1. Introduction
Forests across North America are managed using a variety of different silvicultural strategies, including clear cutting, tree thinning, and prescribed fire [
1]. Forest landscapes in the eastern U.S. have relied on fires for shaping their natural history, and several species evolved in response to direct and indirect effects from fire. The silvicultural practice of prescribed burning reduces the conditions leading to severe wildfires and improves overall forest productivity [
2]. However, improper use of prescribed burning can have detrimental effects such as crown scorch in
Pinus species (e.g., loblolly (
P. taeda), slash (
P. elliottii), and longleaf (
P. palustris)) and can also remove nutrients via high intensity burns and sediment loss through increased erosion exposure to rainfall [
3,
4]. Research involving “burn vs. non-burn” treatments demonstrated an increase in diversity over time of both plants and animals [
5,
6]. While prescribed fires may be overall beneficial for the plant and animal community, they may induce energetically costly behaviors from wildlife to avoid an active fire, or death.
‘Escape responses’ describe the behaviors of wildlife to avoid potential threats, including examples of wildlife avoiding fire [
7,
8]. Using sensory systems and basic survival instincts, many animals demonstrate a realization of immediate danger and avoid it altogether [
9,
10,
11]. Animals are particularly susceptible to fire when they are impaired or non-mobile, as with altricial young (i.e., mouse pups, bird nestlings). Winter habits of bats are not normally considered regarding winter fire management in forests, but eastern red bats (
Lasiurus borealis) have attracted interest because of their unique habit of roosting under the leaf litter and utilizing torpor [
12,
13,
14,
15,
16,
17,
18]. Considering torpor is a state of reduction in activity and metabolism, eastern red bats under the leaf litter are at a unique risk of experiencing the adverse effects of natural or prescribed fires.
Unlike many North American bat species that colonially roost in caves and manmade structures (e.g., barns, houses, and mines), eastern red bats roost solitarily within the foliage in forests and small patches of trees throughout the year [
19]. During the warmer months, eastern red bats are found throughout the eastern and central United States, but in winter are common south of the Ohio and Missouri River Valleys, predominantly in the states surrounding the Gulf of Mexico [
20]. The winter roosting behavior of eastern red bats is thermally dependent, and when temperatures approach or go below 0 °C, they abandon tree roosts in favor of leaf litter on south-facing slopes, which provides a more suitable and stable microclimate [
21,
22,
23,
24,
25,
26]. They remain under the leaf litter until ambient temperatures (T
a) return to approximately 10 °C [
23,
26].
Due to the prevalence of prescribed fires performed by land managers in late fall through early spring, eastern red bats are thought to be a species that may be harmed because of their leaf roosting habits [
25]. Historically, fires have shaped the landscape during the summer months [
27] when eastern red bats are not known to exhibit torpor under leaf litter. Prescribed fires often occur during the months when eastern red bats are torpid in the leaf litter or on leaves or branches of trees [
21]. Low-intensity summer fires can have positive effects on bats [
28,
29], but it is less clear how fires affect bats in winter. While the U.S. Department of Agriculture (USDA) issues state recommendations for a temperature range for setting prescribed fires of 4–21 °C for controlling woody vegetation, it is unclear how bats under leaf litter will respond [
30]. Considering that eastern red bats are experiencing population declines and are a species of conservation concern [
31], it is important to understand if management practices have the potential to cause mortality or detrimental energy consumption in levels harmful to local area populations. Therefore, measurement of eastern red bat ‘escape responses’ to fire conditions at varying weather conditions can inform best management practices.
The ability for bats to detect fire during torpor has been documented [
11]. Gould’s long-eared bat (
Nyctophilus gouldi) detected smoke in a lab setting during deep torpor and initiated an arousal response [
11]. While torpid bats have reduced activity because of lower metabolism, we considered hearing and smell to be the primary senses available to alert eastern red bats to the presence of fire. Eastern red bats have an acute sense of hearing used for echolocation, mainly in the range between 35 and 56 kHz [
32] and capable of responding to noises audible to humans (0.02 to 20 kHz, personal observations). Greater mouse-eared bats (
Myotis myotis) exhibit variable responses to audio-recorded stimuli during shallow torpor through the maternity season [
33]. Other species, such as little brown bats (
Myotis lucifugus), exhibited a decrease in sensitivity to high frequency sounds with decreasing body temperature [
34]. In addition, higher frequency sounds attenuate (lose strength) as a function of distance [
35,
36]. Olfactory communication is used in other species of bats for kin-recognition, as territorial markers, determine reproductive status, and detecting food resources [
37,
38].
With the prevalence of prescribed fires during the winter in areas where eastern red bats occur, the importance of determining physical limitations of behavioral responses to the threat of fire based on ambient weather conditions to assist with improving proper utilization of the forest management tool. Our objective was to determine (1) whether environmental cues associated with fire (i.e., sound, smoke) cause eastern red bats to arouse from torpor and (2) how the behavior (i.e., first response, arousal, flight) of torpid bats is influenced by stimuli associated with fire under controlled-laboratory and experimental field conditions. We provide insight into the sensory abilities of eastern red bats, particularly while torpid, and guidelines for fire management as it relates to reducing direct negative impacts to the species.
2. Materials and Methods
2.1. Study Site
We obtained eastern red bats during the winter months between November through March of 2005–2006 and 2007–2008 in Carter County, Missouri at Peck Ranch Conservation Area (PRCA), a Missouri Department of Conservation managed property. PRCA had a hilly topography and a mixture of pine (
Pinus spp.) and hardwood forests managed with prescribed fires implemented during the winter and early spring to enhance woodland and glade communities by suppressing invading understory plants and reducing litter depths [
39]. Bats were active at PRCA during the winter when daily temperatures were above 10 °C [
26], and were documented to successfully forage during the winter months in southern Missouri [
18].
2.2. Animal Care
Protocols followed guidelines for animal use in research and were approved by the Missouri State University Institutional Animal Care and Use Committee (Protocol # 2009A). We captured eastern red bats for lab trials and transported them to the Missouri State University (Springfield, MO, USA) physiology lab during the winter of 2005–2006. We kept bats in plastic cages (31 × 19 × 17 cm3) with a Styrofoam substrate (29 × 17 × 2 cm3) covered with leaves. Mealworms and water were provided ad libitum. Bats were kept at room temperature (20–25 °C) when they were not being tested and were allowed to fly in a confined space (10.8 × 7.5 × 3 m3) once a week. We released bats at the point of capture after they completed the sequence of trials.
We captured and transported bats to the field lab located in PRCA on the night of capture for field trials performed in 2007–2008. Bats were subjected to trials the following morning, during/after which they were allowed to fly away.
2.3. Laboratory
To determine if torpid eastern red bats would react and arouse from torpor when exposed to smoke, sound, and/or combination of both, each bat was subjected to five laboratory trials. Trials consisted of (1) smoke only, (2) audio recording of fire, (3) combination smoke and audio recording of fire, (4) control air, and (5) control audio recording. Bats were randomly assigned 1 of 5 different trial treatment sequences to avoid any order-effects (i.e., 12345, 54132, 45123, 34251, and 23514). A bat was allowed a minimum of 48 h between trial treatments.
Bats were placed in a test cage that consisted of a plastic base and metal wires for structure and internal plastic mesh that bats could climb and perch. The cage floor was covered with oak (
Quercus sp.) leaves. We placed cages into an environmental chamber to acclimate the bat to the reduced temperature 24 h prior to a trial. Ta was monitored with a digital probe thermometer (RadioShack, Boston, MA, USA) and maintained at 5 ± 0.5 °C throughout all trials. Eastern red bats were assumed to be in a state of “optimum” torpor when T
a was 5 ± 0.5 °C, since the frequency of arousals was less at this parameter when metabolic rates were compared to lower/higher T
a [
16]. Glass-front environmental chambers (
n = 2; Avanti model WC492D 47 × 43 × 83 cm
3) were used for trials. One was used for the control air and sound treatments, and another was used for the smoke and smoke/sound combination trials; this protocol insured that one chamber was not contaminated with residual smoke. Each environmental chamber was constructed to facilitate the movement of air/smoke for the bat arousal trials. We drilled one 4 cm opening into each side of the environmental chambers. On the left side, the opening was centered 17 cm from the top, and on the right side, it was centered 7 cm from the bottom to allow air flow throughout the chambers.
We timed each trial from the onset of an introduction of a stimulus/control to time of first response and arousal as observed by a witness. First response in a trial bat was defined as any movement or increased respiration. Arousal was defined as movement from one location to another (>0.5 cm). To control for the base rate for arousal, we used 60 min as the time limit for all fire stimuli arousal trials based on physical disturbance tests [
17]. We terminated a trial after the bat aroused or the 60 min time limit was reached.
We placed dead oak leaves (approx. 8 g) inside a metal can (3.2 L) with a metal lid outfitted with two openings for smoke trials. A 2.5 cm diameter plastic tube approximately 1 m in length connected one lid opening to the left opening of the environmental chamber (
Figure 1). A hand pump was connected to the other opening in the metal lid, which forced air through the can and pushed smoke through the tube into the environmental chamber. We connected a second tube (same dimensions) on the right side of the environmental chamber to a separate hand pump. At the beginning of each trial, we ignited a fire inside the can, closed the lid and pumped smoke into the test chamber. After 30 s, we closed off the smoke input tube and pumped from the right side of the environmental chamber to remove smoke. We repeated the process every 5 min until trial termination conditions were met. The introduction of smoke/air into the environmental chambers did not influence temperature, as observed via the digital thermometer.
We audio recorded a fire of dead oak leaves and twigs (approximately 10 × 20 cm2) with a power module microphone (Audio-technica, Tokyo, Japan) and duplicated the soundtrack 5 times. Each track was off-set to one another and then joined together into one track, resulting in a final track (the recording of multiple tracks together). We played that recording using a 16-bit mono sound through a speaker that was able to emit sounds of up to 20 kHz (Altec Lansing Multimedia model ACS41) inside the test chamber positioned 17 cm away from a bat until termination conditions were met.
To act as the control for smoke, we used a different hand pump and tubing system (
Figure 1) to force air (approximately 1200 mL) into and out of the environmental chamber every 5 min. To act as a control for the sound of fire, we generated white noise using the Cool Edit Pro Version 2.00 software (Syntrillium Software Corp., Scottsdale, AZ, USA). We set the white noise to play for 60 min using the same procedure as in the audio fire sound test.
2.4. Field
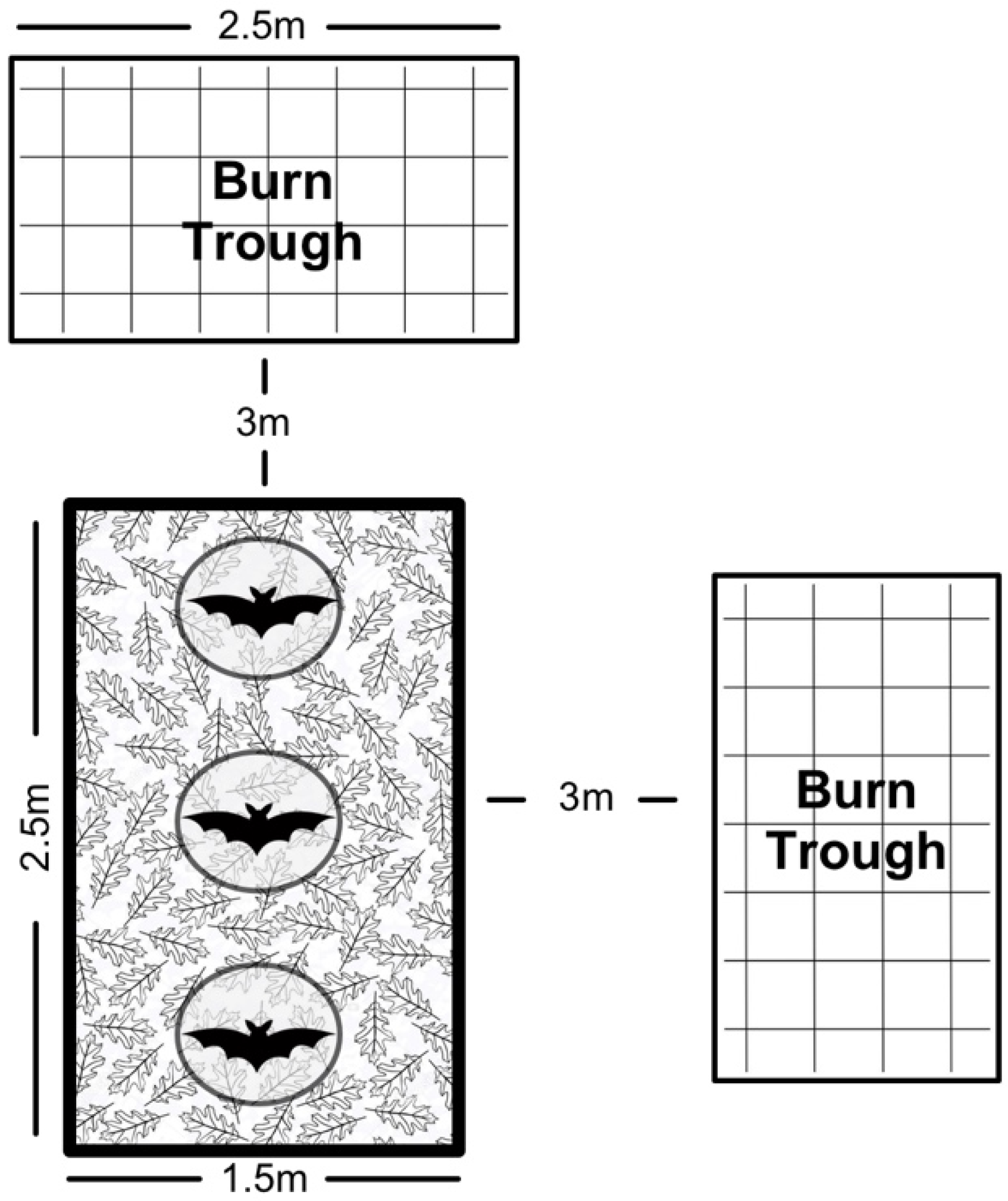
We cleared and leveled an area (2.5 × 1.5 m
2) in a field at PRCA for our field trials. We bordered the area with plastic edging, spread sand over the bare dirt, and used leaf litter to emulate forest ground cover. We placed three circular cone cages (approximately 80 cm in height) 0.2 m apart in a line, held in place by stakes. Outside of the arena, at a distance of approximately 3 m, we positioned two troughs (2.5 × 1.2 × 0.25 m
3; 2 × 1 × 0.25 m
3) composed of expanded metal and square metal tubing (
Figure 2). During trials, we filled the troughs with leaves and covered them with a sheet of expanded metal to contain flaming debris.
An onsite weather logger (Kestrel 4500 Pocket Weather Tracker) placed on a 1.5 m wind vane within 10 m of the burn arena recorded Ta, wind speed/direction, and relative humidity data. We separated weather data for each trial into 3 time periods: (1) 2200 at night of capture through one min before civil sunrise, (2) civil sunrise through one min before the onset of the trial, and (3) onset of the trial until termination.
We monitored and recorded bats with closed-circuit surveillance cameras connected to a video monitor and a recorder stationed 10 m from the fire. Prior to each field trial, roosting positions were classified as either ‘perched’ (hanging from the cage) or ‘prone’ (underneath leaf litter). Perched bats were left undisturbed, with video cameras placed outside of the cages to record their movements. We removed cages over the prone bats and located bats by carefully removing leaf litter to allow for proper camera placement (i.e., head visible).
We used a drip torch to ignite the trough of leaves, emulating a heading fire (flames and smoke going toward the fuel being consumed, and thus toward the bat(s)). Our method provided the greatest amount of smoke exposure going toward a focal bat, and we maintained the fire until the end of the trial. If the wind direction changed during a trial, we ignited the second trough to maintain smoke going in the direction of the bat(s), but only after the first trough’s fire had stopped burning. We defined first response and arousal in prone bats during field trials as we did during laboratory trials. We recorded the latencies to first response and arousal behaviors to the onset of the fire. When we observed a behavior on a recording, the time was marked and subtracted from the onset of the fire to produce latency durations. Additionally, we measured latency to flight, herein defined as the time from the onset of the fire until a bat flew away. Because bats regularly aroused and crawled out of the video frame, we recorded flight latency times by visual observations of bats flying from the ground (
Figure 3). We did not consider perched bats to be in deep states of torpor based on field observations (e.g., head movement during camera setup, etc.), so the first response latencies were not measured. However, we defined arousal in perched bats as any visible head movement or wing movement. Flight response was defined as when wings were unfolded, since bats were still in cages and could not freely fly away. We would carefully tip cages onto their side after a bat was observed to exhibit a flight response to allow free movement away from the burn arena. Trials were terminated after all bats had flown or 60 min after the fire was started.
2.5. Smoke Analyses
We used a carbon monoxide (CO) monitor with two sensors (Sixth Sense, Inc. (Roma, Italy) Eco-Sense 2e electrochemical sensors with a custom electronics signal conditioning board) that sampled every 10 s to compare our results to actual prescribed fires, and to measure for differences between the laboratory and field trial smoke (e.g., Dickinson et al., 2010). For the lab trials, we placed sensors in the same position as bats to replicate the conditions an individual would experience and ran trials to measure CO concentrations. In the field, we placed sensors on the ground between bat cages.
2.6. Analyses
For our laboratory study, we used the Friedman nonparametric test to compare arousal times for the five treatments. Following a significant Friedman’s test, Wilcoxon signed rank tests were used for pairwise comparisons. We used α ≤ 0.05 for indicating significance in all statistical tests.
For our field study, latency values of all three observed behaviors were viewed against each separate time periods weather data and examined for associations among variables. We used Pearson’s product moment correlations (r) to ascertain the strength of relationships. We used α ≤ 0.05 for indicating significance in correlation values. We completed all analyses through Minitab statistical software (Minitab Inc., State College, PA, USA).
3. Results
3.1. Laboratory
We tested 15 male eastern red bats in optimum torpor at Ta 5 ± 0.5 °C for first response and arousal behaviors to fire stimuli and controls through randomized laboratory behavioral trial sequences. Each bat was tested individually through all 5 exposure trials, totaling 75 trials.
3.1.1. Sound Response
No bats responded to the control audio recording. When exposed to the sound of fire, only one individual elicited a first response at 35 min, and we observed no arousals.
3.1.2. Smoke Response
No bats elicited any response during control air trials. In contrast, all bats exposed to fire generated smoke had a first response, with response times varying from 4 s to 5.5 min (
Figure 4). Arousal times (
n = 10) ranged from 11 to 40.5 min (mean ± SE = 36.55 ± 4.98), with five bats not responding within the 60 min time allotment (
Figure 5).
3.1.3. Fire Sound and Smoke Response
All bats had a relatively rapid first response between 4 and 30 s when exposed to both the sound of fire and smoke simultaneously (
Figure 4). Arousal times ranged from 10.5 to 42 min (mean ± SE = 21.92 ± 1.87) (
Figure 5).
3.1.4. Statistical Comparisons
The Friedman nonparametric test indicated a difference in latency to arouse among the five treatments (S = 48.7,
p < 0.05) (
Figure 5). The sound of fire had no effect on arousal times when compared to the sound control (Wilcoxon: z = 3.4,
p = 1). In pairwise comparisons, arousal latencies were faster for the smoke versus smoke control (Wilcoxon: z = 2.8,
p < 0.05). The combination of fire sound and smoke had shorter arousal latencies when compared to smoke alone (Wilcoxon: z = −2.5,
p < 0.05).
3.2. Field
We placed 27 eastern red bats (26 males, 1 female) within the field trial arena. We observed bat behavioral responses to fire stimuli through 10 separate field trials, ranging from 1 to 3 bats per trial. Out of the male bats, 22 were in the prone position, and 5 were in the perched position on the side of the cage. Two prone males flew during the setup of cameras prior to fire ignition and were not considered to be in torpor. Due to technical difficulties with recording equipment, two trials were not recorded for first response or arousal (first trial (12/2/07) and sixth trial (3/1/08)), resulting in five bats (four prone, one perched) not being observed for these behaviors.
Of the 15 prone bats monitored for first response and arousal, 13 exhibited a first response during trials. First responses occurred as soon as 51 s after the ignition of a fire to as late as 40 min after, with an average of 12.5 min (SE ± 2.49) for all individuals that exhibited the behavior. Arousals occurred in 12 individuals, occurring as soon as 3 min 42 s after ignition to 29 min 20 s, with an average of 17.5 min (SE ± 2.18) for all individuals that exhibited the behavior.
Since flight was observed manually, all bats tested (n = 25) were monitored for flight response. Of those, 15 prone individuals (68.2%) exhibited a flight response ranging from as soon as 10 min after ignition to 46 min, with an average of 21.8 min (SE ± 4.20) for all prone bats that exhibited the behavior.
We did not monitor perched bats for first response due to observed shallower states of torpor. Of the five perched bats, only four were video recorded for arousal. Perched bats exhibited arousal behavior between 45 s and 7 min 9 s, with an average of 3.8 min (SE ± 1.37) after fire ignition. Flight occurred from 3 to 26 min, with an average flight latency of 14.4 min (SE ± 4.20).
3.2.1. Smoke Response
Latency to first response, arousal, and flight response were significantly negatively correlated with temperature during all three time periods (
Table 1). First response and arousal latency at time periods 2 and 3 (prior to field trials, during field trials, respectively) were significantly negatively correlated with wind speed, whereas flight response latency was significantly negatively correlated with wind speed during all three time periods. The only significant association between relative humidity and any behavioral response (first) was observed in time period 3 (
Table 1).
3.2.2. Smoke Chemical Concentration
The concentration of CO measured in the environmental chambers fluctuated among trials (n = 3). The average amount of CO was 141.17 (SE ± 4.29), 103.20 (SE ± 3.41), and 60.66 (SE ± 1.54) parts per million (ppm). Maximum exposure at each trial was 375, 291, and 184 ppm, respectively. During the one field trial where CO was monitored, it averaged 1.51 ppm (SE ± 0.14), with a maximum exposure of 40 ppm.
4. Discussion
We determined that eastern red bats are able to arouse from optimum torpor bouts when exposed to cues associated with fire (e.g., smoke and sounds). Eastern red bats are capable of arousing from torpor when exposed to smoke and the additional sound of fire decreases the response time. For our second objective, we described eastern red bats exhibiting decreased latency responses (i.e., first response, arousal, and flight) to increased T
a and wind speeds during the simulated prescribed fire stimuli. Although literature references on escape responses of torpid mammals are limited, there are a variety of species that are capable of this behavior [
11,
40,
41], including other species of bat [
11,
42]. Similar to our findings, torpid Gould’s long-eared bats are capable of arousing from torpor when exposed to smoke stimuli [
11].
Laboratory trial bats had a significantly shorter latency to first response and arousal time compared to controls (
Figure 4 and
Figure 5, respectively). First response latency to smoke alone was <1 min compared to no response within 60 min when exposed to sound alone. Due to the limitations of audio equipment, the sound recording of fire that was emitted from the speaker maxed out at 20 kHz, whereas actual fires have frequencies that range up to 60 kHz [
17]. It is likely that eastern red bats have lower sensitivity to sound during the winter months when temperatures are lower, as seen in other species. For example, little brown bat hearing frequency sensitivity was negatively correlated with temperature, to the point of an inability to detect sounds when T
a and body temperature (T
b) were below 12 °C [
34]. It is unknown how decreasing temperatures affect hearing or smell in eastern red bats. However, when smoke and sound are combined, time to first response decreased slightly. We suggest that the fire sound may act as a catalyst, speeding up the reaction time once smoke is detected. Similarly, exposure to smoke alone significantly decreased the arousal latency to approximately 30 min. Trials that included both smoke and sound resulted in shorter arousal times (
Figure 4 and
Figure 5). This study emphasized the importance of smoke for torpid bats’ awareness to fire. However, lab conditions did not fully encompass the whole range of parameters that are associated with actual fires. Smoke is more variable in nature than the conditions presented in the environmental chambers as measured by the CO monitor [
43]. During all field trials, bats only exhibited a first response after wind blew smoke over the bats. CO monitored during one field trial peaked at 40 ppm, which is low compared to the amount measured by Dickinson et al. (2009) during an early-spring prescribed burn in Ohio (350 to <400 ppm). The wind speed in our field trial when CO was measured as 3.9 km per hour (km/h), which was near the average of all field burn trials (3.6 km/h), and smoke exposure was sporadic, usually lasting less than 1 min. In comparison, peaks and durations of exposure observed in the lab were greater than field measurements, with levels of over 70 ppm lasting longer than 12 min. A higher concentration of CO presumably leads to higher detection probability of fire, and therefore would shorten the latency of behavioral responses. However, since all laboratory trials exhibited longer latencies to first response and arousals it lends credibility to the finding that higher ambient temperatures decreased latency even when CO levels were lower. Our baseline arousal time of 60 min determined during lab trials is related to eastern red bat winter roosting ecology [
23]. Unlike cave-dwelling bats, eastern red bats are constantly exposed to changes in their immediate environment. Considering that arousal is energetically expensive [
44], we postulate that it is more efficient to delay exhibiting an escape response until the potential threat is more imminent. However, an innate response that triggers other senses would provide a selective advantage to a species that, if exposed to the threat of a fire, could escape before local population level mortality would occur. Although eastern red bats face wide fluctuations of temperatures compared to cave roosting species, they are able to remain in torpor until ambient conditions are appropriate for insect activity [
16,
45]. Eastern red bats in PRCA and southwestern Missouri were observed to roost in trees during periods when temperatures exceeded 10 °C [
23,
26]. This same temperature-dependent roosting behavior during the late fall/winter seasons was also observed in Seminole bats (
Lasiurus seminolus) [
24]. Perched bats in this study all flew during field trials compared to the 68.2% of prone bats that flew. This may be explained by bats that roost in trees possibly able to have access and/or assess variables in their surroundings more effectively, resulting in quicker responses than bats located under leaf litter [
46]. However, a bat hidden in leaf litter may feel safer from the threat of fire than an exposed perched bat. Radio-tracked lasurine species roosting in trees in areas that were subsequently managed with prescribed fire were able to arouse and fly to safety [
47]. More studies could be performed to determine response times of other species that may occur in the same forest type as eastern red bats during the winter, but utilize different roosting strategies (e.g., silver-haired bats (
Lasionycteris noctivagans)).
In our field trials, increased temperature and wind speed were correlated with decreased latencies of response behavior in torpid eastern red bats. Bats only exhibited a first response after wind blew smoke over the bats. Wind during the night prior to a trial had a significant, but unexplained correlation with latency behaviors. The occurrence of wind propelling smoke over bats during fires confirmed lab findings that smoke greatly reduces latency time to first response. Latencies of bats that exhibited flight response from prone leaf litter roosts are too long (21.8 min on average) to allow for successful escape from a fire in close proximity. If bats were in natural roosting positions with the chance to passively rewarm before the onset of fire, and because it is likely that CO concentrations are higher during prescribed woodland burns [
34], responses may be quicker and more effectual. The authors have observed bats flying away from leaf litter during backing fires (i.e., smoke blowing away from the direction of fire). Bats in shallower states of torpor or in states of activity due to warm T
a can be assumed not to need smoke to elicit an escape response. The location of roosts that maximize solar exposure [
23,
26] is a factor that would decrease latency times to arousal and thus increase survivorship. As an example, one bat that did not exhibit a flight response during a field trial was left at the burn arena. During the mid-afternoon (14:30), the bat was observed on top of the leaf litter, breathing heavily, with its back towards the sun. It responded to our approach and took flight when we were less than 2 m away. This observation suggests that the bat may have been in a state closer to arousal from torpor while passively rewarming relative to during the fire trial.
Forest managers typically do not start prescribed burns with temperatures below 10 °C, but USDA recommendations [
30] do not preclude starting prescribed fires at lower temperatures. Personal observations by the authors and numerous anecdotal reports indicate that eastern red bats can successfully evade approaching fires even if previous nighttime temperatures were below freezing. For example, temperatures during the night prior to a field trial burn in our study reached 0.8 °C (2 March 2008), with all three bats flying within 18 min when the temperature before onset of the fire averaged 13.6 °C, suggesting that temperatures before the onset of the burn may have a more significant effect on arousals than temperatures during the previous night.
Bats were occasionally observed to awaken and crawl away from their roost locations during burn trials. The only female included in our study was tested for behavioral responses during a field trial. The bat exhibited a first response 1 min 19 s after the fire started, and arousal latency was <8 min, but it exhibited no flight response. The bat crawled approximately 0.5 m away from its prone position to another pile of leaves and remained there throughout the trial. If a bat is aroused due to cues from an oncoming fire, it may be able to move to an area where the fire would not cause injury or death. Burrowing behaviors were never observed in burn trials, but this behavior may be futile based on model results where lethal heat levels are able to penetrate into leaf litter [
25].