Physicochemical, Antioxidant, Microstructural Properties and Bioaccessibility of Dark Chocolate with Plant Extracts
Abstract
:1. Introduction
2. Results and Discussion
2.1. Antioxidant Capacity of Plant Extracts
2.2. Antioxidant Capacity and Total Phenol Content in Chocolates before and after In Vitro Simulated Digestion
2.3. Physicochemical Parameters of Chocolates
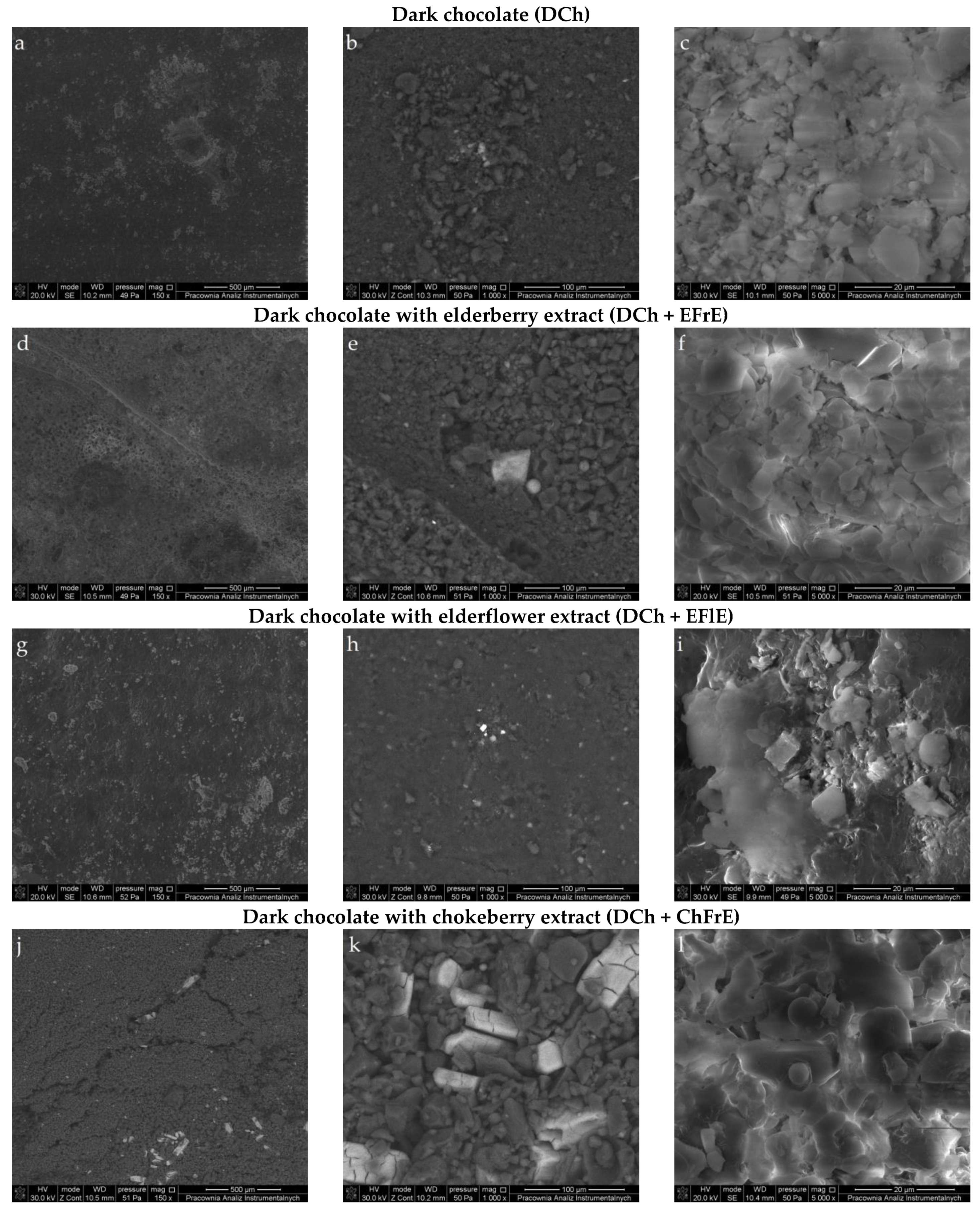
2.4. Microstructural Properties of Chocolates
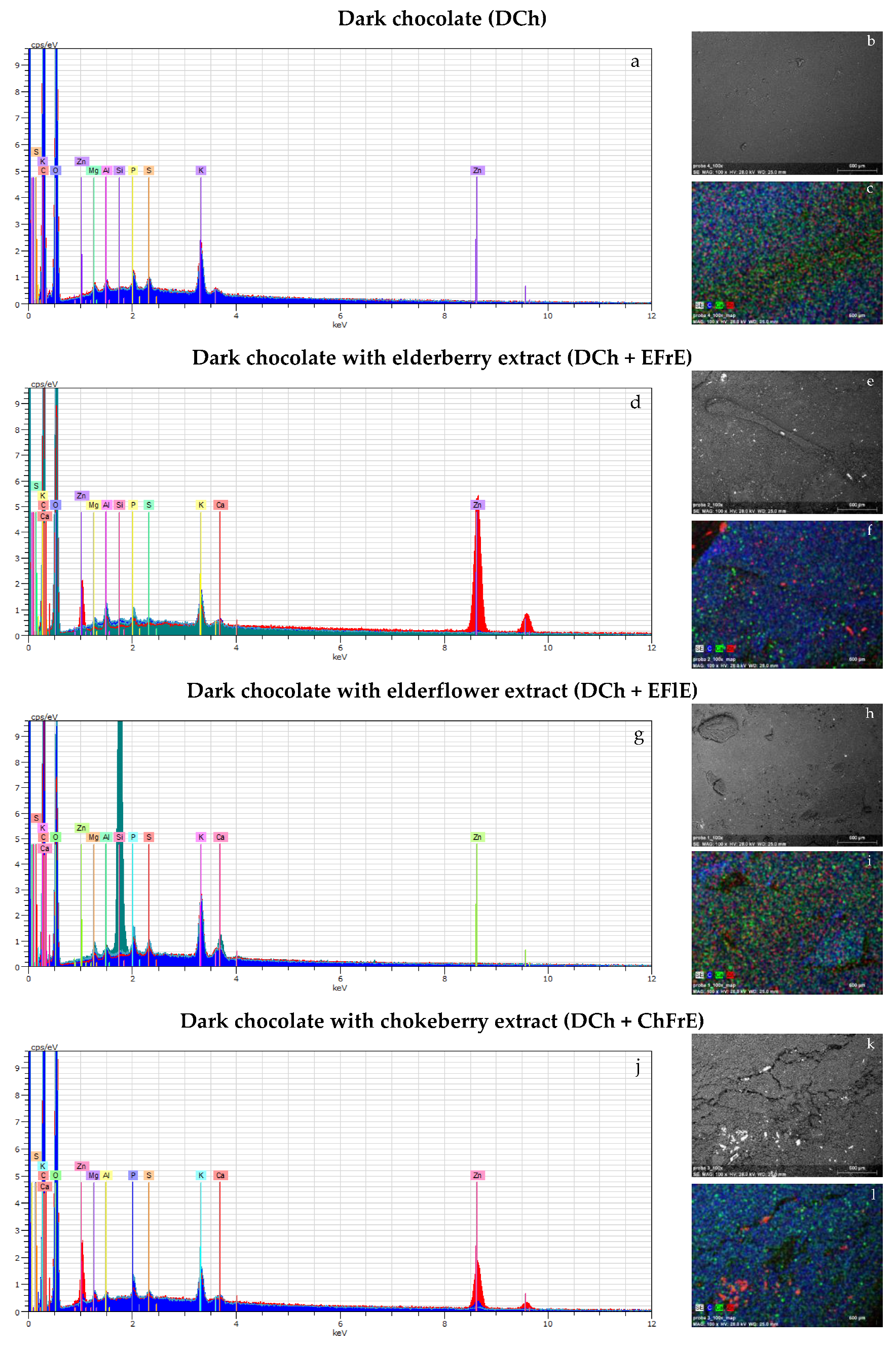
2.5. Energy Spectra Provided by SEM/EDS
3. Materials and Methods
3.1. Chemicals
3.2. Materials
3.3. Ultrasound-Assisted Extraction of Antioxidants from Plants
3.4. Preparation of Chocolates Fortified with Plant Extracts and Extraction Procedure
3.5. In Vitro Simulated Digestion of Chocolates
3.6. Physicochemical Analysis of Chocolates
3.6.1. Moisture and Fat Determination
3.6.2. Viscosity Determination
3.7. Antioxidant Capacity Determination
3.8. Scanning Electron Microscopy with Energy Dispersive X-ray Spectrometer
3.9. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Vinson, J.A.; Proch, J.; Bose, P.; Muchler, S.; Taffera, P.; Shuta, D.; Samman, N.; Agbor., G.A. Chocolate is a powerful ex vivo and in vivo antioxidant, an antiatherosclerotic agent in an animal model, and a significant contributor to antioxidants in the European and American diets. J. Agric. Food Chem. 2006, 54, 8071–8076. [Google Scholar] [CrossRef]
- Komes, D.; Belščak-Cvitanović, A.; Škrabal, S.; Vojvodić, A.; Bušić, A. The influence of dried fruits enrichment on sensory properties of bitter and milk chocolates and bioactive content of their extracts affected by different solvents. LWT Food Sci. Technol. 2013, 58, 360–369. [Google Scholar] [CrossRef]
- Rashid, M.T.; Belščak-Cvitanović, A.; Karača, S.; Ma, H.; Komes, D. Longan (Dimocarpus longan) and lychee (Litchi chinensis): Functional ingredients in chocolate pralines. J. Food Biochem. 2019, 43, e12811. [Google Scholar] [CrossRef] [PubMed]
- Belščak-Cvitanović, A.; Komes, D.; Durgo, K.; Vojvodić, A.; Bušić, A. Nettle (Urtica dioica L.) extracts as functional ingredients for production of chocolates with improved bioactive composition and sensory properties. J. Food Sci. Technol. 2015, 52, 7723–7734. [Google Scholar] [CrossRef] [Green Version]
- Belščak-Cvitanović, A.; Komes, D.; Benković, M.; Karlović, S.; Hečimović, I.; Ježek, D.; Bauman, I. Innovative formulations of chocolates enriched with plant polyphenols from Rubus idaeus L. leaves and characterization of their physical, bioactive and sensory properties. Food Res. Int. 2012, 48, 820–830. [Google Scholar] [CrossRef]
- Martini, S.; Conte, A.; Tagliazucchi, D. Bioaccessibility, bioactivity and cell metabolism of dark chocolate phenolic compounds after in vitro gastro-intestinal digestion. J. Funct. Food. 2018, 49, 424–436. [Google Scholar] [CrossRef] [Green Version]
- Gramza-Michałowska, A.; Kulczyński, B.; Skopiec, M.; Kobus-Cisowska, J.; Brzozowska, A. The effect of yellow tea leaves Camellia sinensis on the quality of stored chocolate confectionery. Appl. Sci. 2021, 11, 4123. [Google Scholar] [CrossRef]
- Sidor, A.; Drożdżyńska, A.; Gramza-Michałowska, A. Black chokeberry (Aronia melanocarpa) and its products as potential health promoting factors-An overview. Trends Food Sci. Technol. 2019, 89, 45–60. [Google Scholar] [CrossRef]
- Domínguez, R.; Zhang, L.; Rocchetti, G.; Lucini, L.; Pateiro, M.; Munekata, P.E.S.; Lorenzo, M.J. Elderberry (Sambucus nigra L.) as potential source of antioxidants. Characterization, optimization of extraction parameters and bioactive properties. Food Chem. 2020, 330, 127266. [Google Scholar] [CrossRef] [PubMed]
- Vujanović, M.; Majkić, T.; Zengin, G.; Beara, I.; Cvetanović, A.; Fawzi, M.M.; Radojković, M. Advantages of contemporary extraction techniques for the extraction of bioactive constituents from black elderberry (Sambucus nigra L.) flowers. Ind. Crop. Prod. 2019, 136, 93–101. [Google Scholar] [CrossRef]
- Zhou, N.; Zhu, W.; Yang, F.; Zhou, K. In vitro gastrointestinal digestion model to monitor the antioxidant properties and bioavailability of phenolic antioxidants from elderberry. React. Oxyg. Species 2016, 2, 421–431. [Google Scholar] [CrossRef]
- Baycar, A.; Konar, N.; Poyrazoglu, E.S.; Goktas, H.; Sagdic, O. Using white spread and compound chocolate as phenolic compound delivering agent: A model study with black carrot extract. J. Food Process. Preserv. 2021, 45, e15392. [Google Scholar] [CrossRef]
- Dala-Paula, B.M.; Deus, V.L.; Tavano, O.L.; Gloria, M.B.A. In vitro bioaccessibility of amino acids and bioactive amines in 70% cocoa dark chocolate: What you eat and what you get. Food Chem. 2021, 343, 128397. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef] [Green Version]
- Arancibia-Avila, P.; Namieśnik, J.; Toledo, F.; Werner, E.; Martinez-Ayala, A.L.; Rocha-Guzmán, N.E.; Gallegos-Infante, J.A.; Gorinstein, S. The influence of different time durations of thermal processing on berries quality. Food Control. 2012, 26, 587–593. [Google Scholar] [CrossRef]
- Jakobek, L.; Šeruga, M.; Novak, I.; Medvidović-Kosanović, M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Dtsch. Lebensm. Rundsch. 2007, 103, 369–378. [Google Scholar]
- Tarko, T.; Duda-Chodak, A.; Sroka, P.; Satora, P.; Semik-Szczurak, D.; Wajda, Ł. Diversity and bioavailability of fruit polyphenols. J. Food Nutr. Res. 2017, 56, 167–178. [Google Scholar]
- Viskelis, P.; Rubinskienė, M.; Bobinaitė, R.; Dambrauskienė, E. Bioactive compounds and antioxidant activity of small fruits in Lithuania. J. Food Agric. Environ. 2010, 8, 259–263. [Google Scholar]
- Dawidowicz, A.L.; Wianowska, D.; Baraniak, B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts). LWT Food Sci. Technol. 2006, 39, 308–315. [Google Scholar] [CrossRef]
- Mikulic-Petkovsek, M.; Ivancic, A.; Schmitzer, V.; Veberic, R.; Stampar, F. Comparison of major taste compounds and antioxidative properties of fruits and flowers of different Sambucus species and interspecific hybrids. Food Chem. 2016, 200, 134–140. [Google Scholar] [CrossRef]
- Młynarczyk, K.; Walkowiak-Tomczak, D.; Staniek, H.; Kidoń, M.; Łysiak, G.P. The content of selected minerals, bioactive compounds, and the antioxidant properties of the flowers and fruit of selected cultivars and wildly growing plants of Sambucus nigra L. Molecules 2020, 25, 876. [Google Scholar] [CrossRef] [Green Version]
- Batista, N.N.; de Andrade, D.P.; Ramos, C.L.; Dias, D.R.; Schwan, R.F. Antioxidant capacity of cocoa beans and chocolate assessed by FTIR. Food Rev. Int. 2016, 90, 313–319. [Google Scholar] [CrossRef]
- Saputro, A.D.; Van de Walle, D.; Aidoo, R.P.; Mensah, M.A.; Delbaere, C.; De Clercq, N.; Van Durme, J.; Dewettinck, K. Quality attributes of dark chocolates formulated with palm sap-based sugar as nutritious and natural alternative sweetener. Eur. Food Res. Technol. 2017, 243, 177–191. [Google Scholar] [CrossRef]
- Praseptiangga, D.; Invicta, S.E.; Khasanah, L.U. Sensory and physicochemical characteristics of dark chocolate bar with addition of cinnamon (Cinnamomum burmannii) bark oleoresin microcapsule. J. Food Sci. Technol. 2019, 56, 4323–4332. [Google Scholar] [CrossRef] [PubMed]
- Chetana, R.; Reddy, S.R.Y.; Negi, P.S. Preparation and properties of probiotic chocolates using yoghurt powder. Food Nutr. Sci. 2013, 4, 276–281. [Google Scholar] [CrossRef] [Green Version]
- Kinta, Y.; Hatta, T. Composition and structure of fat bloom in untempered chocolate. J. Food Sci. 2005, 70, 450–452. [Google Scholar] [CrossRef]
- Burgess, S.; Xiaobing, L.; Holland, J. High spatial resolution energy dispersive X-ray spectrometry in the SEM and the detection of light elements including lithium. Microsc. Anal. 2013, 6, S8–S13. [Google Scholar]
- Hondoh, H.; Yamasaki, K.; Ikutake, M.; Ueno, S. Visualization of oil migration in chocolate using scanning electron microscopy–energy dispersive X-ray spectroscopy. Food Struct. 2016, 8, 8–15. [Google Scholar] [CrossRef]
- Adamson, G.E.; Lazarus, S.A.; Mitchell, A.E.; Prior, R.L.; Cao, G.; Jacobs, P.H.; Kremers, B.G.; Hammerstone, J.F.; Rucker, R.B.; Ritter, K.A.; et al. HPLC method for quantification of procyanidins in cocoa and chocolate samples and correlation to total antioxidant capacity. J. Agric. Food Chem. 1999, 47, 4184–4188. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Tułodziecka, A. Antioxidant capacity of rapeseed extracts obtained by conventional and ultrasound-assisted extraction. J. Am. Oil Chem. Soc. 2014, 91, 2011–2019. [Google Scholar] [CrossRef] [Green Version]
| Sample | DPPH * ± SD (μmol TE/g) | ABTS * ± SD (μmol TE/g) | CUPRAC * ± SD (μmol TE/g) | FRAP * ± SD (μmol TE/g) | TPC * ± SD (mg GA/g) |
|---|---|---|---|---|---|
| EFrE | 1911.6 ± 56.9 a | 2337.1 ± 18.9 a | 5710.4 ± 99.5 a | 229.8 ± 4.5 a | 32.5 ± 1.3 a |
| EFlE | 4765.0 ± 26.6 b | 6258.7 ± 131.2 b | 6224.0 ±54.5 b | 595.2 ± 3.4 b | 81.9 ± 3.9 b |
| ChFrE | 22,269.7 ± 161.4 c | 27,194.2 ± 242.0 c | 113,882.3 ± 303.1 c | 2341.3 ± 10.3 c | 3506 ± 2.3 c |
| Analytical Methods | EFrE | EFlE | ChFrE |
|---|---|---|---|
| DPPH | 100.16 μmol TE/g [16] | 181.07 μmol TE/g [16] | |
| 30–45% [18] | 90–95% [18] | ||
| 50.25–67.69% [19] | 91.95–94.15% [19] | ||
| ABTS | 37.91 μmol TE/g [16] | 78.90 μmol TE/g [16] | |
| 44.02 g/kg [17] | 54.27 g/kg [17] | ||
| 3.20–36.50 mM TE/kg [20] | 44.87–118.26 mM TE/kg [20] | ||
| 397.5–581.3 μmol TE/g [21] | 327.7–421.5 μmol TE/g [21] | ||
| F–C | 4415.33 mg/kg [16] | 7194.40 mg/kg [16] | |
| 80.71 g/kg [17] | 115.15 g/kg [17] | ||
| 700–1050 mg/100 g [18] | 3100–3600 mg GA/100 g [18] | ||
| 2687.6–6831.1 mg GA/kg [20] | 7410–40,137 mg GA/kg [20] | ||
| 5678.8–7087.3 mg ChA/100 g [21] | 6164.4–7561.8 mg ChA/100 g [21] |
| Analytical Method | DCh | DCh + EFrE | DCh + EFlE | DCh + ChFrE | ||||
|---|---|---|---|---|---|---|---|---|
| Ac:H2O:AA Extract | Physiological Extract | Ac:H2O:AA Extract | Physiological Extract | Ac:H2O:AA Extract | Physiological Extract | Ac:H2O:AA Extract | Physiological Extract | |
| DPPH * ± SD (μmol TE/g) | 144.2 ± 5.3 b | 72.2 ± 5.1 a | 149.3 ± 4.6 b | 146.1 ± 1.9 b | 364.3 ± 17.1 c | 151.4 ± 12.5 b | 942.7 ± 31.0 e | 745.6 ± 12.7 d |
| ABTS * ± SD (μmol TE/g) | 433.9 ± 11.5 a,b | 407.0 ± 1.1 a | 672.6 ± 2.5 d | 467.6 ± 1.6 b | 1211.0 ± 8.92 f | 575.8 ± 0.7 c | 3592.0 ± 76.7 g | 755.3 ± 6.4 e |
| CUPRAC * ± SD (μmol TE/g) | 2985.4 ± 14.7 e | 1346.3 ± 19.7 c | 3118.5 ± 7.8 f | 883.1 ± 36.0 a | 3752.3 ± 27.3 g | 1222.9 ± 46.6 b | 12,945.7 ± 132.2 h | 2773.3 ± 53.9 d |
| FRAP * ± SD (μmol TE/g) | 66.5 ± 0.7 d | 29.9 ± 1.3 a | 96.0 ± 1.1 e | 44.3 ± 0.3 b | 102.2 ± 0.8 f | 57.8 ± 0.9 c | 350.7 ± 2.3 h | 133.7 ± 2.0 g |
| F–C * ± SD (mg GA/g) | 11.7 ± 0.5 a,b | 9.8 ± 1.1 a | 12.8 ± 0.4 b | 11.1 ± 2.2 a,b | 17.9 ± 0.5 c | 17.3 ± 1.1 c | 70.9 ± 2.2 e | 67.6 ± 2.8 d |
| Analytical Methods | Plain Chocolates | Enriched Chocolates |
|---|---|---|
| Chemical extracts | ||
| DPPH | 0.044 mmol TE/g for ChP [3] | 0.022–0.031 mmol TE/g for ChP + longan [3] |
| 0.018–0.028 mmol TE/g for ChP + lychee [3] | ||
| 4012 mg TE/100 g for DCh [7] | 4373 mg TE/100 g for DCh + yellow tea extract [7] | |
| 0.08 mg TE/g for CCh [12] | 0.16–0.40 mg TE/g for CCh + black carrot extract [12] | |
| ABTS | 1.91 mmol TE/L for DCh [2] | 2.04 mmol TE/L for DCh + cranberries [2] |
| 0.060 mmol TE/g for ChP [3] | 0.022–0.044 mmol TE/g for ChP + longan [3] | |
| 0.028–0.039 mmol TE/g for ChP + lychee [3] | ||
| 9 mmol/g for DCh [5] | 9–11.5 mmol/g for DCh + red raspberry leaves extract [5] | |
| 11 mmol TE/100 g for DCh [6] | 15.4 mmol TE/100 g for DCh + Sakura green tea leaves [6] | |
| 12.2 mmol TE/100 g for DCh + turmeric powder [6] | ||
| 285 mg TE/100 g for DCh [7] | 386 mg TE/100 g for DCh + yellow tea extract [7] | |
| FRAP | 8.06 mmol Fe(II)/L for DCh [2] | 9.20 mmol Fe(II)/L for DCh + cranberries [2] |
| 13 mmol/g for DCh [5] | 13–14.5 mmol/g for DCh + red raspberry leaves extract [5] | |
| 10.1 mmol TE/100 g for DCh [6] | 15.4 mmol TE/100 g for DCh + Sakura green tea leaves [6] | |
| 10.3 mmol TE/100 g for DCh + turmeric powder [6] | ||
| F–C | 4.8 mg GA/g for DCh [2] | 6.2 mg GA/g for DCh + prunes [2] |
| 10 mg GA/g for ChP [3] | 5–7 mg GA/g for ChP + longan [3] | |
| 4.2–6.1 mg GA/g for ChP + lychee [3] | ||
| 15,425 μmol GA/100 g for DCh [6] | 20,090 μmol GA/100 g for DCh + Sakura green tea leaves [6] | |
| 17,887 μmol GA/100 g for DCh + turmeric powder [6] | ||
| 1760 mg C/100 g for DCh [7] | 2400 mg C/100 g for DCh + yellow tea extract [7] | |
| 56.0 mg GA/kg for CCh [12] | 85.0–117.7 mg GA/kg for CCh + black carrot extract [12] | |
| Physiological extracts | ||
| DPPH | 0.12 mg TE/g for CCh [12] | 0.25–0.56 mg TE/g for CCh + black carrot extract [12] |
| ABTS | 1.8–10 mmol TE/100 g for DCh [6] | 2.2–11.4 mmol TE/100 g for DCh + Sakura green tea leaves [6] |
| 2.1–9.9 mmol TE/100 g for DCh + turmeric powder [6] | ||
| FRAP | 0.9–3.9 mmol TE/100 g for DCh [6] | 1–5.4 mmol TE/100 g for DCh + Sakura green tea leaves [6] |
| 0.8–4 mmol TE/100 g for DCh + turmeric powder [6] | ||
| F–C | 1800–10,100 μmol GA/100 g for DCh [6] | 1850–13,900 μmol GA/100 g for DCh + Sakura green tea leaves [6] |
| 1900–7800 μmol GA/100 g for DCh + turmeric powder [6] | ||
| 70.8 mg GA/kg for CCh [12] | 106.6–287.7 mg GA/kg for CCh + black carrot extract [12] | |
| Sample | Moisture Content ± SD (%) | Fat content ± SD (%) | Viscosity ± SD (mPa⋅s) |
|---|---|---|---|
| DCh | 0.30 ± 0.01 a | 32.91 ± 0.35 d | 2979.24 ± 48.84 a |
| DCh + EFrE | 0.73 ± 0.02 d | 29.69 ± 0.28 b | 4493.28 ± 48.94 c |
| DCh + EFlE | 0.65 ± 0.02 c | 30.78 ± 0.15 c | 3239.72 ± 28.20 b |
| DCh + ChFrE | 0.37 ± 0.02 b | 29.12 ± 0.22 a | 4509.56 ± 28.19 c |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poliński, S.; Kowalska, S.; Topka, P.; Szydłowska-Czerniak, A. Physicochemical, Antioxidant, Microstructural Properties and Bioaccessibility of Dark Chocolate with Plant Extracts. Molecules 2021, 26, 5523. https://doi.org/10.3390/molecules26185523
Poliński S, Kowalska S, Topka P, Szydłowska-Czerniak A. Physicochemical, Antioxidant, Microstructural Properties and Bioaccessibility of Dark Chocolate with Plant Extracts. Molecules. 2021; 26(18):5523. https://doi.org/10.3390/molecules26185523
Chicago/Turabian StylePoliński, Szymon, Sylwia Kowalska, Patrycja Topka, and Aleksandra Szydłowska-Czerniak. 2021. "Physicochemical, Antioxidant, Microstructural Properties and Bioaccessibility of Dark Chocolate with Plant Extracts" Molecules 26, no. 18: 5523. https://doi.org/10.3390/molecules26185523