Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals
Abstract
:1. Introduction
2. Experimental
2.1. Catalyst Preparation
2.2. Catalyst Characterization
2.3. Dehydration of Long-Chain Fatty Alcohol and Product Analysis
3. Results and Discussion
3.1. Characteristics of Catalysts
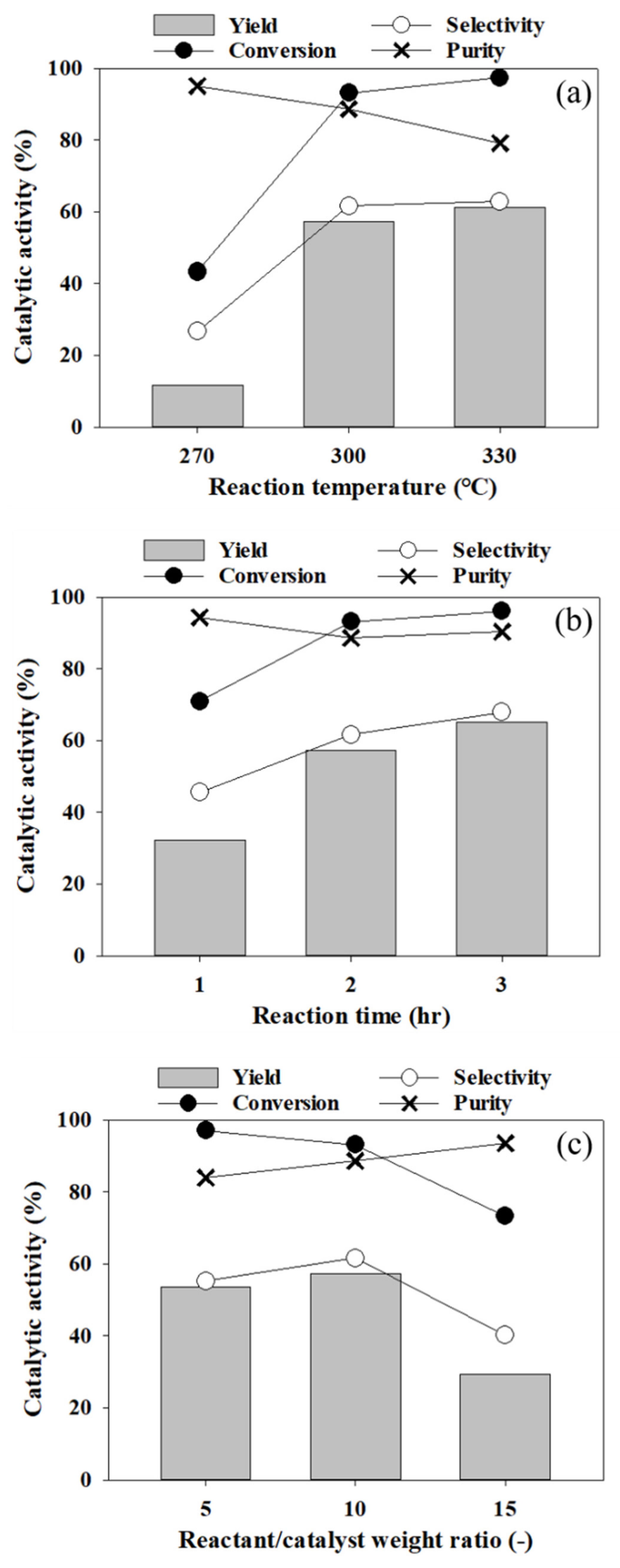
3.2. Dehydration Reaction of 1-Octadecanol
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kunal Ahuja, S.S. Linear Alpha Olefins Market Size By Product (1-Butene, 1-Hexene, 1-Octene, 1-Decene, 1-Dodecene, 1-Tetradecene, 1-Hexadecene, 1-Octadecene), By Application (LLDPE, HDPE, PAO, Detergent Alcohols, Oil Field Chemicals, Lubricants, Plasticizers), Regional Outlook, Competitive Market Share & Forecast, 2017–2024; Global Market Insights Inc.: Selbyville, DE, USA, 2017; p. 175. [Google Scholar]
- Dixon, J.T.; Green, M.J.; Hess, F.M.; Morgan, D.H. Advances in selective ethylene trimerisation–a critical overview. J. Organomet. Chem. 2004, 689, 3641–3668. [Google Scholar] [CrossRef]
- Keim, W. Oligomerization of Ethylene to α-Olefins: Discovery and Development of the Shell Higher Olefin Process (SHOP). Angew. Chem. Int. Ed. 2013, 52, 12492–12496. [Google Scholar] [CrossRef]
- Gao, W.; Gao, R.; Zhao, Y.; Peng, M.; Song, C.; Li, M.; Li, S.; Liu, J.; Li, W.; Deng, Y. Photo-driven syngas conversion to lower olefins over oxygen-decorated Fe5C2 catalyst. Chem 2018, 4, 2917–2928. [Google Scholar] [CrossRef] [Green Version]
- Park, J.C.; Jang, S.; Rhim, G.B.; Lee, J.H.; Choi, H.; Jeong, H.-D.; Youn, M.H.; Lee, D.-W.; Koo, K.Y.; Kang, S.W. A durable nanocatalyst of potassium-doped iron-carbide/alumina for significant production of linear alpha olefins via Fischer-Tropsch synthesis. Appl. Catal. A 2018, 564, 190–198. [Google Scholar] [CrossRef]
- Guo, L.; Cui, Y.; Li, H.; Fang, Y.; Prasert, R.; Wu, J.; Yang, G.; Yoneyama, Y.; Tsubaki, N. Selective formation of linear-alpha olefins (LAOs) by CO2 hydrogenation over bimetallic Fe/Co-Y catalyst. Catal. Commun. 2019. [Google Scholar] [CrossRef]
- Ramachandrarao, B.; Naresh, K.; Panday, A.; Venkateswarlu Choudary, N. A Rapid Py-GC/MS Study for Linear Alpha Olefin Production from Fast Pyrolysis of Wax and Waste Polyethylene. ChemistrySelect 2019, 4, 13245–13249. [Google Scholar] [CrossRef]
- Process for increased olefin yields from heavy feedstocks. Available online: https://patents.google.com/patent/US5906728A/en (accessed on 20 July 2021).
- Serrano, D.P.; Aguado, J.; Escola, J.M. Developing Advanced Catalysts for the Conversion of Polyolefinic Waste Plastics into Fuels and Chemicals. ACS Catal. 2012, 2, 1924–1941. [Google Scholar] [CrossRef]
- Al-Jarallah, A.; Anabtawi, J.; Siddiqui, M.; Aitani, A.; Al-Sa’doun, A. Ethylene dimerization and oligomerization to butene-1 and linear α-olefins: A review of catalytic systems and processes. Catal. Today 1992, 14, 1–121. [Google Scholar] [CrossRef]
- Lutz, E. Shell higher olefins process. J. Chem. Educ. 1986, 63, 202. [Google Scholar] [CrossRef]
- Zhang, L.; Xian, M.; He, Y.; Li, L.; Yang, J.; Yu, S.; Xu, X. A Brønsted acidic ionic liquid as an efficient and environmentally benign catalyst for biodiesel synthesis from free fatty acids and alcohols. Bioresour. Technol. 2009, 100, 4368–4373. [Google Scholar] [CrossRef]
- Shu, Q.; Yang, B.; Yuan, H.; Qing, S.; Zhu, G. Synthesis of biodiesel from soybean oil and methanol catalyzed by zeolite beta modified with La3+. Catal. Commun. 2007, 8, 2159–2165. [Google Scholar] [CrossRef]
- Muanruksa, P.; Winterburn, J.; Kaewkannetra, P. A novel process for biodiesel production from sludge palm oil. MethodsX 2019, 6, 2838–2844. [Google Scholar] [CrossRef] [PubMed]
- Ping, B.T.Y.; Yusof, M. Characteristics and properties of fatty acid distillates from palm oil. Oil Palm Bulle. 2009, 59, 5–11. [Google Scholar]
- Molinder, R.; Almqvist, J. Extractives in the Scandinavian Pulp and Paperindustry: Current and Possible Future Applications; The Research Institute of Sweden: Göteborg, Sweden, 2018; pp. 1–17. [Google Scholar]
- Yelchuri, V.; Srikanth, K.; Prasad, R.; Karuna, M. Olefin metathesis of fatty acids and vegetable oils. J. Chem. Sci. 2019, 131, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Nieres, P.D.; Zelin, J.; Trasarti, A.F.; Apesteguía, C.R. Heterogeneous catalysis for valorisation of vegetable oils via metathesis reactions: Ethenolysis of methyl oleate. Catal. Sci. Technol. 2016, 6, 6561–6568. [Google Scholar] [CrossRef]
- Van der Klis, F.; Le Nôtre, J.; Blaauw, R.; van Haveren, J.; van Es, D.S. Renewable linear alpha olefins by selective ethenolysis of decarboxylated unsaturated fatty acids. Eur. J. Lipid Sci. Technol. 2012, 114, 911–918. [Google Scholar] [CrossRef]
- Gooßen, L.J.; Rodríguez, N. A mild and efficient protocol for the conversion of carboxylic acids to olefins by a catalytic decarbonylative elimination reaction. Chem. Commun. 2004, 724–725. [Google Scholar] [CrossRef] [PubMed]
- Le Nôtre, J.; Scott, E.L.; Franssen, M.C.; Sanders, J.P. Selective preparation of terminal alkenes from aliphatic carboxylic acids by a palladium-catalysed decarbonylation–elimination reaction. Tetrahedron Lett. 2010, 51, 3712–3715. [Google Scholar] [CrossRef]
- Liu, Y.; Kim, K.E.; Herbert, M.B.; Fedorov, A.; Grubbs, R.H.; Stoltz, B.M. Palladium-catalyzed decarbonylative dehydration of fatty acids for the production of linear alpha olefins. Adv. Synth. Catal. 2014, 356, 130–136. [Google Scholar] [CrossRef]
- Chen, S.; Wu, T.; Zhao, C. Conversion of lipid into high-viscosity branched bio-lubricant base oil. Green Chem. 2020, 22, 7348–7354. [Google Scholar] [CrossRef]
- Ali, A.; Zhao, C. Selective synthesis of α-olefins by dehydration of fatty alcohols over alumina–thoria mixed catalysts. Catal. Sci. Technol. 2020, 10, 3701–3708. [Google Scholar] [CrossRef]
- Srinivasan, P.D.; Khivantsev, K.; Tengco, J.M.M.; Zhu, H.; Bravo-Suárez, J.J. Enhanced ethanol dehydration on γ-Al2O3 supported cobalt catalyst. J. Catal. 2019, 373, 276–296. [Google Scholar] [CrossRef]
- Nash, C.P.; Ramanathan, A.; Ruddy, D.A.; Behl, M.; Gjersing, E.; Griffin, M.; Zhu, H.; Subramaniam, B.; Schaidle, J.A.; Hensley, J.E. Mixed alcohol dehydration over Brønsted and Lewis acidic catalysts. Appl. Catal. A 2016, 510, 110–124. [Google Scholar] [CrossRef] [Green Version]
- Kruger, J.S.; Dong, T.; Beckham, G.T.; Biddy, M.J. Integrated conversion of 1-butanol to 1, 3-butadiene. RSC Adv. 2018, 8, 24068–24074. [Google Scholar] [CrossRef] [Green Version]
- Nel, R.J.; de Klerk, A. Dehydration of C5− C12 Linear 1-Alcohols over η-Alumina to Fuel Ethers. Ind. Eng. Chem. Res. 2009, 48, 5230–5238. [Google Scholar] [CrossRef]
- Roy, S.; Mpourmpakis, G.; Hong, D.-Y.; Vlachos, D.G.; Bhan, A.; Gorte, R. Mechanistic study of alcohol dehydration on γ-Al2O3. Acs Catal. 2012, 2, 1846–1853. [Google Scholar] [CrossRef]
- Kim, Y.-e.; Im, H.B.; Jung, U.H.; Park, J.C.; Youn, M.H.; Jeong, H.-D.; Lee, D.-W.; Rhim, G.B.; Chun, D.H.; Lee, K.B. Production of linear α-olefin 1-octene via dehydration of 1-octanol over Al2O3 catalyst. Fuel 2019, 256, 115957. [Google Scholar] [CrossRef]
- Kostestkyy, P.; Yu, J.; Gorte, R.J.; Mpourmpakis, G. Structure–activity relationships on metal-oxides: Alcohol dehydration. Catal. Sci. Technol. 2014, 4, 3861–3869. [Google Scholar] [CrossRef]
- Khom-in, J.; Praserthdam, P.; Panpranot, J.; Mekasuwandumrong, O. Dehydration of methanol to dimethyl ether over nanocrystalline Al2O3 with mixed γ-and χ-crystalline phases. Catal. Commun. 2008, 9, 1955–1958. [Google Scholar] [CrossRef]
- Janlamool, J.; Jongsomjit, B. Catalytic ethanol dehydration to ethylene over nanocrystalline χ-and γ-Al2O3 catalysts. J. Oleo Sci. 2017, 66, 1029–1039. [Google Scholar] [CrossRef] [Green Version]
- Wannaborworn, M.; Praserthdam, P.; Jongsomjit, B. A comparative study of solvothermal and sol-gel-derived nanocrystalline alumina catalysts for ethanol dehydration. J. Nanom. 2015, 16, 429. [Google Scholar] [CrossRef] [Green Version]
- Wu, J.; Liu, H.-J.; Yan, X.; Zhou, Y.-J.; Lin, Z.-N.; Mi, S.; Cheng, K.-K.; Zhang, J.-A. Efficient Catalytic Dehydration of High-Concentration 1-Butanol with Zn-Mn-Co Modified γ-Al2O3 in Jet Fuel Production. Catalysts 2019, 9, 93. [Google Scholar] [CrossRef] [Green Version]
- Phung, T.K.; Busca, G. Ethanol dehydration on silica-aluminas: Active sites and ethylene/diethyl ether selectivities. Catal. Commun. 2015, 68, 110–115. [Google Scholar] [CrossRef]
- He, M.; Wang, M.; Tang, G.; Fang, Y.; Tan, T. From medium chain fatty alcohol to jet fuel: Rational integration of selective dehydration and hydro-processing. Appl. Catal. A 2018, 550, 160–167. [Google Scholar] [CrossRef]
- Mavrič, A.; Valant, M.; Cui, C.; Wang, Z.M. Advanced applications of amorphous alumina: From nano to bulk. J. Non-Cryst. Solids 2019, 521, 119493. [Google Scholar] [CrossRef]
- Magureanu, M.; Dobrin, D.; Mandache, N.B.; Cojocaru, B.; Parvulescu, V.I. Toluene oxidation by non-thermal plasma combined with palladium catalysts. Front. Chem. 2013, 1, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Egorova, S.R.; Zhang, Y.; Mukhamed’yarova, A.N.; Kurbangaleeva, A.Z.; Lamberov, A.A. Application of coarse gibbsite agglomerates to formation of 2D and 3D boehmite particles by the dehydration of the hydrothermal treatment and atmospheric pressure. Surf. Interfaces 2018, 13, 58–64. [Google Scholar] [CrossRef]
- Chen, M.; Lu, W.; Zhu, H.; Gong, L.; Zhao, Z.; Ding, Y. Dehydration of Long-Chain n-Alcohols to Linear α-Olefins Using Sodium-Modified γ-Al2O3. Ind. Eng. Chem. Res. 2020, 59, 4388–4396. [Google Scholar] [CrossRef]
- Parry, E. An infrared study of pyridine adsorbed on acidic solids. Characterization of surface acidity. J. Catal. 1963, 2, 371–379. [Google Scholar]
- Penkova, A.; Bobadilla, L.F.; Romero-Sarria, F.; Centeno, M.A.; Odriozola, J.A. Pyridine adsorption on NiSn/MgO–Al2O3: An FTIR spectroscopic study of surface acidity. Appl. Surf. Sci. 2014, 317, 241–251. [Google Scholar] [CrossRef]
- Haneda, M.; Joubert, E.; Ménézo, J.-C.; Duprez, D.; Barbier, J.; Bion, N.; Daturi, M.; Saussey, J.; Lavalley, J.-C.; Hamada, H. Surface characterization of alumina-supported catalysts prepared by sol–gel method. Part I.—Acid–base properties. Phys. Chem. Chem. Phys 2001, 3, 1366–1370. [Google Scholar] [CrossRef]
- Liu, X.; Truitt, R.E. DRFT-IR Studies of the Surface of γ-Alumina. J. Am. Chem. Soc. 1997, 119, 9856–9860. [Google Scholar] [CrossRef]

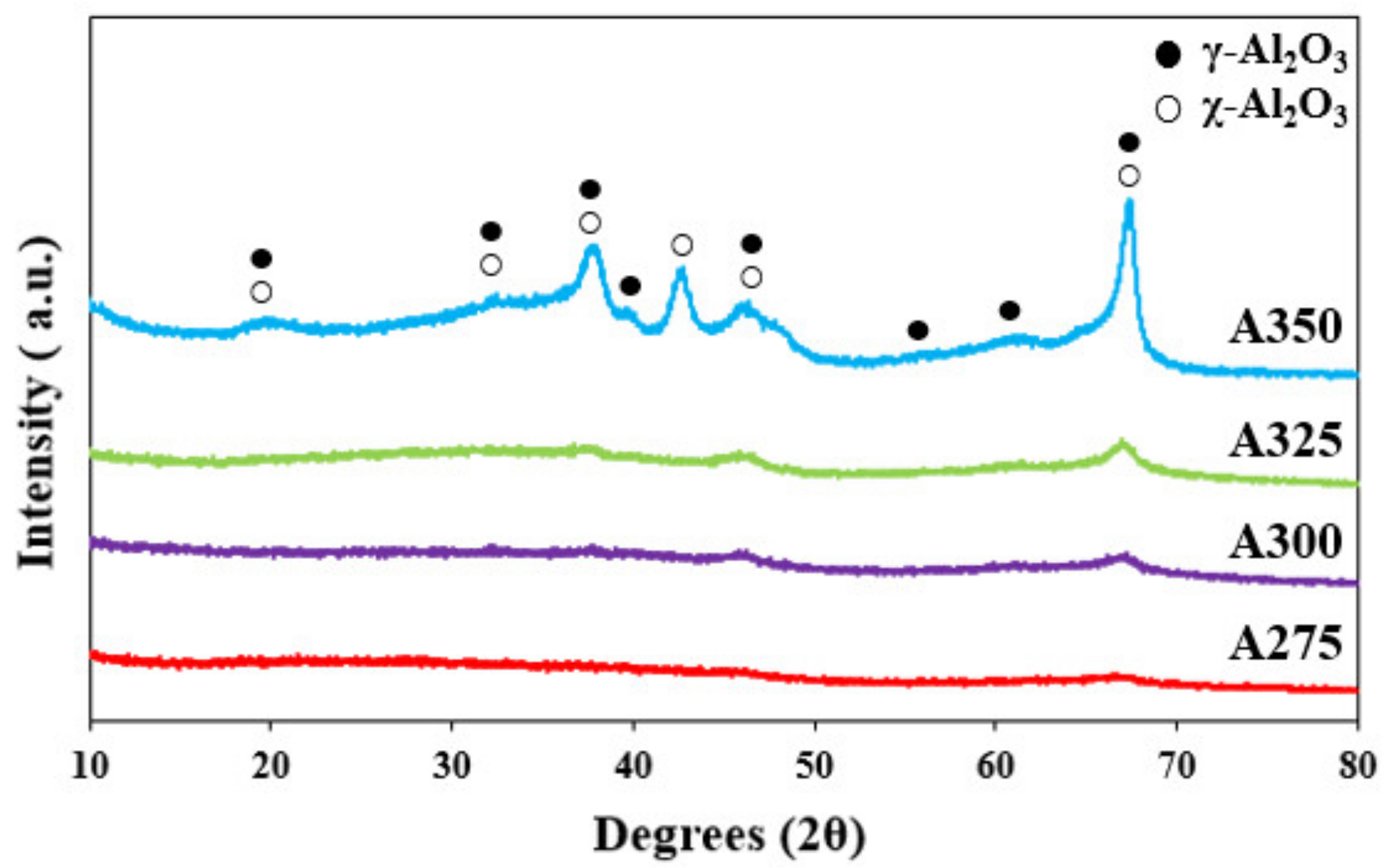

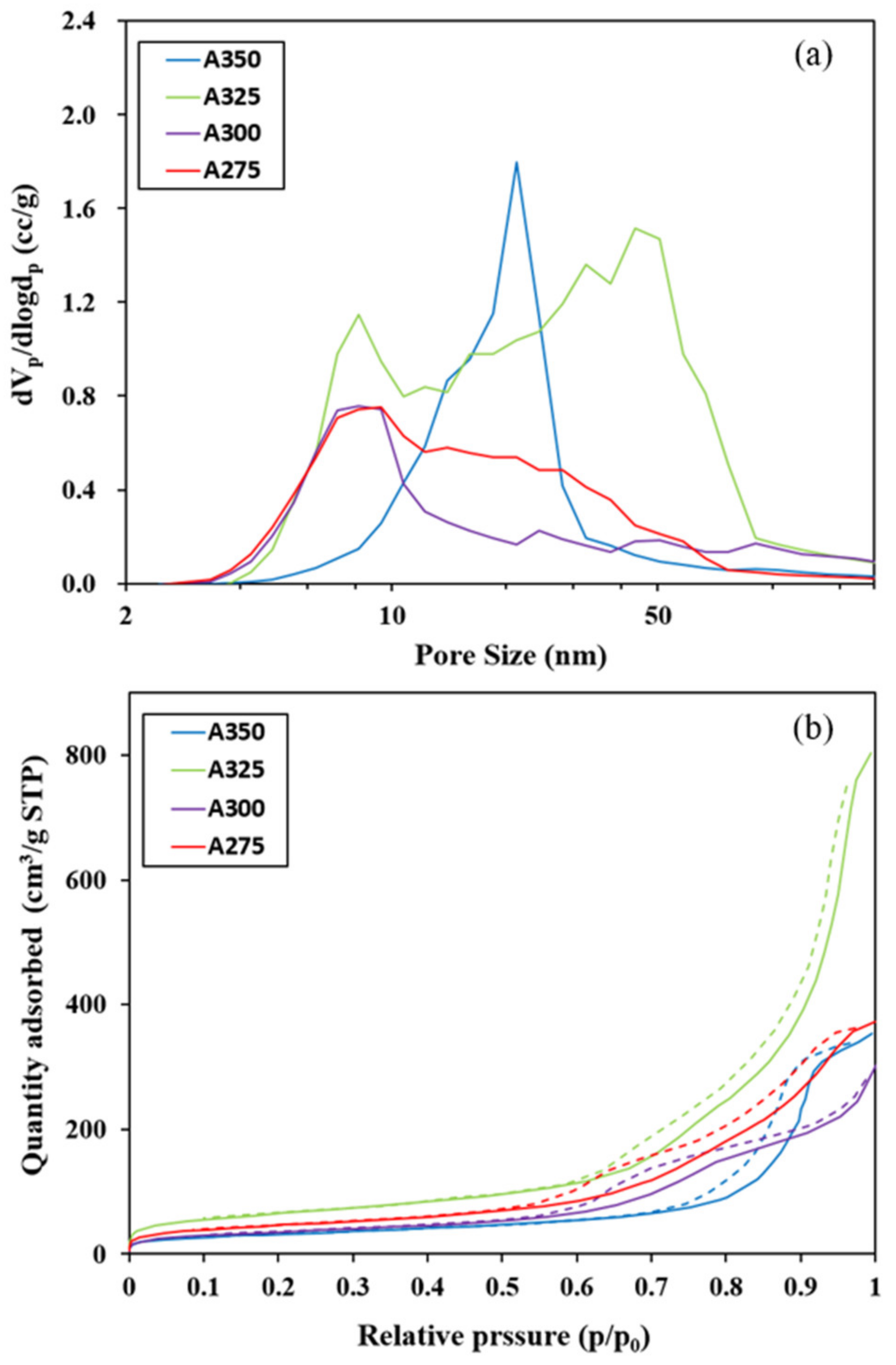
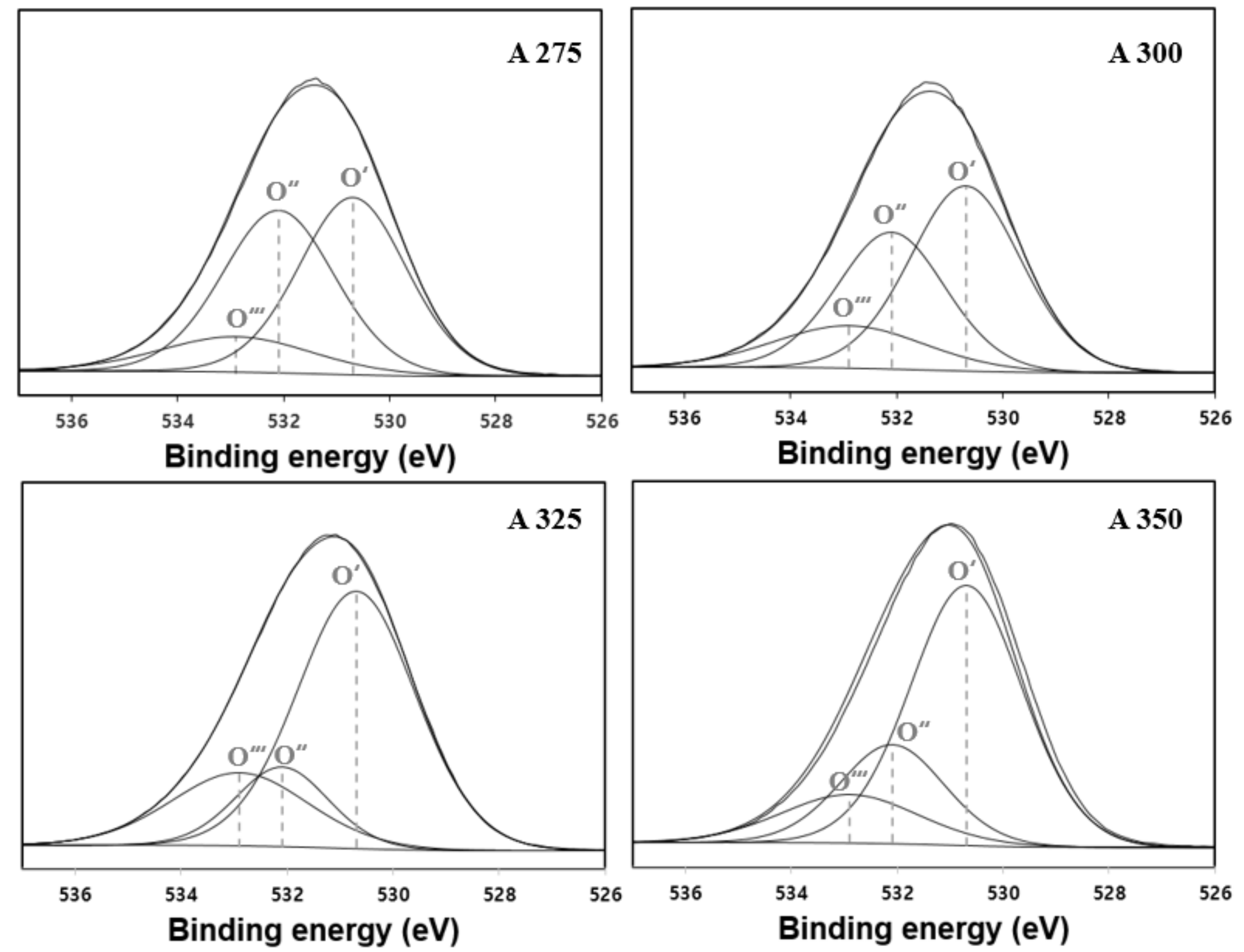
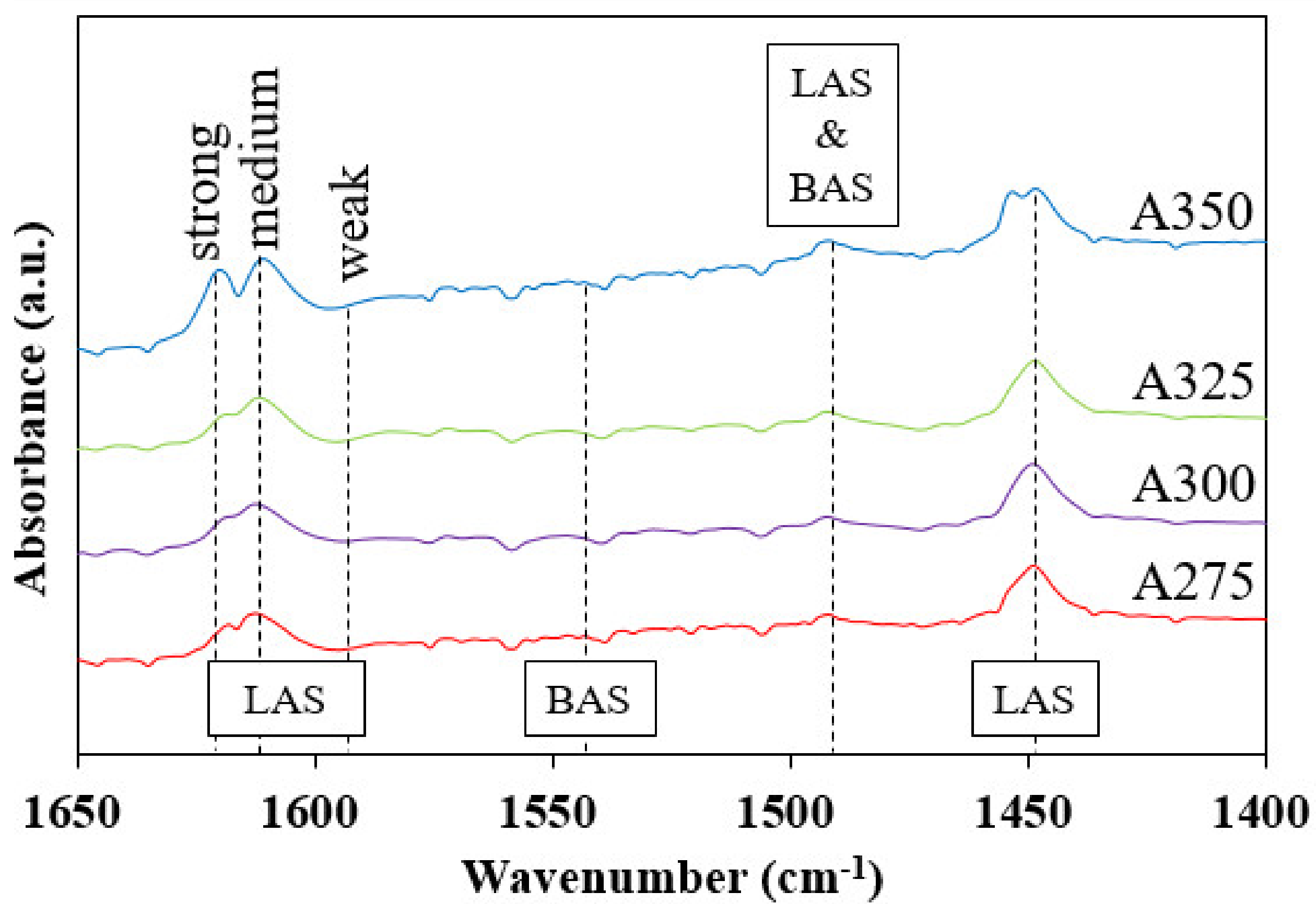


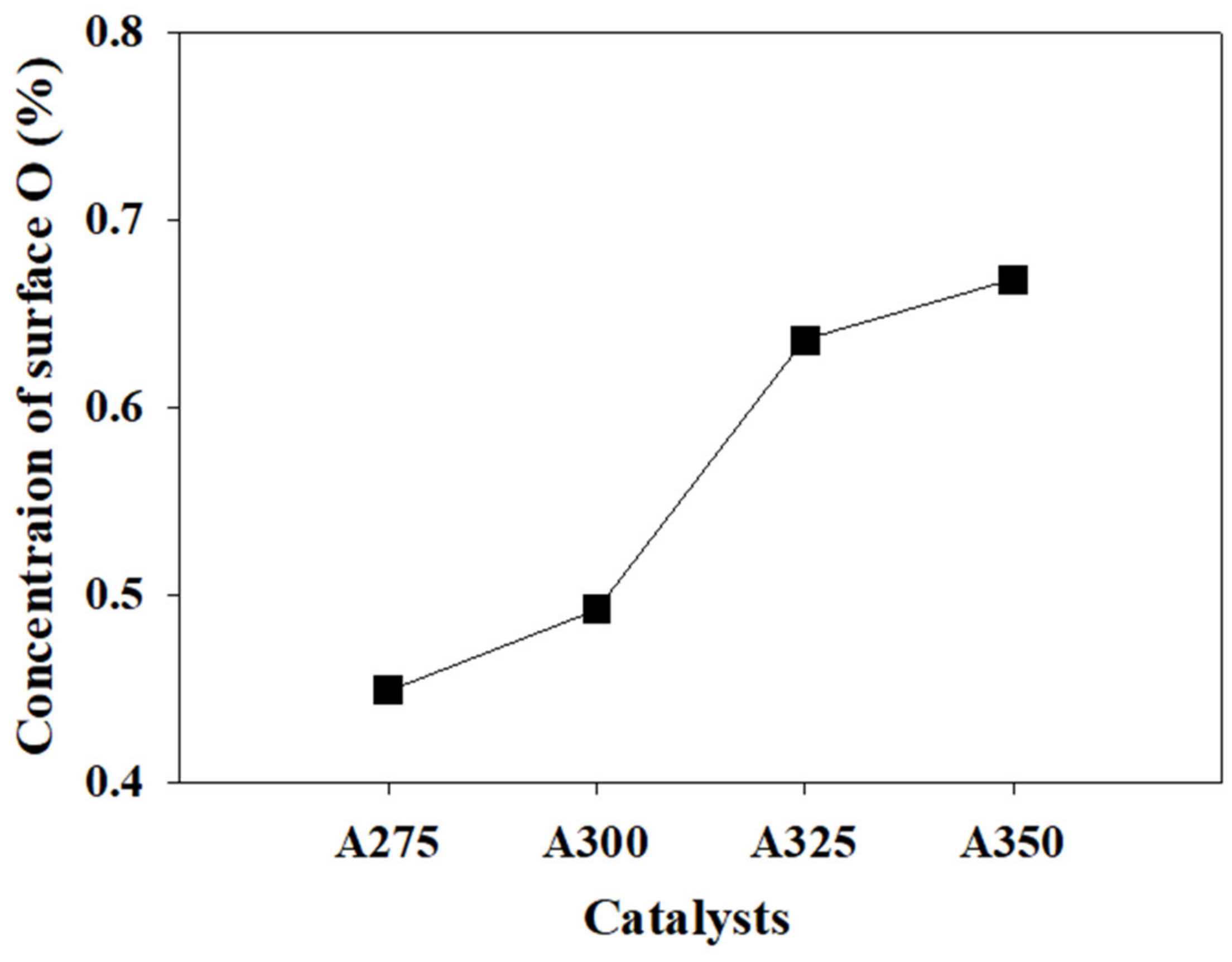
| Catalysts | BET Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Diameter (nm) |
|---|---|---|---|
| A275 | 165.12 | 0.574 | 7.2 |
| A300 | 126.56 | 0.432 | 7.2 |
| A325 | 233.07 | 1.237 | 8.2 |
| A350 | 114.02 | 0.541 | 21.3 |
| Catalysts | Binding Energy for Al2p (eV) | Binding Energy for O1s (eV) | Atomic Concentration (%) | Atomic Ratio (-) | O 1s Peak Area Fraction (%O1s) | O 1s Atomic Concentration (%) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Al 2p | O 1s | Al/O | H2O | OH | O | H2O | OH | O | |||
| A275 | 74.38 | 531.74 | 35.76 | 64.24 | 0.56 | 0.123 | 0.428 | 0.449 | 7.9 | 27.47 | 28.87 |
| A300 | 74.38 | 531.69 | 37.49 | 62.51 | 0.6 | 0.154 | 0.354 | 0.492 | 9.61 | 22.12 | 30.77 |
| A325 | 74.25 | 531.49 | 37.39 | 62.61 | 0.6 | 0.21 | 0.154 | 0.636 | 13.12 | 9.66 | 39.82 |
| A350 | 74.36 | 531.42 | 40.42 | 59.58 | 0.68 | 0.13 | 0.202 | 0.668 | 7.76 | 12.01 | 39.8 |
| Unstandardized Coefficients | Standardized Coefficients | T(p) | Toler-ance(TOL) | Variance Inflation Factor (VIF) | ||
|---|---|---|---|---|---|---|
| B | Std. Error | Β | ||||
| (constant) | 163.26 | 17.81 | 0.00 | 9.17 | 1.0 | 1.0 |
| Temperature | −0.27 | 0.06 | −0.78 | −4.65 ** | 1.0 | 1.0 |
| Reactant/catal. ratio | 0.95 | 0.34 | 0.47 | 2.78 * | 1.0 | 1.0 |
| F(p) | 10.26* | |||||
| Adjusted R2 | 0.776 | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, H.-J.; Choi, I.-H.; Kim, S.-W.; Hwang, K.-R. Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals. Polymers 2021, 13, 2850. https://doi.org/10.3390/polym13172850
Lee H-J, Choi I-H, Kim S-W, Hwang K-R. Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals. Polymers. 2021; 13(17):2850. https://doi.org/10.3390/polym13172850
Chicago/Turabian StyleLee, Hye-Jin, Il-Ho Choi, Seung-Wook Kim, and Kyung-Ran Hwang. 2021. "Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals" Polymers 13, no. 17: 2850. https://doi.org/10.3390/polym13172850
APA StyleLee, H.-J., Choi, I.-H., Kim, S.-W., & Hwang, K.-R. (2021). Selective Production of Bio-Based Linear Alpha-Olefin from Wasted Fatty Alcohol on Al2O3 for Bio-Based Chemicals. Polymers, 13(17), 2850. https://doi.org/10.3390/polym13172850






