Studying the Complex Formation of Sulfonatocalix[4]naphthalene and Meloxicam towards Enhancing Its Solubility and Dissolution Performance
Abstract
:1. Introduction
2. Materials and Methods
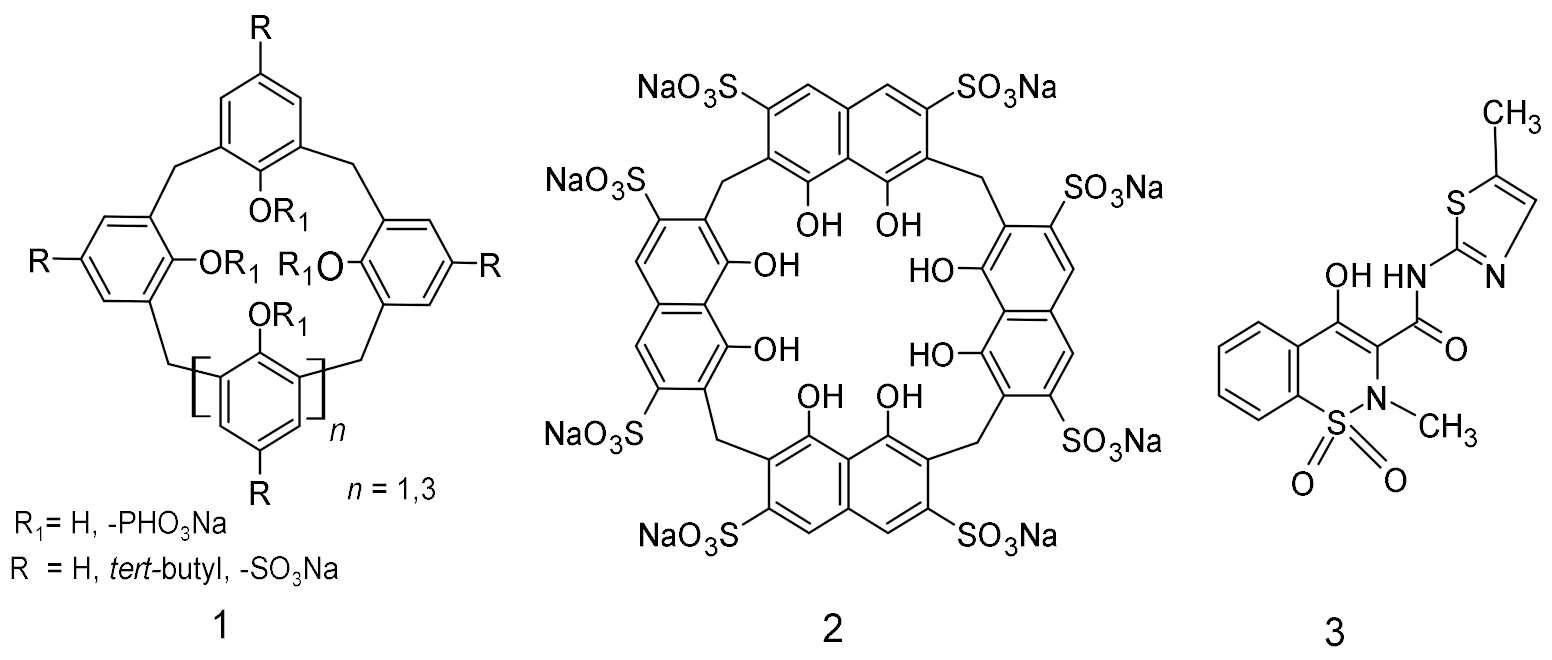
2.1. Materials
2.2. Methods
2.2.1. Phase Solubility Studies
2.2.2. Preparation of Inclusion Complexes
Physical Mixture
Kneading
Co-Evaporation
2.2.3. Characterization of Solid Systems
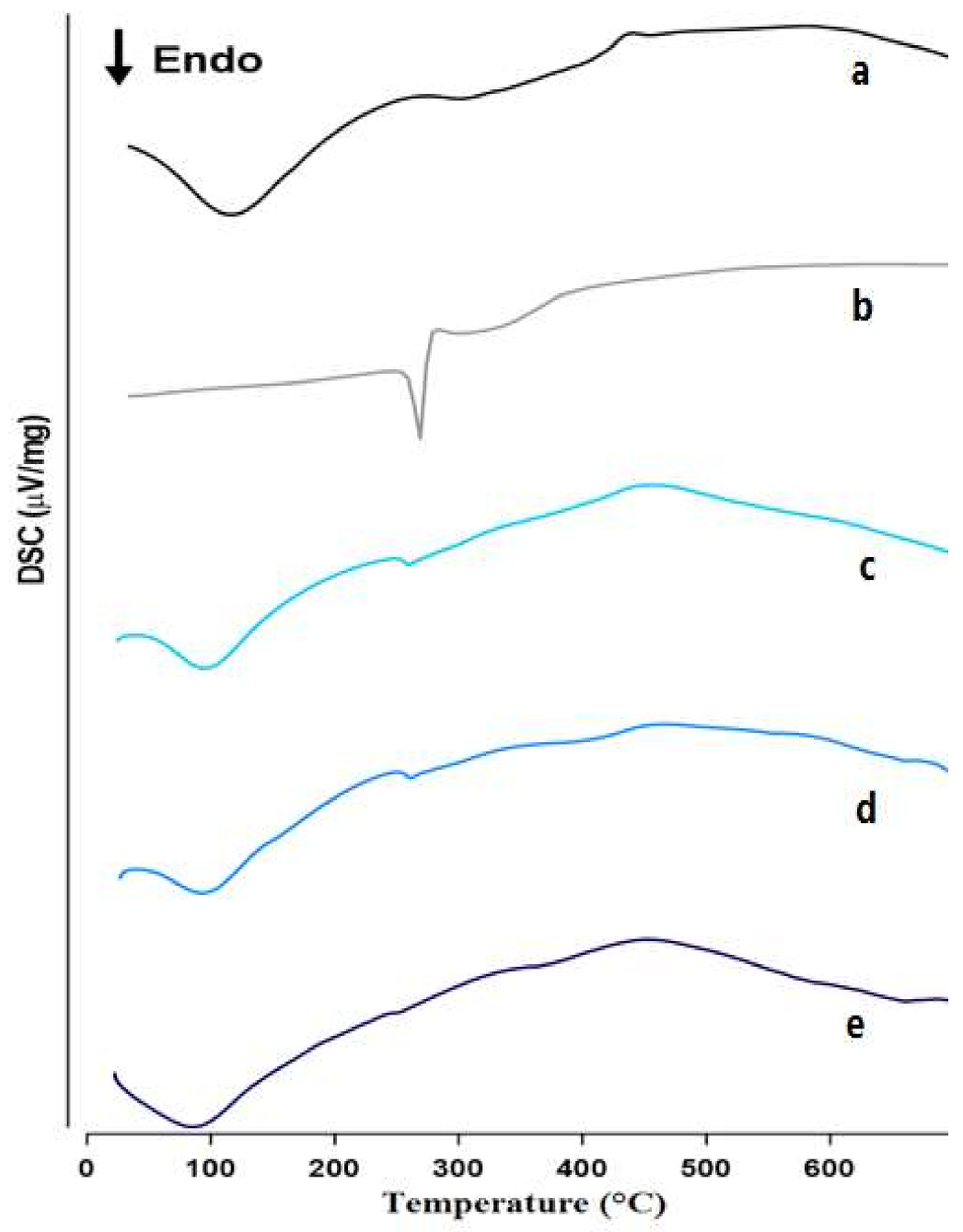
Differential Scanning Calorimetry (DSC)
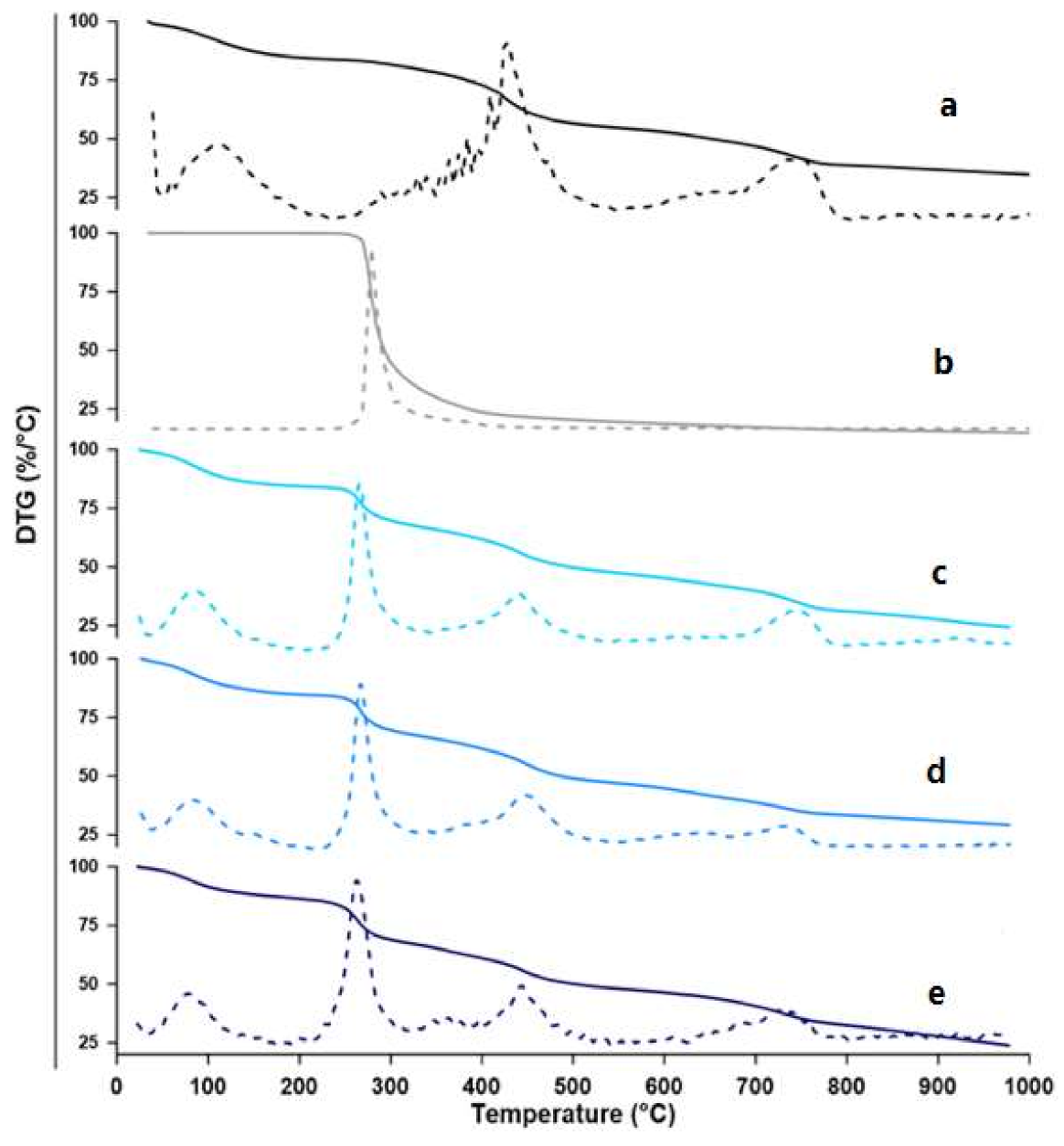
Thermogravimetric Analysis (TGA), and Derivative Thermogravimetry (DTG)
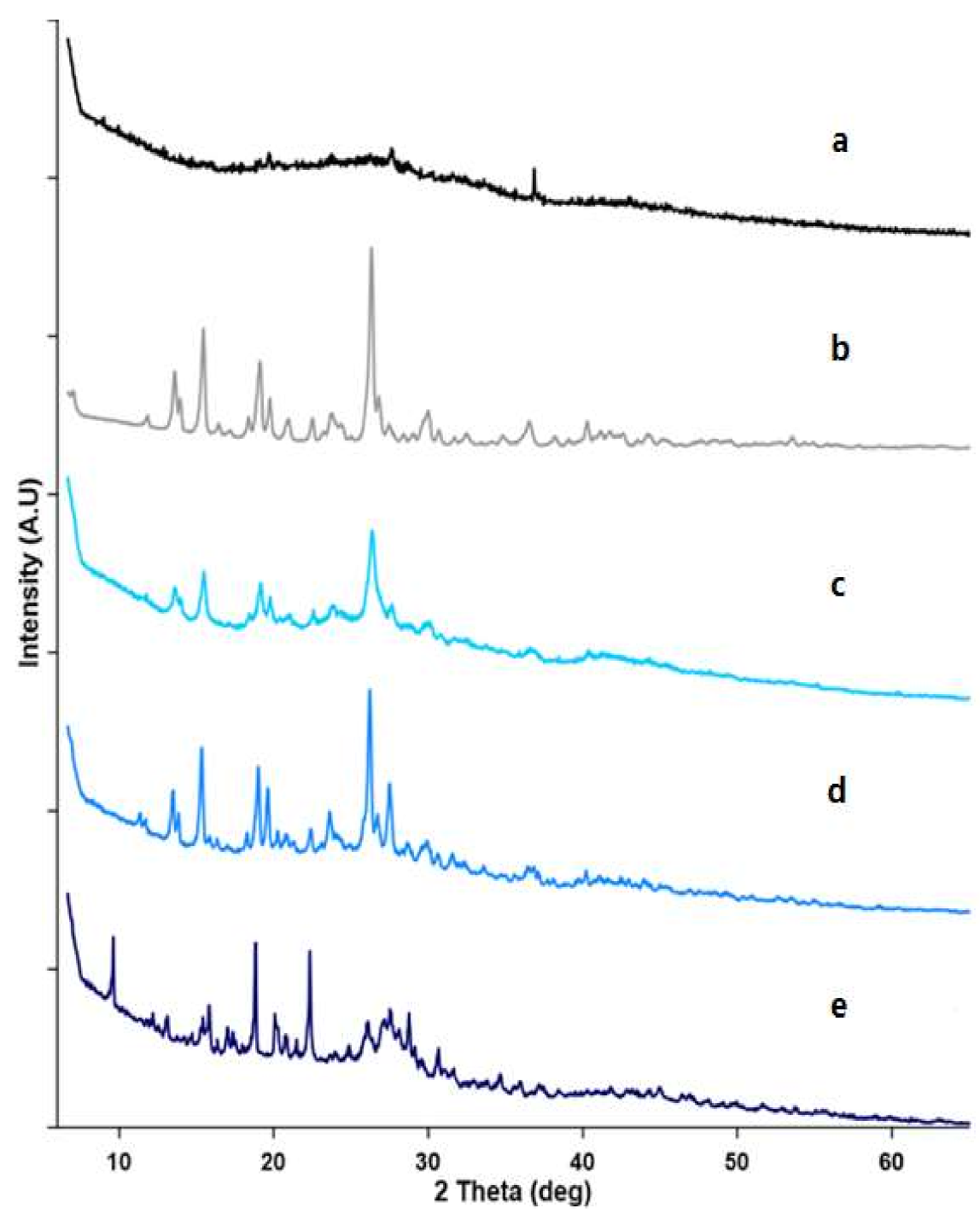
Powder X-ray Diffraction (PXRD)
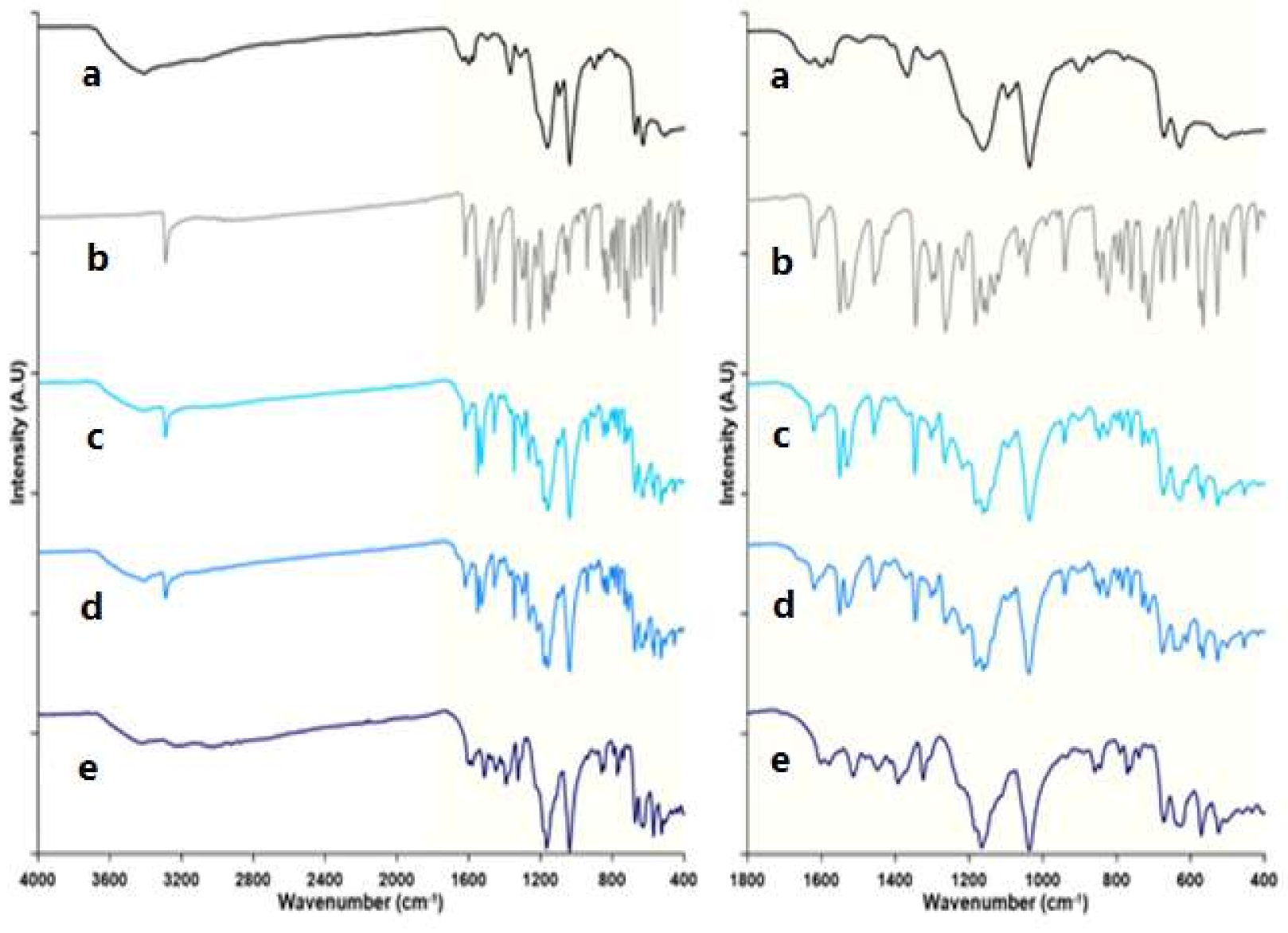
Fourier-Transform Infrared Spectroscopy (FT-IR)
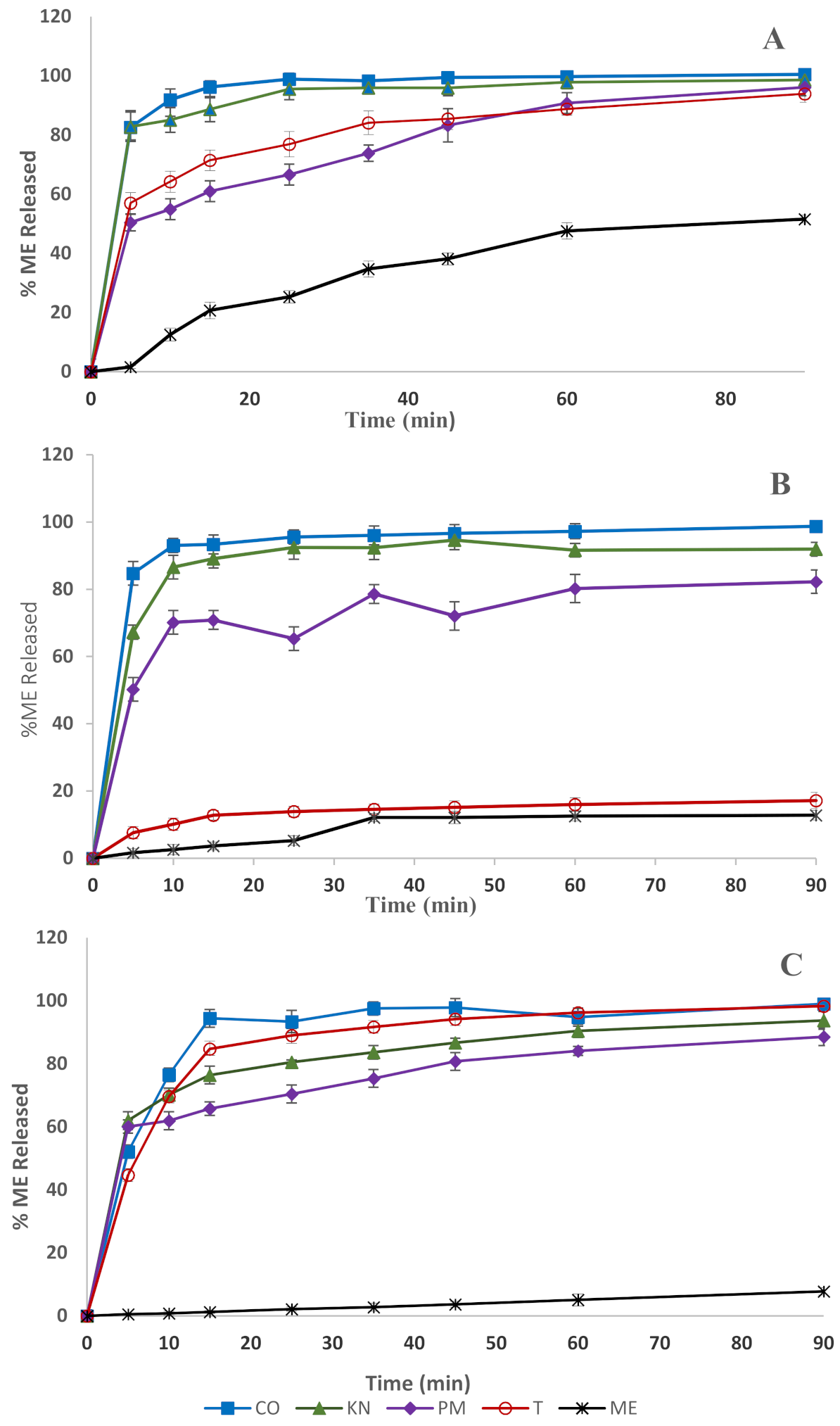
2.2.4. Dissolution Studies
2.2.5. Statistical Analysis
3. Results and Discussion
3.1. Phase Solubility Studies
3.2. Thermal Analysis
3.2.1. DSC
3.2.2. TGA
3.2.3. Powder X-ray Diffractometry (PXRD)
3.2.4. Infrared Spectroscopy
3.3. Dissolution Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Desiraju, G.R. Chemistry Beyond the Molecule. Nature 2001, 412, 397–400. [Google Scholar] [CrossRef] [PubMed]
- Amabilino, D.B.; Gale, P.A. Supramolecular Chemistry Anniversary. Chem. Soc. Rev. 2017, 46, 2376–2377. [Google Scholar] [CrossRef] [PubMed]
- Lu, D.; Huang, Q.; Wang, S.; Wang, J.; Huang, P.; Du, P. The Supramolecular Chemistry of Cycloparaphenylenes and Their Analogs. Front. Chem. 2019, 7, 668. [Google Scholar] [CrossRef] [PubMed]
- Mokhtari, B.; Pourabdollah, K. Applications of Calixarene Nano-Baskets in Pharmacology. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 1–15. [Google Scholar] [CrossRef]
- Chehardoli, G.; Bahmani, A. The Role of Crown Ethers in Drug Delivery. Supramol. Chem. 2019, 31, 221–238. [Google Scholar] [CrossRef]
- Liu, Y.; Han, B.H.; Chen, Y.T. Molecular Recognition and Complexation Thermodynamics of Dye Guest Molecules by Modified Cyclodextrins and Calixarenesulfonates. J. Phys. Chem. B 2002, 106, 4678–4687. [Google Scholar] [CrossRef]
- Gutsche, C.D. Properties of the Calixarenes from p-Tert-Butylphenol. J. Am. Chem. Soc. 1981, 103, 3782–3792. [Google Scholar] [CrossRef]
- Gutsche, C.D.; Nam, K.C. Calixarenes. 22. Synthesis, Properties, and Metal Complexation of Aminocalixarenes. J. Am. Chem. Soc. 1988, 110, 6153–6162. [Google Scholar] [CrossRef]
- Alhujran, T.A.; Dawe, L.N.; Georghiou, P.E. Synthesis of Functionalized Acenaphthenes and a New Class of Homooxacalixarenes. Org. Lett. 2012, 14, 3530–3533. [Google Scholar] [CrossRef]
- AlHujran, T.A.; Dawe, L.N.; Collins, J.; Georghiou, P.E. Synthesis and Clathrates of Oligomeric 2-O-Naphthoide Macrocycles. J. Org. Chem. 2011, 76, 971–973. [Google Scholar] [CrossRef]
- Bayrakc, M.; Ertul, Ş.; Yilmaz, M. Phase Solubility Studies of Poorly Soluble Drug Molecules by Using O -Phosphorylated Calixarenes as Drug-Solubilizing Agents. J. Chem. Eng. Data 2012, 57, 233–239. [Google Scholar] [CrossRef]
- Crini, G. Review: A History of Cyclodextrins. Chem. Rev. 2014, 114, 10940–10975. [Google Scholar] [CrossRef]
- Matencio, A.; Caldera, F.; Cecone, C.; López-nicolás, J.M.; Trotta, F. Cyclic Oligosaccharides as Active Drugs, an Updated Review. Pharmaceuticals 2020, 13, 281. [Google Scholar] [CrossRef]
- Carneiro, S.B.; Duarte, F.Í.C.; Heimfarth, L.; Quintans, J.D.S.S.; Quintans-Júnior, L.J.; Júnior, V.F.D.V.; De Lima, Á.A.N. Cyclodextrin-Drug Inclusion Complexes: In Vivo and in Vitro Approaches. Int. J. Mol. Sci. 2019, 20, 642. [Google Scholar] [CrossRef] [Green Version]
- Yang, W.; De Villiers, M.M. The Solubilization of the Poorly Water Soluble Drug Nifedipine by Water Soluble 4-Sulphonic Calix[n]Arenes. Eur. J. Pharm. Biopharm. 2004, 58, 629–636. [Google Scholar] [CrossRef]
- Yang, W.; de Villiers, M.M. Aqueous Solubilization of Furosemide by Supramolecular Complexation with 4-Sulphonic Calix[n]Arenes. J. Pharm. Pharmacol. 2004, 56, 703–708. [Google Scholar] [CrossRef]
- Español, E.S.; Villamil, M.M. Calixarenes: Generalities and Their Role in Improving the Solubility, Biocompatibility, Stability, Bioavailability, Detection, and Transport of Biomolecules. Biomolecules 2019, 9, 90. [Google Scholar] [CrossRef] [Green Version]
- Bayrakcı, M.; Ertul, Ş.; Yılmaz, M. Solubilizing Effect of the P-Phosphonate Calix[n]Arenes towards Poorly Soluble Drug Molecules Such as Nifedipine, Niclosamide and Furosemide. J. Incl. Phenom. Macrocycl. Chem. 2012, 74, 415–423. [Google Scholar] [CrossRef]
- Menon, S.K.; Mistry, B.R.; Joshi, K.V.; Modi, N.R.; Shashtri, D. Evaluation and Solubility Improvement of Carvedilol: PSC[n]Arene Inclusion Complexes with Acute Oral Toxicity Studies. J. Incl. Phenom. Macrocycl. Chem. 2012, 73, 295–303. [Google Scholar] [CrossRef]
- Shahgaldian, P.; Da Silva, E.; Coleman, A.W. A First Approach to the Study of Calixarene Solid Lipid Nanoparticle (SLN) Toxicity. J. Incl. Phenom. 2003, 46, 175–177. [Google Scholar] [CrossRef]
- Da Silva, E.; Shahgaldian, P.; Coleman, A.W. Haemolytic Properties of Some Water-Soluble Para-Sulphonato-Calix-[n]- Arenes. Int. J. Pharm. 2004, 273, 57–62. [Google Scholar] [CrossRef]
- Schuette, J.M.; Ndou, T.T.; Warner, I.M. Cyclodextrin-Induced Asymmetry of Achiral Nitrogen Heterocycles. J. Phys. Chem. 1992, 96, 5309–5314. [Google Scholar] [CrossRef]
- Castro, R.; Godínez, L.A.; Criss, C.M.; Kaifer, A.E. Host Properties of α-Cyclodextrin and a Water-Soluble Calix[6]Arene Probed with Dimeric Bipyridinium Guests. J. Org. Chem. 1997, 62, 4928–4935. [Google Scholar] [CrossRef]
- Poh, B.L.; Lim, C.S.; Khoo, K.S. A Water-Soluble Cyclic Tetramer from Reacting Chromotropic Acid with Formaldehyde. Tetrahedron Lett. 1989, 30, 1005–1008. [Google Scholar] [CrossRef]
- Poh, B.L.; Lim, C.H.; Tan, C.M.; Wong, W.M. 1H NMR Study on the Complexation of Phenols with Cyclotetrachromotropylene in Aqueous Solution. Tetrahedron 1993, 49, 7259–7266. [Google Scholar] [CrossRef]
- Poh, C.B.; Tan, C.M. Contribution of Guest-Host CH-π Interaction to the Stability of Complexes Formed from Cyclotetrachromotropylene as Host and Alcohols and Sugars as Guests in Water. Tetrahedron 1993, 49, 9581–9592. [Google Scholar] [CrossRef]
- Poh, B.L.; Tan, C.M. Cyclotetrachromotropylene Plays Host to α-, β-, and γ-Cyclodextrin in Water. Tetrahedron Lett. 1994, 35, 6387–6390. [Google Scholar] [CrossRef]
- Jin, C.; Zhao, C.; Shen, D.; Dong, W.; Liu, H.; He, Z. Evaluating Bioequivalence of Meloxicam Tablets: Is in-Vitro Dissolution Test Overdiscriminating? J. Pharm. Pharmacol. 2018, 70, 250–258. [Google Scholar] [CrossRef]
- Goldman, A.P.; Williams, C.S.; Sheng, H.; Lamps, L.W.; Williams, V.P.; Pairet, M.; Morrow, J.D.; Dubois, R.N. Meloxicam Inhibits the Growth of Colorectal Cancer Cells. Carcinogenesis 1998, 19, 2195–2199. [Google Scholar] [CrossRef]
- Pairet, M.; Van Ryn, J.; Schierok, H.; Mauz, A.; Trummlitz, G.; Engelhardt, G. Differential Inhibition of Cyclooxygenases-1 and -2 by Meloxicam and Its 4’-Isomer. Inflamm. Res. 1998, 47, 270–276. [Google Scholar] [CrossRef]
- Ahmed, M.; Khanna, D.; Furst, D.E. Meloxicam in Rheumatoid Arthritis. Expert Opin. Drug Metab. Toxicol. 2005, 1, 739–751. [Google Scholar] [CrossRef] [PubMed]
- Aoki, T.; Yamaguchi, H.; Naito, H.; Shiiki, K.; Izawa, K.; Ota, Y.; Sakamoto, H.; Kaneko, A. Premedication with Cyclooxygenase-2 Inhibitor Meloxicam Reduced Postoperative Pain in Patients after Oral Surgery. Int. J. Oral Maxillofac. Surg. 2006, 35, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Yakushiji, K.; Matsunaga, S.; Yamauchi, Y.; Seto, Y.; Sato, H.; Onoue, S. Amorphous Solid Dispersion of Meloxicam Enhanced Oral Absorption in Rats With Impaired Gastric Motility. J. Pharm. Sci. 2018, 107, 446–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weyna, D.R.; Cheney, M.L.; Shan, N.; Hanna, M.; Zaworotko, M.J.; Sava, V.; Song, S.; Sanchez-Ramos, J.R. Improving Solubility and Pharmacokinetics of Meloxicam via Multiple-Component Crystal Formation. Mol. Pharm. 2012, 9, 2094–2102. [Google Scholar] [CrossRef]
- Shoormeij, Z.; Taheri, A.; Homayouni, A. Preparation and Physicochemical Characterization of Meloxicam Orally Fast Disintegration Tablet Using Its Solid Dispersion. Braz. J. Pharm. Sci. 2017, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Karagianni, A.; Kachrimanis, K.; Nikolakakis, I. Co-Amorphous Solid Dispersions for Solubility and Absorption Improvement of Drugs: Composition, Preparation, Characterization and Formulations for Oral Delivery. Pharmaceutics 2018, 10, 98. [Google Scholar] [CrossRef] [Green Version]
- Salem, L.H.; El-Feky, G.S.; Fahmy, R.H.; El Gazayerly, O.N.; Abdelbary, A. Coated Lipidic Nanoparticles as a New Strategy for Enhancing Nose-to-Brain Delivery of a Hydrophilic Drug Molecule. J. Pharm. Sci. 2020, 109, 2237–2251. [Google Scholar] [CrossRef]
- Chacko, I.A.; Ghate, V.M.; Dsouza, L.; Lewis, S.A. Lipid Vesicles: A Versatile Drug Delivery Platform for Dermal and Transdermal Applications. Colloids Surf. B Biointerfaces 2020, 195, 111262. [Google Scholar] [CrossRef]
- Loh, Z.H.; Samanta, A.K.; Sia Heng, P.W. Overview of Milling Techniques for Improving the Solubility of Poorly Water-Soluble Drugs. Asian J. Pharm. Sci. 2014, 10, 255–274. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.M.; Roberts, M.; El Hamd, M.A.; Abdellatif, A.A.H.; Younis, M.A. Glibenclamide Mini-Tablets with an Enhanced Pharmacokinetic and Pharmacodynamic Performance. AAPS PharmSciTech 2018, 19, 2948–2960. [Google Scholar] [CrossRef]
- Sarumane, C.; Titwan, A.; Sirithunyalug, J.; Okonogi, S.; Wolschann, P.; Viernstein, H. Enhancement of Solubility and Dissolution of Meloxicam by Cyclodextrin Complexation Materials. Starch Update 2005. In Proceedings of the 3rd Conference on Starch Technology, National Center for Genetic Engineering and Biotechnology (BIOTEC), Bangkok, Thailand, 4–5 November 2005; pp. 357–363. [Google Scholar]
- Obaidat, A.A.; Khanfar, R.A.; Khawam, M.N. The Effect of β-Cyclodextrin on the Solubility and Dissolution Rate of Meloxicam and Investigation of the Driving Force for Complexation Using Molecular Modeling. J. Incl. Phenom. Macrocycl. Chem. 2009, 63, 273–279. [Google Scholar] [CrossRef]
- Mader, W.J.; Higuchi, T. Phase Solubility Analysis. Crit. Rev. Anal. Chem. 1970, 1, 193–215. [Google Scholar] [CrossRef]
- Saokham, P.; Muankaew, C.; Jansook, P.; Loftsson, T. Solubility of Cyclodextrins and Drug/Cyclodextrin Complexes. Molecules 2018, 23, 1161. [Google Scholar] [CrossRef] [Green Version]
- Hassan, A.F.; Helmy, S.A.; Donia, A. MCM-41 for Meloxicam Dissolution Improvement. J. Braz. Chem. Soc. 2015, 26, 1367–1378. [Google Scholar]
- Abranches, P.A.S.; Varejão, E.V.V.; Da Silva, C.M.; De Fátima, A.; Magalhães, T.F.F.; Da Silva, D.L.; De Resende-Stoianoff, M.A.; Reis, F.S.; Nascimento, J.S.; De Almeida, W.B.; et al. Complexes of Fluconazole with Sodium P-Sulfonatocalix[n]Arenes: Characterization, Solubility and Antifungal Activity. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Newman, A.; Zografi, G. Commentary: Considerations in the Measurement of Glass Transition Temperatures of Pharmaceutical Amorphous Solids. AAPS PharmSciTech 2020, 21, 26. [Google Scholar] [CrossRef] [Green Version]
- Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Sol, V.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning. Polymers 2019, 12, 5. [Google Scholar] [CrossRef] [Green Version]
- Tawfeek, H.M.; Faisal, W.; Soliman, G.M. Enalapril Maleate Orally Disintegrating Tablets: Tableting and in Vivo Evaluation in Hypertensive Rats. Pharm. Dev. Technol. 2018, 23, 496–503. [Google Scholar] [CrossRef]
- Tawfeek, H.M.; Hassan, Y.A.; Aldawsari, M.F.; Fayed, M.H. Enhancing the Low Oral Bioavailability of Sulpiride via Fast Orally Disintegrating Tablets: Formulation, Optimization and in Vivo Characterization. Pharmaceuticals 2020, 13, 446. [Google Scholar] [CrossRef]
- Ki, H.M.; Choi, H.K. The Effect of Meloxicam/Ethanolamine Salt Formation on Percutaneous Absorption of Meloxicam. Arch. Pharm. Res. 2007, 30, 215–221. [Google Scholar] [CrossRef]
- Katkov, I.I.; Levine, F. Prediction of the Glass Transition Temperature of Water Solutions: Comparison of Different Models. Cryobiology 2004, 49, 62–82. [Google Scholar] [CrossRef]
- Garnero, C.; Aloisio, C.; Longhi, M. Ibuprofen-Maltodextrin Interaction: Study of Enantiomeric Recognition and Complex Characterization. Pharmacol. Pharm. 2013, 4, 18–30. [Google Scholar] [CrossRef] [Green Version]
- Doile, M.M.; Fortunato, K.A.; Schmücker, I.C.; Schucko, S.K.; Silva, M.A.S.; Rodrigues, P.O. Physicochemical Properties and Dissolution Studies of Dexamethasone Acetate-β-Cyclodextrin Inclusion Complexes Produced by Different Methods. AAPS PharmSciTech 2008, 9, 314–321. [Google Scholar] [CrossRef] [Green Version]
- Etman, M.; Shekedef, M.; Nada, A.; Ismail, A. In Vitro and In Vivo Evaluation of Tablets Containing Meloxicam-PEG 6000 Ball-Milled Co-Ground Mixture. J. Appl. Pharm. Sci. 2017, 7, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.L.; Wang, H.B.; Chen, Q.; Yang, W.C.; Yang, G.F. Preparation and Characterization of Inclusion Complex of Iprodione and β-Cyclodextrin to Improve Fungicidal Activity. J. Agric. Food Chem. 2007, 55, 3535–3539. [Google Scholar] [CrossRef]
- Kumara, P.; Mohanb, C.; Uma Shankara, M.K.S.; Gulatia, M. Physiochemical Characterization and Release Rate Studies of Solid Dispersions of Ketoconazole with Pluronic F127 and PVP K-30. Iran J. Pharm. Res. 2011, 10, 685–694. [Google Scholar] [CrossRef]
- Khan, C.A.; Rhodes, C.T. The Concept of Dissolution Efficiency. J. Pharm. Pharmac. 1975, 27, 48–49. [Google Scholar] [CrossRef]
- Naidu, N.B.; Chowdary, K.P.R.; Murthy, K.V.R.; Satyanarayana, V.; Hayman, A.R.; Becket, G. Physicochemical Characterization and Dissolution Properties of Meloxicam-Cyclodextrin Binary Systems. J. Pharm. Biomed. Anal. 2004, 35, 75–86. [Google Scholar] [CrossRef]
- Miclea, L.M.; Vlaia, L.; Vlaia, V.; Hǎdǎrugǎ, D.I.; Mircioiu, C. Preparation and Characterisation of Inclusion Complexes of Meloxicam and α-Cyclodextrin and β-Cyclodextrin. Farmacia 2010, 58, 583–593. [Google Scholar]
- Ali, H.R.H.; Saleem, I.Y.; Tawfeek, H.M. Insight into Inclusion Complexation of Indomethacin Nicotinamide Cocrystals. J. Incl. Phenom. Macrocycl. Chem. 2016, 84, 179–188. [Google Scholar] [CrossRef]
- Rajendrakumar, K.; Madhusudan, S.; Pralhad, T. Cyclodextrin Complexes of Valdecoxib: Properties and Anti-Inflammatory Activity in Rat. Eur. J. Pharm. Biopharm. 2005, 60, 39–46. [Google Scholar] [CrossRef] [PubMed]

| Media | Type of Phase Solubility Diagram | Stability Constant ± S.D (M−1) | Increasing of Solubility (St/So) * |
|---|---|---|---|
| Phosphate buffer pH 7.4 | AL | 1159.739 ± 0.084 | 23.993 |
| Distilled water | AL | 653.256 ± 0.167 | 5.695 |
| 0.1 M HCl pH 1.2 | AL | 555.953 ± 0.021 | 4.369 |
| Methods | ED25 ± SD, n = 3 | Fold Increase | RDR10 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| pH 7.4 | pH 1.2 | D.W | pH 7.4 | pH 1.2 | D.W | pH 7.4 | pH 1.2 | D.W | |
| CO | 20.86 ± 0.93 | 19.00 ± 0.35 | 18.76 ± 0.26 | 5.38 | 23.34 | 65.28 | 7.31 | 36.05 | 90.07 |
| KN | 19.73 ± 0.92 | 19.24 ± 0.68 | 16.47 ± 0.47 | 5.09 | 23.63 | 57.30 | 6.76 | 33.56 | 82.61 |
| PM | 13.24 ± 0.76 | 14.82 ± 0.72 | 14.50 ± 0.43 | 3.42 | 18.21 | 50.47 | 4.37 | 27.19 | 72.89 |
| Commercial ME | 15.38 ± 0.43 | 2.74 ± 0.053 | 17.11 ± 0.47 | 3.97 | 3.37 | 59.55 | 5.11 | 3.92 | 81.95 |
| ME powder | 3.88 ± 0.46 | 0.81 ± 0.29 | 0.29 ± 0.12 | - | - | - | - | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Hujran, T.A.; Magharbeh, M.K.; Al-Gharabli, S.; Haddadin, R.R.; Al Soub, M.N.; Tawfeek, H.M. Studying the Complex Formation of Sulfonatocalix[4]naphthalene and Meloxicam towards Enhancing Its Solubility and Dissolution Performance. Pharmaceutics 2021, 13, 994. https://doi.org/10.3390/pharmaceutics13070994
Al Hujran TA, Magharbeh MK, Al-Gharabli S, Haddadin RR, Al Soub MN, Tawfeek HM. Studying the Complex Formation of Sulfonatocalix[4]naphthalene and Meloxicam towards Enhancing Its Solubility and Dissolution Performance. Pharmaceutics. 2021; 13(7):994. https://doi.org/10.3390/pharmaceutics13070994
Chicago/Turabian StyleAl Hujran, Tayel A., Mousa K. Magharbeh, Samer Al-Gharabli, Rula R. Haddadin, Manal N. Al Soub, and Hesham M. Tawfeek. 2021. "Studying the Complex Formation of Sulfonatocalix[4]naphthalene and Meloxicam towards Enhancing Its Solubility and Dissolution Performance" Pharmaceutics 13, no. 7: 994. https://doi.org/10.3390/pharmaceutics13070994






