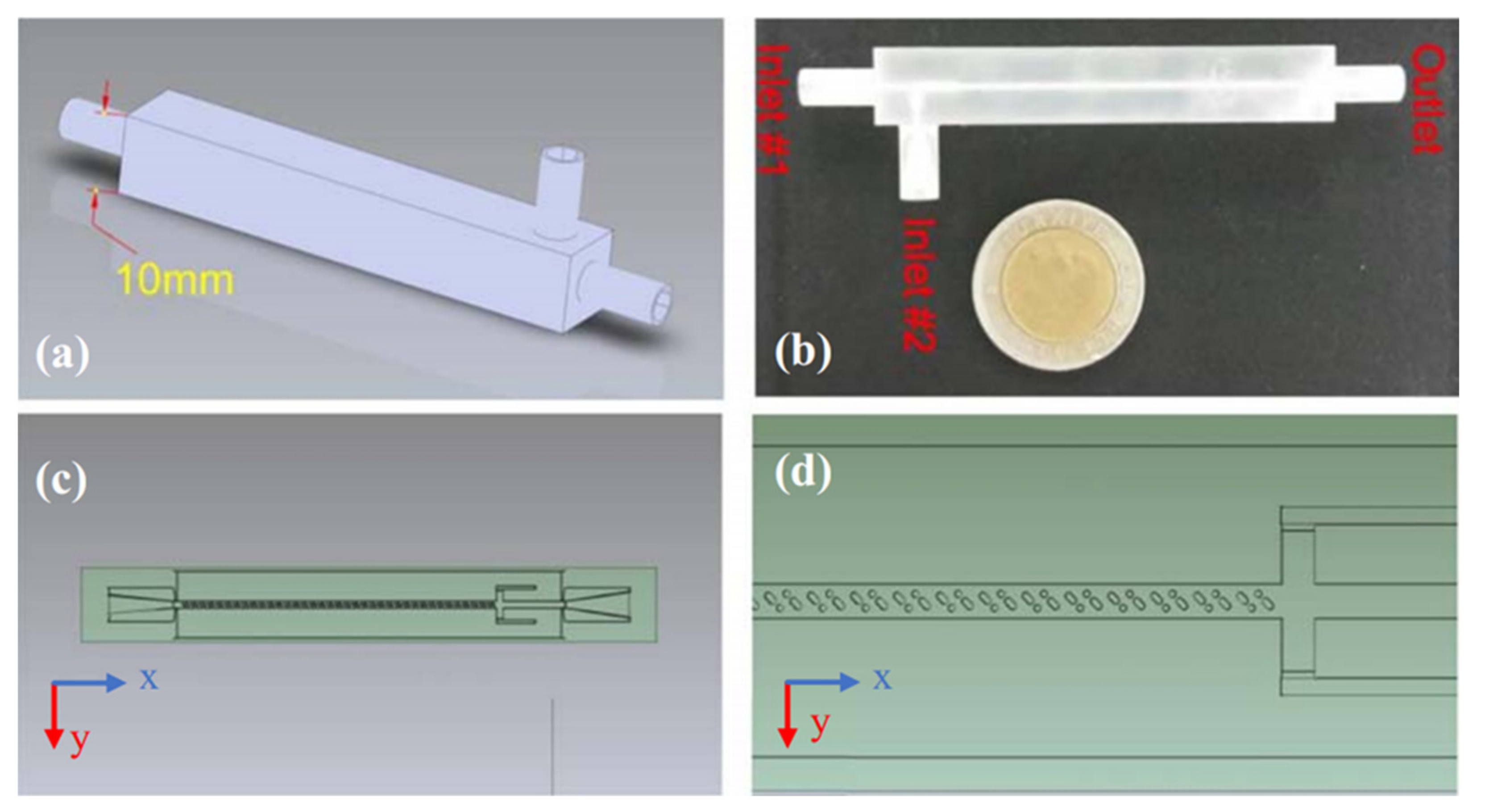
3.3. Microspheres Encapsulating Magnetic Nanoparticles
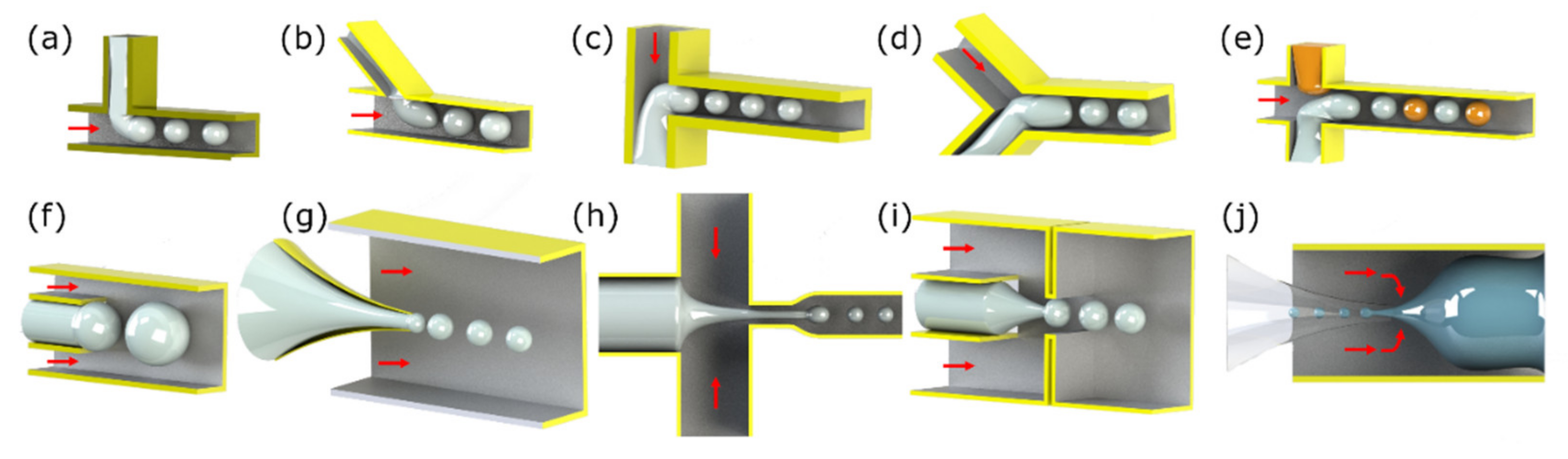
4. Particle Manipulation
4.1. Particle Manipulation with an External Permanent Magnet or Electromagnet
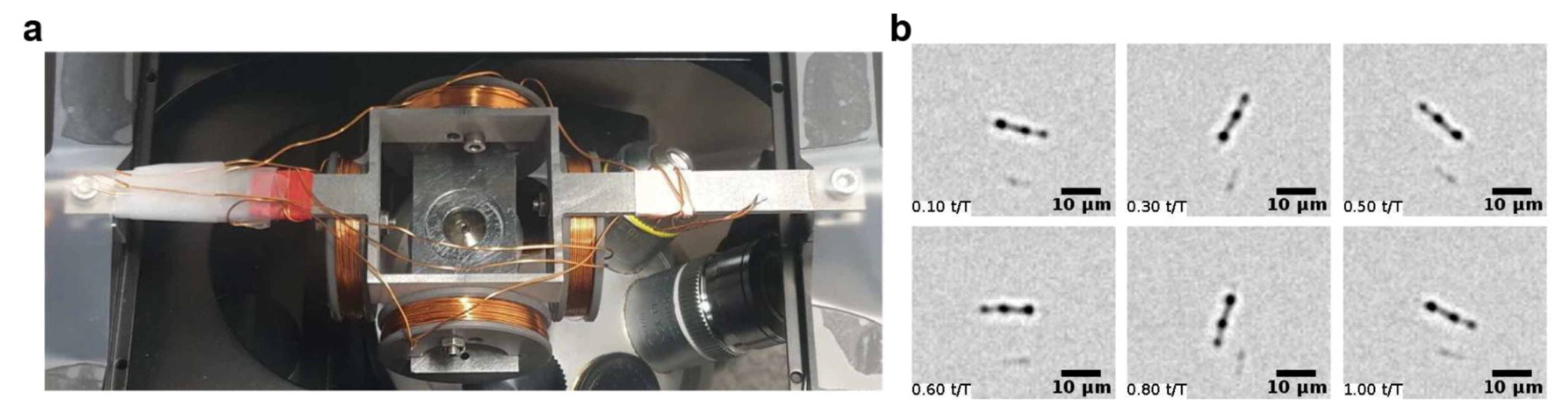
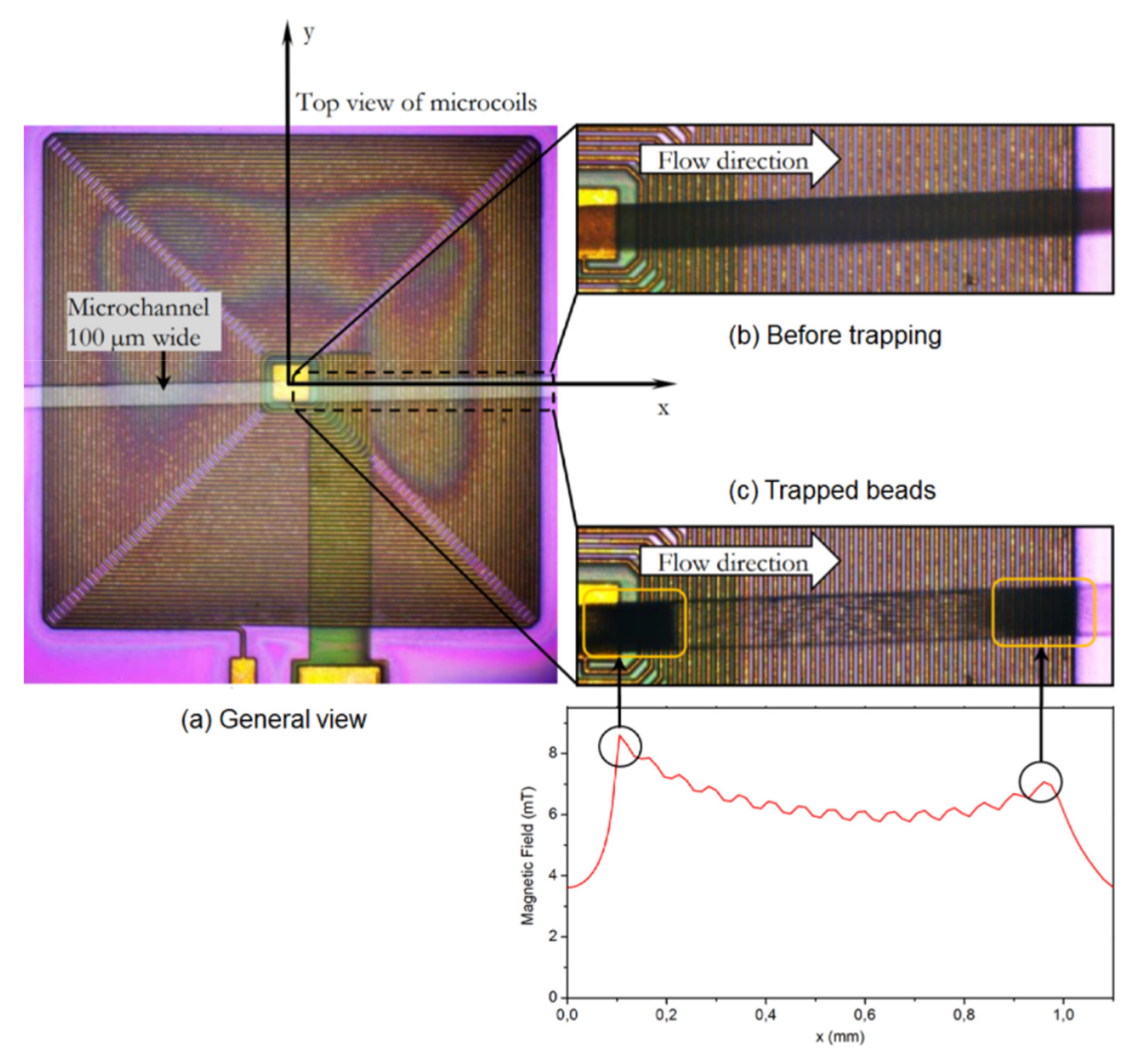
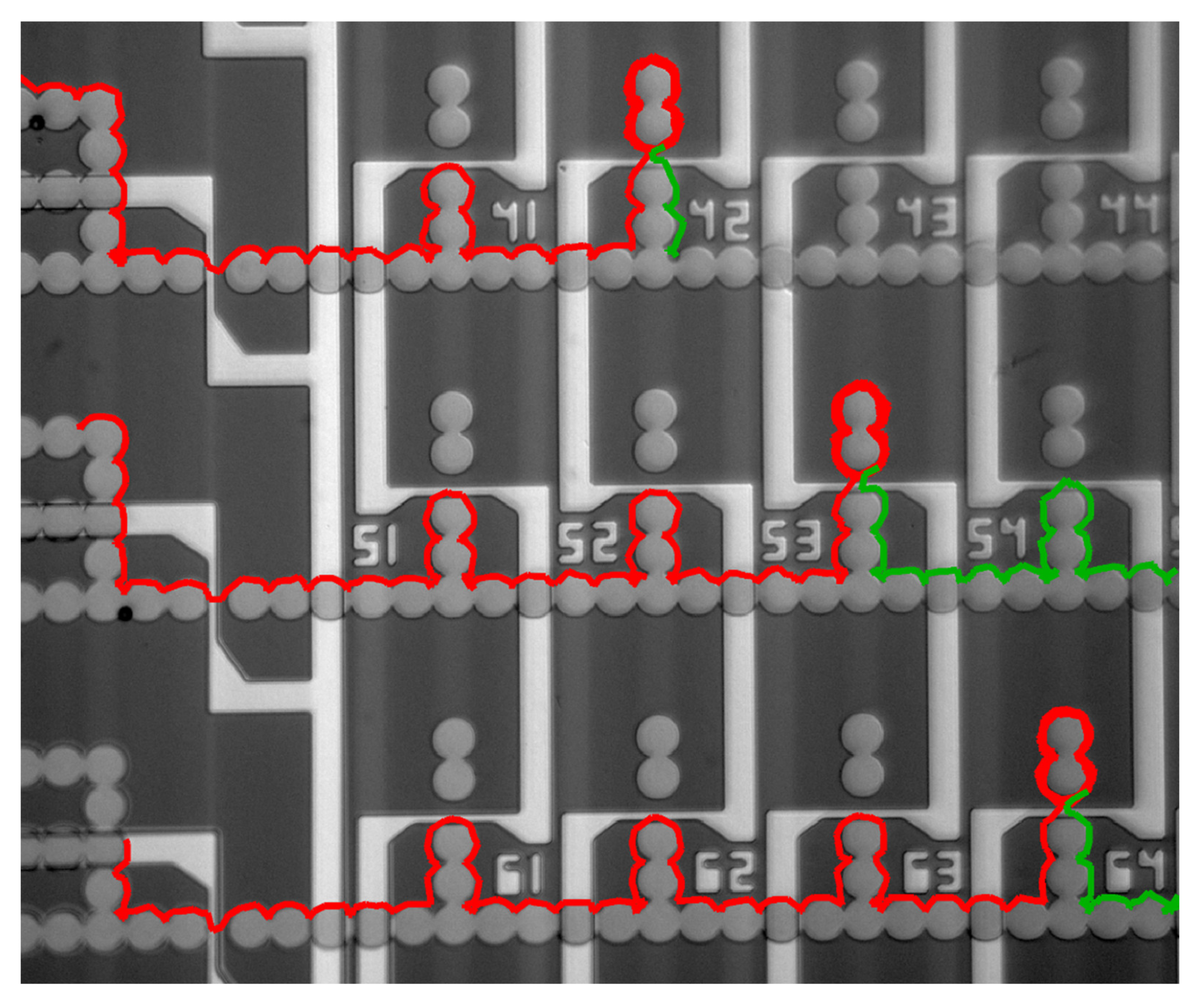
4.2. Particle Manipulation with Embedded Micro-Wires and Micro-Coils
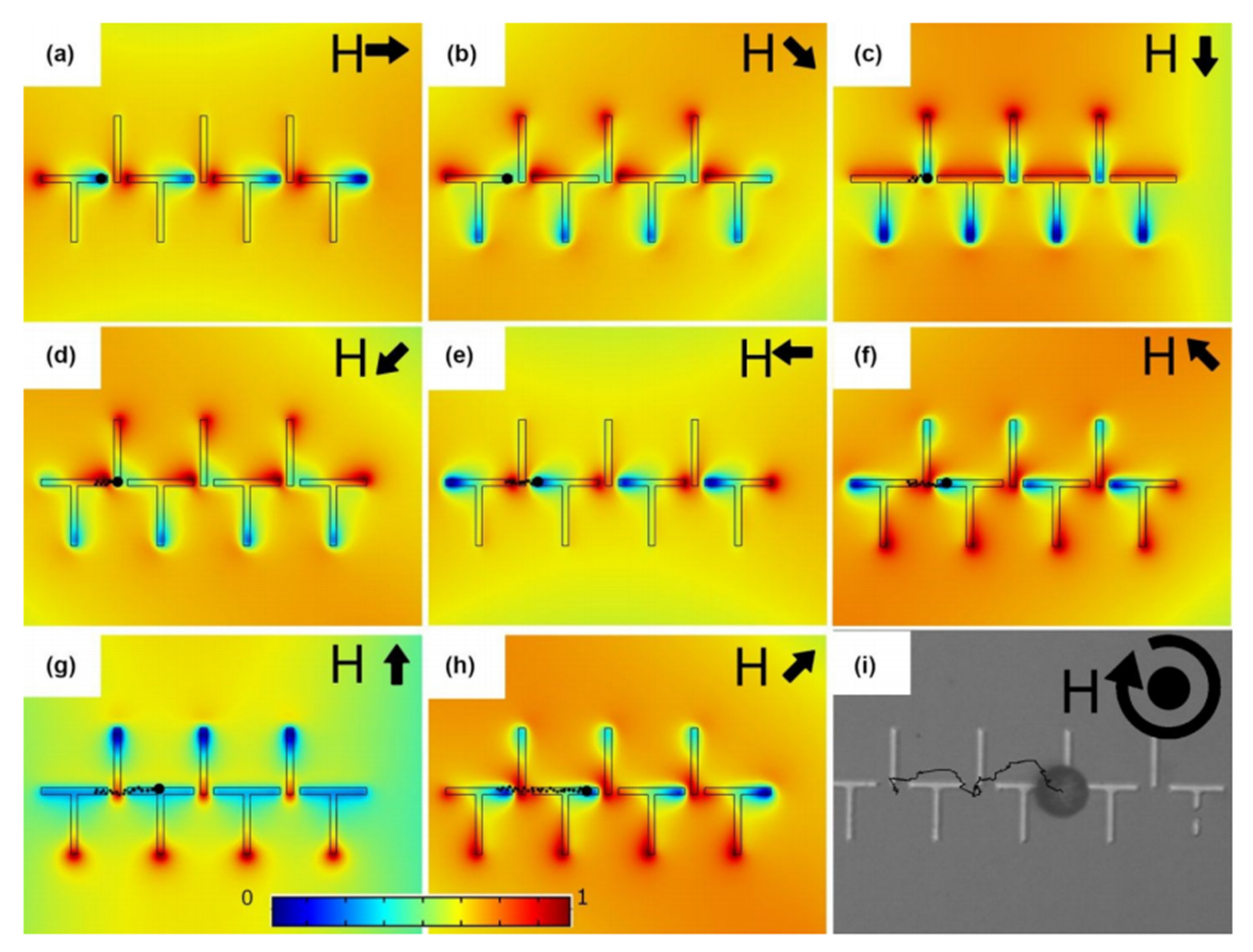
4.3. Particle Manipulation with Embedded Magnetic Thin Films
5. Detection and Characterization
5.1. Anisotropic Magnetoresistive Sensor
5.2. Giant Magnetoresistive Sensors
5.3. Tunneling Magnetoresistive Sensors
5.4. Magnetorelaxometry-Based Sensors
5.5. Other Magnetic Sensors
6. Conclusions and Future Works (Summary)
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Baig, N.; Kammakakam, I.; Falath, W. Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges. Mater. Adv. 2021, 2, 1821–1871. [Google Scholar] [CrossRef]
- Pankhurst, Q.A.; Connolly, J.; Jones, S.K.; Dobson, J. Applications of magnetic nanoparticles in biomedicine. J. Phys. D Appl. Phys. 2003, 36, R167–R181. [Google Scholar] [CrossRef] [Green Version]
- Reddy, L.H.; Arias, J.L.; Nicolas, J.; Couvreur, P. Magnetic Nanoparticles: Design and Characterization, Toxicity and Biocompatibility, Pharmaceutical and Biomedical Applications. Chem. Rev. 2012, 112, 5818–5878. [Google Scholar] [CrossRef]
- Kudr, J.; Haddad, Y.A.E.; Richtera, L.; Heger, Z.; Cernak, M.; Adam, V.; Zitka, O. Magnetic Nanoparticles: From Design and Synthesis to Real World Applications. Nanomaterials 2017, 7, 243. [Google Scholar] [CrossRef]
- Akbarzadeh, A.; Samiei, M.; Davaran, S. Magnetic nanoparticles: Preparation, physical properties, and applications in biomedicine. Nanoscale Res. Lett. 2012, 7, 144. [Google Scholar] [CrossRef] [Green Version]
- Khurshid, H.; Nemati, Z.; Iglesias, Ó.; Alonso, J.; Phan, M.-H.; Srikanth, H. Hollow Magnetic Nanoparticles. In New Trends in Nanoparticle Magnetism; Peddis, D., Laureti, S., Fiorani, D., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 137–158. ISBN 978-3-030-60472-1. [Google Scholar]
- Das, R.; Masa, J.; Kalappattil, V.; Nemati, Z.; Rodrigo, I.; Garaio, E.; García, J.; Phan, M.-H.; Srikanth, H. Iron Oxide Nanorings and Nanotubes for Magnetic Hyperthermia: The Problem of Intraparticle Interactions. Nanomaterials 2021, 11, 1380. [Google Scholar] [CrossRef] [PubMed]
- Lavorato, G.C.; Das, R.; Masa, J.A.; Phan, M.-H.; Srikanth, H. Hybrid magnetic nanoparticles as efficient nanoheaters in biomedical applications. Nanoscale Adv. 2021, 3, 867–888. [Google Scholar] [CrossRef]
- Liu, Z.; Fontana, F.; Python, A.; Hirvonen, J.T.; Santos, H.A. Microfluidics for Production of Particles: Mechanism, Methodology, and Applications. Small 2020, 16, e1904673. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Jiang, X. Microfluidic Synthesis of Functional Nanoparticles. In Nanotechnology and Microfluidics; Jiang, X., Bai, C., Liu, M., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2019; pp. 319–345. ISBN 978-352-781-834-1. [Google Scholar]
- Zhang, L.; Chen, Q.; Ma, Y.; Sun, J. Microfluidic Methods for Fabrication and Engineering of Nanoparticle Drug Delivery Systems. ACS Appl. Bio Mater. 2019, 3, 107–120. [Google Scholar] [CrossRef]
- Kulkarni, M.B.; Goel, S.G. Microfluidic devices for synthesizing nanomaterials—A review. Nano Express 2020, 1, 032004. [Google Scholar] [CrossRef]
- Amreen, K.; Goel, S. Review—Miniaturized and Microfluidic Devices for Automated Nanoparticle Synthesis. ECS J. Solid State Sci. Technol. 2021, 10, 017002. [Google Scholar] [CrossRef]
- Cha, J.; Lee, I. Single-cell network biology for resolving cellular heterogeneity in human diseases. Exp. Mol. Med. 2020, 52, 1798–1808. [Google Scholar] [CrossRef]
- Su, X.; Zhao, L.; Shi, Y.; Zhang, R.; Long, Q.; Bai, S.; Luo, Q.; Lin, Y.; Zou, X.; Ghazanfar, S.; et al. Clonal evolution in liver cancer at single-cell and single-variant resolution. J. Hematol. Oncol. 2021, 14, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gantner, P.; Pagliuzza, A.; Pardons, M.; Ramgopal, M.; Routy, J.; Fromentin, R.; Chomont, N. Single-cell TCR sequencing reveals phenotypically diverse clonally expanded cells harboring inducible HIV proviruses during ART. Nat. Commun. 2020, 11, 4089. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zou, T.H.; Xuan, B.; Yan, Y.; Yan, T.; Shen, C.; Fang, J.Y. Single cell transcriptome revealed SARS-CoV-2 entry genes enriched in colon tissues and associated with coronavirus infection and cytokine production. Signal Transduct. Target Ther. 2020, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Giouroudi, I.; Kokkinis, G. Recent Advances in Magnetic Microfluidic Biosensors. Nanomaterials 2017, 7, 171. [Google Scholar] [CrossRef] [Green Version]
- Su, D.; Wu, K.; Saha, R.; Peng, C.; Wang, J. Advances in Magnetoresistive Biosensors. Micromachines 2019, 11, 34. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.T.; Garu, P.; Li, C.H.; Chang, W.C.; Chen, B.W.; Sung, S.Y.; Lee, C.M.; Chen, J.Y.; Hsieh, T.F.; Sheu, W.J.; et al. Magnetoresistive Biosensors for Direct Detection of Magnetic Nanoparticle Conjugated Biomarkers on a Chip. SPIN 2019, 9, 1940002. [Google Scholar] [CrossRef]
- Giouroudi, I.; Keplinger, F. Microfluidic biosensing systems using magnetic nanoparticles. Int. J. Mol. Sci. 2013, 14, 18535–18556. [Google Scholar] [CrossRef] [Green Version]
- Pushkarev, A.; Orlov, A.; Znoyko, S.; Bragina, V.; Nikitin, P. Rapid and Easy-to-Use Method for Accurate Characterization of Target Binding and Kinetics of Magnetic Particle Bioconjugates for Biosensing. Sensors 2021, 21, 2802. [Google Scholar] [CrossRef]
- Gessner, I.; Fries, J.W.U.; Brune, V.; Mathur, S. Magnetic nanoparticle-based amplification of microRNA detection in body fluids for early disease diagnosis. J. Mater. Chem. B 2021, 9, 9–22. [Google Scholar] [CrossRef]
- Chen, Y.-T.; Kolhatkar, A.G.; Zenasni, O.; Xu, S.; Lee, T.R. Biosensing Using Magnetic Particle Detection Techniques. Sensors 2017, 17, 2300. [Google Scholar] [CrossRef]
- Spaldin, N.A. Magnetic Materials: Fundamentals and Applications, 2nd ed.; Cambridge University Press: Cambridge, UK, 2010. [Google Scholar]
- Dennis, C.L.; Ivkov, R. Physics of heat generation using magnetic nanoparticles for hyperthermia. Int. J. Hyperth. 2013, 29, 715–729. [Google Scholar] [CrossRef] [PubMed]
- Wysin, G.M. Magnetism theory: Spin models. In Magnetic Excitations and Geometric Confinement Theory and Simulations; IOP Publishing: Bristol, UK, 2015; p. 21247. [Google Scholar]
- Linderoth, S.; Khanna, S. Superparamagnetic behaviour of ferromagnetic transition metal clusters. J. Magn. Magn. Mater. 1992, 104–107, 1574–1576. [Google Scholar] [CrossRef]
- Wahajuddin, S.A. Superparamagnetic iron oxide nanoparticles: Magnetic nanoplatforms as drug carriers. Int. J. Nanomed. 2012, 7, 3445–3471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edel, J.B.; Fortt, R.; Demello, J.C.; Demello, A.J. Microfluidic routes to the controlled production of nanoparticles. Chem. Commun. 2002, 10, 1136–1137. [Google Scholar] [CrossRef]
- Aşık, M.D.; Kaplan, M.; Çetin, B.; Sağlam, N. Synthesis of iron oxide core chitosan nanoparticles in a 3D printed microfluidic device. J. Nanoparticle Res. 2021, 23, 1–11. [Google Scholar] [CrossRef]
- Thu, V.T.; Mai, A.N.; Tam, L.T.; Van Trung, H.; Thu, P.T.; Tien, B.Q.; Thuat, N.T.; Lam, T.D. Fabrication of PDMS-Based Microfluidic Devices: Application for Synthesis of Magnetic Nanoparticles. J. Electron. Mater. 2016, 45, 2576–2581. [Google Scholar] [CrossRef]
- Song, Y.; Modrow, H.; Henry, L.L.; Saw, C.K.; Doomes, E.E.; Palshin, V.; Hormes, A.J.; Kumar, C.S.S.R. Microfluidic Synthesis of Cobalt Nanoparticles. Chem. Mater. 2006, 18, 2817–2827. [Google Scholar] [CrossRef]
- Song, Y.; Henry, L.L.; Yang, W. Stable Amorphous Cobalt Nanoparticles Formed by an in Situ Rapidly Cooling Microfluidic Process. Langmuir 2009, 25, 10209–10217. [Google Scholar] [CrossRef]
- Ohannesian, N.; De Leo, C.T.; Martirosyan, K.S. Dextran coated superparamagnetic iron oxide nanoparticles produced by microfluidic process. Mater. Today Proc. 2019, 13, 397–403. [Google Scholar] [CrossRef]
- Wang, J.; Zhao, K.; Shen, X.; Zhang, W.; Ji, S.; Song, Y.; Zhang, X.; Rong, R.; Wang, X. Microfluidic synthesis of ultra-small magnetic nanohybrids for enhanced magnetic resonance imaging. J. Mater. Chem. C 2015, 3, 12418–12429. [Google Scholar] [CrossRef]
- Shen, X.; Song, Y.; Li, S.; Li, R.; Ji, S.; Li, Q.; Duan, H.; Xu, R.; Yang, W.; Zhao, K.; et al. Spatiotemporal-resolved nanoparticle synthesis via simple programmed microfluidic processes. RSC Adv. 2014, 4, 34179–34188. [Google Scholar] [CrossRef]
- Ding, S.; Attia, M.F.; Wallyn, J.; Taddei, C.; Serra, C.A.; Anton, N.; Kassem, M.; Schmutz, M.; Er-Rafik, M.; Messaddeq, N.; et al. Microfluidic-Assisted Production of Size-Controlled Superparamagnetic Iron Oxide Nanoparticles-Loaded Poly(methyl methacrylate) Nanohybrids. Langmuir 2018, 34, 1981–1991. [Google Scholar] [CrossRef]
- Hasani-Sadrabadi, M.M.; Dashtimoghadam, E.; Bahlakeh, G.; Majedi, F.S.; Keshvari, H.; Van Dersarl, J.J.; Bertsch, A.; Panahifar, A.; Renaud, P.; Tayebi, L.; et al. On-chip synthesis of fine-tuned bone-seeking hybrid nanoparticles. Nanomedicine 2015, 10, 3431–3449. [Google Scholar] [CrossRef] [PubMed]
- Javed, S.; Alshehri, S.; Shoaib, A.; Ahsan, W.; Sultan, M.H.; Alqahtani, S.S.; Shakeel, F. Microfluidic Electroporation-Facilitated Synthesis of Erythrocyte Membrane-Coated Magnetic Nanoparticles for Enhanced Imaging-Guided Cancer Therapy. ACS Nano 2017, 11, 3496–3505. [Google Scholar]
- Liu, C.; Zhang, W.; Li, Y.; Chang, J.; Tian, F.; Zhao, F.; Sun, J. Microfluidic Sonication To Assemble Exosome Membrane-Coated Nanoparticles for Immune Evasion-Mediated Targeting. Nano Lett. 2019, 19, 7836–7844. [Google Scholar] [CrossRef]
- Kumar, K.; Nightingale, A.M.; Krishnadasan, S.H.; Kamaly, N.; Wylenzinska-Arridge, M.; Zeissler, K.; Branford, W.R.; Ware, E.; deMello, A.J.; deMello, J.C. Direct synthesis of dextran-coated superparamagnetic iron oxide nanoparticles in a capillary-based droplet reactor. J. Mater. Chem. 2012, 22, 4704–4708. [Google Scholar] [CrossRef]
- Kašpar, O.; Koyuncu, A.H.; Hubatová-Vacková, A.; Balouch, M.; Tokárová, V. Influence of channel height on mixing efficiency and synthesis of iron oxide nanoparticles using droplet-based microfluidics. RSC Adv. 2020, 10, 15179–15189. [Google Scholar] [CrossRef]
- Yao, J.; Lin, F.; Kim, H.S.; Park, J. The Effect of Oil Viscosity on Droplet Generation Rate and Droplet Size in a T-Junction Microfluidic Droplet Generator. Micromachines 2019, 10, 808. [Google Scholar] [CrossRef] [Green Version]
- Ushikubo, F.Y.; Birribilli, F.S.; Oliveira, D.R.; Cunha, R.L. Y- and T-junction microfluidic devices: Effect of fluids and interface properties and operating conditions. Microfluid. Nanofluidics 2014, 17, 711–720. [Google Scholar] [CrossRef]
- Huang, D.; Wang, K.; Wang, Y.; Sun, H.; Liang, X.; Meng, T. Precise control for the size of droplet in T-junction microfluidic based on iterative learning method. J. Frankl. Inst. 2020, 357, 5302–5316. [Google Scholar] [CrossRef]
- Garstecki, P.; Fuerstman, M.J.; Stone, H.A.; Whitesides, G.M. Formation of droplets and bubbles in a microfluidic T-junction—scaling and mechanism of break-up. Lab Chip 2006, 6, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Xu, W.; Xu, F.; Lu, W.; Hu, L.; Zhou, J.; Zhang, C.; Jiang, Z. Microfluidic droplet formation in co-flow devices fabricated by micro 3D printing. J. Food Eng. 2021, 290, 110212. [Google Scholar] [CrossRef]
- Cramer, C.; Fischer, P.; Windhab, E.J. Drop formation in a co-flowing ambient fluid. Chem. Eng. Sci. 2004, 59, 3045–3058. [Google Scholar] [CrossRef]
- Yin, Z.; Huang, Z.; Lin, X.; Gao, X.; Bao, F. Droplet Generation in a Flow-Focusing Microfluidic Device with External Mechanical Vibration. Micromachines 2020, 11, 743. [Google Scholar] [CrossRef]
- Dewandre, A.; Rivero-Rodriguez, J.; Vitry, Y.; Sobac, B.; Scheid, B. Microfluidic droplet generation based on non-embedded co-flow-focusing using 3D printed nozzle. Sci. Rep. 2020, 10, 1–17. [Google Scholar] [CrossRef]
- Yaghmur, A.; Ghazal, A.; Ghazal, R.; Dimaki, M.; Svendsen, W.E. A hydrodynamic flow focusing microfluidic device for the continuous production of hexosomes based on docosahexaenoic acid monoglyceride. Phys. Chem. Chem. Phys. 2019, 21, 13005–13013. [Google Scholar] [CrossRef] [PubMed]
- Lashkaripour, A.; Rodriguez, C.; Mehdipour, N.; Mardian, R.; McIntyre, D.; Ortiz, L.; Campbell, J.; Densmore, D. Machine learning enables design automation of microfluidic flow-focusing droplet generation. Nat. Commun. 2021, 12, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Salazar-Alvarez, G.; Muhammed, M.; Zagorodni, A.A. Novel flow injection synthesis of iron oxide nanoparticles with narrow size distribution. Chem. Eng. Sci. 2006, 61, 4625–4633. [Google Scholar] [CrossRef]
- Bemetz, J.; Wegemann, A.; Saatchi, K.; Haase, A.; Häfeli, U.O.; Niessner, R.; Gleich, B.; Seidel, M. Microfluidic-Based Synthesis of Magnetic Nanoparticles Coupled with Miniaturized NMR for Online Relaxation Studies. Anal. Chem. 2018, 90, 9975–9982. [Google Scholar] [CrossRef] [PubMed]
- Siavashy, S.; Soltani, M.; Ghorbani-Bidkorbeh, F.; Fallah, N.; Farnam, G.; Mortazavi, S.A.; Shirazi, F.H.; Tehrani, M.H.; Hamedi, M.H. Microfluidic platform for synthesis and optimization of chitosan-coated magnetic nanoparticles in cisplatin delivery. Carbohydr. Polym. 2021, 265, 118027. [Google Scholar] [CrossRef] [PubMed]
- Frenz, L.; El Harrak, A.; Pauly, M.; Griffiths, A.D.; Baret, J.-C.; Bégin-Colin, S. Droplet-Based Microreactors for the Synthesis of Magnetic Iron Oxide Nanoparticles. Angew. Chem. Int. Ed. 2008, 47, 6817–6820. [Google Scholar] [CrossRef]
- Wahab, M.A.; Erdem, E.Y. Chitosan Coated Iron-Oxide Nanoparticle Synthesis Using a Droplet Based Microfluidic Reactor. In Proceedings of the 20th International Conference on Solid-State Sensors, Actuators and Microsystems & Eurosensors XXXIII (Transducers & Eurosensors XXXIII), Berlin, Germany, 23–27 June 2019; pp. 2330–2332. [Google Scholar]
- Jiao, M.; Zeng, J.; Jing, L.; Liu, C.; Gao, M. Flow Synthesis of Biocompatible Fe3O4 Nanoparticles: Insight into the Effects of Residence Time, Fluid Velocity, and Tube Reactor Dimension on Particle Size Distribution. Chem. Mater. 2015, 27, 1299–1305. [Google Scholar] [CrossRef]
- Abou Hassan, A.; Sandre, O.; Cabuil, V.; Tabeling, P. Synthesis of iron oxide nanoparticles in a microfluidic device: Preliminary results in a coaxial flow millichannel. Chem. Commun. 2008, 15, 1783–1785. [Google Scholar] [CrossRef] [Green Version]
- Abou-Hassan, A.; Neveu, S.; Dupuis, V.; Cabuil, V. Synthesis of cobalt ferrite nanoparticles in continuous-flow microreactors. RSC Adv. 2012, 2, 11263–11266. [Google Scholar] [CrossRef]
- Suryawanshi, L.; Sonawane, H.; Bhanvase, A.; Ashokkumar, M.; Pimplapure, S.; Gogate, R. Synthesis of iron oxide nanoparticles in a continuous flow spiral microreactor and Corning® advanced flow™ reactor. Green Process. Synth. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Abou-Hassan, A.; Bazzi, R.; Cabuil, V. Multistep continuous-flow microsynthesis of magnetic and fluorescent gamma-Fe2O3@SiO2 core/shell nanoparticles. Angew. Chem. Int. Ed. Engl. 2009, 48, 7180–7183. [Google Scholar] [CrossRef]
- Forigua, A.; Kirsch, R.L.; Willerth, S.M.; Elvira, K.S. Recent advances in the design of microfluidic technologies for the manufacture of drug releasing particles. J. Control Release 2021, 333, 258–268. [Google Scholar] [CrossRef]
- Liu, D.; Zhang, H.; Herranz-Blanco, B.; Mäkilä, E.; Lehto, V.-P.; Salonen, J.; Hirvonen, J.; Santos, H.A. Microfluidic Assembly of Monodisperse Multistage pH-Responsive Polymer/Porous Silicon Composites for Precisely Controlled Multi-Drug Delivery. Small 2014, 10, 2029–2038. [Google Scholar] [CrossRef]
- Maher, S.; Santos, A.; Kumeria, T.; Kaur, G.; Lambert, M.; Forward, P.; Evdokiou, A.; Losic, D. Multifunctional microspherical magnetic and pH responsive carriers for combination anticancer therapy engineered by droplet-based microfluidics. J. Mater. Chem. B. 2017, 5, 4097–4109. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Ju, X.-J.; Zou, X.-Y.; Xie, R.; Wang, W.; Liu, Y.-M.; Chu, L.-Y. Multi-Stimuli-Responsive Microcapsules for Adjustable Controlled-Release. Adv. Funct. Mater. 2014, 24, 3312–3323. [Google Scholar] [CrossRef]
- Hwang, D.K.; Dendukuri, D.; Doyle, P.S. Microfluidic-based synthesis of non-spherical magnetic hydrogel microparticles. Lab Chip 2008, 8, 1640–1647. [Google Scholar] [CrossRef] [PubMed]
- Macosko, E.Z.; Basu, A.; Satija, R.; Nemesh, J.; Shekhar, K.; Goldman, M.; Tirosh, I.; Bialas, A.R.; Kamitaki, N.; Martersteck, E.M.; et al. Highly Parallel Genome-wide Expression Profiling of Individual Cells Using Nanoliter Droplets. Cell 2015, 161, 1202–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mantri, M.; Scuderi, G.J.; Abedini-Nassab, R.; Wang, M.F.Z.; McKellar, D.; Shi, H.; Grodner, B.; Butcher, J.T.; De Vlaminck, I. Spatiotemporal single-cell RNA sequencing of developing chicken hearts identifies interplay between cellular differentiation and morphogenesis. Nat. Commun. 2021, 12, 1771. [Google Scholar] [CrossRef]
- Zhang, X.; Li, T.; Liu, F.; Chen, Y.; Yao, J.; Li, Z.; Huang, Y.; Wang, J. Comparative Analysis of Droplet-Based Ultra-High.-Throughput Single-Cell RNA-Seq Systems. Mol. Cell 2019, 73, 130–142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Zhang, C.; You, S.; Liu, H.; Zhang, L.; Liu, W.; Guo, S.-S.; Zhao, X.-Z. Microfluidic synthesis of multiferroic Janus particles with disk-like compartments. Appl. Phys. Lett. 2016, 108, 073504. [Google Scholar] [CrossRef]
- Ma, J.; Lee, S.M.; Yi, C.; Li, C.W. Controllable synthesis of functional nanoparticles by microfluidic platforms for biomedical applications—a review. Lab Chip 2017, 17, 209–226. [Google Scholar] [CrossRef] [PubMed]
- Di Carlo, D.; Wu, L.Y.; Lee, L.P. Dynamic single cell culture array. Lab Chip 2006, 6, 1445–1449. [Google Scholar] [CrossRef]
- Narayanamurthy, V.; Nagarajan, S.; Firus Khan, A.a.Y.; Samsuri, F.; Sridhar, T.M. Microfluidic hydrodynamic trapping for single cell analysis: Mechanisms, methods and applications. Anal. Methods 2017, 9, 3751–3772. [Google Scholar] [CrossRef]
- Sohrabi Kashani, A.; Packirisamy, M. Efficient Low Shear Flow-based Trapping of Biological Entities. Sci. Rep. 2019, 9, 5511. [Google Scholar] [CrossRef] [Green Version]
- Saucedo-Espinosa, M.A.; LaLonde, A.; Gencoglu, A.; Romero-Creel, M.F.; Dolas, J.R.; Lapizco-Encinas, B.H. Dielectrophoretic manipulation of particle mixtures employing asymmetric insulating posts. Electrophoresis 2016, 37, 282–290. [Google Scholar] [CrossRef]
- Yafouz, B.; Kadri, N.A.; Ibrahim, F. Dielectrophoretic manipulation and separation of microparticles using microarray dot electrodes. Sensors 2014, 14, 6356–6369. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.; Ding, W.; Nieto-Vesperinas, M.; Ding, X.; Rahman, M.; Zhang, T.; Lim, C.; Qiu, C.W. Optical manipulation from the microscale to the nanoscale: Fundamentals, advances and prospects. Light Sci. Appl. 2017, 6, e17039. [Google Scholar] [CrossRef] [PubMed]
- Bruce, G.D.; Rodríguez-Sevilla, P.; Dholakia, K. Initiating revolutions for optical manipulation: The origins and applications of rotational dynamics of trapped particles. Adv. Phys. X 2021, 6, 1838322. [Google Scholar]
- Gong, Z.; Pan, Y.-L.; Videen, G.; Wang, C. Optical trapping and manipulation of single particles in air: Principles, technical details, and applications. J. Quant. Spectrosc. Radiat. Transf. 2018, 214, 94–119. [Google Scholar] [CrossRef]
- Baudoin, M.; Thomas, J.L.; Sahely, R.A.; Gerbedoen, J.C.; Gong, Z.; Sivery, A.; Matar, O.B.; Smagin, N. Spatially selective manipulation of cells with single-beam acoustical tweezers. Nat. Commun. 2020, 11, 4244. [Google Scholar] [CrossRef]
- Ohiri, K.A.; Kelly, S.T.; Motschman, J.D.; Lin, K.H.; Wood, K.C.; Yellen, B.B. An acoustofluidic trap and transfer approach for organizing a high density single cell array. Lab Chip 2018, 18, 2124–2133. [Google Scholar] [CrossRef]
- Gómez-Pastora, J.; Karampelas, I.H.; Bringas, E.; Furlani, E.P.; Ortiz, I. Numerical Analysis of Bead Magnetophoresis from Flowing Blood in a Continuous-Flow Microchannel: Implications to the Bead-Fluid Interactions. Sci. Rep. 2019, 9, 7265. [Google Scholar] [CrossRef] [Green Version]
- Cao, Q.; Han, X.; Li, L. Numerical analysis of magnetic nanoparticle transport in microfluidic systems under the influence of permanent magnets. J. Phys. D Appl. Phys. 2012, 45, 465001. [Google Scholar] [CrossRef]
- Wu, J.; Yan, Q.; Xuan, S.; Gong, X. Size-selective separation of magnetic nanospheres in a microfluidic channel. Microfluid. Nanofluidics 2017, 21, 47. [Google Scholar] [CrossRef]
- Munir, A.; Zhu, Z.; Wang, J.; Zhou, H.S. Experimental investigation of magnetically actuated separation using tangential microfluidic channels and magnetic nanoparticles. IET Nanobiotechnol. 2014, 8, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-H.; Lien, K.-Y.; Wu, J.-J.; Lee, G.-B. A magnetic bead-based assay for the rapid detection of methicillin-resistant Staphylococcus aureus by using a microfluidic system with integrated loop-mediated isothermal amplification. Lab Chip 2011, 11, 1521–1531. [Google Scholar] [CrossRef]
- Shanko, E.S.; van de Burgt, Y.; Anderson, P.D.; den Toonder, J.M.J. Microfluidic Magnetic Mixing at Low Reynolds Numbers and in Stagnant Fluids. Micromachines 2019, 10, 731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Yang, L.; Zhang, L. Pattern generation and motion control of a vortex-like paramagnetic nanoparticle swarm. Int. J. Robot. Res. 2018, 37, 912–930. [Google Scholar] [CrossRef]
- Yu, J.; Wang, B.; Du, X.; Wang, Q.; Zhang, L. Ultra-extensible ribbon-like magnetic microswarm. Nat. Commun. 2018, 9, 3260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Y.; Wu, H.; Xiong, Q.; Ji, B.; Yi, H.; Duan, H.; Mao, H. Size-Controllable Magnetic Iron Oxide Nanorods for Biomarker Targeting and Improving Microfluidic Mixing. ACS Appl. Bio. Mater. 2019, 2, 3362–3371. [Google Scholar] [CrossRef]
- Gires, Y.; Thampi, M.; Weiss, M. Miniaturized magnetic stir bars for controlled agitation of aqueous microdroplets. Sci. Rep. 2020, 10, 10911. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.S.; Lee, H.; Westervelt, R.M. Microelectromagnets for the control of magnetic nanoparticles. Appl. Phys. Lett. 2001, 79, 3308–3310. [Google Scholar] [CrossRef]
- Silverio, V.; Amaral, M.; Gaspar, J.; Cardoso, S.; Freitas, P.P. Manipulation of Magnetic Beads with Thin Film Microelectromagnet Traps. Micromachines 2019, 10, 607. [Google Scholar] [CrossRef] [Green Version]
- Lefebvre, O.; Cao, H.H.; Cortés Francisco, M.; Woytasik, M.; Dufour-Gergam, E.; Ammar, M. Reusable Embedded Microcoils for Magnetic Nano-Beads Trapping in Microfluidics: Magnetic Simulation and Experiments. Micromachines 2020, 11, 257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lefebvre, O.; Smadja, C.; Martincic, E.; Woytasik, M.; Ammar, M. Integration of microcoils for on-chip immunosensors based on magnetic nanoparticles capture. Sens. Bio-Sensing Res. 2017, 13, 115–121. [Google Scholar] [CrossRef] [Green Version]
- Devkota, J.; Kokkinis, G.; Berris, T.; Jamalieh, M.; Cardoso, S.; Cardoso, F.; Srikanth, H.; Phan, M.H.; Giouroudi, I. A novel approach for detection and quantification of magnetic nanomarkers using a spin valve GMR-integrated microfluidic sensor. RSC Adv. 2015, 5, 51169–51175. [Google Scholar] [CrossRef]
- Gooneratne, C.P.; Kodzius, R.; Li, F.; Foulds, I.G.; Kosel, J. On-Chip Magnetic Bead Manipulation and Detection Using a Magnetoresistive Sensor-Based Micro-Chip: Design Considerations and Experimental Characterization. Sensors 2016, 16, 1369. [Google Scholar] [CrossRef] [PubMed]
- Silverio, V.; López-Martínez, M.J.; Franco, F.; Amaral, M.; Gaspar, J.; Cardoso, S.; Freitas, P.P. On-Chip Magnetic Nanoparticle Manipulation and Trapping for Biomedical Applications. IEEE Trans. Magn. 2017, 53, 1–6. [Google Scholar] [CrossRef]
- Donolato, M.; Dalslet, B.T.; Hansen, M.F. Microstripes for transport and separation of magnetic particles. Biomicrofluidics 2012, 6, 24110–241106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monticelli, M.; Torti, A.; Cantoni, M.; Petti, D.; Albisetti, E.; Manzin, A.; Guerriero, E.; Sordan, R.; Gervasoni, G.; Carminati, M.; et al. On-Chip Magnetic Platform for Single-Particle Manipulation with Integrated Electrical Feedback. Small 2016, 12, 921–929. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, E.; Beach, G.S.D. Architecture for Directed Transport of Superparamagnetic Microbeads in a Magnetic Domain Wall Routing Network. Sci. Rep. 2017, 7, 10139. [Google Scholar] [CrossRef]
- Lim, B.; Reddy, V.; Hu, X.; Kim, K.; Jadhav, M.; Abedini-Nassab, R.; Noh, Y.W.; Lim, Y.T.; Yellen, B.B.; Kim, C. Magnetophoretic circuits for digital control of single particles and cells. Nat. Commun. 2014, 5, 3846. [Google Scholar] [CrossRef] [Green Version]
- Abedini-Nassab, R.; Joh, D.Y.; Van Heest, M.A.; Yi, J.S.; Baker, C.; Taherifard, Z.; Margolis, D.M.; Garcia, J.V.; Chilkoti, A. Characterizing the Switching Thresholds of Magnetophoretic Transistors. Adv. Mater. 2015, 27, 6176–6180. [Google Scholar] [CrossRef]
- Abedini-Nassab, R. Magnetophoretic Circuit Biocompatibility. J. Mech. Med. Biol. 2020, 20, 2050050. [Google Scholar] [CrossRef]
- Hu, X.; Abedini-Nassab, R.; Lim, B.; Yang, Y.; Howdyshell, M.; Sooryakumar, R.; Yellen, B.B.; Kim, C. Dynamic trajectory analysis of superparamagnetic beads driven by on-chip micromagnets. J. Appl. Phys. 2015, 118, 203904. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedini-Nassab, R.; Shourabi, R. Bends in magnetophoretic conductors. AIP Adv. 2019, 9, 125121. [Google Scholar] [CrossRef]
- Abedini-Nassab, R. Magnetomicrofluidic Platforms for Organizing Arrays of Single-Particles and Particle-Pairs. J. Microelectromechanical Syst. 2019, 28, 732–738. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Mahdaviyan, N. A Microfluidic Platform Equipped With Magnetic Nano Films for Organizing Bio-Particle Arrays and Long-Term Studies. IEEE Sens. J. 2020, 20, 9668–9676. [Google Scholar] [CrossRef]
- Hu, X.; Lim, B.; Torati, S.R.; Ding, J.; Novosad, V.; Im, M.Y.; Reddy, V.; Kim, K.; Jung, E.; Shawl, A.I.; et al. Autonomous Magnetic Microrobots by Navigating Gates for Multiple Biomolecules Delivery. Small 2018, 14, e1800504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Lim, B.; Yoon, J.; Kim, K.; Torati, S.R.; Kim, C. Magnetophoretic Decoupler for Disaggregation and Interparticle Distance Control. Adv. Sci. 2021, 8, 2100532. [Google Scholar] [CrossRef]
- Abedini-Nassab, R.; Joh, D.Y.; Van Heest, M.; Baker, C.; Chilkoti, A.; Murdoch, D.M.; Yellen, B.B. Magnetophoretic Conductors and Diodes in a 3D Magnetic Field. Adv. Funct. Mater. 2016, 26, 4026–4034. [Google Scholar] [CrossRef] [Green Version]
- Abedini-Nassab, R.; Joh, D.Y.; Albarghouthi, F.; Chilkoti, A.; Murdoch, D.M.; Yellen, B.B. Magnetophoretic transistors in a tri-axial magnetic field. Lab Chip 2016, 16, 4181–4188. [Google Scholar] [CrossRef] [Green Version]
- Abedini-Nassab, R.; Bahrami, S. Synchronous control of magnetic particles and magnetized cells in a tri-axial magnetic field. Lab Chip 2021, 21, 1998–2007. [Google Scholar] [CrossRef]
- Chiang, C.-Y.; Jeng, J.-T.; Lai, B.-L.; Luong, V.S.; Lu, C.-C. Tri-axis magnetometer with in-plane giant magnetoresistance sensors for compass application. J. Appl. Phys. 2015, 117, 17A321. [Google Scholar] [CrossRef]
- Li, W.; Wang, J. Magnetic Sensors for Navigation Applications: An. Overview. J. Navig. 2014, 67, 263–275. [Google Scholar] [CrossRef]
- Včelák, J.; Ripka, P.; Zikmund, A. Precise Magnetic Sensors for Navigation and Prospection. J. Supercond. Nov. Magn. 2015, 28, 1077–1080. [Google Scholar] [CrossRef]
- Jogschies, L.; Klaas, D.; Kruppe, R.; Rittinger, J.; Taptimthong, P.; Wienecke, A.; Rissing, L.; Wurz, M.C. Recent Developments of Magnetoresistive Sensors for Industrial Applications. Sensors 2015, 15, 28665–28689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jia, Z.; Misra, R.D.K. Magnetic sensors for data storage: Perspective and future outlook. Mater. Technol. 2011, 26, 191–199. [Google Scholar] [CrossRef]
- Lόpez-Mir, L.; Frontera, C.; Aramberri, H.; Bouzehouane, K.; Cisneros-Fernández, J.; Bozzo, B.; Balcells, L.; Martínez, B. Anisotropic sensor and memory device with a ferromagnetic tunnel barrier as the only magnetic element. Sci. Rep. 2018, 8, 861. [Google Scholar] [CrossRef] [PubMed]
- Lin, G.; Makarov, D.; Schmidt, O.G. Magnetic sensing platform technologies for biomedical applications. Lab Chip 2017, 17, 1884–1912. [Google Scholar] [CrossRef]
- Gaster, R.S.; Xu, L.; Han, S.J.; Wilson, R.J.; Hall, D.A.; Osterfeld, S.J.; Yu, H.; Wang, S.X. Quantification of protein interactions and solution transport using high-density GMR sensor arrays. Nat. Nanotechnol. 2011, 6, 314–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, B.; Wang, K.; Xu, H.; Qin, Q.; Yang, J.; Zheng, W.; Jin, Q.; Cui, D. Development of magnetic sensor technologies for point-of-care testing: Fundamentals, methodologies and applications. Sens. Actuators A Phys. 2020, 312, 112130. [Google Scholar] [CrossRef]
- Murzin, D.; Mapps, D.J.; Levada, K.; Belyaev, V.; Omelyanchik, A.; Panina, L.; Rodionova, V. Ultrasensitive Magnetic Field Sensors f Ultrasensitive Magnetic Field Sensors for Biomedical Applications. Sensors 2020, 20, 1569. [Google Scholar] [CrossRef] [Green Version]
- De Ranieri, E.; Rushforth, A.W.; Výborný, K.; Rana, U.; Ahmad, E.; Campion, R.P.; Foxon, C.T.; Gallagher, B.L.; Irvine, A.C.; Wunderlich, J.; et al. Lithographically and electrically controlled strain effects on anisotropic magnetoresistance in (Ga,Mn)As. New J. Phys. 2008, 10, 065003. [Google Scholar] [CrossRef]
- Quynh, L.K.; Tu, B.D.; Dang, D.X.; Viet, D.Q.; Hien, L.T.; Huong Giang, D.T.; Duc, N.H. Detection of magnetic nanoparticles using simple AMR sensors in Wheatstone bridge. J. Sci. Adv. Mater. Devices 2016, 1, 98–102. [Google Scholar] [CrossRef] [Green Version]
- Hien, L.T.; Quynh, L.K.; Huyen, V.T.; Tu, B.D.; Hien, N.T.; Phuong, D.M.; Nhung, P.H.; Giang, D.T.H.; Duc, N.H. DNA-magnetic bead detection using disposable cards and the anisotropic magnetoresistive sensor. Advances in Natural Sciences. Nanosci. Nanotechnol. 2016, 7, 045006. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.; Du, W.; Yu, J.; Zhang, H.; Su, H. A flexible way to modulate the detection range of anisotropic magnetoresistance by exchange bias field. J. Magn. Magn. Mater. 2019, 476, 469–471. [Google Scholar] [CrossRef]
- Giouroudi, I.; Hristoforou, E. Perspective: Magnetoresistive sensors for biomedicine. J. Appl. Phys. 2018, 124, 030902. [Google Scholar] [CrossRef]
- Parkin, S.S.P.; More, N.; Roche, K.P. Oscillations in exchange coupling and magnetoresistance in metallic superlattice structures: Co/Ru, Co/Cr, and Fe/Cr. Phys. Rev. Lett. 1990, 64, 2304–2307. [Google Scholar] [CrossRef]
- Wang, T.; Chen, Y.; Wang, B.; He, Y.; Li, H.; Liu, M.; Rao, J.; Wu, Z.; Xie, S.; Luo, J. A giant magnetoimpedance-based separable-type method for supersensitive detection of 10 magnetic beads at high frequency. Sens. Actuators A Phys. 2019, 300, 111656. [Google Scholar] [CrossRef]
- Su, D.; Wu, K.; Krishna, V.D.; Klein, T.; Liu, J.; Feng, Y.; Perez, A.M.; Cheeran, M.C.; Wang, J.P. Detection of Influenza a Virus in Swine Nasal Swab Samples With a Wash-Free Magnetic Bioassay and a Handheld Giant Magnetoresistance Sensing System. Front. Microbiol. 2019, 10, 1077. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Wang, W.; Yu, L.; Wu, K.; Boylan, K.L.M.; Vogel, R.I.; Skubitz, A.P.N.; Wang, J.P. Development of a multiplexed giant magnetoresistive biosensor array prototype to quantify ovarian cancer biomarkers. Biosens. Bioelectron. 2019, 126, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Ng, E.; Le, A.K.; Nguyen, M.H.; Wang, S.X. Early Multiplexed Detection of Cirrhosis using Giant Magnetoresistive Biosensors with Protein Biomarkers. ACS Sens. 2020, 5, 3049–3057. [Google Scholar] [CrossRef] [PubMed]
- Fodil, K.; Denoual, M.; Dolabdjian, C.; Treizebre, A.; Senez, V. In-flow detection of ultra-small magnetic particles by an integrated giant magnetic impedance sensor. Appl. Phys. Lett. 2016, 108, 173701. [Google Scholar] [CrossRef]
- Lin, G.; Baraban, L.; Han, L.; Karnaushenko, D.; Makarov, D.; Cuniberti, G.; Schmidt, O.G. Magnetoresistive emulsion analyzer. Sci. Rep. 2013, 3, 2548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deak, J.G.; Zhou, Z.; Shen, W. Tunneling magnetoresistance sensor with pT level 1/f magnetic noise. AIP Adv. 2017, 7, 056676. [Google Scholar] [CrossRef]
- Lei, H.; Wang, K.; Ji, X.; Cui, D. Contactless Measurement of Magnetic Nanoparticles on Lateral Flow Strips Using Tunneling Magnetoresistance (TMR) Sensors in Differential Configuration. Sensors 2016, 16, 2130. [Google Scholar] [CrossRef] [Green Version]
- Albon, C.; Weddemann, A.; Auge, A.; Rott, K.; Hütten, A. Tunneling magnetoresistance sensors for high resolutive particle detection. Appl. Phys. Lett. 2009, 95, 023101. [Google Scholar] [CrossRef]
- Jin, Z.; Koo, T.M.; Kim, M.S.; Al-Mahdawi, M.; Oogane, M.; Ando, Y.; Kim, Y.K. Highly-sensitive magnetic sensor for detecting magnetic nanoparticles based on magnetic tunnel junctions at a low static field. AIP Adv. 2021, 11, 015046. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, Y.; Zhan, Q.; Liu, J.P.; Li, R.-W. Rapid detection of Escherichia coli O157:H7 using tunneling magnetoresistance biosensor. AIP Adv. 2017, 7, 056658. [Google Scholar] [CrossRef] [Green Version]
- Mu, X.-H.; Liu, H.-F.; Tong, Z.-Y.; Du, B.; Liu, S.; Liu, B.; Liu, Z.-W.; Gao, C.; Wang, J.; Dong, H. A new rapid detection method for ricin based on tunneling magnetoresistance biosensor. Sens. Actuators B Chem. 2019, 284, 638–649. [Google Scholar] [CrossRef]
- Sharma, P.; Albisetti, E.; Massetti, M.; Scolari, M.; La Torre, C.; Monticelli, M.; Leone, M.; Damin, F.; Gervasoni, G.; Ferrari, G.; et al. Integrated platform for detecting pathogenic DNA via magnetic tunneling junction-based biosensors. Sens. Actuators B Chem. 2017, 242, 280–287. [Google Scholar] [CrossRef] [Green Version]
- Ikeda, S.; Hayakawa, J.; Ashizawa, Y.; Lee, Y.M.; Miura, K.; Hasegawa, H.; Tsunoda, M. Tunnel magnetoresistance of 604% at 300K by suppression of Ta diffusion in CoFeB∕MgO∕CoFeB pseudo-spin-valves annealed at high temperature. Appl. Phys. Lett. 2008, 93, 082508. [Google Scholar] [CrossRef]
- Jaufenthaler, A.; Kornack, T.; Lebedev, V.; Limes, M.E.; Körber, R.; Liebl, M.; Baumgarten, D. Pulsed Optically Pumped Magnetometers: Addressing Dead Time and Bandwidth for the Unshielded Magnetorelaxometry of Magnetic Nanoparticles. Sensors 2021, 21, 1212. [Google Scholar] [CrossRef]
- Wiekhorst, F.; Steinhoff, U.; Eberbeck, D.; Trahms, L. Magnetorelaxometry assisting biomedical applications of magnetic nanoparticles. Pharm. Res. 2012, 29, 1189–1202. [Google Scholar] [CrossRef] [Green Version]
- Huang, C.-C.; Zhou, X.; Hall, D.A. Giant Magnetoresistive Biosensors for Time-Domain Magnetorelaxometry: A Theoretical Investigation and Progress toward an Immunoassay. Sci. Rep. 2017, 7, 45493. [Google Scholar] [CrossRef]
- Flynn, E.R. Magnetic Relaxometry: A Comparison to Magnetoencephalography. In Magnetoencephalography: From Signals to Dynamic Cortical Networks; Supek, S., Aine, C.J., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 1343–1355. [Google Scholar]
- Buchner, M.; Höfler, K.; Henne, B.; Ney, V.; Ney, A. Tutorial: Basic principles, limits of detection, and pitfalls of highly sensitive SQUID magnetometry for nanomagnetism and spintronics. J. Appl. Phys. 2018, 124, 161101. [Google Scholar] [CrossRef] [Green Version]
- Enpuku, K.; Minotani, T.; Gima, T.; Kuroki, Y.; Itoh, Y.; Yamashita, M.; Katakura, Y.; Kuhara, S. Detection of Magnetic Nanoparticles with Superconducting Quantum Interference Device (SQUID) Magnetometer and Application to Immunoassays. Jpn. J. Appl. Phys. 1999, 38, L1102–L1105. [Google Scholar] [CrossRef]
- Škrátek, M.; Dvurečenskij, A.; Kluknavský, M.; Barta, A.; Bališ, P.; Mičurová, A.; Cigáň, A.; Eckstein-Andicsová, A.; Maňka, J.; Bernátová, I. Sensitive SQUID Bio-Magnetometry for Determination and Differentiation of Biogenic Iron and Iron Oxide Nanoparticles in the Biological Samples. Nanomaterials 2020, 10, 1993. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Skucha, K.; Megens, M.; Boser, B. A CMOS Hall-Effect Sensor for the Characterization and Detection of Magnetic Nanoparticles for Biomedical Applications. IEEE Trans. Magn. 2011, 47, 3449–3451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, C.; Park, J.; Mun, J.K.; Lim, Y.; Min, J.; Lim, J.W.; Kang, D.M.; Ahn, H.K.; Shin, T.H.; Cheon, J.; et al. Integrated microHall magnetometer to measure the magnetic properties of nanoparticles. Lab Chip 2017, 17, 4000–4007. [Google Scholar] [CrossRef]
- Schütt, J.; Illing, R.; Volkov, O.; Kosub, T.; Granell, P.N.; Nhalil, H.; Fassbender, J.; Klein, L.; Grosz, A.; Makarov, D. Two Orders of Magnitude Boost in the Detection Limit of Droplet-Based Micro-Magnetofluidics with Planar Hall Effect Sensors. ACS Omega 2020, 5, 20609–20617. [Google Scholar] [CrossRef]
- Ender, F.; Weiser, D.; Vitéz, A.; Sallai, G.; Németh, M.; Poppe, L. In-situ measurement of magnetic nanoparticle quantity in a microfluidic device. Microsyst. Technol. 2017, 23, 3979–3990. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, C. Ferromagnetic Metal Particle Detection Including Calculation of Particle Magnetic Permeability Based on Micro Inductive Sensor. IEEE Sens. J. 2021, 21, 447–454. [Google Scholar] [CrossRef]
- Balk, A.L.; Mair, L.O.; Guo, F.; Hangarter, C.; Mathai, P.P.; McMichael, R.D.; Stavis, S.M.; Unguris, J. Quantitative magnetometry of ferromagnetic nanorods by microfluidic analytical magnetophoresis. J. Appl. Phys. 2015, 118, 093904. [Google Scholar] [CrossRef]
- Grob, D.T.; Wise, N.; Oduwole, O.; Sheard, S. Magnetic susceptibility characterisation of superparamagnetic microspheres. J. Magn. Magn. Mater. 2018, 452, 134–140. [Google Scholar] [CrossRef]
- Quynh, L.K.; Tu, B.D.; Anh, C.V.; Duc, N.H.; Phung, A.T.; Dung, T.T.; Giang, D.T.H. Design Optimization of an Anisotropic Magnetoresistance Sensor for Detection of Magnetic Nanoparticles. J. Electron. Mater. 2019, 48, 997–1004. [Google Scholar] [CrossRef]
- Fu, A.; Hu, W.; Xu, L.; Wilson, R.J.; Yu, H.; Osterfeld, S.J.; Gambhir, S.S.; Wang, S.X. Protein-functionalized synthetic antiferromagnetic nanoparticles for biomolecule detection and magnetic manipulation. Angew. Chem. Int. Ed. Engl. 2009, 48, 1620–1624. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, B.; Li, Y.; Jing, Y.; Xing, C.; Slaton, J.; Wang, J.-P. A Three-Layer Competition-Based Giant Magnetoresistive Assay for Direct Quantification of Endoglin from Human Urine. Anal. Chem. 2011, 83, 2996–3002. [Google Scholar] [CrossRef]
- Su, D.; Wu, K.; Wang, J.-P. Large-area GMR bio-sensors based on reverse nucleation switching mechanism. J. Magn. Magn. Mater. 2019, 473, 484–489. [Google Scholar] [CrossRef]
- Shen, W.; Schrag, B.D.; Carter, M.J.; Xie, J.; Xu, C.; Sun, S.; Xiao, G. Detection of DNA labeled with magnetic nanoparticles using MgO-based magnetic tunnel junction sensors. J. Appl. Phys. 2008, 103, 07A306. [Google Scholar] [CrossRef]
- Ludwig, F.; Mäuselein, S.; Heim, E.; Schilling, M. Magnetorelaxometry of magnetic nanoparticles in magnetically unshielded environment utilizing a differential fluxgate arrangement. Rev. Sci. Instrum. 2005, 76, 106102. [Google Scholar] [CrossRef]
- Johnson, C.; Adolphi, N.L.; Butler, K.L.; Debbie, M.L.; Larson, R.; Schwindt, P.D.; Flynn, E.R. Magnetic Relaxometry with an Atomic Magnetometer and SQUID Sensors on Targeted Cancer Cells. J. Magn. Magn. Mater. 2012, 324, 2613–2619. [Google Scholar] [CrossRef] [Green Version]



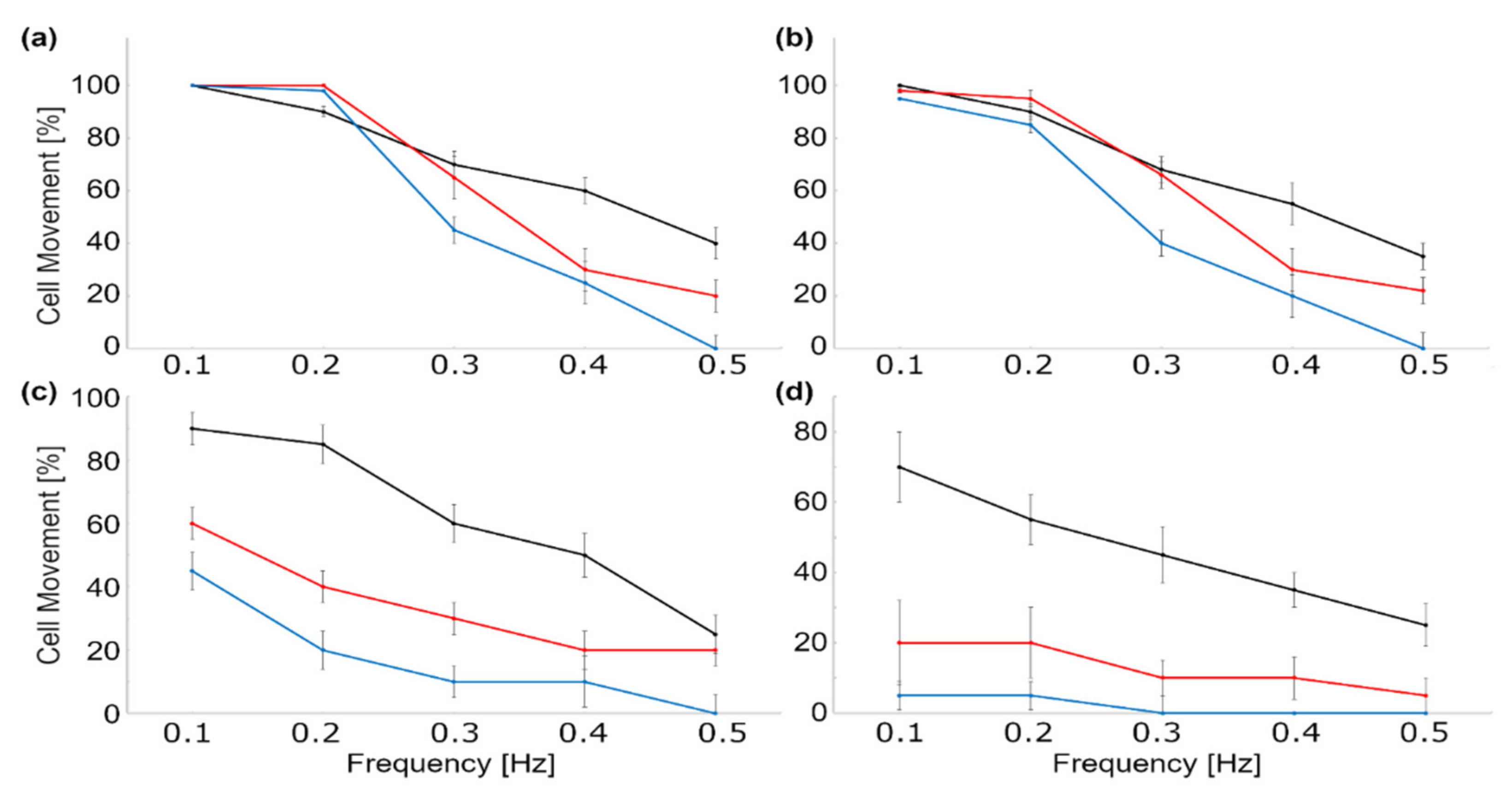

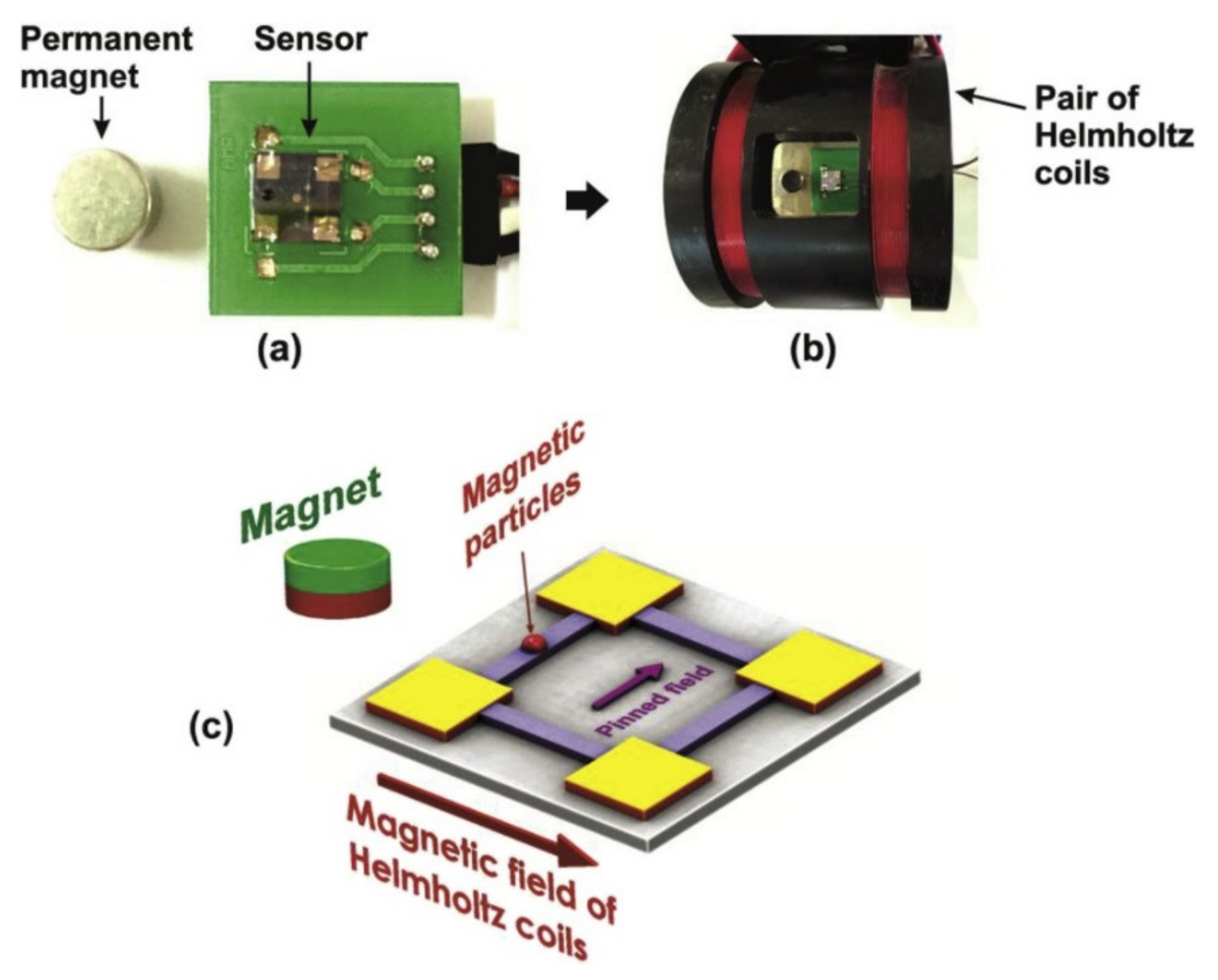
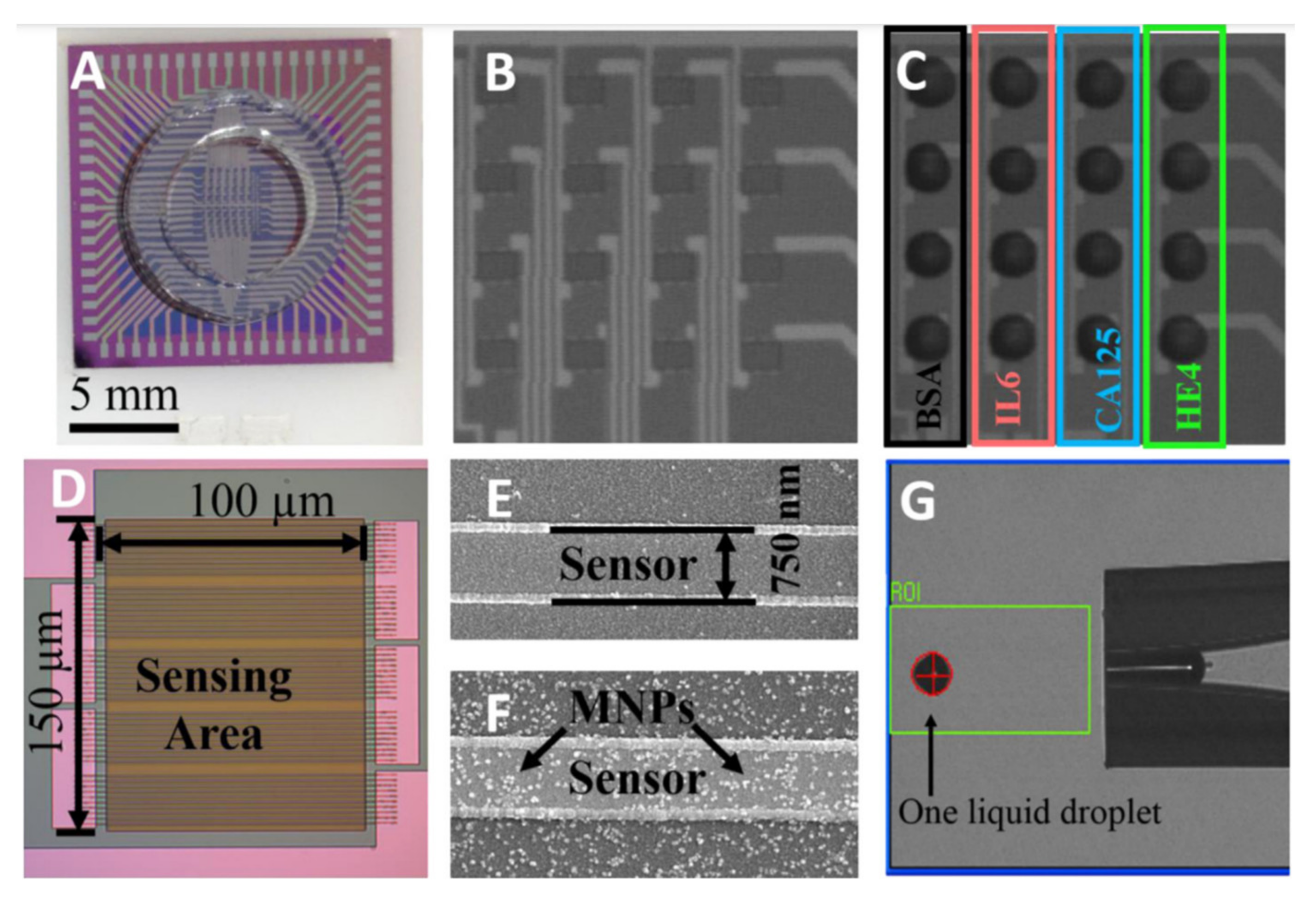
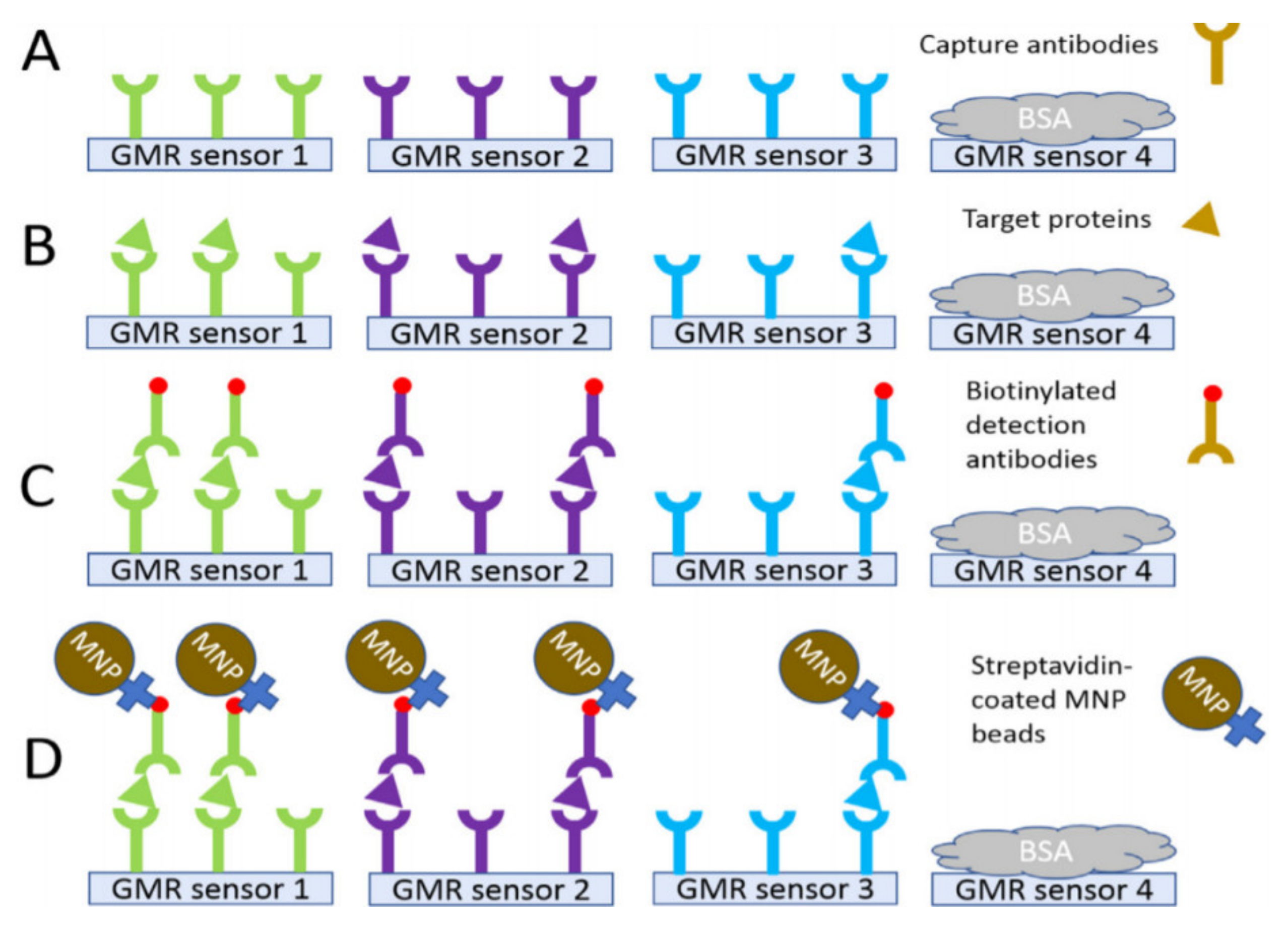

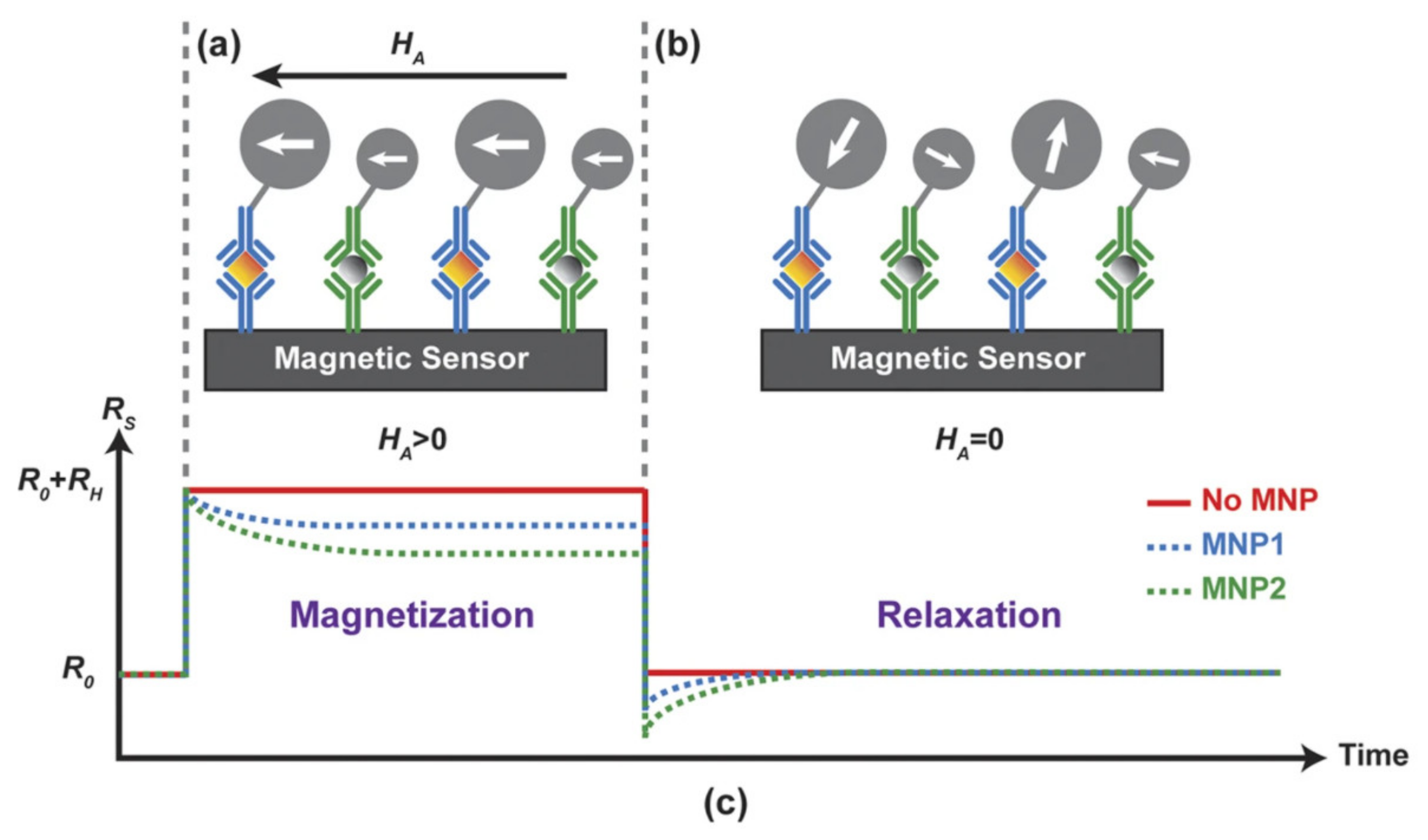
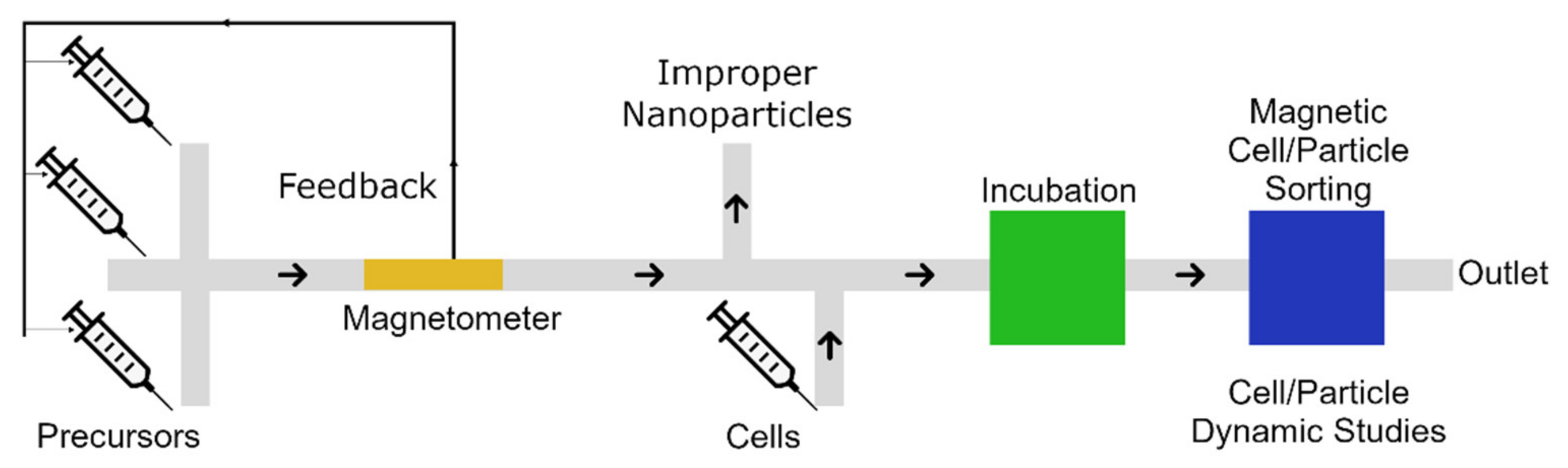
| Materials | Method | Size (nm) | Ref. |
|---|---|---|---|
| Fe3O4 | Continuous-flow | 5–6 | [59,60] |
| Fe3O4 | Droplet-based | 4 | [57] |
| Dextran coated Fe3O4 | Continuous-flow | 10 | [35] |
| CoFe2O4 | Continuous-flow | 6 | [61] |
| Fe2O3 | Continuous-flow | 6 | [62] |
| Fe2O3 | Droplet-based | 4.7–6 | [43] |
| γ-Fe2O3@SiO2 | Continuous-flow | 50 | [63] |
| Iron oxide @ chitosan | Continuous-flow | 190 | [31] |
| Iron oxide @ chitosan | Droplet-based | 104 | [56] |
| Synthesis Method | Advantages | Challenges |
|---|---|---|
| Conventional reactors | - Simple setup | - Low efficiency; - Poor control on parameters; - Low reproducibility; - Agglomeration. |
| Continuous-flow microreactors | - Simple design; - Relatively simple fabrication; - High throughput; - Good control over parameters, and change of parameters in microseconds; - Sufficient millisecond mixing; - Uniform particle size; - High reproducibility; - Large surface/volume ratio; - Low sample consumption (as low as nanoliters); - Potential for non-spherical particle synthesis; - Potential for automation. | - Channel clogging; - Limitation for heat required protocols; - Taylor dispersion effect; - Poor solvent compatibility; - Sometimes expensive tools. |
| Droplet-based microreactors | - High throughput (thousands per second); - Uniform and tunable particle size, with polydispersity index as low as 0.024 [73] and sizes of 3.6 nm up to the micrometer range; - Excellent control over parameters, and change of parameters in microseconds; - Sufficient millisecond mixing, followed by as low as 2 ms particle formation; - Very high reproducibility; - Large surface/volume ratio; - Very low sample consumption, (as low as picoliters); - Potential for the synthesis of complicated particles with shells; - Enclosed reaction environment; - Potential for automation. | - Poor solvent compatibility; - Sometimes expensive tools. |
| Manipulation Method | Advantages | Disadvantages |
|---|---|---|
| With external permanent magnet or electromagnet | - Simple design; - Low price, (as low as a few USD). | - Lack of precise control. |
| With embedded micro-wires and micro-coils | - Control on particles in various sections of the chip; - Independent of external coils or magnets. | - Lack of precise control over individual particles; - Complicated wiring system. |
| With embedded magnetic thin films | - Precise control over individual particles; - Potential to manipulate many individual particles in parallel (thousands of particles); - Potential for automation; - High throughput. | - Sometimes expensive (a few hundred USD). |
| Sensor | Advantages | Disadvantages | Examples |
|---|---|---|---|
| AMR | - Small operating field; - Linear operation; - Simple fabrication. | - Fragile at high temperatures; - Low MR ratio. | - 50 nm Fe3O4 chitosan nanoparticles were detected (detection limit: magnetic moments of 0.56 μemu) [160]. - 50 nm Fe3O4 chitosan nanoparticles were detected (detection limit of 1 μemu) [127]. |
| GMR | - Moderate MR ratio; - Simple fabrication; - Linear operation. | - Noise at low frequencies. | - 100 nm FeCo nanoparticles were used for detecting DNA with sensitivity of 10 pM [161]. - 200 nm magnetic/polymer beads were used to detect proteins (detection limitation of 7.4 pg/mL) [134]. - 12.8 nm FeCo nanoparticles were used for detecting endoglin (as few as 1000 copies and concentration of 83 fM) [162]. - 4.5 μm beads were detected (as few as 10 beads) [132]. - 50nm Fe2O3 nanoparticles were to detect Immunoglobulin G protein (140 ng/mL limitation) [163]. |
| TMR | - High MR value; - Low power consumption. | - Large noise; - Complicated fabrication. | - 250 nm streptavidin coated magnetic beads were used to detect DNA (sensitivity below the nM range) [144]. - 16 nm and 50 nm magnetic nanoparticles were used to detect 2.5 μM DNA with signal-to-noise ratios of 25 and 12, respectively [164]. - 200 nm Fe3O4 beads were detected (sensitivity to detect 500 ng at concentrations of 0.01 mg/m) [141]. |
| MRX | - Avoids the high dynamic range requirement; - Potential for detecting two different particles. | - Fe3O4 magnetic nanoparticles were detected (volume of 150μL, 100 nmol Fe) [165]. - 22.4 nm Fe3O4 magnetic nanoparticles were used to detect breast cancer cells (with ability to detect fewer than 100 thousands cells) [166]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abedini-Nassab, R.; Pouryosef Miandoab, M.; Şaşmaz, M. Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review. Micromachines 2021, 12, 768. https://doi.org/10.3390/mi12070768
Abedini-Nassab R, Pouryosef Miandoab M, Şaşmaz M. Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review. Micromachines. 2021; 12(7):768. https://doi.org/10.3390/mi12070768
Chicago/Turabian StyleAbedini-Nassab, Roozbeh, Mahrad Pouryosef Miandoab, and Merivan Şaşmaz. 2021. "Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review" Micromachines 12, no. 7: 768. https://doi.org/10.3390/mi12070768
APA StyleAbedini-Nassab, R., Pouryosef Miandoab, M., & Şaşmaz, M. (2021). Microfluidic Synthesis, Control, and Sensing of Magnetic Nanoparticles: A Review. Micromachines, 12(7), 768. https://doi.org/10.3390/mi12070768






