The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression
Abstract
:1. Introduction
2. Results
2.1. Body Weight, Food Intake and Locomotor Activity
2.2. Blood Parameters
2.3. Gene Expression
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Design
4.2. Diets
4.3. Plasma Assays
4.4. Expression and Purification of Mouse FGF21
4.5. Relative Quantitation Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Swinburn, B.A.; Sacks, G.; Hall, K.D.; McPherson, K.; Finegood, D.T.; Moodie, M.L.; Gortmaker, S.L. The global obesity pandemic: Shaped by global drivers and local environments. Lancet 2011, 378, 804–814. [Google Scholar] [CrossRef]
- May, C.E.; Dus, M. Confection confusion: Interplay between diet, taste, and nutrition. Trends Endocrinol. Metab. 2021, 32, 95–105. [Google Scholar] [CrossRef]
- Spinelli, S.; Monteleone, E. Food preferences and obesity. Endocrinol. Metab. 2021, 36, 209–219. [Google Scholar] [CrossRef]
- Martínez-Garza, Ú.; Torres-Oteros, D.; Yarritu-Gallego, A.; Marrero, P.F.; Haro, D.; Relat, J. Fibroblast growth factor 21 and the adaptive response to nutritional challenges. Int. J. Mol. Sci. 2019, 20, 4692. [Google Scholar] [CrossRef] [Green Version]
- Von Holstein-Rathlou, S.; Bondurant, L.D.; Peltekian, L.; Naber, M.C.; Yin, T.C.; Claflin, K.E.; Urizar, A.I.; Madsen, A.N.; Ratner, C.; Holst, B.; et al. FGF21 mediates endocrine control of simple sugar intake and sweet taste preference by the liver. Cell Metab. 2016, 23, 335–343. [Google Scholar] [CrossRef] [Green Version]
- Kharitonenkov, A.; Adams, A.C. Inventing new medicines: The FGF21 story. Mol. Metab. 2014, 3, 221–229. [Google Scholar] [CrossRef]
- Kharitonenkov, A.; Shiyanova, T.L.; Koester, A.; Ford, A.M.; Micanovic, R.; Galbreath, E.J.; Sandusky, G.E.; Hammond, L.J.; Moyers, J.S.; Owens, R.A.; et al. FGF-21 as a novel metabolic regulator. J. Clin. Invest. 2005, 115, 1627–1635. [Google Scholar] [CrossRef] [Green Version]
- Coskun, T.; Bina, H.A.; Schneider, M.A.; Dunbar, J.D.; Hu, C.C.; Chen, Y.; Moller, D.E.; Kharitonenkov, A. Fibroblast growth factor 21 corrects obesity in mice. Endocrinology 2008, 149, 6018–6027. [Google Scholar] [CrossRef]
- Xu, J.; Lloyd, D.J.; Hale, C.; Stanislaus, S.; Chen, M.; Sivits, G.; Vonderfecht, S.; Hecht, R.; Li, Y.S.; Lindberg, R.A.; et al. Fibroblast growth factor 21 reverses hepatic steatosis, increases energy expenditure, and improves insulin sensitivity in diet-induced obese mice. Diabetes 2009, 58, 250–259. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.W.; Lee, J.E.; Cha, J.J.; Hyun, Y.Y.; Kim, J.E.; Lee, M.H.; Song, H.K.; Nam, D.H.; Han, J.Y.; Han, S.Y.; et al. Fibroblast growth factor 21 improves insulin resistance and ameliorates renal injury in db/db mice. Endocrinology 2013, 154, 3366–3376. [Google Scholar] [CrossRef] [Green Version]
- Larson, K.R.; Chaffin, A.T.B.; Goodson, M.L.; Fang, Y.; Ryan, K.K. Fibroblast growth factor-21 controls dietary protein intake in male mice. Endocrinology 2019, 160, 1069–1080. [Google Scholar] [CrossRef] [PubMed]
- von Holstein-Rathlou, S.; Gillum, M.P. Fibroblast growth factor 21: An endocrine inhibitor of sugar and alcohol appetite. J. Physiol. 2019, 597, 3539–3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talukdar, S.; Owen, B.M.; Song, P.; Hernandez, G.; Zhang, Y.; Zhou, Y.; Scott, W.T.; Paratala, B.; Turner, T.; Smith, A.; et al. FGF21 regulates sweet and alcohol preference. Cell Metab. 2016, 23, 344–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, A.Y.; Workalemahu, T.; Paynter, N.P.; Rose, L.M.; Giulianini, F.; Tanaka, T.; Ngwa, J.S.; Qi, Q.; Curhan, G.C.; Rimm, E.B.; et al. Novel locus including FGF21 is associated with dietary macronutrient intake. Hum. Mol. Genet. 2013, 22, 1895–1902. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Ngwa, J.S.; Van Rooij, F.J.A.; Zillikens, M.C.; Wojczynski, M.K.; Frazier-Wood, A.C.; Houston, D.K.; Kanoni, S.; Lemaitre, R.N.; Luan, J.N.A.; et al. Genome-wide meta-analysis of observational studies shows common genetic variants associated with macronutrient intake. Am. J. Clin. Nutr. 2013, 97, 1395–1402. [Google Scholar] [CrossRef] [Green Version]
- Søberg, S.; Sandholt, C.H.; Jespersen, N.Z.; Toft, U.; Madsen, A.L.; von Holstein-Rathlou, S.; Grevengoed, T.J.; Christensen, K.B.; Bredie, W.L.P.; Potthoff, M.J.; et al. FGF21 Is a sugar-induced hormone associated with sweet intake and preference in humans. Cell Metab. 2017, 25, 1045–1053. [Google Scholar] [CrossRef] [Green Version]
- Baldini, G.; Phelan, K.D. The melanocortin pathway and control of appetite-progress and therapeutic implications. J. Endocrinol. 2019, 241, R1–R33. [Google Scholar] [CrossRef]
- Geng, L.; Lam, K.S.L.; Xu, A. The therapeutic potential of FGF21 in metabolic diseases: From bench to clinic. Nat. Rev. Endocrinol. 2020, 16, 654–667. [Google Scholar] [CrossRef]
- Talukdar, S.; Kharitonenkov, A. FGF19 and FGF21: In NASH we trust. Mol. Metab. 2021, 46, 101152. [Google Scholar] [CrossRef]
- Véniant, M.M.; Komorowski, R.; Chen, P.; Stanislaus, S.; Winters, K.; Hager, T.; Zhou, L.; Wada, R.; Hecht, R.; Xu, J. Long-acting FGF21 has enhanced efficacy in diet-induced obese mice and in obese rhesus monkeys. Endocrinology 2012, 153, 4192–4203. [Google Scholar] [CrossRef] [Green Version]
- Baruch, A.; Wong, C.; Chinn, L.W.; Vaze, A.; Sonoda, J.; Gelzleichter, T.; Chen, S.; Lewin-Koh, N.; Morrow, L.; Dheerendra, S.; et al. Antibody-mediated activation of the FGFR1/Klothoβ complex corrects metabolic dysfunction and alters food preference in obese humans. Proc. Natl. Acad. Sci. USA 2020, 117, 28992–29000. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F.; Arnold, A.P.; Reue, K. A guide for the design of pre-clinical studies on sex differences in metabolism. Cell Metab. 2017, 25, 1216–1230. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.A. Applying the new SABV (sex as a biological variable) policy to research and clinical care. Physiol. Behav. 2018, 187, 2–5. [Google Scholar] [CrossRef]
- Lee, Y.H.; Kim, S.H.; Kim, S.N.; Kwon, H.J.; Kim, J.D.; Oh, J.Y.; Jung, Y.S. Sex-specific metabolic interactions between liver and adipose tissue in MCD diet-induced non-alcoholic fatty liver disease. Oncotarget 2016, 7, 46959. [Google Scholar] [CrossRef] [Green Version]
- Chukijrungroat, N.; Khamphaya, T.; Weerachayaphorn, J.; Songserm, T.; Saengsirisuwan, V. Hepatic FGF21 mediates sex differences in high-fat high-fructose diet-induced fatty liver. Am. J. Physiol.-Endocrinol. Metab. 2017, 313, E203–E212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparin, F.R.S.; Carreño, F.O.; Mewes, J.M.; Gilglioni, E.H.; Pagadigorria, C.L.S.; Natali, M.R.M.; Utsunomiya, K.S.; Constantin, R.P.; Ouchida, A.T.; Curti, C.; et al. Sex differences in the development of hepatic steatosis in cafeteria diet-induced obesity in young mice. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 2495–2509. [Google Scholar] [CrossRef] [PubMed]
- Bazhan, N.; Jakovleva, T.; Feofanova, N.; Denisova, E.; Dubinina, A.; Sitnikova, N.; Makarova, E. Sex differences in liver, adipose tissue, and muscle transcriptional response to fasting and refeeding in mice. Cells 2019, 8, 1529. [Google Scholar] [CrossRef] [Green Version]
- Bazhan, N.; Jakovleva, T.; Balyibina, N.; Dubinina, A.; Denisova, E.; Feofanova, N.; Makarova, E. Sex dimorphism in the Fgf21 gene expression in liver and adipose tissues is dependent on the metabolic condition. Online J. Biol. Sci. 2019, 19, 28–36. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Xie, Y.; Berglund, E.D.; Colbert Coate, K.; He, T.T.; Katafuchi, T.; Xiao, G.; Potthoff, M.J.; Wei, W.; Wan, Y.; et al. The starvation hormone, fibroblast growth factor-21, extends lifespan in mice. Elife 2012, 2012, e00065. [Google Scholar] [CrossRef]
- Makarova, E.N.; Yakovleva, T.V.; Balyibina, N.Y.; Baranov, K.O.; Denisova, E.I.; Dubinina, A.D.; Feofanova, N.A.; Bazhan, N.M. Pharmacological effects of fibroblast growth factor 21 are sex-specific in mice with the lethal yellow (Ay) mutation. Vavilovskii Zhurnal Genet. Selektsii 2020, 24, 200. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Stanislaus, S.; Chinookoswong, N.; Lau, Y.Y.; Hager, T.; Patel, J.; Ge, H.; Weiszmann, J.; Lu, S.C.; Graham, M.; et al. Acute glucose-lowering and insulin-sensitizing action of FGF21 in insulin-resistant mouse models-Association with liver and adipose tissue effects. Am. J. Physiol.-Endocrinol. Metab. 2009, 297, E1105–E1114. [Google Scholar] [CrossRef] [Green Version]
- Kharitonenkov, A.; Larsen, P. FGF21 reloaded: Challenges of a rapidly growing field. Trends Endocrinol. Metab. 2011, 22, 81–86. [Google Scholar] [CrossRef]
- Markan, K.R.; Naber, M.C.; Ameka, M.K.; Anderegg, M.D.; Mangelsdorf, D.J.; Kliewer, S.A.; Mohammadi, M.; Potthoff, M.J. Circulating FGF21 is liver derived and enhances glucose uptake during refeeding and overfeeding. Diabetes 2014, 63, 4057–4063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruigrok, S.R.; Kotah, J.M.; Kuindersma, J.E.; Speijer, E.; van Irsen, A.A.S.; la Fleur, S.E.; Korosi, A. Adult food choices depend on sex and exposure to early-life stress: Underlying brain circuitry, adipose tissue adaptations and metabolic responses. Neurobiol. Stress 2021, 15, 100360. [Google Scholar] [CrossRef]
- Medrikova, D.; Jilkova, Z.M.; Bardova, K.; Janovska, P.; Rossmeisl, M.; Kopecky, J. Sex differences during the course of diet-induced obesity in mice: Adipose tissue expandability and glycemic control. Int. J. Obes. 2012, 36, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Ingvorsen, C.; Karp, N.A.; Lelliott, C.J. The role of sex and body weight on the metabolic effects of high-fat diet in C57BL/6N mice. Nutr. Diabetes 2017 74 2017, 7, e261. [Google Scholar] [CrossRef]
- Stubbins, R.E.; Holcomb, V.B.; Hong, J.; Núñez, N.P. Estrogen modulates abdominal adiposity and protects female mice from obesity and impaired glucose tolerance. Eur. J. Nutr. 2012, 51, 861–870. [Google Scholar] [CrossRef] [PubMed]
- Camporez, J.P.; Lyu, K.; Goldberg, E.L.; Zhang, D.; Cline, G.W.; Jurczak, M.J.; Dixit, V.D.; Petersen, K.F.; Shulman, G.I. Anti-inflammatory effects of oestrogen mediate the sexual dimorphic response to lipid-induced insulin resistance. J. Physiol. 2019, 597, 3885–3903. [Google Scholar] [CrossRef]
- Rudnicki, M.; Abdifarkosh, G.; Rezvan, O.; Nwadozi, E.; Roudier, E.; Haas, T.L. Female mice have higher angiogenesis in perigonadal adipose tissue than males in response to high-fat diet. Front. Physiol. 2018, 9, 1452. [Google Scholar] [CrossRef] [Green Version]
- MacCannell, A.D.V.; Futers, T.S.; Whitehead, A.; Moran, A.; Witte, K.K.; Roberts, L.D. Sexual dimorphism in adipose tissue mitochondrial function and metabolic flexibility in obesity. Int. J. Obes. 2021, 45, 1773–1781. [Google Scholar] [CrossRef]
- Yau, W.W.; Singh, B.K.; Lesmana, R.; Zhou, J.; Sinha, R.A.; Wong, K.A.; Wu, Y.; Bay, B.H.; Sugii, S.; Sun, L.; et al. Thyroid hormone (T3) stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Autophagy 2019, 15, 131–150. [Google Scholar] [CrossRef] [Green Version]
- Kaikaew, K.; Grefhorst, A.; Visser, J.A. Sex differences in brown adipose tissue function: Sex hormones, glucocorticoids, and their crosstalk. Front. Endocrinol. 2021, 12, 357. [Google Scholar] [CrossRef]
- Pawlak, M.; Lefebvre, P.; Staels, B. Molecular mechanism of PPARα action and its impact on lipid metabolism, inflammation and fibrosis in non-alcoholic fatty liver disease. J. Hepatol. 2015, 62, 720–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, C.F.; Ku, H.C.; Lin, H. Pgc-1α as a pivotal factor in lipid and metabolic regulation. Int. J. Mol. Sci. 2018, 19, 3447. [Google Scholar] [CrossRef] [Green Version]
- Stern, J.H.; Rutkowski, J.M.; Scherer, P.E. Adiponectin, leptin, and fatty acids in the maintenance of metabolic homeostasis through adipose tissue crosstalk. Cell Metab. 2016, 23, 770–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solon-Biet, S.M.; Cogger, V.C.; Pulpitel, T.; Heblinski, M.; Wahl, D.; McMahon, A.C.; Warren, A.; Durrant-Whyte, J.; Walters, K.A.; Krycer, J.R.; et al. Defining the nutritional and metabolic context of FGF21 using the geometric framework. Cell Metab. 2016, 24, 555–565. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owen, B.M.; Ding, X.; Morgan, D.A.; Coate, K.C.; Bookout, A.L.; Rahmouni, K.; Kliewer, S.A.; Mangelsdorf, D.J. FGF21 acts centrally to induce sympathetic nerve activity, energy expenditure, and weight loss. Cell Metab. 2014, 20, 670–677. [Google Scholar] [CrossRef] [Green Version]
- Fletcher, J.A.; Linden, M.A.; Sheldon, R.D.; Meers, G.M.; Morris, E.M.; Butterfield, A.; Perfield, J.W.; Rector, R.S.; Thyfault, J.P. Fibroblast growth factor 21 increases hepatic oxidative capacity but not physical activity or energy expenditure in hepatic peroxisome proliferator-activated receptor γ coactivator-1α-deficient mice. Exp. Physiol. 2018, 103, 408–418. [Google Scholar] [CrossRef]
- Schlein, C.; Talukdar, S.; Heine, M.; Fischer, A.W.; Krott, L.M.; Nilsson, S.K.; Brenner, M.B.; Heeren, J.; Scheja, L. FGF21 lowers plasma triglycerides by accelerating lipoprotein catabolism in white and brown adipose tissues. Cell Metab. 2016, 23, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Flippo, K.H.; Potthoff, M.J. Metabolic messengers: FGF21. Nat. Metab. 2021, 3, 309–317. [Google Scholar] [CrossRef]
- BonDurant, L.D.; Ameka, M.; Naber, M.C.; Markan, K.R.; Idiga, S.O.; Acevedo, M.R.; Walsh, S.A.; Ornitz, D.M.; Potthoff, M.J. FGF21 regulates metabolism through adipose-dependent and -independent mechanisms. Cell Metab. 2017, 25, 935–944. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuda, H.; Iritani, N.; Sugimoto, T.; Ikeda, H. Transcriptional regulation of fatty acid synthase gene by insulin/glucose, polyunsaturated fatty acid and leptin in hepatocytes and adipocytes in normal and genetically obese rats. Eur. J. Biochem. 1999, 260, 505–511. [Google Scholar] [CrossRef] [PubMed]
- Deng, T.; Shan, S.; Li, P.P.; Shen, Z.F.; Lu, X.P.; Cheng, J.; Ning, Z.Q. Peroxisome proliferator-activated receptor-γ transcriptionally up-regulates hormone-sensitive lipase via the involvement of specificity protein-1. Endocrinology 2006, 147, 875–884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakamura, M.T.; Yudell, B.E.; Loor, J.J. Regulation of energy metabolism by long-chain fatty acids. Prog. Lipid Res. 2014, 53, 124–144. [Google Scholar] [CrossRef] [PubMed]
- Stevanovic, D.M.; Hebert, A.J.; Desai, B.N.; Singhal, G.; Adams, A.C.; Flier, J.S.; Maratos-Flier, E. Fibroblast Growth Factor 21 (FGF21) creates sugar-specific taste aversion to fructose through action in the brain in mice. bioRxiv 2020. [Google Scholar] [CrossRef]
- Jensen-Cody, S.O.; Flippo, K.H.; Claflin, K.E.; Yavuz, Y.; Sapouckey, S.A.; Walters, G.C.; Usachev, Y.M.; Atasoy, D.; Gillum, M.P.; Potthoff, M.J. FGF21 signals to glutamatergic neurons in the ventromedial hypothalamus to suppress carbohydrate intake. Cell Metab. 2020, 32, 273–286. [Google Scholar] [CrossRef]
- Hill, C.M.; Laeger, T.; Dehner, M.; Albarado, D.C.; Clarke, B.; Wanders, D.; Burke, S.J.; Collier, J.J.; Qualls-Creekmore, E.; Solon-Biet, S.M.; et al. FGF21 signals protein status to the brain and adaptively regulates food choice and metabolism. Cell Rep. 2019, 27, 2934–2947. [Google Scholar] [CrossRef] [Green Version]
- Flippo, K.H.; Jensen-Cody, S.O.; Claflin, K.E.; Potthoff, M.J. FGF21 signaling in glutamatergic neurons is required for weight loss associated with dietary protein dilution. Sci. Rep. 2020, 10, 19521. [Google Scholar] [CrossRef]
- Hagan, M.M.; Rushing, P.A.; Benoit, S.C.; Woods, S.C.; Seeley, R.J. Opioid receptor involvement in the effect of AgRP-(83-132) on food intake and food selection. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2001, 280, R814–R821. [Google Scholar] [CrossRef]
- Hu, S.; Wang, L.; Yang, D.; Li, L.; Togo, J.; Wu, Y.; Liu, Q.; Li, B.; Li, M.; Wang, G.; et al. Dietary fat, but not protein or carbohydrate, regulates energy intake and causes adiposity in mice. Cell Metab. 2018, 28, 415–431. [Google Scholar] [CrossRef] [Green Version]
- Allard, C.; Bonnet, F.; Xu, B.; Coons, L.; Albarado, D.; Hill, C.; Fagherazzi, G.; Korach, K.S.; Levin, E.R.; Lefante, J.; et al. Activation of hepatic estrogen receptor-α increases energy expenditure by stimulating the production of fibroblast growth factor 21 in female mice. Mol. Metab. 2019, 22, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Badakhshi, Y.; Shao, W.; Liu, D.; Tian, L.; Pang, J.; Gu, J.; Hu, J.; Jin, T. Estrogen-Wnt signaling cascade regulates expression of hepatic fibroblast growth factor 21. Am. J. Physiol. Metab. 2021, 321, E292–E304. [Google Scholar] [CrossRef]
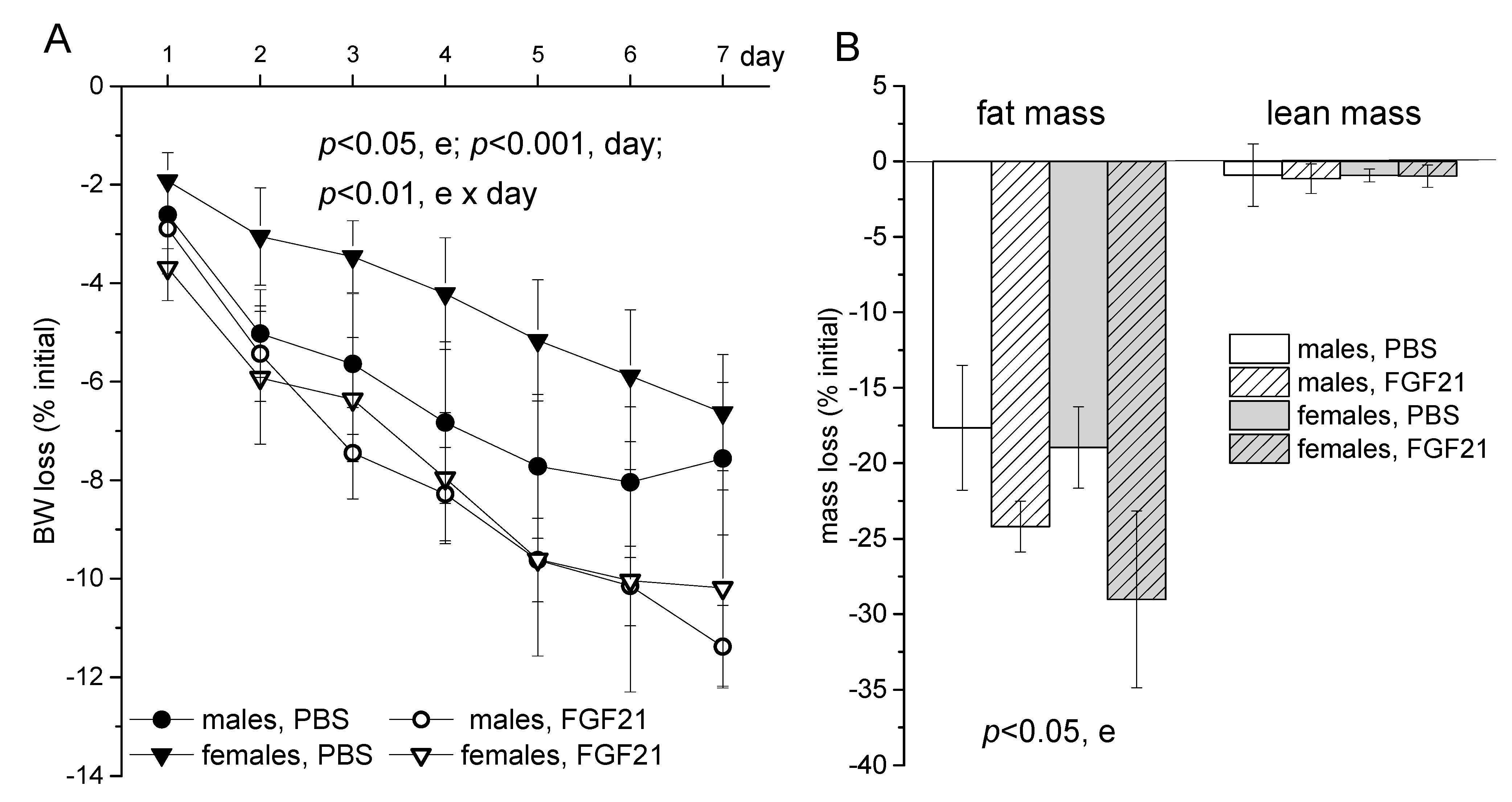
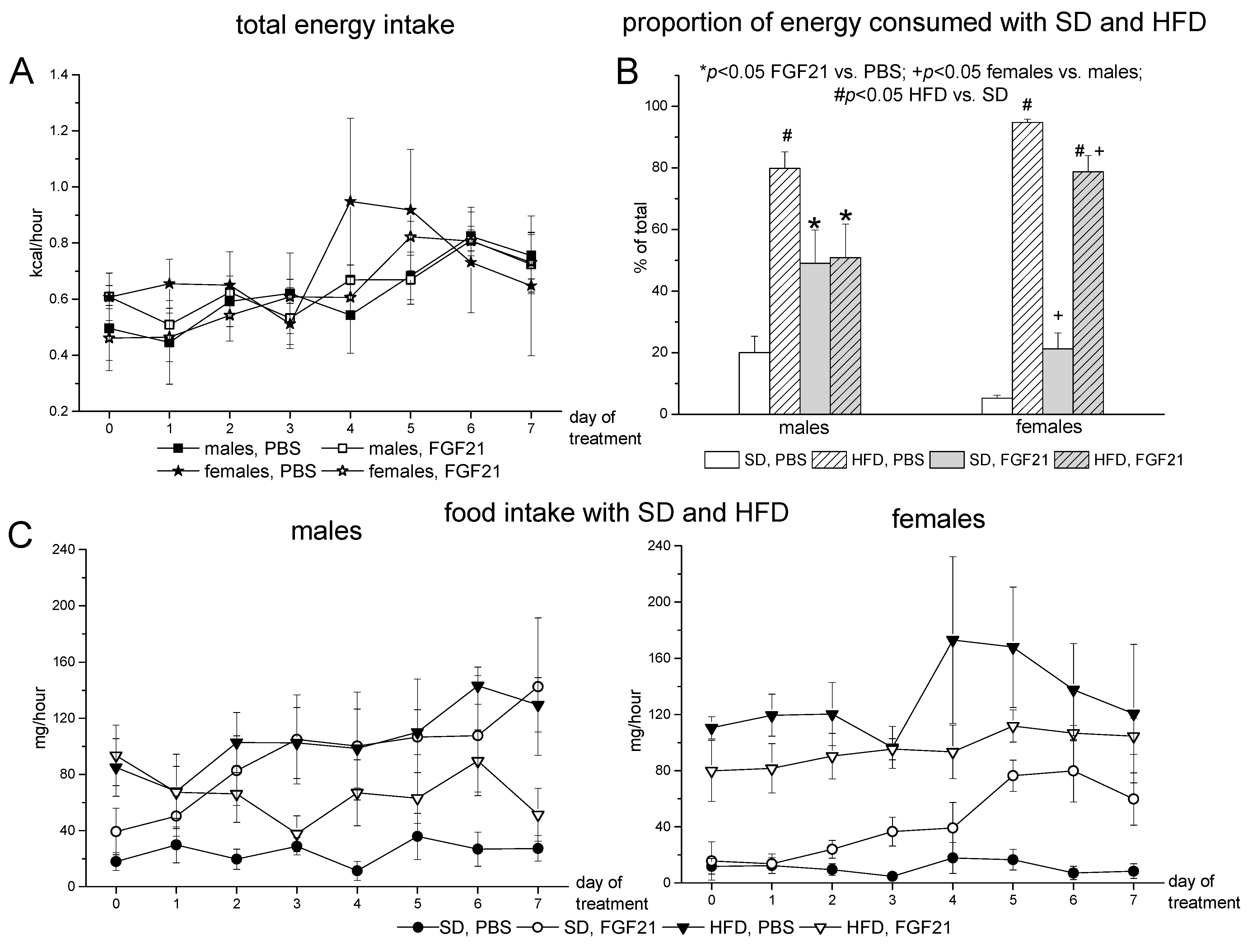


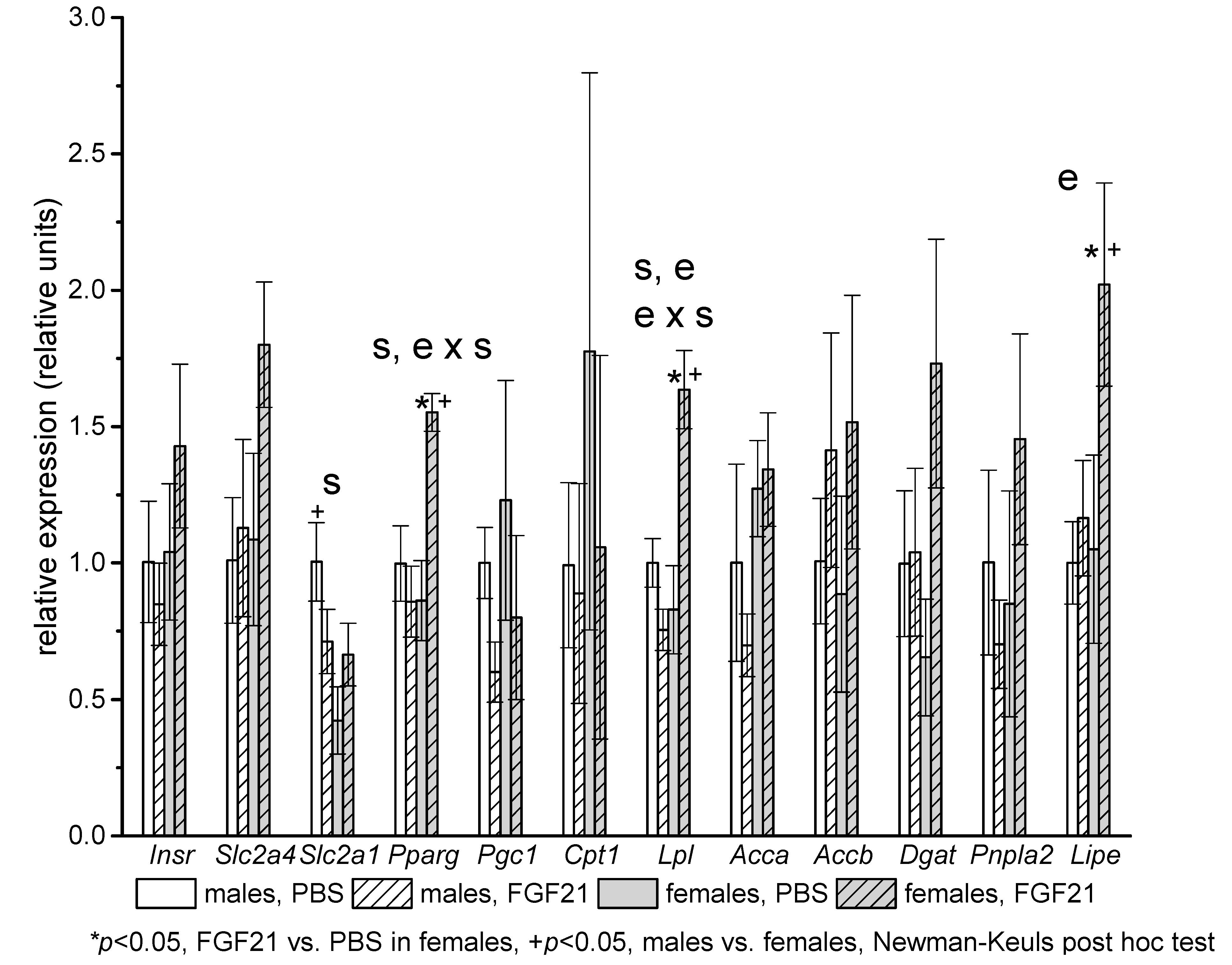

| Males | Females | p ANOVA | |||
|---|---|---|---|---|---|
| PBS (n = 7) | FGF21 (n = 7) | PBS (n = 5) | FGF21 (n = 5) | ||
| BW day 0 (g) | 41.9 ± 0.6 | 43.0 ± 0.7 | 31.6 ± 0.4 *** | 32.4 ± 1.5 *** | < 0.001, s |
| BW day 7 (g) | 38.7 ± 0.7 | 38.1 ± 0.4 | 29.5 ± 0.5 *** | 29.1 ± 1.6 *** | < 0.001, s |
| Fat day 0 (g) | 15.5 ± 0.5 | 15.7 ± 0.5 | 10.1 ± 0.5 *** | 11.2 ± 1.5 ** | < 0.001, s |
| Fat index day 0 | 0.37 ± 0.01 | 0.364 ± 0.007 | 0.32 ± 0.01 | 0.34 ± 0.03 | < 0.05, s |
| Fat day 7 (g) | 12.7 ± 0.58 | 11.9 ± 0.4 | 8.2 ± 0.3 ** | 8.15 ± 1.53 ** | < 0.001, s |
| Fat index day 7 | 0.33 ± 0.01 | 0.312 ± 0.008 | 0.28 ± 0.01 | 0.27 ± 0.03 | < 0.05, s |
| Liver (g) | 1.34 ± 0.06 | 1.38 ± 0.07 | 0.98 ± 0.02 *** | 1.0 ± 0.04 *** | < 0.001, s |
| Liver index | 0.035 ± 0.001 | 0.036 ± 0.002 | 0.033 ± 0.001 | 0.035 ± 0.002 | NS |
| iBAT (g) | 0.118 ± 0.007 | 0.114 ± 0.009 | 0.082 ± 0.004 | 0.083 ± 0.007 | <0.001, s |
| iBAT index | (3.04 ± 0.18) × 10−3 | (3.0 ± 0.24) × 10−3 | (2.8 ± 0.14) × 10−3 | (2.8 ± 0.11) × 10−3 | NS |
| Males | Females | p ANOVA | |||
|---|---|---|---|---|---|
| PBS (n = 6) | FGF21 (n = 6) | PBS (n = 5) | FGF21 (n = 5) | ||
| FFA (mM) | 0.54 ± 0.18 | 0.63 ± 0.22 | 0.50 ± 0.11 | 0.49 ± 0.05 | ns |
| Triglycerides (mM) | 1.03 ± 0.05 | 0.92 ± 0.04 | 0.93 ± 0.08 | 0.91 ± 0.09 | ns |
| Cholesterol (mM) | 4.69 ± 0.26 | 3.53 ± 0.25 * | 3.46 ± 0.18 + | 2.72 ± 0.09 *+ | p < 0.001, sp < 0.001, e |
| Glucose (mM) | 15.9 ± 1.0 | 13.7 ± 0.8 | 14.1 ± 0.9 | 11.6 ± 1.0 | p = 0.05, sp < 0.05, e |
| Insulin (ng/mL) | 6.9 ± 0.6 | 5.2 ± 0.5 * | 3.5 ± 0.4 + | 1.8 ± 0.5 *+ | p < 0.001, sp < 0.01, e |
| Adiponectin(mkg/mL) | 4.9 ± 0.3 | 4.5 ± 0.3 | 6.5 ± 0.2 + | 6.1 ± 0.5 + | p < 0.001, s |
| Leptin (ng/mL) | 18.5 ± 3.9 | 14.3 ± 2.3 | 7.5 ± 0.9 + | 6.3 ± 1.7 | p < 0.01, s |
| Mouse Age (Weeks) | Experiment (Days) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 12–28 | 28–38 | −2 | −1 | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
| Obesity development (consumption of SD + HFD) | Selection of obese mice | Adaptation to Pheno-Master cages | Fat and lean mass | Injections of PBS or FGF21 | Fat and lean mass atDecapitation | |||||||
| Body weight, SD and HFD intake, locomotor activity | ||||||||||||
| Protein | Gene | Gene Expression Assay ID |
|---|---|---|
| Acetyl-coenzyme A carboxylase alpha | Acca | Mm01304285_m1 |
| Acetyl-coenzyme A carboxylase beta | Accb | Mm01204683_m1 |
| Agouti related neuropeptide | Agrp | Mm00475829_g1 |
| Beta-actin | Actb | Mm00607939_s1 |
| Carnitine palmitoyltransferase 1a | Cpt1a | Mm01231183_m1 |
| Carnitine palmitoyltransferase 1b | Cpt1b | Mm00487191_g1 |
| Corticotropin releasing hormone | Crh | Mm01293920_s1 |
| Diglyceride acyltransferase | Dgat | Mm00515643_m1 |
| Deiodinase, iodothyronine, type II | Dio2 | Mm00515664_m1 |
| Fatty acid synthase | Fasn | Mm00662319_m1 |
| Fibroblast growth factor 21 | Fgf21 | Mm00840165_g1 |
| Glucose-6-phosphatase, catalytic | G6pc | Mm00839363_m1 |
| Glucokinase | Gck | Mm00439129_m1 |
| Insulin receptor | Insr | Mm01211875_m1 |
| Leptin receptor | Lepr | Mm00440181_m1 |
| Lipase, hormone sensitive | Lipe | Mm00495359_m1 |
| Lipoprotein lipase | Lpl | Mm00434764_m1 |
| Neuropeptide Y | Npy | Mm01410146_m1 |
| Patatin-like phospholipase domain containing 2 (adipocyte triglyceride lipase, ATGL) | Pnpla2 | Mm00503040_m1 |
| Peroxisome proliferative activated receptor, gamma, coactivator 1 alpha | Ppargc1a (Pgc1) | Mm01208835_m1 |
| Peroxisome proliferator activated receptor alpha | Ppara | Mm0040939_m1 |
| Peroxisome proliferator activated receptor gamma | Pparg | Mm00440940_m1 |
| Phosphoenolpyruvate carboxykinase 1, cytosolic | Pck1 | Mm01247058_m1 |
| Pro-opiomelanocortin | Pomc | Mm00435874_m1 |
| Pyruvate kinase liver and red blood cell | Pklr | Mm00443090_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 1 (GLUT1) | Slc2a1 | Mm00441480_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 2 (GLUT2) | Slc2a2 | Mm00446229_m1 |
| Solute carrier family 2 (facilitated glucose transporter), member 4 (GLUT4) | Slc2a4 | Mm00436615_m1 |
| Uncoupling protein 1 (mitochondrial, proton carrier) | Ucp1 | Mm01244861_m1 |
| Uncoupling protein 3 (mitochondrial, proton carrier) | Ucp3 | Mm01163394_m1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makarova, E.; Kazantseva, A.; Dubinina, A.; Jakovleva, T.; Balybina, N.; Baranov, K.; Bazhan, N. The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression. Int. J. Mol. Sci. 2021, 22, 10561. https://doi.org/10.3390/ijms221910561
Makarova E, Kazantseva A, Dubinina A, Jakovleva T, Balybina N, Baranov K, Bazhan N. The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression. International Journal of Molecular Sciences. 2021; 22(19):10561. https://doi.org/10.3390/ijms221910561
Chicago/Turabian StyleMakarova, Elena, Antonina Kazantseva, Anastasia Dubinina, Tatiana Jakovleva, Natalia Balybina, Konstantin Baranov, and Nadezhda Bazhan. 2021. "The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression" International Journal of Molecular Sciences 22, no. 19: 10561. https://doi.org/10.3390/ijms221910561
APA StyleMakarova, E., Kazantseva, A., Dubinina, A., Jakovleva, T., Balybina, N., Baranov, K., & Bazhan, N. (2021). The Same Metabolic Response to FGF21 Administration in Male and Female Obese Mice Is Accompanied by Sex-Specific Changes in Adipose Tissue Gene Expression. International Journal of Molecular Sciences, 22(19), 10561. https://doi.org/10.3390/ijms221910561






