Sex Hormone-Binding Globulin (SHBG) in Cerebrospinal Fluid Does Not Discriminate between the Main FTLD Pathological Subtypes but Correlates with Cognitive Decline in FTLD Tauopathies
Abstract
:1. Introduction
2. Methods
2.1. Humans CSF Samples
2.2. CSF Biomarker Analysis
2.3. Statistical Analysis
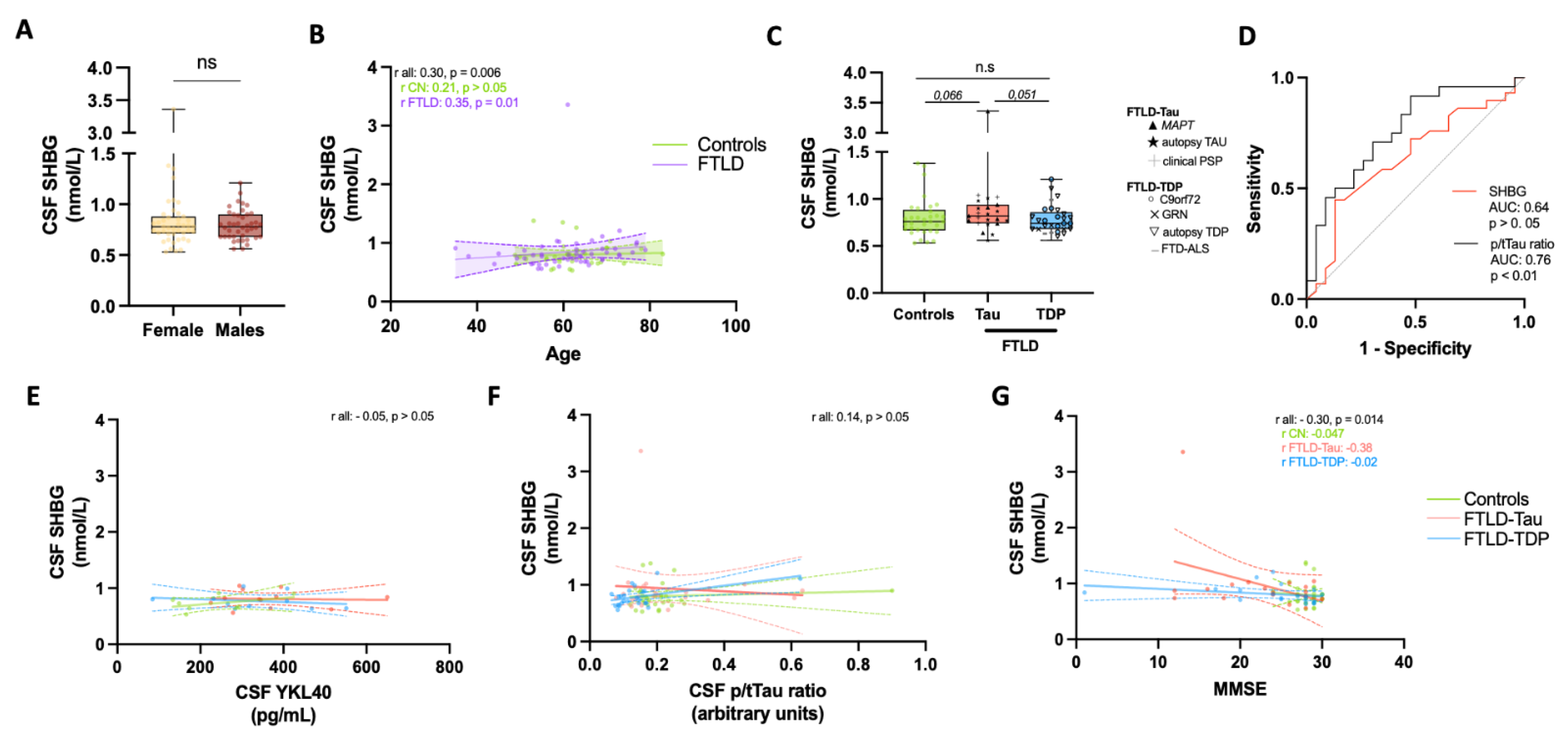
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Irwin, D.J.; Cairns, N.J.; Grossman, M.; McMillan, C.T.; Lee, E.B.; Van Deerlin, V.M.; Lee, V.M.-Y.; Trojanowski, J.Q. Frontotemporal lobar degeneration: Defining phenotypic diversity through personalized medicine. Acta Neuropathol. 2014, 129, 469–491. [Google Scholar] [CrossRef] [PubMed]
- Swift, I.J.; Sogorb-Esteve, A.; Heller, C.; Synofzik, M.; Otto, M.; Graff, C.; Galimberti, D.; Todd, E.; Heslegrave, A.J.; Van Der Ende, E.L.; et al. Fluid biomarkers in frontotemporal dementia: Past, present and future. J. Neurol. Neurosurg. Psychiatry 2020, 92, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Hu, W.T.; Watts, K.; Grossman, M.; Glass, J.; James, J.; Hales, C.; Shelnutt, M.; Van Deerlin, V.; John, Q.; Levey, A.I. Reduced CSF p-Tau 181 to Tau ratio is a biomarker for FTLD-TDP Study Sponsorship: Author Disclosure. Neurology 2013, 81, 1945–1952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teunissen, C.E.; Elias, N.; Koel-Simmelink, M.J.A.; Durieux-Lu, S.; Malekzadeh, A.; Pham, T.V.; Beccari, T.; Meeter, L.H.H.; Dopper, E.G.P.; van Swieten, J.C.; et al. Novel diagnostic cerebrospinal fluid protein biomarkers for pathologic subtypes of frontotemporal dementia identified by proteomics. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2016, 2, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Dye, R.V.; Miller, K.J.; Singer, E.J.; Levine, A.J. Hormone replacement therapy and risk for neurodegenerative diseases. Int. J. Alzheimer’s Dis. 2012, 2012, 258454. [Google Scholar] [CrossRef] [Green Version]
- Hansberg-Pastor, V.; González-Arenas, A.; Piña-Medina, A.G.; Camacho-Arroyo, I. Sex hormones regulate cytoskeletal proteins involved in brain plasticity. Front. Psychiatry 2015, 6, 165. [Google Scholar] [CrossRef] [Green Version]
- Höglinger, G.U.; Respondek, G.; Stamelou, M.; Kurz, C.; Josephs, K.A.; Lang, A.E.; Mollenhauer, B.; Müller, U.; Nilsson, C.; Whitwell, J.L.; et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov. Disord. 2017, 32, 853–864. [Google Scholar] [CrossRef]
- Del Campo, M.; Mollenhauer, B.; Bertolotto, A.; Engelborghs, S.; Hampel, H.; Simonsen, A.H.; Kapaki, E.; Kruse, N.; Le Bastard, N.; Lehmann, S.; et al. Recommendations to standardize preanalytical confounding factors in Alzheimer’s and Parkinson’s disease cerebrospinal fluid biomarkers: An update. Biomark. Med. 2012, 6, 419–430. [Google Scholar] [CrossRef]
- Rascovsky, K.; Hodges, J.R.; Knopman, D.; Mendez, M.F.; Kramer, J.H.; Neuhaus, J.; van Swieten, J.C.; Seelaar, H.; Dopper, E.G.P.; Onyike, C.U.; et al. Sensitivity of revised diagnostic criteria for the behavioural variant of frontotemporal dementia. Brain 2011, 134, 2456–2477. [Google Scholar] [CrossRef]
- Litvan, I.; Agid, Y.; Calne, D.; Campbell, G.; Dubois, B.; Duvoisin, R.C.; Goetz, C.G.; Golbe, L.I.; Grafman, J.; Growdon, J.H.; et al. Clinical research criteria for the diagnosis of progressive supranuclear palsy (Steele-Richardson-Olszewski syndrome): Report of the NINDS-SPSP international workshop. Neurology 1996, 47, 1–9. [Google Scholar] [CrossRef]
- del Campo, M.; Galimberti, D.; Elias, N.; Boonkamp, L.; Pijnenburg, Y.A.; van Swieten, J.; Watts, K.; Paciotti, S.; Beccari, T.; Hu, W.; et al. Novel CSF biomarkers to discriminate FTLD and its pathological subtypes. Ann. Clin. Transl. Neurol. 2018, 5, 1163–1175. [Google Scholar] [CrossRef]
- Del Campo, M.; Jongbloed, W.; Twaalfhoven, H.A.; Veerhuis, R.; Blankenstein, M.A.; Teunissen, C.E. Facilitating the Validation of Novel Protein Biomarkers for Dementia: An Optimal Workflow for the Development of Sandwich Immunoassays. Front. Neurol. 2015, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Irwin, D.J.; Lleó, A.; Xie, S.X.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. Ante mortem cerebrospinal fluid tau levels correlate with postmortem tau pathology in frontotemporal lobar degeneration. Ann. Neurol. 2017, 82, 247–258. [Google Scholar] [CrossRef]
- Lleó, A.; Irwin, D.J.; Illán-Gala, I.; McMillan, C.T.; Wolk, D.A.; Lee, E.B.; Van Deerlin, V.M.; Shaw, L.M.; Trojanowski, J.Q.; Grossman, M. A 2-Step Cerebrospinal Algorithm for the Selection of Frontotemporal Lobar Degeneration Subtypes. JAMA Neurol. 2018, 75, 738–745. [Google Scholar] [CrossRef] [Green Version]
- Heller, C.; Foiani, M.S.; Moore, K.; Convery, R.; Bocchetta, M.; Neason, M.; Cash, D.M.; Thomas, D.; Greaves, C.V.; Woollacott, I.O.C.; et al. Plasma glial fibrillary acidic protein is raised in progranulin-associated frontotemporal dementia. J. Neurol. Neurosurg. Psychiatry 2020, 91, 263–270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, C.N.; Wu, Y.; Odeh, H.M.; Gendron, T.F.; Jansen-West, K.; Del Rosso, G.; Yue, M.; Jiang, P.; Gomes, E.; Tong, J.; et al. C9orf72 poly(GR) aggregation induces TDP-43 proteinopathy. Sci. Transl. Med. 2020, 12. [Google Scholar] [CrossRef]
- Zhang, J.; Velmeshev, D.; Hashimoto, K.; Huang, Y.H.; Hofmann, J.W.; Shi, X.; Chen, J.; Leidal, A.M.; Dishart, J.G.; Cahill, M.K.; et al. Neurotoxic microglia promote TDP-43 proteinopathy in progranulin deficiency. Nature 2020, 588, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Boxer, A.L.; Gold, M.; Feldman, H.; Boeve, B.F.; Dickinson, S.L.-J.; Fillit, H.; Ho, C.; Paul, R.; Pearlman, R.; Sutherland, M.; et al. New directions in clinical trials for frontotemporal lobar degeneration: Methods and outcome measures. Alzheimer’s Dement. 2020, 16, 131–143. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Su, B.J.; Shen, X.N.; Bi, Y.L.; Tan, C.C.; Li, J.Q.; Cao, X.P.; Dong, Q.; Tan, L.; Yu, J.T. Plasma sex hormone-binding globulin predicts neurodegeneration and clinical progression in prodromal Alzheimer’s disease. Aging 2020, 12, 14528–14541. [Google Scholar] [CrossRef] [PubMed]
- Hoskin, E.K.; Tang, M.X.; Manly, J.J.; Mayeux, R. Elevated sex-hormone binding globulin in elderly women with Alzheimer’s disease. Neurobiol. Aging 2004, 25, 141–147. [Google Scholar] [CrossRef]
- Xu, J.; Xia, L.-L.; Song, N.; Chen, S.-D.; Wang, G. Testosterone, Estradiol, and Sex Hormone-Binding Globulin in Alzheimer’s Disease: A Meta-Analysis. Curr. Alzheimer Res. 2015, 13, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Johansson, P.; Johansson, J.O.; Labrie, F.; Mattsson, N.; Hansson, O.; Blennow, K.; Zetterberg, H.; Wallin, A.; Ohlsson, C.; Svensson, J. Mild dementia is associated with increased adrenal secretion of cortisol and precursor sex steroids in women. Clin. Endocrinol. 2011, 75, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Lin, K.A.; Rundel, C.; Doraiswamy, P.M. Serum SHBG Levels are not Associated with Longitudinal Cognitive Decline in Mild Cognitive Impairment. J. Alzheimer’s Dis. 2017, 55, 1123–1130. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.J.; Xu, W.; Tan, C.C.; Tan, L. Blood-based biomarkers in hypothalamic-pituitary axes for the risk of dementia or cognitive decline: A systematic review and meta-analysis. Aging 2020, 12, 20350–20365. [Google Scholar] [CrossRef] [PubMed]
- Ishii, M.; Iadecola, C. Metabolic and Non-Cognitive Manifestations of Alzheimers Disease: The Hypothalamus as Both Culprit and Target of Pathology. Cell Metab. 2015, 22, 761–776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vercruysse, P.; Vieau, D.; Blum, D.; Petersén, Å.; Dupuis, L. Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front. Mol. Neurosci. 2018, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Piguet, O.; Petersén, Å.; Yin Ka Lam, B.; Gabery, S.; Murphy, K.; Hodges, J.R.; Halliday, G.M. Eating and hypothalamus changes in behavioral-variant frontotemporal dementia. Ann. Neurol. 2011, 69, 312–319. [Google Scholar] [CrossRef]
- Miyagawa, T.; Brushaber, D.; Syrjanen, J.; Kremers, W.; Fields, J.; Forsberg, L.K.; Heuer, H.W.; Knopman, D.; Kornak, J.; Boxer, A.; et al. Use of the CDR® plus NACC FTLD in mild FTLD: Data from the ARTFL/LEFFTDS consortium. Alzheimer’s Dement. 2020, 16, 79–90. [Google Scholar] [CrossRef]
| n (F/M) | Age (Mean, SD) | MMSE * (Mean, SD) | CSF p/tTau † | CSF YKL40 ‡ (ng/mL) | SHBG (nmol/L) | Subgroups | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| CON | 33 (17/16) | 62 (8) | 28 (1) | 0.19 (0.06) | 234 (138) | 0.8 (0.21) | na | |||
| FTD | 52 (26/26) | 61 (10) | 24 (6) § | 0.16 (0.08) § | 349 (133) § | 0.85 (0.21) | na | |||
| FTLD-Tau | 23 (16/7) | 60 (13) | 23 (6) § | 0.19 (0.07) | 363 (109) § | 0.93 (0.20) | 10 Autopsy, 8 MAPT, 5 cPSP | |||
| FTLD-TDP | 29 (10/19) | 62 (6) | 24 (7) § | 0.12 (0.06) §,¶ | 332 (166) § | 0.78 (0.18) | 13 Autopsy, 9 C9orf72, 3 GRN, 4 FTD-ALS | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campo, M.d.; Pijnenburg, Y.A.L.; Chen-Plotkin, A.; Irwin, D.J.; Grossman, M.; Twaalfhoven, H.A.M.; Hu, W.T.; Meeter, L.H.; van Swieten, J.; Vermunt, L.; et al. Sex Hormone-Binding Globulin (SHBG) in Cerebrospinal Fluid Does Not Discriminate between the Main FTLD Pathological Subtypes but Correlates with Cognitive Decline in FTLD Tauopathies. Biomolecules 2021, 11, 1484. https://doi.org/10.3390/biom11101484
Campo Md, Pijnenburg YAL, Chen-Plotkin A, Irwin DJ, Grossman M, Twaalfhoven HAM, Hu WT, Meeter LH, van Swieten J, Vermunt L, et al. Sex Hormone-Binding Globulin (SHBG) in Cerebrospinal Fluid Does Not Discriminate between the Main FTLD Pathological Subtypes but Correlates with Cognitive Decline in FTLD Tauopathies. Biomolecules. 2021; 11(10):1484. https://doi.org/10.3390/biom11101484
Chicago/Turabian StyleCampo, Marta del, Yolande A. L. Pijnenburg, Alice Chen-Plotkin, David J. Irwin, Murray Grossman, Harry A. M. Twaalfhoven, William T. Hu, Lieke H. Meeter, John van Swieten, Lisa Vermunt, and et al. 2021. "Sex Hormone-Binding Globulin (SHBG) in Cerebrospinal Fluid Does Not Discriminate between the Main FTLD Pathological Subtypes but Correlates with Cognitive Decline in FTLD Tauopathies" Biomolecules 11, no. 10: 1484. https://doi.org/10.3390/biom11101484
APA StyleCampo, M. d., Pijnenburg, Y. A. L., Chen-Plotkin, A., Irwin, D. J., Grossman, M., Twaalfhoven, H. A. M., Hu, W. T., Meeter, L. H., van Swieten, J., Vermunt, L., Martens, F., Heijboer, A. C., & Teunissen, C. E. (2021). Sex Hormone-Binding Globulin (SHBG) in Cerebrospinal Fluid Does Not Discriminate between the Main FTLD Pathological Subtypes but Correlates with Cognitive Decline in FTLD Tauopathies. Biomolecules, 11(10), 1484. https://doi.org/10.3390/biom11101484






