Abstract
Although the first published works on electrodeposition dates from more than one century ago (1905), the uses of this technique in the recovery of metals are attracting an increasing interest from the scientific community in the recent years. Moreover, the intense use of metals in electronics and the necessity to assure a second life of these devices in a context of circular economy, have increased the interest of the scientific community on electrodeposition, with almost 3000 works published per year nowadays. In this review, we aim to revise the most relevant and recent publications in the application of electrodeposition for metal recovery. These contributions have been classified into four main groups of approaches: (1) treatment and reuse of wastewater; (2) use of ionic liquids; (3) use of bio-electrochemical processes (microbial fuel cells and microbial electrolysis cells) and (4) integration of electrodeposition with other processes (bioleaching, adsorption, membrane processes, etc.). This would increase the awareness about the importance of the technology and would serve as a starting point for anyone that aims to start working in the field.
1. Introduction
Electrodeposition (ED) has been studied as one of the key electrochemical technologies for more than a century. According to the Scopus database, the first article about electrodeposition was published in 1905 [1] and studied the electrodeposition of copper upon iron. Since then, the scientific community has added new materials, methods, conditions and many other elements in order to improve this technique and its results and to widen the range of potential applications. This growing interest has caused an exponential increase in the number of publications regarding electrodeposition. Thus, Figure 1 shows the number of works published, according to the Scopus database, related to the terms “electrodeposition” or “electrochemical deposition”.
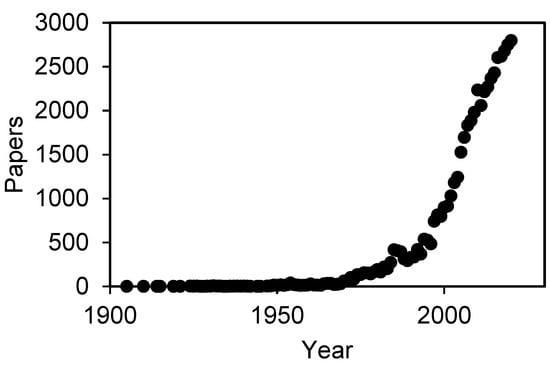
Figure 1.
Number of papers published related to “electrodeposition” appearing in either abstract, title or keywords.
As it can be seen in Figure 1, there is an exponential increase in the number of articles published in this topic, reaching a total amount of almost 56,000 papers and 3000 articles in the recent 2020 (1771 in 2021 until 23 July). This evolution means that, although electrodeposition is a mature technology, the range of applications in which this technique can be applied is widening, increasing the interest of the scientific community on its development.
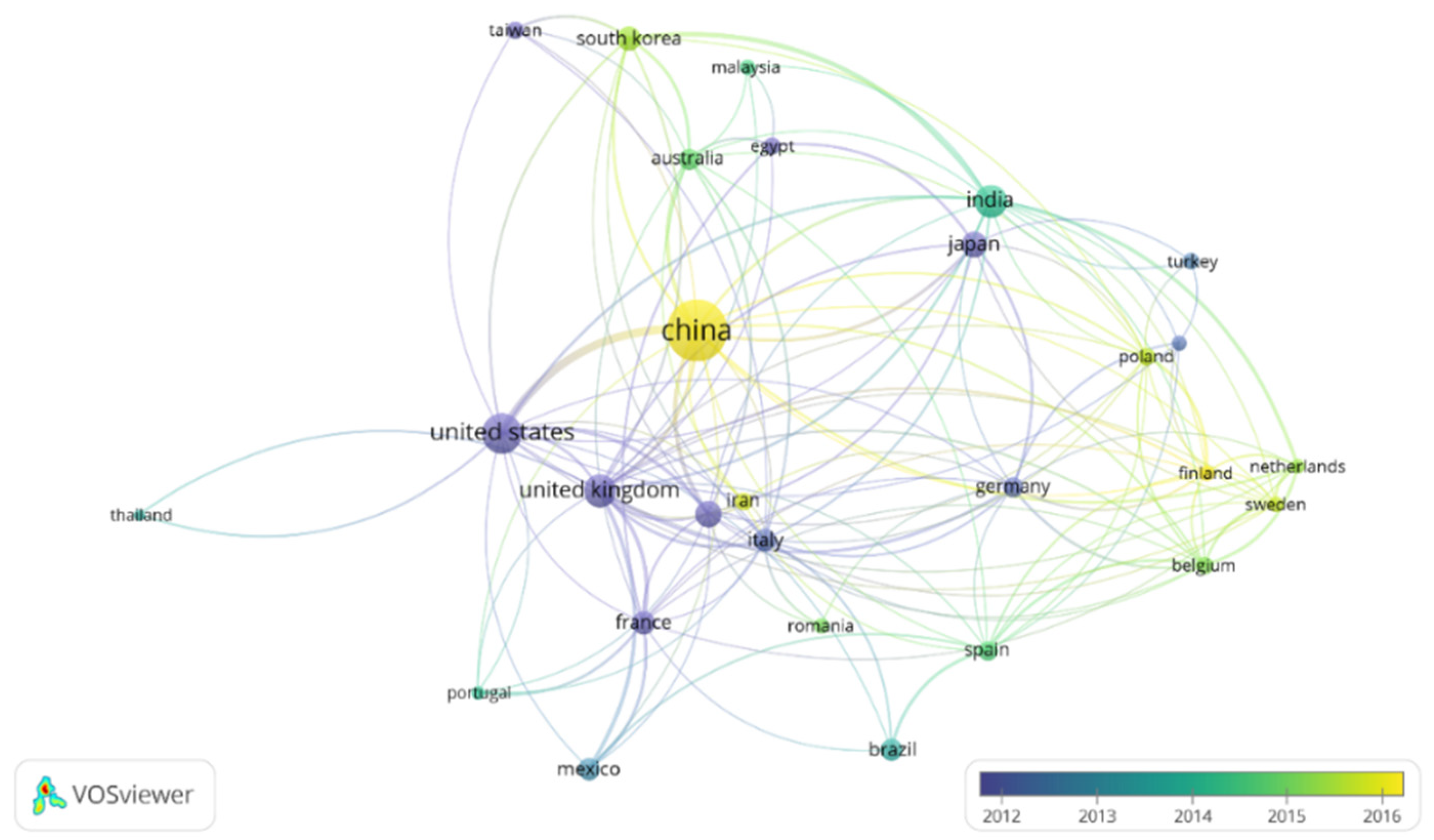
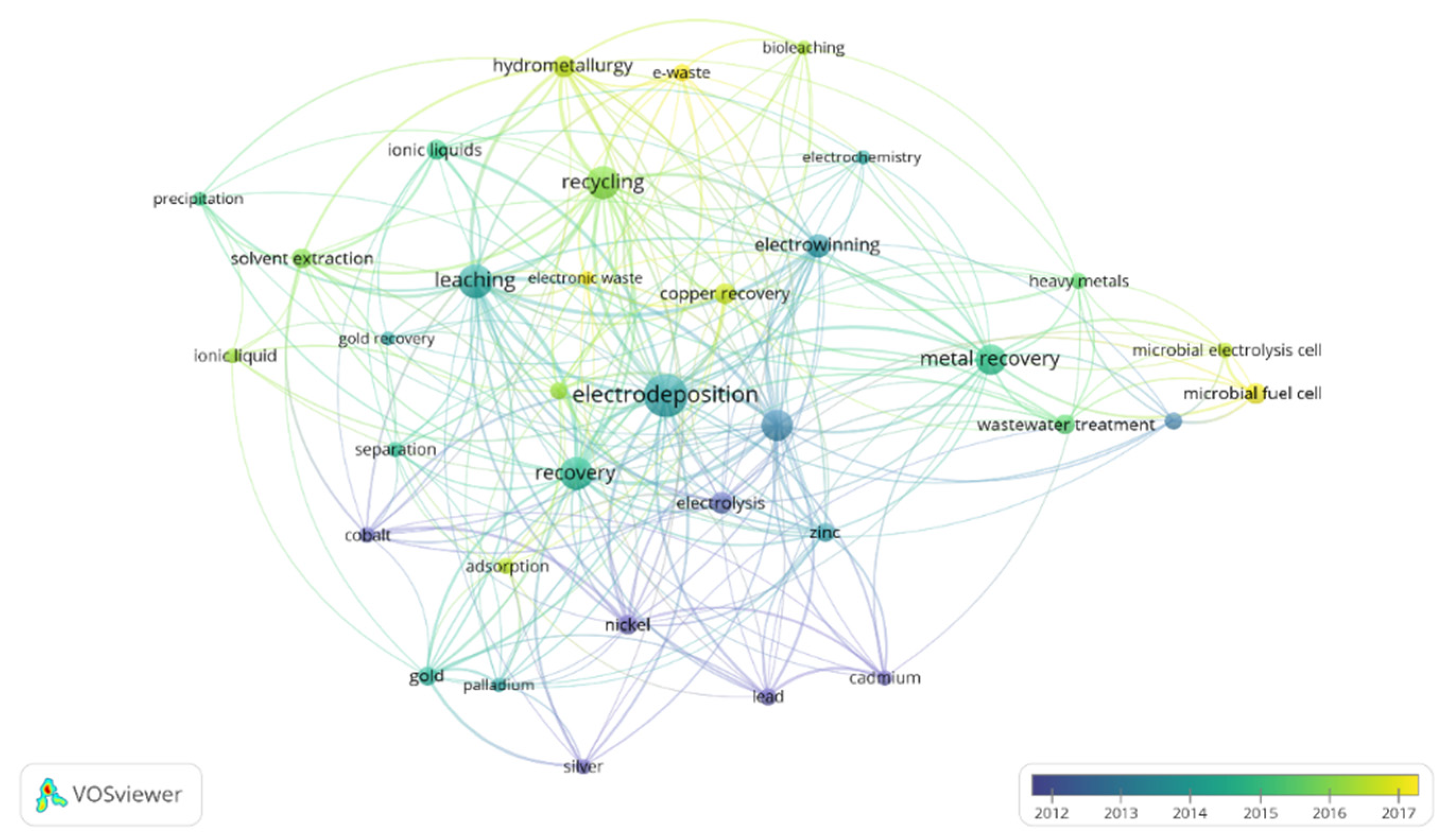
In order to focus on the topic of this review, the term “metal recovery” was included to refine this search. The results of this refined search in the Scopus database were analyzed by the software VOSviewer, a free software developed by Nees Jan van Eck and Ludo Waltman at Leiden University’s Centre for Science and Technology Studies (CWTS). Two figures were created in order to show the countries that have devoted more interest to this topic (Figure 2) and the most used keywords (Figure 3). Moreover, the connections between papers are also shown in these figures. In the case of Figure 2, it was restricted to countries with a minimum of 13 publications, meanwhile for Figure 3, a minimum number of occurrences of an author keyword of 13 was also used in order to limit the complexity of the figures.
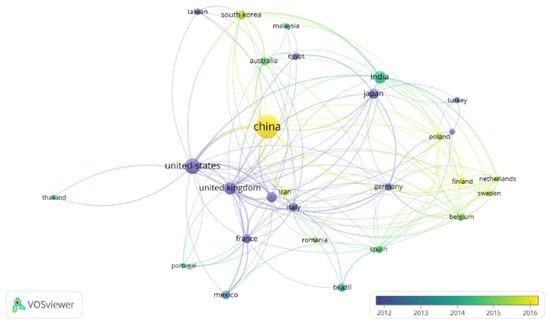
Figure 2.
Publication density of countries by years including the term “electrodeposition” and “metal recovery”. Minimum number of publications by country: 13. Figure made with the software VOSviewer.
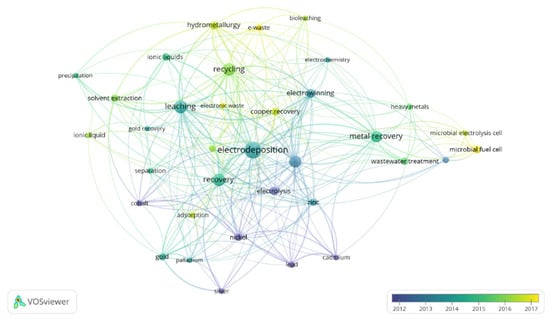
Figure 3.
Keywords of papers published per year including the terms “electrodeposition” and “metal recovery”. Minimum number of keyword occurrence: 13. Figure made with the software VOSviewer.
Almost 1300 papers were found for this topic. As it can be observed in Figure 2, China appears as the country which has devoted the highest interest in the development of electrodeposition for metal recovery. It is followed by the United States, United Kingdom, India and Japan. Regarding Figure 3, apart from electrodeposition and metal recovery (terms included in the search restrictions), recycling, leaching, different metals, ionic liquids, microbial electrolysis cell, microbial fuel cell and wastewater treatment are among the most common topics in this research field.
The use of electrodeposition for the recovery of metal ions is a topic closely related to environmental applications, as the presence of heavy metals represents an environmental issue of increasing concern, due to the massive use of metals in electronics and other applications and its inherent bioaccumulation and potential risks for the human health [2,3,4,5,6]. If this treatment is performed by electrodeposition, the removal of heavy metals is performed together with its recovery, working on the field of circular economy, an essential part of the main research programs worldwide, including Horizon Europe of the European Commission.
Based on this, the main aim of this paper is to review the most recent and relevant applications of electrodeposition, focusing on the recovery of metals. According to the most common topics of recent research work, the articles have been classified into four different groups: (1) treatment and reuse of wastewater; (2) use of ionic liquids; (3) use of bio-electrochemical processes (microbial fuel cells and microbial electrolysis cells) and (4) integration of electrodeposition with other processes (bioleaching, adsorption, electrodialysis, etc.).
2. Treatment and Reuse of Wastewater by Electrodeposition
Wastewater from industries, hospitals and domestic applications are an environmental issue of increasing concern nowadays [7,8,9,10]. As it is stated in several of the United Nations Global Development Goals and it all the research programs worldwide, it is compulsory to look for water treatments in order to obtain clean water without metals, plastics and microplastics, pesticides and other pollutants [11,12,13].
In this field, electrodeposition serves as a plausible technology to recover a wide spectrum of metals and to remove them from the target effluents to be treated. Table 1 gathers some of the most recent works in this field, including the main objective of the work, the effluent treated and the main conclusions obtained.
Generally, the articles published in the field are divided between those treating real wastewater [14,15,16,17] and those that elaborate a synthetic one [18,19,20,21,22] in order to simulate real effluents to set the material required for the installation, operational conditions, parameters to measure, elements to quantify and the equipment to use for it.
The common presence of copper in both electronic or deplating wastewaters joined to its high value of reduction potential (+0.34 V vs. SHE) and its consequent facile electrodeposition. This metal is the most commonly studied in papers regarding the recovery of metals by electrodeposition, although the recovery of other metals such as Co, Ni, Pd, Pb, Zn or Te is also evaluated, as can be observed in Table 1.
In the recent papers published about metal recovery by electrodeposition, it is possible to find several approaches to enhance the overall behavior of the process. The first interesting approach is coupling the cathodic recovery of target metals to the anodic oxidation of organic matter, as it is the case of the work of Gu et al. [14], who studied the reduction of chemical oxygen demand (COD) by oxidation of plastic deplating wastewater, obtaining a reduction of COD from 1360 mg L−1 to 378 mg L−1 after the electrochemical oxidation and a subsequent oxidation by H2O2. In the same line, Gu et al. [16] evaluated the simultaneous removal of phenol and recovery of several heavy metals from petrochemical wastewaters.

Table 1.
Most recent articles published for treatment and reuse of wastewater (WW) by electrodeposition for metal recovery.
Table 1.
Most recent articles published for treatment and reuse of wastewater (WW) by electrodeposition for metal recovery.
| Objective | Water Composition/Process Conditions | Conclusions | Ref. |
|---|---|---|---|
| Remove Ag+ by precipitation, Cu2+ by electrodeposition and reduce COD by oxidation of real wastewater from plastic deplating. | WW Composition: 5600 mg·L−1 Ag+; 9069 mg·L−1 Cu2+; 1360.00 mg·L−1 COD. Anode: Ruthenium- Iridium-Titanium (Ru-Ir-Ti) net. Cathode materials: copper sheet, titanium sheet, Ru-Ir-Ti net and graphite sheet. Current densities: from 35 to 95 mA/cm2. Temperatures: from 15 to 45 °C. Applied potential: 3.5–5.5 V. | Cu2+ recover: up to 89.3% in 80 min; purity Cu0 98.34% by ICP. Optimal conditions: Copper sheet as cathode; current density: 75 mA/cm2; 1.5 cm electrode distance; 15 °C. | [14] |
| Compare Cu electrodeposition vs. Cu precipitation for treating the depleted solutions obtained in the biomachining of copper pieces. | WW composition: 9.0 g Fe2+ L−1; 10.0 g Cu2+ L−1; pH 1.8. Electrodes: Pt Winkler. Temperature: 24 °C Constant voltage (10 V) vs. constant current (2 A) compared. | Both alternatives need previous Fe removal and allows Cu recovery (>98%) at reasonable cost. Constant voltage operation preferred with an estimated cost of 1.43 €·kg−1 Cu. | [18] |
| Removing Cu(II), Co(II), Ni(II) and Ag(I) from wastewater while generating high-power electric energy. | Synthetic WW: CuSO4 (0.0005–0.3 M); CoSO4 (0.01–0.1 M); NiSO4 (0.01–0.1 M; (NH4)2SO4 1 M. Electrodes. Cathode: copper; Anode: zinc Cathode: stirred with an egg-shaped stir bar Temperature: 18–23 °C. Applied potential: 0.13–0.32 V. | Cu recovery: >90% Optimal energy density of 4888 Whm−3 Optimal power density of 546 Wm−2 | [19] |
| Study and optimization of copper recovery with pulsed electrodeposition. | Real WW from jewelry industry: 0.21 mg·L−1 silver, 0.79 mg·L−1 nickel, 430 mg·L−1 copper, <15 ppm gold and 8.3397 ± 0.66 g·L−1 cyanide; pH 1.74. pulsed electrodeposition (30 min) Rotating cathode, Pattern 616 A—Princeton Applied Research Anode: hollow cylindrical platinum mesh | 33.59% copper removal (with 10 ms pulse, 190 mA, 70 rpm, and 37 °C) with a deposition efficiency of 84.36% in 30 min. | [15] |
| Recovery of electric energy, water, and metals through an autonomous electrochemical-osmotic system (EOS). |
Synthetic WW from copper-laden: CuSO4. Electrode: Hydrophilic carbon fiber Anode: iron plate Cathode: carbon fiber Polyamide thin-film composite (TFC) FO, asymmetric TFC FO membrane and seawater reverse osmosis membrane. 10 mV at open circuit and variation of cell voltage from 0.2 to 0.8 V. |
Maximum electric power density of 10.5 W·m−2 using a spontaneous Fe/Cu2+ galvanic cell. 98.6% recovery Cu. Electric energy production of 3.3 kW·h using a 1.0 m2 area membrane produces 1 kg copper and 100 L of water in 122 h of operation. | [20] |
| Recovery of Cu with jet electrodeposition at high current density and efficiency. | Synthetic electroplating WW: 500 mg·L−1 Cu2+, 3.15 g·L−1 SO42−; pH 1. Anode: RuO2- coated Ti tube. Cathode: pure Ti sheet. Applied voltages: −1 to 0 V. |
97.4% recovery of Cu2+ with current efficiency of 76.3% and current density of 12 A·dm−2. Total energy consumption: 5.6 kW·h·kg−1. | [21] |
| Recovery of heavy metals and simultaneous removal of phenol from multicomponent acidic media. | Real WW from petrochemical refinery. 500 mg·L−1 Cu2+; 100 mg·L−1 Zn2+; 200 mg·L−1 Pb2+; 50 mg·L−1 Cd2+; 50 mg·L−1 phenol Anode: IrO2- Ta2O5 coated Ti tube. Cathode: stainless steel. Cyclone electrochemical reactor system. Applied potential: −0.44 to −0.40 V |
100% Cu recovery. Noticeable recovery of Zn, Pb and Cd and removal of phenol Energy consumption of 0.012 kW·h for a 5 L batch | [16] |
| Electrochemical recovery of copper and nickel from acid pickling solutions at pilot scale. | Aqueous water acid pickling solutions: Ni (6–19 g·L−1); Cu (2–74.1 g·L−1); COD (1250–40000 mg·L−1); H2O2 (0–5 mg·L−1); pH (1–6); Conductivity (20.3–232 mS·cm−1) Anode materials: DSA®, graphite and lead. Cathode materials: Stainless steel and brass. Current density: 3–30 A·dm−2. Temperature: 25–50 °C. Applied potential: 0.1–0.89 V. |
Optimal removal of Cu: 100% laboratory; 40% pilot. Very low Ni removal (<20%). Optimum energy consumption: 2 kWh·kg−1 Cu. Promising results for Cu recovery from cost analysis. | [23] |
| Copper recovery from treated wood waste by sulfuric acid extraction and electrodeposition. |
Real WW from wood waste: copper quaternary (1890 mg·kg−1 Cu) and copper azole (1890 mg·kg−1 Cu) Intensities from 1 to 10 A. Anode: titanium coated with iridium oxide (Ti/IrO2). Cathode: stainless steel or copper. |
92% Cu deposition at 10 A for 90 min. 65 US$·tn−1 of profit for wood waste treatment using electrodeposition according to an economic analysis. | [17] |
| Tellurium recovery from spent Te electrolytes by cyclone electrowinning. |
Composition of Te electrolytes: 40.02, 4.96, 4.64 and 0.52 g/L of Te, Sn, S and As, respectively and 39.00, 8.00, <1, <1 and 132.91 mg/L of Se, Mg, Pb, Cu and NaOH, respectively. Anode: IrO2–Ta2O5-coated titanium. Cathode: Titanium (Ti) sheet or stainless steel sheet (316 L SS). Applied potential: 1.8–2.15 V. |
82.89% of maximum. 99.90% purity of Te deposits, 95.61% of current efficiency and 1810.58 kWh·tn−1 energy consumption. | [24] |
| To recover Co and Ni through cementation (electrochemical deposition) with Al powder as electron donor. |
Synthetic sulphate and chloride solutions: 1 mM Co/Ni; 4 pH. Process: 40 mm of shaking amplitude and 120 min−1 of shaking frequency at 25 °C for 24 h of operation. |
Recovery of 52–56% Co and 61–71% Ni from sulphate solutions. From chloride solutions: with 0.1 g AC dosage and adding Al a recovery of 61–70% Co and 70–99.9% Ni recovery from chloride solutions. | [22] |
| To recover palladium from spent heterogenous catalyst from petrochemical industries. |
Acid solution (HCl/H2O2) with heterogeneous catalyst LD-265 (Pd/Al2O3). Three-electrode glass electrochemical cell. Electrodes: flat plate graphite. | 99.07% Pd recovery with a purity of 94.02% | [25] |
| To recover copper by electro-electrodialysis (EED) from ammonia solutions. |
Cu+2 concentrations from 0.01 to 0.1 mol·dm−3. Current density from 200 to 500 A·m−2. Anode: titanium mesh coated with ruthenium oxide. Cathode: stainless steel. Applied potential: 2–4 V. |
Almost 80% current efficiency in Cu recovery. Ammonia complexing agent can be reused. | [26] |
| To obtain high-purity copper deposits from complex mixtures by electrodeposition with a centrifuge electrode. | Synthetic WW: 2–9 g·L−1 Cu2+, 2–9 g·L−1 Ni2+, 5–40 g·L−1 H2SO4 and 1.5 g·L−1 sodium dodecylsulfonate (SDS). Bipolar centrifuge Ti electrode. Cathode face (Ti); anode face (IrO2-Ta2O5) Room temperature. Potential: 0.8–1.0 V. |
99.9% copper purity obtained. The rest of metals can be also separated. | [27] |
| To recover uranium from aqueous solution into magnetite (Fe3O4) formed by iron anode dissolution and electrodeposition. | Synthetic WW: 0.5 to 10 mg·L−1 U; pH 1.6–10. Adsorbent: Fe3O4 formed at the cathode (graphite) from iron (anode) dissolution. Sorption capacity: 53.6 mg·g−1. Room temperature. Electrode gap from 4 to 19 cm. Potential: 10–30 V. | Maximum removal of U (88%) at pH: 2.6, 30 V, 8–10 cm electrode gap. | [28] |
| To recover gold from cyanide solutions using a static batch electrochemical reactor operating in an electrogenerative mode. | Cathode: three-dimensional cathodes: porous graphite and reticulated vitreous carbon (RVC) and two-dimensional cathode materials: copper and stainless steel plates). Anode: zinc. Synthetic WW: KAu(CN)2 with 500 mg·L−1 Au in 0.041 M sodium cyanide solution. Anolyte: 0.10 M sodium cyanide solution. Cathode potential vs. SHE: −0.1–(−0.05) V. |
>90% of gold can be recovered in 3 h of experiment for the cathodes studied. More than 99% gold was recovered in 1 h of operation using activated RVC. | [29] |
The second way to improve the performance of electrodeposition is by proposing novel reactor concepts with the aim of maximizing mass transfer or enhancing the reuse of the treated effluents. One interesting approach is that used by Campenedo de Morais Nepel and coworkers [15], who propose a pulsed electrodeposition with a rotating electrode in order to improve the removal of copper and the quality of the deposit. An additional system was designed by Ning and coworkers [21], who proposed a jet design (jet electrodeposition) that maximizes mass transfer by a direct injection of the solution on the cathode surface or Wang et al., who propose a centrifuge electrode to enhance the recovery of copper into high-purity solids from complex matrixes [27]. With this approach, it is possible to work at very high current densities (and thus at high recovery rates) maintaining also high recovery percentages and current efficiencies. A similar objective is obtained with the design of cyclone cells, that promote high flow rate of electrolyte on the cathode surface in order to enhance mass transfer of the process [24]. An additional interesting approach is the so-called electro-electrodialysis (EED), that combines the proper use of ion exchange membranes inside an electrochemical cell in order to allow the recovery of the metal and the simultaneous reuse of a solution of interest [26]. Finally, Lu and coworkers proposed a system that promotes the adsorption of uranium on magnetite (Fe3O4), that is produced on a graphite cathode from the Fe(II) dissolved from an iron anode [28]. Specific details about the metal recovery percentages and efficiencies can be consulted in Table 1.
Additionally, a very recent approach to upgrade the potential uses of electrodeposition for metal recovery is coupling this process with the generation of energy. This is the case of the work of Wang et al. [19], who propose to apply the process called bimetallic thermally regenerative electro-deposition battery (B-TREB), which uses waste heat to regenerate an ammonia solution that is used as the anolyte of a spontaneous galvanic cell that produce energy by oxidation of zinc and electrodeposition of copper. The similar aim is reached by using an electrochemical-osmotic system (EOS), that take advantage of the high salinity of a copper containing wastewater to produce energy by promoting a spontaneous electrochemical reaction involving the electrodeposition of copper and the oxidation or iron. Moreover, by placing an osmosis membrane between electrodes, it is possible to produce reclaimed water in the anodic compartment [20].
3. Use of Ionic Liquids in Electrodeposition
Ionic liquids (ILs) can be defined as organic ionic salts that are liquid at ambient or near ambient temperature. Among its properties, it is worth noting their low or negligible volatility, high thermal and chemical stability, high ionic conductivity, high solubility, low flammability, moderate viscosity and high polarity [30]. This interesting combination of properties have attracted an exponential increasing interest of the scientific community, with a growing number or articles published and an increasing spectrum of potential applications [31]. Among these applications, the low volatility and high electrical conductivity of ionic liquids, their potential applications electrochemical processes is continuously increasing, including their use as electrolyte in batteries and fuel cells, electrode materials for batteries/supercapacitors and carbon precursors for electrode catalysts [31,32,33,34,35].
Electrodeposition is one of these potential applications of ionic liquids in electrochemistry. The use of ILs in electrodeposition mainly tends to enhance the recovery yield of heavy metals due to the high conductivity of ILs together with their wide electrochemical window, that prevent the concurrence of hydrogen evolution [36].
Thus, Table 2 resumes the most recent and relevant papers published in the field, including the main objective, the most relevant process conditions and conclusions reached from these works and focusing mainly on those works devoted to the recovery of metals.
As it can be observed, ionic liquids are mainly used to replace aqueous environments in order to enhance the recovery percentage of high value-added metals, as gold, palladium, copper or platinum and increasing current efficiency. In general terms, using a certain percentage of ILs enhances the recovery of the target metal and allows obtaining this metal as a power of a controlled size or morphology [37,38,39,40], including some works regarding the formation of metal nanotubes [41]. It is also common to observe that the metal recovery does not improve when the concentration of the IL increases above a given threshold.
The use of ILs not only improves the recovery and morphology of the target metal, but also increases the percentage of metal extracted from mineral ores or from electronic waste as waste printed circuits or used mobile phones [37,38,42]. In this line, an approach has been done when using mixtures of ILs to prepare a deep eutectic solvent mixture, which can be used to efficiently extract metals from mineral power and further recover them by electrodeposition [43].
Although the use of ILs in electrodeposition is gaining an increasing interest, some issues should still be solved, as is the case of the low thermal and chemical stability of ILs, which hinder its reusability and thus increase its cost and environmental impact, and the high viscosity of ILs, which may lead to low mass transfer coefficients and, consequently, to mass transfer limitations [44,45].
The cost of ionic liquids is also an additional key aspect to consider for its use in the recovering of metals by electrodeposition. Although there is not a comprehensive study about the cost/benefit of using ionic liquids combined with electrodeposition, some works have presented partial conclusions on this topic. Thus, Abbott et al. conclude that the high cost and viscosity of ionic liquids make them better suited for the concentration of metals from large volumes of aqueous metal solutions to reduced volumes of ionic liquids concentrated in the target metals to be recovered [36]. Additionally, Magina et al. pointed out in their recent review about the challenges and opportunities in the use of ionic liquids, that the high cost of ionic liquids is one of the main drawbacks for the use of these group of compounds [31]. In the same line, Parmentier et al. concluded that the use of tetraoctylphosphonium oleate for cobalt concentration was more expensive in both CAPEX and OPEX than a conventional process using ion exchange resins, although they pointed out that it could be a promising alternative for the recovery of precious metals or when the brine disposal is a matter of concern [46].
To sum up, although the use of ionic liquids is a promising alternative, a detailed study of the environmental and cost issues should be carefully performed for any application to be developed.

Table 2.
Use of ionic liquids for metal recovery.
Table 2.
Use of ionic liquids for metal recovery.
| Objective | Process Conditions | Conclusions | Ref. |
|---|---|---|---|
| Evaluating the properties of N,N,N-Dimethylbutylammonium Methanesulfonate [DMBA][MS] for Pb electrodeposition from Pb-acid batteries. | Synthetic Pb solutions (from 0.025 M to 0.1 M Pb) with increasing water contents (from 20 to 60%). Cathode: copper foil. Anode: lead wire. Stable electrode potential −0.510 vs. SHE. | Electrochemical properties of IL strongly depend on water content and acid to base ratio. The morphology of the deposit depends on the potential. | [47] |
| Studying the deposition of Ta nanotube arrays by porous anodic alumina (PAA) assisted electrodeposition using 1-butyl-1-methylpyrrolidinium bis(trifluoro-methylsulfonyl) imide ([BMP]Tf2N as solvent. | Cathodes: Sitall substrate with Ni/Cr adhesion layer and sputter-deposited gold layer on the top/ PAA template (100 nm wide/1 μm length pores). Anode: platinum wire. Potentiostatic deposition at −1.4 V at 200 °C. | The main component of nanotubes was tantalum pentoxide. Synthesis of nanotubes up to 900–1000 nm long and diameters of 100 nm, characterized as semiconducting. | [41] |
| Comparing the efficiency of five new imidazolium ionic liquids for the extraction of Pt (IV) from mixed metal solutions and its further electrodeposition. | Pt extraction experiments from mixed metal solutions with Zn2+, Fe3+, Cu2+, Ni2+ and Rh(III) (2 mM each). Cathode for electrodeposition: Cu film (10 × 10 mm). Potentiostatic deposition at −1.75 V. | ILs successfully extract Pt from mixed metal solutions. Pure Pt coating and Pt nanoparticles with diameters of 2–10 nm were identified as the electrolytic products. | [48] |
| Improving the recovery of copper from waste printed circuit boards (WPCBs) by replacing H2SO4 by the ionic liquid [BSO3HPy]∙HSO4. | WPCBs powders dried and microwave-digested: 20.6% Cu; 4.55% Zn; 4.3% Al; 3.73% Fe; 2.36% Pb; 0.42% Sn. IL: [BSO3HPy]·HSO4. Slurry electrolytic system. Cathode: titanium net. Anode: graphite rod. Working conditions: 0.5 A for 3 h. | At the optimal conditions (10% H2SO4 was replaced by IL) the recovery rate, current efficiency, purity and particle size of copper powders were 90.94%, 70.68%, 81.69% and 2.30 μm, respectively. | [37] |
| Enhancing the total metal recovery from waste printed circuit boards (WPCBs) by replacing H2SO4 by the ionic liquid [MIm] HSO4. | WPCB metal-enriched scraps dried and microwave-digested: 71.96% Cu; 2.92% Pb; 2.43% Fe; 1.22% Al. IL: [MIm]HSO4. Slurry electrolysis system. Cathode: copper plate. Anode: ruthenium-plated titanium plate. Working conditions: 1.624 A (80 mA·cm−2), for 4 h. | H2SO4 substitution by IL increase recovery rate of Al, Fe, Sn and Zn but decreases that of Cu, Mg, Ni, and Pb. For 80% H2SO4 replaced: total metal recovery: 85%; purity: 89%; current efficiency: 52%; particle size of cathode metal powder; 3.77 μm. | [38] |
| To study copper leaching from waste mobile phones with different ILs and evaluate the influence of several operational variables. | Waste printed circuit boards (PCBs): 83.31% Cu; 3.35% Fe; 12.44% Zn. ILs: [Bmim]Cl; [Emim]Cl; [Bmin][BF4]; [Bmim][PF6]. Slurry electrolysis system. Cathode: titanium plate. Anode: carbon rod. | [Emim]Cl and [Bmim][PF6] are the best ILs for Cu leaching. Copper leaching efficiency was improved with the addition of H2O2. Maximal Cu recovery of 92.65% with a current efficiency of 79.99%. | [42] |
| To improve the permeability of gold from aurocyanide solutions with an ionic liquid-based polymer inclusion membrane (PIM) technology integrated with an electrodeposition unit. | [A336][SCN] as the extractant with PVDF as support. ED separated from the feed solution (50 ppm Au+) by a PIM. Cathode: copper. Anode: graphite. | 98.6% Au transported and 96.4% deposited. A constant voltage (1.5 V) applied to the stripping solution enhances Au permeability. The PIM shows outstanding stability. | [49] |
| Extraction and recovery of neodymium from acidic medium using extraction by novel undiluted and non-fluorinated ILs and electrodeposition. | Nd acid solutions with concentrations between 5·× 10−4 and 5 ×·10−2 M. ILs: 1-octyl-1-methylpiperidinium octylphos- phitePip18-OP, 1-octyl-1 methylmorpholiniumoctylphosphiteMo r18-OP and 1-octyl-1-methylpyrrolidinium octylphosphitePyr18- OP. Potentiostatic electrolysis at −2 V. Cathode: copper disk electrode. Anode: neodymium rod. | Current efficiency higher than 83%. 48% purity in Nd deposits: crystallite rods of 3–70 µm in length and 0.5–30 µm diameter. | [39] |
| Extract gold from ore in bmimHS04. | Ore: Si02 (81%w/w), CaS04 (10%w/w), Fe203 (6%w/w), Al203 (2.5%w/w), Mn02 (0.3% w/w) and Ti02 (0.2%w/w); 42 g·tonne−1 Au. IL: 1 -butyl-3-methylimidazolium hydrogen sulfate (bmimHS04). Current density: 5–200 mA·dm−2. Cathode and anode: platinum. | Gold extraction in the presence of thiourea is high yielding (86%). Current efficiency of 30% for the 20% bmimHS04 solution at a current density of 5 mA·dm−2. | [50] |
| Use ionic liquids as deep eutectic solvents (DES), for the recovery of different metals by electrodeposition. | Dissolution of a mineral powder (multielemental: Cu, Fe, Pb, Ni, Zn, Al, Au, Co, Mn, Ag and Cr). IL: Choline chloride, malonic acid and ethylene glycol were used to make the hybrid DESs. Electrode: titanium. Anode: platinum. | With a small concentration of DES added, the recovery of Cu, Fe, Pb, Ni, Zn, Al, Au, Co and Mn increased. Ag and Cr recovery increases with a high quantity of DES. | [43] |
| Removal of iron and boron by ionic liquid extraction and recovery of neodymium by ED from voice coil motors (VCMs) waste. | Nd-Fe-B magnets from VCMs. ILs: phosphonium: tri-n-butyl phosphate (TBP) and triethylpentylphosphonium bis(trifluoromethylsulfonyl)amide ([P2225][TFSA]) Cathode: Cu substrate. Anode: prismatic Nd-Fe-B rod. | %Recovery yield Fe: 95,1% with −1.75 V applied. %Recovery yield Nd: 75% with −3.25 V applied 100% B removed. | [51] |
| To study gold(I) recovery from alkaline cyanide solutions by using hydrophobic imidazolium-based ionic liquids as extractants. | ILs: 1-butyl-3-methylimidazolium hexafluorophosphate ([C4mim][PF6]), 1-hexyl-3-methylimidazolium hexafluorophosphate ([C6mim][PF6]), 1-octyl-3-methylimidazolium hexafluorophosphate ([C8mim][PF6]), and 1-octyl-3-methyl- imidazolium bis(trifluoromethylsulfonyl)imide ([C8mim][Tf2N]). Cathode: copper. Anode: platinum foil. Potentiostatic electrodeposition at −1.2 V. | Recovery of 92% with 16 h electrolysis. Excellent stability of the IL | [52] |
| Recover palladium, ruthenium and rhodium by electrodeposition controlling the morphology and composition of the deposits. | IL: 1-butyl-3-methylimidazolium chloride (bmimCl). Electrodeposition of platinoids from bmimCl. Cathode: Stainless steel. | Recovery Pd: 51% with −1.2 V applied from bmimCl. Recovery Rh: 26.6% with −1.7 V applied from bmimCl. Recovery Ru: 10,1% with −1.0 V applied from bmimPF6. | [40] |
4. Use of Bio-Electrochemical Processes
The bio-electrochemical process is defined as a system where the electrochemical reactions are taking place with the contribution of a living system. In most of the cases, the living systems are based on microorganisms, but also plants and higher organisms can be used. In the bio-electrochemical processes, the living organisms mainly contribute to the oxidation processes but can also contribute to the reduction processes.
Depending on reaction spontaneity of the bio-electrochemical systems, these systems can be defined as Microbial Fuel Cells (MFCs) or Microbial Electrolysis Cells (MECs). On the one hand, MFCs carry out spontaneous oxidative and reductive half reactions, therefore exerting a net energy flow. From the oxidative and the reductive processes, chemicals can be oxidized and reduced, leading to the removal of pollutants, the recovery of precious chemical, such as metals, etc. On the other hand, MECs carry out non-spontaneous oxidative and reductive half reactions, being necessary and an energy supply to carry out the reactions and causing, therefore, a net energy consumption [53,54].
When using bio-electrochemical systems for metal recovery, single, dual or multiple electrochemical deposition cells can be used. The single configuration is the simplest. In this configuration, the air acts as the final electron acceptor, its availability on the cathode being necessary [55]. In the dual configuration, the anode and cathode are electrically connected by an external conductor, whereas both compartments are separated by a membrane [55,56,57,58,59,60]. Finally, the multiple configuration requires the participation of several units electrically connected in series or parallel and hydraulically connected individual, cascade, etc. With the multiple-cells configuration, a higher energy production and chemical transformations can be obtained. Nevertheless, it has multiple electrical and hydraulic connections, so this can hinder the assembly [55,61,62].
In the literature, during the last years different new models or configurations have been developed with the aim to improve the performance of the bio-electrochemical systems. These modifications are very important because, depending on the shape (cylindrical, rectangular, etc.), the size, the superficial characteristics, and many other parameters, the mass transfer can be enhanced, leading to an increase in the performance yields.
In Table 3, the most relevant publications related to the metal recovery by using bio-electrochemical processes are presented. In these publications, the main operational parameters and performance of the bioelectrochemical systems have been studied.

Table 3.
Bio-electrochemical processes for metal recovery.
Nowadays, the bio-electrochemical systems seem to be a very interesting option of metal recovery from effluents. This is because these systems allow us to reach simultaneously two objectives: on the one hand, the metal recovery and on the other hand, the energy generation. The bioelectrochemical processes can be coupled with other technologies or processes, like coupling with a thermoelectric generation [63] or electrocoagulation to increase the pH and remove iron [63,68]. With the last, more than 94 and 99% Fe was recovered by electrocoagulation and MFC, respectively. Both obtained great results, but the MFC configuration yielded better results [68].
In the scientific studies, different substrates were fed to the microbial culture of the anodic chamber, those presenting higher yield in terms of electricity generation and metal recovery being the most biodegradable [65,73]. In other cases, real wastewaters have been coupled with the metals recovery in both synthetic and real AMD effluents [59] obtaining high recovery yields of the metals presenting the highest reduction potentials, mainly Cd, Cu, Fe, Al, Zn and Pb [64,66]. The coupling between anode and cathode has also been implemented with the objective of the antibiotic removal from hospital wastewater saving 478.88 Wh·m−3 of energy [67].
When using electrically enhanced systems, microbial electrolysis cells, higher metal recovery yields were obtained. These systems have the advantage that they can be done in situ [71]. Novel flow-by fixed bed bio-electrochemical reactors are also under development, allowing us to reach higher metal recoveries and energy generation efficiencies [69,70].
In conclusion, to obtain better removals and recoveries of metal is necessary to continue studying the initial concentration, molar ratios of elements from wastewaters, pH of catholyte, applied cell potential, flow regimes, inter alia as it can be proved with the scientific articles mentioned above [62,63,64,65,66,67,68,69,70,71,72].
5. Integration of Electrodeposition with Other Processes
The last approach that will be covered in this review is the combination of electrodeposition with other technologies. In general terms, the main aim of combining other technologies with electrodeposition is either transferring the metal from a solid to a liquid phase or improving the process of metal recovery by a previous stage of concentration/purification. Table 4 shows a selection of recent combination of electrodeposition with other technologies for metal recovery.
As it can be observed, the use of leaching or bioleaching to extract the metal from a solid to a liquid phase and thus allowing its recovery by electrodeposition is a common approach. Bioleaching consists in the extraction of metals from their ores using living organisms [74]. This technology has been extensively studied in order to enhance the metal recovery from many different solid matrixes polluted with metals [75,76,77,78,79,80,81]. Therefore, the coupling of bioleaching with electrodeposition has been extensively evaluated to recover copper from printed circuit boards, obtaining very high copper purities in the final deposits and metal recoveries ranging from 75.8% to 92.85% [82,83,84]. Additionally, this leaching can be also performed chemically, as it is the case of the work published by Wang et al., who propose the combination of chemical leaching (by a combination of acid and H2O2 addition) to obtain ultra-pure Ag deposits from spent silver oxide button batteries [85].

Table 4.
Integration of electrodeposition with other processes for metal recovery.
Table 4.
Integration of electrodeposition with other processes for metal recovery.
| Objective | Process Conditions (Regarding Electrodeposition Stage) | Conclusions (Regarding Electrodeposition Stage) | Ref. |
|---|---|---|---|
| To extract copper from Printed Circuit Boards (PDBs) waste by using bioleaching and electrodeposition sequentially. | Bioleaching solution (mg·L−1): Cu: 2903; Ni:17; Fe: 400; Zn: 19; Mn: 6; Al: 180; Mg: 56; Na: 17 Cathode: 316 stainless steel sheet. Anode: titanium coated with mixed metal oxide (IrO2-Ta2O5). Current density: 10 mA·cm−2. Electrowinning at 2 V cell voltage on average. | 75.8% copper from PCBs (highest pulp density) was recovered with more than 99% purity as copper foil with a current efficiency of 80.6%. The optimum time for electrodeposition operation is 3 h. | [82] |
| To recover metals (Zn, Pb and Cu) from PCDs of cell phone chargers by bioleaching and electrodeposition. | PCBs: 10.8, 68.0, and 710.9 mg·L−1 of Zn, Pb, and Cu, respectively. Cathode: steel plate. Anode: copper plate. Potential: 2.6–2.9 V; Intensity: 0.19–0.25 A; Time 2–3 h. | 92.85% recovery of Cu. The use of non-pulverized PCBs for bioleaching of metals is recommended. | [83] |
| To recover high-purity copper from PCBs by bioleaching, solvent extraction and electrodeposition. | Bioleaching solution (g·L−1): Cu: 2.196; Fe: 2.791; Mn, Pb, Sn, Zn, Al, Ni:<0.012 Cathode: stainless steel. Anode: lead–calcium–tin. Potential: 1.9–2.0 V; current density: 200–250 A·m−2; Time: 24 h | Specific energy consumption 1.8–1.9 kWh·kg−1 Cu Current efficiency of 93%. >99.83% copper metal purity. | [84] |
| To recover silver from spent silver oxide batteries by acid leaching and electrodeposition. | Raw material: spent silver oxide button batteries (SR44). Cathode and anode: glassy carbon. Potentiostatic ED: −0.6 to −0.05 V. | Ultra-pure silver (>99.98% wt.) is obtained. 98.5% metal recovery with 98.7% energy efficiency. | [85] |
| Recover Ni(II) ions from real nickel plating wastewater, with a pilot-scale fixed-bed ion exchange resin and subsequent electrodeposition. | Real WW from nickel electroplating: 500 mg·L−1 Ni(II). Anode: RuO2/IrO2 titanium plate. Cathode: titanium plate. Current density: 50–500 A·m−2. Temperature: 30–70 °C. pH: 1–5. Time: 8 h. |
Ni recovery: 95.6%. Current efficiency: 95.8%. Energy consumption: 25.05 kWh per ton electroplating wastewater. Total cost: ¥ 80000 per ton electroplating wastewater. | [86] |
| To develop a membrane capacitive deionization (MCDI) system for water desalination and metal recovery. | Real saltwater: 500 mg·L−1 CuCl2 and ZnCl2. Anode and cathode: activated carbon fiber attached onto the graphite sheet. Voltages: 0.4, 0.8. 1.0 and 1.2 V for desalination. |
Recovery Cu2+: 42.8%. Adsorption capacity: 108.7 mg·g−1 Cu2+; 122.6 mg·g−1 Zn2+. Current efficiency: From 24.1% to 36.5%. Energy consumption: from 1.24 Wh·g−1 to 1.65 Wh·g−1 | [87] |
| To recover Cu(II) from wastewater through an ion exchange process coupled with electrodeposition. | Wastewater: from 98 to 266 mg·L−1 Cu(II). Room temperature. Anode: platinum wire. Cathode: copper plates. Scanning potential: −0.7 to −0.5 V vs. Ag/AgCl. |
Energy consumption: 0.6 kWh kg−1 in electrolysis. Copper purity (96.38%). Cu recovery: 65% as electrolyzed copper. | [88] |
| To study the recovery of Cu(II) by a combination of polymer enhanced ultrafiltration (PEUF) and electrodeposition. | Synthetic nitrate solution: 160 mg·L−1 Cu for PEUF stage; 800 mg·L−1 for electrodeposition. Rotating graphite cathode (60 × 60 mm square size; 10 mm thickness). Anode: Cylindrical porous graphite. Intensity: 0.15 A; pH: 2. |
Electrodeposition is a viable via for polymer regeneration in PEUF. 100% copper recovery. Polymer used in PEUF is not affected by electrodeposition. | [89] |
Another process with results that are interesting for enhancing the efficiency of electrodeposition technique is ion exchange. The main aim of using an ion exchange resin is to increase the concentration of the metal to be recovered in order to increase the efficiency of the subsequent electrodeposition stage. Thus, when using an ion exchange, it works in cycles of operation (production of a treated solution)–regeneration. This latter regeneration stage is performed by adding an acid stream, and it produces a concentrated metal solution that is further treated by electrodeposition. With this approach, it is possible to recover nickel with very high current efficiency (95.6%) [86] and to recover Cu from wastewater with high purity (96.38%) and low energy consumption (0.6 kWh·kg−1 Cu) [88].
Further approaches have been done including the combination of membrane processes with electrodeposition. In this group, it is worth noting the combination of a membrane capacitive deionization with electrodeposition in order to simultaneously promote the desalination of a water stream with the recovery of copper, concluding that the recovery of copper is possible by the combination of technologies proposed [87]. Another example of combination of membrane technologies and electrodeposition was proposed by Camarillo et al., who combined the so-called polymer enhanced ultrafiltration technique (PEUF) with electrodeposition as an efficient process for efficient copper recovery [89]. In this latter work, PEUF uses a water-soluble polymer to recover and concentrate Cu(II) by an ultrafiltration membrane. This concentrated is further treated by electrodeposition, a technology that allows us to recover copper and to simultaneously regenerate the polymer, that can be used in a further PEUF stage.
To sum up, great recovering percentages, acceptable energy consumption and high metal purities can be obtained from both solid and liquid wastes by properly combining electrodeposition with leaching or concentration techniques.
6. Conclusions
This review covers the most relevant and recent uses of electrodeposition for metal recovery. From the information included in this this review, the following conclusions can be deduced:
- The scientific interest in the use of electrodeposition is continuously growing since it was first reported in 1904.
- The use of electrodeposition in water treatment and reuse is probably the most important topic regarding the use of the technology for metal recovery, with the most recent works mainly devoted to the development of novel reactor configurations of enhanced mass transfer characteristics.
- When using electrodeposition combined with ionic liquids, it is possible to obtain an elevated yield of value-added metals recovery and a controlled morphology and size of the deposits. The cost, stability and reusability of ILs is a matter of improvement for the development of the technology.
- The attention devoted to the use of bio-electrodeposition systems has increased within the last years, as the selection of the reactor configuration, operational conditions and source of the inoculum are critical in order to obtain the best performance of these systems.
- Electrodeposition is commonly coupled to other technologies that allow either extracting the metals from a solid phase or concentrating them in the liquid phase, leading to an upgrade of the metal recovering while saving energy.
Funding
This research was funded by Junta de Comunidades de Castilla-La Mancha’s project SBPLY/19/180501/000254 and from the Spanish Ministry of Science and Innovation through the project PID2019-107282RB-I00.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Brown, O.W.; Mathers, F.C. Electrodeposition of Copper upon Iron. J. Phys. Chem. 1906, 10, 39–51. [Google Scholar] [CrossRef] [Green Version]
- Isroni, W.; Bahri, A.S.; Maulida, N. Assessing bioaccumulation of Pb and Cu of mangroves in Sarinah island, Indonesia. Ecol. Environ. Conserv. 2020, 26, 1584–1586. [Google Scholar]
- Hu, J.; Liu, J.; Li, J.; Lv, X.; Yu, L.; Wu, K.; Yang, Y. Metal contamination, bioaccumulation, ROS generation, and epigenotoxicity influences on zebrafish exposed to river water polluted by mining activities. J. Hazard. Mater. 2021, 405, 124150. [Google Scholar] [CrossRef]
- Pradesh, A. Bioaccumulation of heavy metals in edible marine fish from coastal areas of Nellore. GSC Biol. Pharm. Sci. 2020, 10, 18–24. [Google Scholar]
- Hong, Y.-J.; Liao, W.; Yan, Z.-F.; Bai, Y.-C.; Feng, C.-L.; Xu, Z.-X.; Xu, D.-Y. Progress in the Research of the Toxicity Effect Mechanisms of Heavy Metals on Freshwater Organisms and Their Water Quality Criteria in China. J. Chem. 2020, 2020, 1–12. [Google Scholar] [CrossRef]
- Abalaka, S.E.; Enem, S.I.; Idoko, I.S.; Sani, N.A.; Tenuche, O.Z.; Ejeh, S.A.; Sambo, W.K. Heavy Metals Bioaccumulation and Health Risks with Associated Histopathological Changes in Clarias gariepinus from the Kado Fish Market, Abuja, Nigeria. J. Heal. Pollut. 2020, 10, 200602. [Google Scholar] [CrossRef] [PubMed]
- Zandaryaa, S.; Frank-Kamenetsky, D. A source-to-sea approach to emerging pollutants in freshwater and oceans: Pharmaceuticals in the Baltic Sea region. Water Int. 2021, 46, 1–16. [Google Scholar] [CrossRef]
- Laffoley, D.; Baxter, J.M. Ocean Deoxygenation: Everyone’s Problem: Causes, Impacts, Consequences and Solutions; IUCN: Gland, Switzerland, 2019. [Google Scholar]
- Eganhouse, R.P.; Kaplan, I.R. Extractable organic matter in municipal wastewaters. 1. Petroleum hydrocarbons: Temporal variations and mass emission rates to the ocean. Environ. Sci. Technol. 1982, 16, 180–186. [Google Scholar] [CrossRef]
- Tschinkel, P.F.S.; Melo, E.S.P.; Pereira, H.S.; Silva, K.R.N.; Arakaki, D.G.; Lima, N.V.; Fernandes, M.R.; Leite, L.C.S.; Melo, E.S.P.; Melnikov, P.; et al. The Hazardous Level of Heavy Metals in Different Medicinal Plants and Their Decoctions in Water: A Public Health Problem in Brazil. BioMed Res. Int. 2020, 2020, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papapostolou, C.M.; Kondili, E.M.; Zafirakis, D.P.; Tzanes, G. Sustainable water supply systems for the islands: The integration with the energy problem. Renew. Energy 2020, 146, 2577–2588. [Google Scholar] [CrossRef]
- Nakagawa, Y. Taking a Future Generation’s Perspective as a Facilitator of Insight Problem-Solving: Sustainable Water Supply Management. Sustainability 2020, 12, 1000. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Lin, T.; Chen, W. Occurrence and removal of microplastics in an advanced drinking water treatment plant (ADWTP). Sci. Total. Environ. 2020, 700, 134520. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.-N.; Liang, J.; Chen, C.; Li, K.; Zhou, W.; Jia, J.; Sun, T. Treatment of real deplating wastewater through an environmental friendly precipitation-electrodeposition-oxidation process: Recovery of silver and copper and reuse of wastewater. Sep. Purif. Technol. 2020, 248, 117082. [Google Scholar] [CrossRef]
- Nepel, T.C.D.M.; Landers, R.; Vieira, M.G.A.; Neto, A.F.D.A. Metallic copper removal optimization from real wastewater using pulsed electrodeposition. J. Hazard. Mater. 2020, 384, 121416. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Sun, Z.H.; Hu, J.; Lei, H.; Jin, W. Simultaneous Phenol Detoxification and Dilute Metal Recovery in Cyclone Electrochemical Reactor. Ind. Eng. Chem. Res. 2019, 58, 12642–12649. [Google Scholar] [CrossRef]
- Janin, A.; Coudert, L.; Mercier, G.; Blais, J.-F. Copper extraction and recovery from alkaline copper quaternary and copper azole treated wood using sulfuric acid leaching and ion exchange or electrodeposition. J. Clean. Prod. 2021, 279, 123687. [Google Scholar] [CrossRef]
- Santaolalla, A.; García, J.; Rojo, N.; Barona, A.; Gallastegui, G. Viability of two alternatives for treating waste solutions from the biomachining process. J. Clean. Prod. 2020, 270, 122549. [Google Scholar] [CrossRef]
- Wang, W.; Shu, G.; Tian, H.; Zhu, X. Removals of Cu(II), Ni(II), Co(II) and Ag(I) from wastewater and electricity generation by bimetallic thermally regenerative electro-deposition batteries. Sep. Purif. Technol. 2020, 235, 116230. [Google Scholar] [CrossRef]
- Sun, M.; Qin, M.; Wang, C.; Weng, G.-M.; Huo, M.-X.; Taylor, A.D.; Qu, J.; Elimelech, M. Electrochemical-Osmotic Process for Simultaneous Recovery of Electric Energy, Water, and Metals from Wastewater. Environ. Sci. Technol. 2020, 54, 8430–8442. [Google Scholar] [CrossRef]
- Ning, D.; Yang, C.; Wu, H. Ultrafast Cu2+ recovery from waste water by jet electrodeposition. Sep. Purif. Technol. 2019, 220, 217–221. [Google Scholar] [CrossRef]
- Choi, S.; Jeon, S.; Park, I.; Ito, M.; Hiroyoshi, N. Enhanced Cementation of Co2+ and Ni2+ from Sulfate and Chloride Solutions Using Aluminum as Electron Donor and Conductive Particles as an Electron Pathway. Metals 2021, 11, 248. [Google Scholar] [CrossRef]
- Collivignarelli, M.C.; Abbà, A.; Bestetti, M.; Crotti, B.M.; Miino, M.C. Electrolytic Recovery of Nickel and Copper from Acid Pickling Solutions Used to Treat Metal Surfaces. Water Air Soil Pollut. 2019, 230, 101. [Google Scholar] [CrossRef]
- Tian, Q.; Li, J.; Guo, X.; Li, D.; Yang, Y.; Xu, Z.; Li, W. Efficient Electrochemical Recovery of Tellurium from Spent Electrolytes by Cyclone Electrowinning. J. Sustain. Met. 2021, 7, 27–45. [Google Scholar] [CrossRef]
- Ghalehkhondabi, V.; Fazlali, A.; Daneshpour, F. Electrochemical extraction of palladium from spent heterogeneous catalysts of a petrochemical unit using the leaching and flat plate graphite electrodes. Sep. Purif. Technol. 2021, 258, 117527. [Google Scholar] [CrossRef]
- Garrido, B.; Cifuentes, G.; Fredes, P.; Pino, E.; Calderón, C.; Cifuentes-Cabezas, M. Copper Recovery from Ammonia Solutions Through Electro-Electrodialysis (EED). Front. Chem. 2021, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Gong, X.; Wang, Z. Sustainable electrochemical recovery of high-purity Cu powders from multi-metal acid solution by a centrifuge electrode. J. Clean. Prod. 2018, 204, 41–49. [Google Scholar] [CrossRef]
- Lu, B.-Q.; Li, M.; Zhang, X.-W.; Huang, C.-M.; Wu, X.-Y.; Fang, Q. Immobilization of uranium into magnetite from aqueous solution by electrodepositing approach. J. Hazard. Mater. 2018, 343, 255–265. [Google Scholar] [CrossRef]
- Yap, C.; Mohamed, N. An electrogenerative process for the recovery of gold from cyanide solutions. Chemosphere 2007, 67, 1502–1510. [Google Scholar] [CrossRef]
- Armand, M.; Endres, F.; MacFarlane, D.; Ohno, H.; Scrosati, B. Ionic-liquid materials for the electrochemical challenges of the future. Nat. Mater. 2009, 8, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Magina, S.; Barros-Timmons, A.; Ventura, S.P.M.; Evtuguin, D.V. Evaluating the hazardous impact of ionic liquids—Challenges and opportunities. J. Hazard. Mater. 2021, 412, 125215. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wang, Z.; Shi, L.; Jungsuttiwong, S.; Yuan, S. Ionic liquids for high performance lithium metal batteries. J. Energy Chem. 2021, 59, 320–333. [Google Scholar] [CrossRef]
- Ye, W.; Wang, H.; Ning, J.; Zhong, Y.; Hu, Y. New types of hybrid electrolytes for supercapacitors. J. Energy Chem. 2021, 57, 219–232. [Google Scholar] [CrossRef]
- Barrulas, R.V.; Zanatta, M.; Casimiro, T.; Corvo, M.C. Advanced porous materials from poly(ionic liquid)s: Challenges, applications and opportunities. Chem. Eng. J. 2021, 411, 128528. [Google Scholar] [CrossRef]
- Vasilyev, D.V.; Dyson, P.J. The Role of Organic Promoters in the Electroreduction of Carbon Dioxide. ACS Catal. 2021, 11, 1392–1405. [Google Scholar] [CrossRef]
- Abbott, A.; Frisch, G.; Hartley, J.; Ryder, K. Processing of metals and metal oxides using ionic liquids. Green Chem. 2011, 13, 471–481. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, M.; Tan, Q.; Wang, B.; Chen, S. Recovery of copper from WPCBs using slurry electrolysis with ionic liquid [BSO3HPy]∙HSO4. Hydrometallurgy 2018, 175, 150–154. [Google Scholar] [CrossRef]
- Qi, Y.; Yi, X.; Zhang, Y.; Meng, F.; Shu, J.; Xiu, F.; Sun, Z.; Sun, S.; Chen, M. Effect of ionic liquid [MIm]HSO4 on WPCB metal-enriched scraps refined by slurry electrolysis. Environ. Sci. Pollut. Res. 2019, 26, 33260–33268. [Google Scholar] [CrossRef]
- Zarrougui, R.; Mdimagh, R.; Raouafi, N. Highly efficient and eco-friendly extraction of neodymium using, undiluted and non-fluorinated ionic liquids. Direct electrochemical metal separation. Sep. Purif. Technol. 2017, 175, 87–98. [Google Scholar] [CrossRef]
- Jayakumar, M.; Venkatesan, K.; Sudha, R.; Srinivasan, T.; Rao, P.V. Electrodeposition of ruthenium, rhodium and palladium from nitric acid and ionic liquid media: Recovery and surface morphology of the deposits. Mater. Chem. Phys. 2011, 128, 141–150. [Google Scholar] [CrossRef]
- Simunkova, H.; Lednický, T.; Whitehead, A.; Kalina, L.; Simunek, P.; Hubalek, J. Tantalum-based nanotube arrays via porous-alumina-assisted electrodeposition from ionic liquid: Formation and electrical characterization. Appl. Surf. Sci. 2021, 548, 149264. [Google Scholar] [CrossRef]
- He, J.; Yang, J.; Tariq, S.M.; Duan, C.; Zhao, Y. Comparative investigation on copper leaching efficiency from waste mobile phones using various types of ionic liquids. J. Clean. Prod. 2020, 256, 120368. [Google Scholar] [CrossRef]
- Mendes, T.; Nano, G.; Magagnin, L. Electrochemical Recovery of Metals in Deep Eutectic Solvents. ECS Trans. 2013, 53, 63–67. [Google Scholar] [CrossRef]
- Quijada-Maldonado, E.; Olea, F.; Sepúlveda, R.; Castillo, J.; Cabezas, R.; Merlet, G.; Romero, J. Possibilities and challenges for ionic liquids in hydrometallurgy. Sep. Purif. Technol. 2020, 251, 117289. [Google Scholar] [CrossRef]
- Park, J.; Jung, Y.; Kusumah, P.; Lee, J.; Kwon, K.; Lee, C.K. Application of Ionic Liquids in Hydrometallurgy. Int. J. Mol. Sci. 2014, 15, 15320–15343. [Google Scholar] [CrossRef] [Green Version]
- Parmentier, D.; Paradis, S.; Metz, S.J.; Wiedmer, S.; Kroon, M.C. Continuous process for selective metal extraction with an ionic liquid. Chem. Eng. Res. Des. 2016, 109, 553–560. [Google Scholar] [CrossRef] [Green Version]
- Tan, S.-Y.; Bedoya-Lora, F.E.; Hallett, J.P.; Kelsall, G.H. Evaluation of N,N,N-Dimethylbutylammonium Methanesulfonate Ionic liquid for electrochemical recovery of lead from lead-acid batteries. Electrochim. Acta 2021, 376, 137893. [Google Scholar] [CrossRef]
- Chen, M.; Li, S.; Jin, C.; Shao, M.; Huang, Z. Selective recovery of platinum by combining a novel reusable ionic liquid with electrodeposition. Sep. Purif. Technol. 2021, 259, 118204. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Z.; Wang, Y.; Liu, M.; Li, S.; Tang, L.; Wang, S.; Yang, X.; Ji, S. Improved transport of gold(I) from aurocyanide solution using a green ionic liquid-based polymer inclusion membrane with in-situ electrodeposition. Chem. Eng. Res. Des. 2020, 153, 136–145. [Google Scholar] [CrossRef]
- Whitehead, J.A.; Lawrance, G.A.; Owen, M.P.; McCluskey, A. A New Route to Precious Metal Recovery and Subsequent Electrodeposition Using Ionic Liquids. ECS Proc. 2004, 2004–2024, 901–910. [Google Scholar] [CrossRef]
- Matsumiya, M.; Yamada, T.; Kikuchi, Y.; Kawakami, S. Removal of Iron and Boron by Solvent Extraction with Ionic Liquids and Recovery of Neodymium Metal by Direct Electrodeposition. Solvent Extr. Ion Exch. 2016, 34, 522–534. [Google Scholar] [CrossRef]
- Yang, X.; Yang, R.; Shi, D.; Wang, S.; Chen, J.; Guo, H. Hydrophobic ionic liquids as novel extractants for gold(I) recovery from alkaline cyanide solutions. J. Chem. Technol. Biotechnol. 2014, 90, 1102–1109. [Google Scholar] [CrossRef]
- Rabaey, J.; Angenent, K.; Schroder, L.; Keller, U. Bioelectrochemical Systems; International Water Association: London, UK, 2010. [Google Scholar]
- Hamelers, H.V.M.; Ter Heijne, A.; Sleutels, T.; Jeremiasse, A.W.; Strik, D.P.B.T.B.; Buisman, C.J.N. New applications and performance of bioelectrochemical systems. Appl. Microbiol. Biotechnol. 2010, 85, 1673–1685. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total. Environ. 2021, 754, 142429. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Li, H.; Gu, T. A state of the art review on microbial fuel cells: A promising technology for wastewater treatment and bioenergy. Biotechnol. Adv. 2007, 25, 464–482. [Google Scholar] [CrossRef]
- Janicek, A.; Fan, Y.; Liu, H. Design of microbial fuel cells for practical application: A review and analysis of scale-up studies. Biofuels 2014, 5, 79–92. [Google Scholar] [CrossRef]
- Slate, A.J.; Whitehead, K.A.; Brownson, D.; Banks, C.E. Microbial fuel cells: An overview of current technology. Renew. Sustain. Energy Rev. 2019, 101, 60–81. [Google Scholar] [CrossRef]
- Tran, T.K.; Kim, N.; Le, Q.C.; Nguyen, M.T.; Leu, H.J.; Thi, K.N.V. Electrochemical preparation and characterization of polyaniline enhanced electrodes: An application for the removal of cadmium metals in industrial wastewater. Mater. Chem. Phys. 2021, 261, 124221. [Google Scholar] [CrossRef]
- Min, B.; Logan, B.E. Continuous Electricity Generation from Domestic Wastewater and Organic Substrates in a Flat Plate Microbial Fuel Cell. Environ. Sci. Technol. 2004, 38, 5809–5814. [Google Scholar] [CrossRef]
- Choudhury, P.; Uday, U.S.P.; Mahata, N.; Tiwari, O.N.; Ray, R.N.; Bandyopadhyay, T.K.; Bhunia, B. Performance improvement of microbial fuel cells for waste water treatment along with value addition: A review on past achievements and recent perspectives. Renew. Sustain. Energy Rev. 2017, 79, 372–389. [Google Scholar] [CrossRef]
- Wu, S.; Li, H.; Zhou, X.; Liang, P.; Zhang, X.; Jiang, Y.; Huang, X. A novel pilot-scale stacked microbial fuel cell for efficient electricity generation and wastewater treatment. Water Res. 2016, 98, 396–403. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Hou, S.; Huo, Q.; Chai, L.; Qiu, G.; Zeng, W. Sequentially recover heavy metals from smelting wastewater using bioelectrochemical system coupled with thermoelectric generators. Ecotoxicol. Environ. Saf. 2020, 205, 111174. [Google Scholar] [CrossRef]
- Vélez-Pérez, L.; Ramirez-Nava, J.; Hernández-Flores, G.; Talavera-Mendoza, O.; Escamilla-Alvarado, C.; Poggi-Varaldo, H.; Solorza-Feria, O.; López-Díaz, J. Industrial acid mine drainage and municipal wastewater co-treatment by dual-chamber microbial fuel cells. Int. J. Hydrog. Energy 2020, 45, 13757–13766. [Google Scholar] [CrossRef]
- Ai, C.; Yan, Z.; Hou, S.; Zheng, X.; Zeng, Z.; Amanze, C.; Dai, Z.; Chai, L.; Qiu, G.; Zeng, W. Effective Treatment of Acid Mine Drainage with Microbial Fuel Cells: An Emphasis on Typical Energy Substrates. Minerals 2020, 10, 443. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Z.; He, Z. Selective recovery of lead and zinc through controlling cathodic potential in a bioelectrochemically-assisted electrodeposition system. J. Hazard. Mater. 2020, 386, 121941. [Google Scholar] [CrossRef]
- Wu, D.; Lu, D.; Sun, F.; Zhou, Y. Process optimization for simultaneous antibiotic removal and precious metal recovery in an energy neutral process. Sci. Total. Environ. 2019, 695, 133914. [Google Scholar] [CrossRef]
- Foudhaili, T.; Rakotonimaro, T.V.; Neculita, C.M.; Coudert, L.; Lefebvre, O.P. Comparative efficiency of microbial fuel cells and electrocoagulation for the treatment of iron-rich acid mine drainage. J. Environ. Chem. Eng. 2019, 7, 103149. [Google Scholar] [CrossRef]
- Saad, D.R.; Alismaeel, Z.T.; Abbar, A.H. Cobalt Removal from Simulated Wastewaters Using a Novel Flow-by Fixed Bed Bio-electrochemical Reactor. Chem. Eng. Process. Process. Intensif. 2020, 156, 108097. [Google Scholar] [CrossRef]
- Dávila-Pulido, G.I.; Flores-Álvarez, J.M.; Uribe-Salas, A.; López, F. Copper removal from a cyanidation liquor by electrowinning using batch and continuous flow cells. Can. Met. Q. 2019, 59, 17–25. [Google Scholar] [CrossRef]
- Jiang, Q.; Song, X.; Liu, J.; Shao, Y.; Feng, Y. In-situ Cu(II) enrichment and recovery from low-strength copper-laden wastewater using a novel electrically enhanced microbial copper recovery cell (MCRC). Chem. Eng. J. 2020, 382, 122788. [Google Scholar] [CrossRef]
- Kaur, A.; Boghani, H.C.; Milner, E.M.; Kimber, R.L.; Michie, I.S.; Daalmans, R.; Dinsdale, R.M.; Guwy, A.J.; Head, I.M.; Lloyd, J.R.; et al. Bioelectrochemical treatment and recovery of copper from distillery waste effluents using power and voltage control strategies. J. Hazard. Mater. 2019, 371, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Song, P.; Gai, R.; Yan, C.; Jiao, Y.; Yin, D.; Cai, L.; Zhang, L. Recovering platinum from wastewater by charring biofilm of microbial fuel cells (MFCs). J. Saudi Chem. Soc. 2019, 23, 338–345. [Google Scholar] [CrossRef]
- Bioleaching (Biomining) Advantages, Process & More|Anglo American. Available online: https://www.angloamerican.com/futuresmart/stories/our-industry/mining-explained/mining-terms-explained-a-to-z/bioleaching-definition-and-process (accessed on 15 April 2021).
- Santaolalla, A.; Gutierrez, J.; Gallastegui, G.; Barona, A.; Rojo, N. Immobilization of Acidithiobacillus ferrooxidans in bacterial cellulose for a more sustainable bioleaching process. J. Environ. Chem. Eng. 2021, 9, 105283. [Google Scholar] [CrossRef]
- Hosseinzadeh, F.; Rastegar, S.O.; Ashengroph, M. Bioleaching of rare earth elements from spent automobile catalyst as pretreatment method to improve Pt and Pd recovery: Process optimization and kinetic study. Process. Biochem. 2021, 105, 1–7. [Google Scholar] [CrossRef]
- Keshavarz, S.; Faraji, F.; Rashchi, F.; Mokmeli, M. Bioleaching of manganese from a low-grade pyrolusite ore using Aspergillus niger: Process optimization and kinetic studies. J. Environ. Manag. 2021, 285, 112153. [Google Scholar] [CrossRef]
- Wang, X.; Ma, L.; Wu, J.; Xiao, Y.; Tao, J.; Liu, X. Effective bioleaching of low-grade copper ores: Insights from microbial cross experiments. Bioresour. Technol. 2020, 308, 123273. [Google Scholar] [CrossRef]
- Sajjad, W.; Zheng, G.; Din, G.; Ma, X.; Rafiq, M.; Xu, W. Metals Extraction from Sulfide Ores with Microorganisms: The Bioleaching Technology and Recent Developments. Trans. Indian Inst. Met. 2019, 72, 559–579. [Google Scholar] [CrossRef]
- Yu, Z.; Han, H.; Feng, P.; Zhao, S.; Zhou, T.; Kakade, A.; Kulshrestha, S.; Majeed, S.; Li, X. Recent advances in the recovery of metals from waste through biological processes. Bioresour. Technol. 2020, 297, 122416. [Google Scholar] [CrossRef]
- Srichandan, H.; Mohapatra, R.K.; Parhi, P.K.; Mishra, S. Bioleaching approach for extraction of metal values from secondary solid wastes: A critical review. Hydrometallurgy 2019, 189, 105122. [Google Scholar] [CrossRef]
- Baniasadi, M.; Graves, J.; Ray, D.A.; De Silva, A.L.; Renshaw, D.; Farnaud, S. Closed-Loop Recycling of Copper from Waste Printed Circuit Boards Using Bioleaching and Electrowinning Processes. Waste Biomass Valorization 2021, 12, 3125–3136. [Google Scholar] [CrossRef]
- Joshi, V.; Shah, N.; Wakte, P.; Dhakephalkar, P.; Dhakephalkar, A.; Khobragade, R.; Naphade, B.; Shaikh, S.; Deshmukh, A.; Adhapure, N. Comparative bioleaching of metals from pulverized and non-pulverized PCBs of cell phone charger: Advantages of non-pulverized PCBs. Environ. Sci. Pollut. Res. 2017, 24, 28277–28286. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wang, J.; Bai, J.; Mao, W. Production of High Purity Copper from Bio-Leaching Solutions of Waste Printed Circuit Boards. In Proceedings of the 2010 4th International Conference on Bioinformatics and Biomedical Engineering, Chengdu, China, 18–20 June 2010; pp. 1–4. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, C.; Yliniemi, K.; Lundström, M. Recovery of High-Purity Silver from Spent Silver Oxide Batteries by Sulfuric Acid Leaching and Electrowinning. ACS Sustain. Chem. Eng. 2020, 8, 15573–15583. [Google Scholar] [CrossRef]
- Li, T.; Xiao, K.; Yang, B.; Peng, G.; Liu, F.; Tao, L.; Chen, S.; Wei, H.; Yu, G.; Deng, S. Recovery of Ni(II) from real electroplating wastewater using fixed-bed resin adsorption and subsequent electrodeposition. Front. Environ. Sci. Eng. 2019, 13, 91. [Google Scholar] [CrossRef]
- Wang, C.; Chen, L.; Liu, S. Activated carbon fiber for adsorption/electrodeposition of Cu (II) and the recovery of Cu (0) by controlling the applied voltage during membrane capacitive deionization. J. Colloid Interface Sci. 2019, 548, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Romero-Cano, L.A.; García-Rosero, H.; Baldenegro-Pérez, L.A.; Marín, F.C.; González-Gutiérrez, L.V. Coupled Adsorption and Electrochemical Process for Copper Recovery from Wastewater Using Grapefruit Peel. J. Environ. Eng. 2020, 146, 04020100. [Google Scholar] [CrossRef]
- Camarillo, R.; Llanos, J.; García-Fernández, L.; Perez, A.; Cañizares, P. Treatment of copper (II)-loaded aqueous nitrate solutions by polymer enhanced ultrafiltration and electrodeposition. Sep. Purif. Technol. 2010, 70, 320–328. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).