Abstract
Leaf pubescence is an important trait closely associated with plant adaptability to specialized habitats. Baimaomai (BMM) is a wheat (Triticum aestivum L.) landrace originating from the high-altitude, drought-prone environment of Sichuan Province, China with long, dense leaf pubescence. A population of 234 recombinant inbred lines (F10) developed from the cross between Chuanmai104 (CM104), which lacks leaf pubescence, and BMM with pubescent leaves, was used to conduct a phenotypic evaluation of leaf pubescence. Three quantitative trait loci (QTLs) were detected on chromosome arms 7BS, 3DL and 3AL using a high-density wheat 50K single-nucleotide polymorphism array in four environments. The QTLs were designated QLp.saas-7BS, QLp.saas-3DL and QLp.saas-3AL. QLp.saas-3AL, derived from BMM, and QLp.saas-3DL, derived from CM104, were new minor-effect loci. QLp.saas-7BS, derived from BMM, was a novel major-effect locus detected in all environments and was localized in a 0.48 Mb interval on chromosome arm 7BS based on the wheat ‘Chinese Spring’ reference genome. QLp.saas-7BS explained up to 40.77% of the total phenotypic variance. KASP markers tightly linked to QLp.saas-7BS were developed and verified. The present results provide valuable information for further fine mapping, cloning, and marker-assisted selection with QLp.saas-7BS in wheat.
1. Introduction
Leaf pubescence is an adaptive morphological trait and a common characteristic of angiosperms [1,2]. Leaf pubescence plays an important role in plant growth, development, and environmental adaptation. Pubescence refers to the presence of trichomes, which are ubiquitous in plants and comprise uni- or multi-cellular outgrowths from the epidermis [3,4]. Some trichomes perform a secretory function and may secrete a variety of chemicals, such as terpenoids and phenylpropanoids, which are important for plant–animal [5] or plant–fungal interactions [6].
With regard to resistance to biotic stresses, leaf pubescence may improve plant resistance to insects by affecting the preferences and behavior of diverse insects [7], such as cereal leaf beetle [8,9], yellow sugarcane aphid and greenbug [10], and bird cherry-oat aphid in wheat [11]. The degree and density of leaf pubescence is associated with variation in the degree of resistance or susceptibility to various insect pests [12,13,14]. In addition, pubescence may serve as a barrier to infection by foliar pathogens [15]. The presence of trichomes may reduce the relative humidity at the leaf surface, which is unfavorable for germination of fungal spores [15]. Some trichome-related plant proteins are reported to play an essential role in resistance to foliar pathogens, such as TRICHOME BIREFRINGENCE (TBR)-like proteins in rice [16] and α-1,3-glucanase in Arabidopsis thaliana [17].
In addition, plant leaf pubescence is important in the response to abiotic stresses. Many studies have observed the important protective value of leaf pubescence under drought [18,19], thermal stress [2,20,21], and intense solar radiation [20,21]. Drought also leads to increased pubescence production in plants as an adaptative response [3]. Many plants growing in semi-arid environments maintain water contents by foliar absorption of water aided by pubescence [6]. For example, pubescence reduces evaporation and regulates plant tissue temperature by increasing the thickness of the epidermis and the content of long-chain fatty acids [22]. Pubescence also plays an important role in moisture absorption from dew which decreases the water potential of drought-stressed leaves, and contributes to plant photosynthetic performance under water stress [23,24,25]. The density of leaf pubescence is an important component of adaptation for drought tolerance as revealed by genetic analysis of leaf pubescence density and water status traits in soybean [26]. Notably, leaf pubescence plays an important role in plant development and productivity because it influences photosynthesis and therefore transpiration, respiration, and grain-yield formation [7,23,27,28].
In wheat, pubescence traits are expressed in various tissues and are regulated by different genes. For instance, glume pubescence is controlled by the gene Hg, also named Hg1 (weakly hairy phenotype), located on chromosome arm 1AS [29,30]. The gene Hg2 (very hairy phenotype) was localized to chromosome 2BL of synthetic hexaploid wheat using a bulked segregant analysis and RNA-sequencing approach in a F2:3 population. Luo et al. [31] detected 37 differentially expressed genes and 39 single-nucleotide polymorphisms (SNPs) in the Hg gene region on chromosome 1AS by transcriptome analysis. A hairy leaf sheath in bread wheat introgressed from Aegilops tauschii Coss. is mainly controlled by the quantitative trait locus (QTL) QLsh.saas-4D, which is significantly positively associated with yield and yield-related traits, including grain yield, grain weight, spike number per square meter, and grain weight per spike [32]. Hairiness of the leaf margins and auricles in wheat are regulated by the QTLs QHl.ipk-4B and QHl.ipk-4D on chromosome 4BL, and QPa.ipk-4B and QPa.ipk-4D on chromosome 4DL, respectively [33].
Numerous genes that regulate leaf pubescence in wheat have been identified. The dominant gene Hl1 is localized on chromosome 4BL [33,34]. A second dominant gene, Hl2, was localized on chromosome 7BS in the pubescent Chinese cultivar ‘Hong-Mang-Mai’ grown on the arid Loess Plateau [35]. A third pubescence-related gene, Hl3, was detected by genetic analysis but its precise genomic position remains unknown [36]. Hl1 and Hl3 more strongly affect initiation and development of leaf pubescence, whereas Hl2 affects leaf trichome length [36]. A major gene, Hl2aesp, on chromosome 7BS, regulates the leaf hair length, which was introgressed from Ae. speltoides Tausch into common wheat [37]. Hl2aesp is considered to be an allele of Hl2 [33]. In addition, a gene on chromosome 7D of the cultivar ‘Novosibirskaya 67’ increases pubescence density [38]. More recently, Simonov et al. [39] used an introgression line obtained by crossing ‘S29’ with tetraploid Triticum timopheevii (Zhuk.) Zhuk. to identify and map the novel dominant gene Hltt to the distal region of chromosome 5AL, which controls the formation of long trichomes. The Hltt gene exerts a suppressive effect both on dominant and recessive alleles of Hl1 and Hl3 in different genetic backgrounds. In contrast, Hl1 and Hl3 may enhance the effect of Hltt in specific genetic backgrounds, which results in formation of an abundance of extremely long trichomes [39].
In the present study, we used Baimaomai (BMM), a hexaploid wheat landrace originating from a specialized environment characterized by high altitude, intense radiation, drought, and aridity in the western plateau region of Sichuan, China. BMM exhibits a number of specific physiological traits, including long and dense pubescence on the leaves. The aim of the study was to map QTLs for leaf pubescence of BMM using a genetically isolated population derived from crosses between Chuanmai104 (CM104; non-pubescent leaves) and BMM, and high-throughput SNP microarray technology. Linkage markers linked to the major QTLs were developed and verified. The results will contribute to an improved understanding of the genetic control of leaf pubescence in wheat.
2. Materials and Methods
2.1. Plant Materials
Baimaomai, a hexaploid wheat landrace with long, dense pubescence on the leaves, was originally collected from the western plateau region of Sichuan, China. Chuanmai104, an elite commercial wheat cultivar that lacks leaf pubescence, was developed by the Crop Research Institute, Sichuan Academy of Agricultural Sciences (CRI-SAAS) and released in 2012 [40]. The leaf pubescence phenotypes of BMM and CM104 are shown in Figure 1. A recombinant inbred line (RIL) mapping population comprising 234 F10 lines was developed from the cross between CM104 and BMM, and was used to map QTLs associated with leaf pubescence. The RIL population is hereafter referred to as CB-RILs. In addition, 110 F9 RILs were developed from the cross between 14Pin16 (a hexaploid wheat with non-pubescent leaves) and BMM, hereafter abbreviated as PB-RILs which were intended for marker validation. A total of 62 lines, comprising CM104, 14Pin16, BMM, 48 CB-RILs, and 11 PB-RILs, were used to validate the developed kompetitive allele-specific PCR (KASP) markers closely linked to the major QTL QLp.saas-7BS (Supplementary Table S1). All materials were provided by CRI-SAAS.
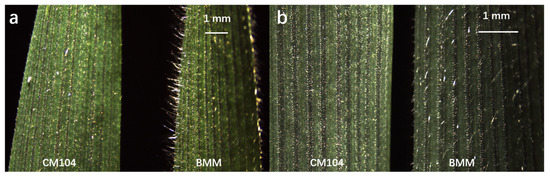
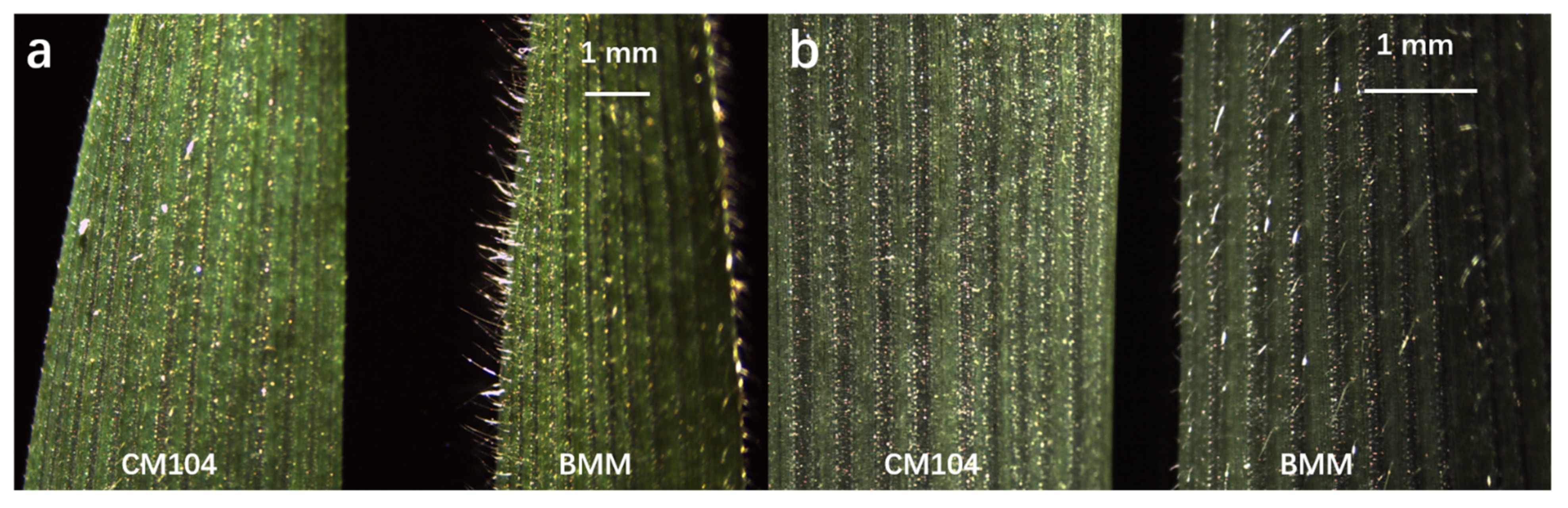
Figure 1.
Leaf pubescence phenotypes of the parents Chuanmai104 (CM104) and Baimaomai (BMM) observed with a stereomicroscope at the heading stage: (a) the lateral observation of leaf surface; (b) the frontal observation of leaf surface.
2.2. Phenotypic Evaluation
The RILs and parents were planted in four environments comprising Pixian (30°81′ N, 103°88′ E) in 2019–2020 (2020PX) and 2020–2021 (2021PX), and Guanghan (30°99′ N, 104°25′ E) in 2019–2020 (2020GH) and 2020–2021 (2021GH) in Sichuan Province of China. The RILs and parents in all environments were sown in random blocks. Each line was planted in a single 1 m long row, and with 25 cm inter-row spacing between RILs. The sowing density was 10 seeds per row with 10 cm spacing between plants in these specific areas. Field management followed conventional practices used in wheat production. Five representative flag leaves per line were selected for phenotypic trait measurement at the heading stage. The trichome length and density of leaf pubescence were observed in an area of 1 cm2 in the center of the flag leaves using a Leica EZ4 HD stereo microscope (Leica Microsystems company, Wetzlar, Germany; Figure 1). The phenotypic traits were scored using a scale from 0 to 3, with 0 representing no pubescence, 1 for short and sparse pubescence, 2 for pubescence of moderate length and density, and 3 for the longest and most dense pubescence. The statistics of phenotypic data were conducted using Microsoft Excel, and the correlation analysis among the different environments were conducted using the software IBM SPSS Statistics 25.0 (International business machines corporation, Armonk, NY, USA).
2.3. QTL Mapping and Candidate Genes Prediction
The genetic linkage map used in this study was constructed using 3800 polymorphic SNP markers from the wheat 50K array developed by CapitalBio Corporation (Beijing, China) and synthesized by Affymetrix [40]. The QTL analysis was conducted by inclusive composite interval mapping using QTL IciMapping 4.1 software [40]. The QTLs were mapped at a logarithm of odds threshold of 2.5 based on 1000 permutations and a walk speed of 1.0 cM, with p = 0.001, by stepwise regression. The QTL effect was estimated as the proportion of the total phenotypic variance explained by the QTL.
Based on the flanking sequences, the physical distance between the marker interval of each QTL was determined using the Chinese Spring IWGSC RefSeq v2.1 genome assembly accessed on the International Wheat Genome Sequencing Consortium (IWGSC) website (http://www.wheatgenome.org/) [41]. According to the physical location of the flanking sequences, the candidate genes for the major QTL QLp.saas-7BS were predicted with reference to IWGSC RefSeq v2.1, the wild emmer reference genome (Zavitan WEWSeq v2.0), and durum wheat reference genome (Svevo RefSeq Rel. 1.0) accessible in the Triticeae Multi-omics Center database (http://202.194.139.32/).
2.4. Marker Development and QTL Validation
Based on the QTL mapping results, we converted the flanking SNP markers AX-86172908 and AX-86175290 tightly linked to the major QTL QLp.saas-7BS into KASP markers, designated KASP-AX-86172908 and KASP-AX-86175290, respectively. Forty-four lines randomly selected from the RIL population and BMM were used to perform genotyping using two sets of KASP markers, respectively. The reaction mixture for genotype detection comprised 0.75 μL DNA, 2.85 μL deionized water, 5 μL KASP Master Mix (LGC Biosearch Technologies, Hoddesdon, UK), and 1.4 μL primers. Reactions were conducted using the CFX96 Real-Time PCR Detection System (Bio-Rad, Hercules, CA, USA). The primers for KASP-AX-86172908 (FAM: GAAGGTGACCAAGTTCATGCTTGCACAACCAGTCAGCAGAAA; HEX: GAAGGTCGGAGTCAACGGATTTGCACAACCAGTCAGCAGAAG; Reverse: TCGGGATATTGAATTCTTGAGCTAC) and KASP-AX-86175290 (FAM: GAAGGTGACCAAGTTCATGCTTGCAGTGAAGTTATTAACACC; HEX: GAAGGTCGGAGTCAACGGATTTGCAGTGAAGTTATTAACACT; Reverse: ATATGCAGTACAGATCTTTCACAG) were designed according to the sequence of AX-86172908 and AX-86175290, respectively. The lines were divided into two categories based on the genotype of the two sets of KASP markers: the first category comprised lines with homozygous alleles from BMM, and the second category comprised lines with homozygous alleles from CM104. The significance of differences between the two categories (p < 0.05) was statistically analyzed using IBM SPSS Statistics 25 software.
3. Results
3.1. Phenotypic Evaluation
The leaf pubescence of CM104 (with non-pubescent leaves) was scored as 0 and BMM (with the longest and highest-density leaf pubescence) was scored as 3 (Figure 1, Table 1). Thus, the length and density of leaf pubescence differed significantly between CM104 and BMM. The 234 RILs differed in pubescence phenotypes ranging in scale from 0 to 3 in the four environments at the heading stage (Table 1). The phenotype of each line was consistent across all environments. Correlation coefficients among the different environments were significant (p < 0.01) and ranged from 0.4815 to 0.9139.

Table 1.
LP density of the parents and recombinant inbred lines at the heading stage.
3.2. QTL Mapping for Leaf Pubescence
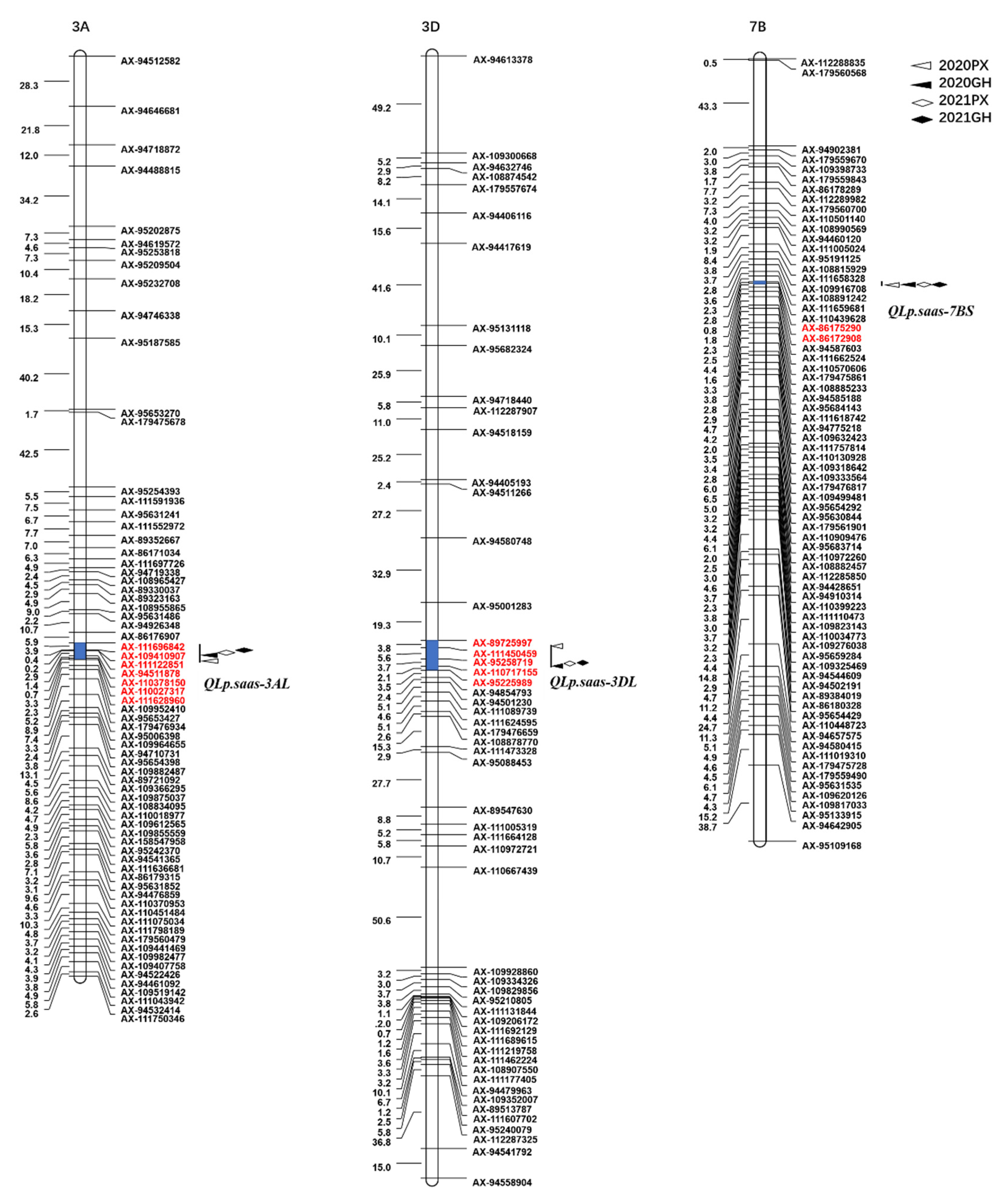
Three putative QTLs associated with leaf pubescence were detected and localized on chromosomes 7BS, 3AL, and 3DL, and were designated QLp.saas-7BS, QLp.saas-3AL, and QLp.saas-3DL, respectively (Figure 2, Table 2). QLp.saas-7BS and QLp.saas-3AL were derived from BMM, whereas QLp.saas-3DL was derived from CM104.
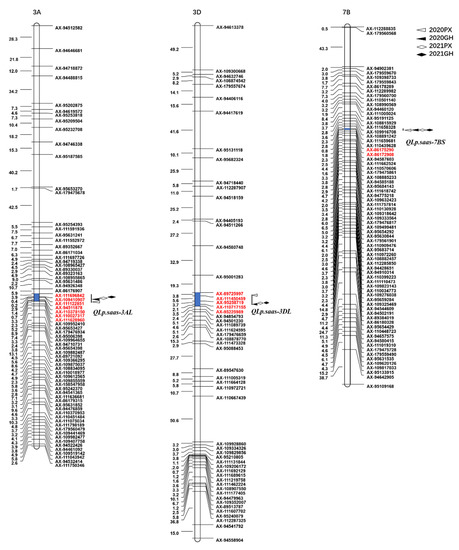
Figure 2.
Mapping of QTLs associated with leaf pubescence in the CM104 × BMM recombinant inbred lines population grown in four environments; 2020PX, 2021PX, 2020GH, and 2021GH represent the four environments: Pixian in 2020, Pixian in 2021, Guanghan in 2020, and Guanghan 2021, respectively.

Table 2.
QTLs for leaf pubescence by inclusive composite interval mapping in the recombinant inbred lines population of Chuanmai104 × Baimaomai in four environments. The physical position of markers is in relation to the Chinese Spring reference genome (IWGSC RefSeq v2.1).
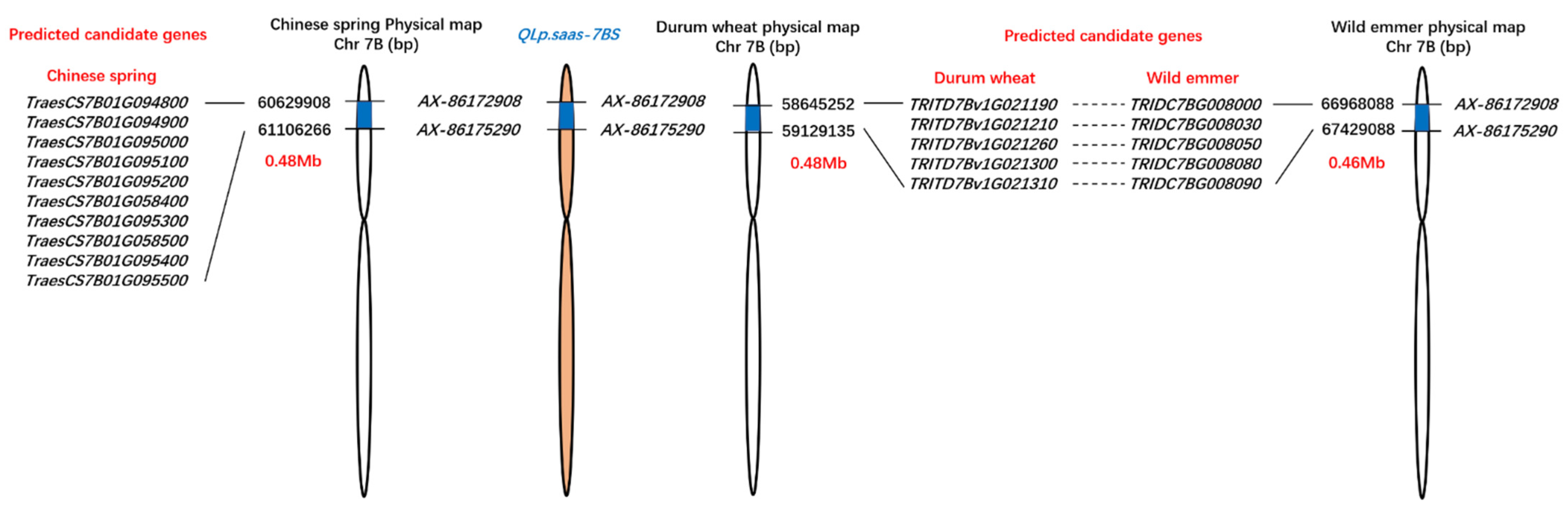
As a stable major locus, QLp.saas-7BS was detected in all four environments and explained 14.16–40.77% of the total phenotypic variance (Table 2). Based on the Chinese Spring IWGSC RefSeq v2.1 reference genome [41], the major allele of QLp.saas-7BS was localized in a 0.48 Mb genomic region between the markers AX-86175290 and AX-86172908 on the short arm of chromosome 7B; the genetic distance between the markers was 0.8 cM. On the basis of the physical position of the flanking sequences of the SNP markers, 10 candidate genes were located in this interval of the Chinese Spring genome.
With reference to the genome sequence of durum wheat (Svevo RefSeq Rel. 1.0), QLp.saas-7BS was localized in a 0.48 Mb interval between the physical locations 58.65 Mb and 59.13 Mb on chromosome 7BS. Five candidate genes were located in this interval. Furthermore, QLp.saas-7BS was localized to a 0.46 Mb interval between the physical positions 66.97 Mb and 67.43 Mb on chromosome 7BS of the wild emmer reference genome (Zavitan WEWSeq v2.0). Five candidate genes were also located in this interval (Figure 3).
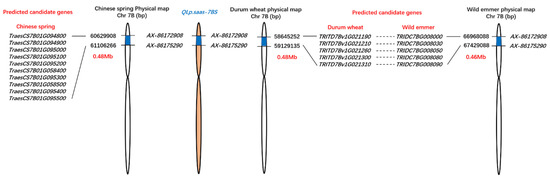
Figure 3.
Physical map and candidate genes of the major-effect QTL QLp.saas-7BS in relation to the Chinese Spring, wild emmer, and durum wheat reference genome sequences accessed in the Triticeae Multi-omics Center database (http://202.194.139.32/).
A minor-effect locus, QLp.saas-3AL, derived from BMM was identified in all environments and was localized on the long arm of chromosome 3A. This QTL explained 3.07–4.44% of the total phenotypic variance. The minimum physical distance of QLp.saas-3AL was mapped to a 3.16 Mb region between AX-110027317 and AX-111628960 in 2020PX, and the maximum physical distance was localized to a 10.60 Mb region between AX-94511878 and AX-110378150 in 2020GH. In addition, QLp.saas-3AL was detected in 2021PX and 2021GH, and the physical interval was 3.4 Mb between AX-109410907 and AX-111122851, and 5.92 Mb between AX-111696842 and AX-109410907, respectively (Table 2, Figure 2).
An additional minor locus, QLp.saas-3DL, derived from CM104, was detected in all environments and mapped on the long arm of chromosome 3D, and explained 3.64–6.46% of the total phenotypic variance. The physical interval of QLp.saas-3DL was localized to a 4.92 Mb genome region between AX-89725997 and AX-95258719 in 2021PX and 2021GH. However, in 2020PX, the physical interval of QLp.saas-3DL was localized to the 10.33 Mb genome region between AX-111450459 and AX-110717155 in 2020PX, and to the 7.78 Mb region between AX-95258719 and AX-95225989 in 2020GH (Table 2, Figure 2).
3.3. Validation of the Novel Major QTL QLp.saas-7BS
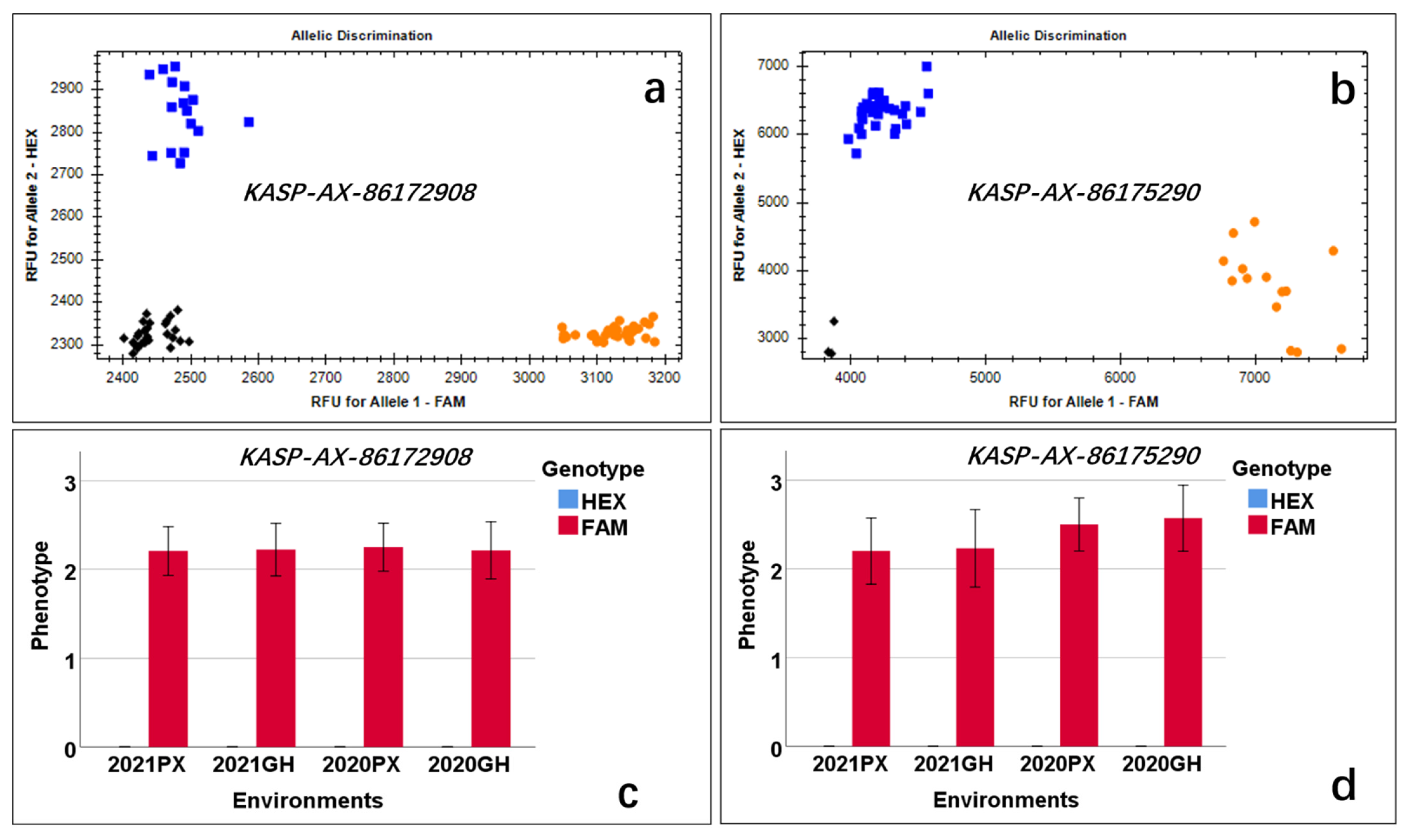
A total of 62 lines randomly selected from among the CB-RIL and PB-RIL populations were used to evaluate the effects of QLp.saas-7BS. The newly developed markers KASP-AX-86172908 and KASP-AX-86175290 tightly linked to QLp.saas-7BS were used to evaluate the alleles derived from BMM. Based on the presence or absence of the allele from BMM, all 62 tested lines showed polymorphism by detection with the two markers and were classified into two categories (Figure 4, Table S1). The difference between the two categories was significant (p < 0.05) (Figure 4).
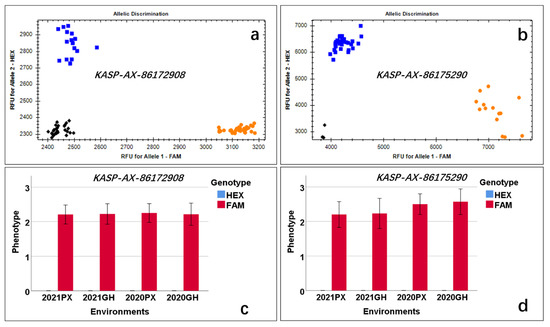
Figure 4.
Allelic discrimination of the markers KASP-AX-86172908 and KASP-AX-86175290. Blue squares represent the lines lacking leaf pubescence (HEX fluorescence), orange circles represent the lines with leaf pubescence (FAM fluorescence), and black diamonds represent the negative control. (a) Allelic discrimination of KASP-AX-86172908 included 16 lines without leaf pubescence (blue squares) and 28 lines with leaf pubescence (orange circles). (b) Allelic discrimination of KASP-AX-86175290 included 30 lines without leaf pubescence (blue squares) and 14 lines with leaf pubescence (orange circles). (c,d) Effects of QLp.saas-7BS in validation lines detected by KASP-AX-86172908 and KASP-AX-86175290, respectively; the y-axis is the phenotype score, and the x-axis is the four environments; the phenotype score of lines without pubescence was 0, which is represented by a blue bar (HEX); the phenotype score of other lines is represented by a red bar (FAM fluorescence).
4. Discussion
4.1. QLp.saas-7BS Is a Novel Major QTL
The dominant gene Hl2 was previously localized on chromosome 7B in the pubescent Chinese wheat cultivar ‘Hong-Mang-Mai’ by monosomic and phenotypic analysis of F1 plants and the F2 population developed from the cross between ‘Hong-Mang-Mai’ and ‘Chinese Spring’. The Hl2 gene was subsequently mapped to the short arm of chromosome 7B by telosomic and phenotypic analysis of BC1F1 populations developed from the backcross between monotelodisomic F1 hybrids and ‘Chinese Spring’ [35]. However, the precise physical position of Hl2 remains unclear. Pshenichnikova et al. [37] identified a major gene, Hl2aesp, on chromosome 7BS that controls the length of leaf trichomes using the same method. The gene Hl2aesp was introgressed from Ae. speltoides into common wheat. Based on an allelism test of F2 individuals from the cross between ‘Hong-Mang-Mai’ carrying Hl2 and the wheat/Ae. speltoides introgression line 102/00I carrying Hl2aesp, Hl2aesp was concluded to be allelic to Hl2 and was localized to the interval between Xgwm255 and Xgwm400 [33,36]. On the basis of marker sequences and the Chinese Spring IWGSC RefSeq v2.1 genome assembly [41], Hl2aesp was approximately localized to a more distal position within the 26.8–34.3 Mb interval on the short arm of chromosome 7B. In addition to chromosome 7B, QTLs associated with leaf pubescence have been identified on chromosomes 7A [42] and 7D [38]. Therefore, it is speculated that a series of homoeologous genes regulating leaf pubescence might be present on homoeologous group 7 chromosomes [38].
In the present study, QLp.saas-7BS, a major QTL derived from BMM, was localized to chromosome 7BS in a 0.48 Mb interval between the markers AX-86175290 (60.63 Mb) and AX-86172908 (61.11 Mb) in the Chinese Spring IWGSC RefSeq v2.1 genome [41]. The results showed that QLp.saas-7BS was closer to the centromere than Hl2aesp, which was closer to the distal end of 7BS. Therefore, it is speculated that QLp.saas-7BS might be a novel major QTL and distinct from Hl2aesp, although the relationship among these genes requires further study. Effective KASP markers tightly linked to QLp.saas-7BS were developed and verified, which would be helpful to conduct marker-assisted selection for this morphological trait in the future.
4.2. Candidate Genes Located in the Interval for QLp.saas-7BS
Based on the Chinese Spring IWGSC RefSeq v2.1 reference genome, the physical interval of QLp.saas-7BS contained 10 candidate genes (Figure 3). Four of these genes, namely TraesCS7B01G094800, TraesCS7B01G094900, TraesCS7B01G095000, and TraesCS7B01G058400, were annotated to encode a glutamate receptor, which is associated with various physiological and developmental processes [43]. It is speculated that the development of leaf pubescence is regulated by glutamate receptor signaling. In addition, a plant glutamate receptor may contribute to the ability to respond to an attack from a pest or pathogen [44], and enhances the heat tolerance of maize seedlings [45]. Interestingly, one candidate gene, TraesCS7B01G058500, was annotated to encode a mannitol transporter, which contributes to the plant response to drought stress [46,47]. A mannitol transporter is also associated with resistance to biotic and abiotic stresses [48]. TraesCS7B01G095500 was annotated to encode a pentatricopeptide repeat (PPR) superfamily protein, which are mainly localized in chloroplasts and mitochondria and are reported to be involved in the regulation of plant growth and development under stresses [40]. Among the other candidate genes, TraesCS7B01G095400 was annotated to encode a DNA polymerase, which is responsible for DNA synthesis during the process of DNA replication and the transfer genetic information between generations. Three candidate genes, namely TraesCS7B01G095100, TraesCS7B01G095200, and TraesCS7B01G095300, were annotated to encode a retrotransposon protein, which represents a complex fraction of repetitive DNA present in most eukaryotes [49] and is involved in generation of mutations through insertions near or within genes [50]. These candidate genes located in the physical interval of QLp.saas-7BS will be evaluated and validated for the association to the leaf pubescence in further studies.
Furthermore, QLp.saas-7BS was localized to a narrow physical interval on chromosome 7BS of durum wheat (0.48 Mb) and wild emmer (0.46 Mb). The reference genomes for durum wheat (Svevo RefSeq Rel. 1.0) and wild emmer (Zavitan WEWSeq v2.0) each contained five candidate genes in the target interval (Figure 3). These genes will contribute to fine mapping and cloning of the novel major QTL QLp.saas-7BS in the future.
4.3. Other QTLs
Taketa et al. [35] detected a highly significant segregation ratio for a critical monosomic combination from a monosomic analysis of F2 plants derived from the cross between Chinese Spring 3D monosomics and Hong-Mang-Mai, but the monosomic analysis was contrary to the results of phenotypic analysis. Therefore, it was considered that chromosome 3D did not carry a gene for leaf pubescence [35]. Interestingly, a stable and minor QTL, QLp.saas-3DL, derived from CM104, was also identified on chromosome 3DL in all four environments in the present study (Table 2). However, the leaf blade of CM104 is non-pubescent (Figure 1), but has been reported to show leaf sheath hairiness when introgressed from Ae. tauschii [32]. The possibility of a relationship between leaf sheath hairiness and QLp.saas-3DL requires further investigation in the future. Furthermore, the relationship between chromosome 3D and leaf pubescence remains uncertain and requires further study.
To date, genes controlling leaf pubescence on chromosome 3A have not been reported previously in wheat. QLp.saas-3AL, as a stable and minor QTL, was first reported to be localized on chromosome 3AL derived from BMM (Table 2). In barley (Hordeum vulgare L.), the gene Pub on chromosome 3HL is responsible for leaf pubescence [36]. The collinearity between QLp.saas-3AL and Pub would be crucial warranting further research to understand the functions of both loci.
Leaf pubescence, as an important adaptive trait, reflects resistance to biotic and abiotic stresses, such as insect and pathogen attack, drought, and radiation, and can also enhance photosynthetic efficiency. In the present study, three gene loci associated with leaf pubescence were identified in wheat, comprising the major QTL QLp.saas-7BS and two minor QTLs QLp.saas-3AL and QLp.saas-3DL. The present results will contribute to a better understanding of the formation and development of leaf pubescence in wheat, and the newly developed markers will be useful to detect the QLp.saas-7BS related to leaf pubescence in the future.
5. Conclusions
Leaf pubescence is an important phenotypic trait closely associated with plant adaptability. Three QTLs related to leaf pubescence were detected in this study, namely QLp.saas-3AL, QLp.saas-3DL, and QLp.saas-7BS. The QTLs QLp.saas-3AL and QLp.saas-3DL are minor-effect loci. QLp.saas-7BS derived from BMM is a novel major-effect locus that was detected in all four environments and mapped to a 0.48 Mb interval on chromosome arm 7BS, and explained up to 40.77% of the total phenotypic variance. We also developed and verified KASP markers tightly linked to QLp.saas-7BS. These results will be helpful for marker-assisted selection, fine mapping, and cloning of genes associated with leaf pubescence in wheat.
6. Patents
A patent application has been submitted in China for the primer sequences of all the KASP markers in this study.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11112237/s1, Table S1: The total of 62 lines were used to validate the developed KASP markers including KASP-AX-86172908 and KASP-AX-86175290.
Author Contributions
Data curation, Z.L., Q.W. and H.W.; formal analysis, F.Y., S.L., M.Y. and J.L. (Jiangtao Luo); validation, J.Z., Q.D., H.L. and G.D.; resources, N.Y.; writing—original draft, Z.L. and Q.W.; writing—review and editing, J.L. (Jun Li) and W.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Key Research and Development Program of Science and Technology Project of Sichuan Province (2021YFYZ0020), the Accurate Identification Project of Crop Germplasm from Sichuan Provincial Finance Department, the Program of Chinese Agriculture Research System (CARS-03), and the Science and Technology Project of Sichuan Province (2020YFSY0049).
Acknowledgments
We thank Robert McKenzie for improving the language of this manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Solereder, H. Systematic anatomy of dicotyledons: A handbook for laboratories of pure and applied botany. Nature 1908, 79, 211–212. [Google Scholar]
- Ehleringer, J.; Bjorkman, O.; Mooney, H.A. Leaf Pubescence: Effects on Absorptance and Photosynthesis in a Desert Shrub. Science 1976, 192, 376–377. [Google Scholar] [CrossRef] [PubMed]
- Wood, W.; Gore, J.; Catchot, A.; Cook, D.; Dodds, D.; Krutz, J. Effect of leaf pubescence on tarnished plant bug (Hemiptera: Miridae) ability to cause damage and yield loss in cotton. J. Cotton Sci. 2017, 21, 122–127. [Google Scholar]
- Xie, Y.; Yu, X.; Jiang, S.; Xiao, K.; Wang, Y.; Li, L.; Wang, F.; He, W.; Cai, Q.; Xie, H.; et al. OsGL6, a conserved AP2 domain protein, promotes leaf trichome initiation in rice. Biochem. Biophys. Res. Commun. 2020, 522, 448–455. [Google Scholar] [CrossRef]
- Oguchi, R.; Onoda, Y.; Terashima, I.; Tholen, D. Leaf anatomy and function of chapter 5. In The Leaf: A Platform for Performing Photosynthesis, Advances in Photosynthesis and Respiration Including Bioenergy and Related Processes, 2nd ed.; Adams, W.W., III, Terashima, I., Eds.; Springer Nature Switzerland AG: Gewerbestrasse, Switzerland, 2018; Volume 44, pp. 98–128. [Google Scholar]
- Pandey, P.; Irulappan, V.; Bagavathiannan, M.V.; Senthil-kumar, M. Impact of combined abiotic and biotic stresses on plant growth and avenues for crop improvement by exploiting physio-morpholgical traits. Front. Plant Sci. 2017, 8, 537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nawab, N.N.; Khan, I.A.; Khan, A.A.; Amjad, M. Characterization and inheritance of cotton leaf pubescence. Pak. J. Bot. 2011, 43, 649–658. [Google Scholar]
- Ringlund, K.; Everson, E.H. Leaf pubescence in common wheat, Triticum aestivum L., and resistance to the cereal leaf beetle, Oulema melanopus (L.). Crop Sci. 1968, 8, 705–710. [Google Scholar] [CrossRef]
- Webster, J.A.; Smith, D.H.; Rathke, J.E.; Cress, C.E. Resistance to cereal leaf beetle in wheat: Density and length of leaf-surface pubescence in four wheat lines. Crop Sci. 1975, 15, 199–202. [Google Scholar] [CrossRef]
- Webster, J.A.; Inayatullah, C.; Hamissou, M.; Mirkes, K.A. Leaf pubescence effects in wheat on yellow sugarcane aphids and greenbugs (Hmoptera: Aphididae). J. Econ. Entomol. 1994, 87, 231–240. [Google Scholar] [CrossRef]
- Roberts, J.J.; Foster, J.E. Effect of leaf pubescence in wheat on the bird cherry oat aphid (Homoptera: Aphidae). J. Econ. Entomol. 1983, 76, 1320–1322. [Google Scholar] [CrossRef]
- Mergher, R.L.; Smith, C.W.; Smith, W.J. Preference of Gossypium genotypes to Bemisisa argentifolii (Homoptera: Aleyrodidae). J. Econ. Entomol. 1997, 90, 1046–1052. [Google Scholar] [CrossRef]
- Haider, S.S.; Hassan, M.W.; Jamil, M. Comparison of wheat varieties (Triticum aestivum L.) for aphid (Homoptera: Aphididae) infestation in relation to physico-morphic traits sown under semi arid climatic conditions. J. Agric. Sci. 2017, 3, 42–51. [Google Scholar]
- Konyspaevna, S.K. Spring wheat resistance against cereal leaf beetle (Oulema melanopus Z.) in relation to leaf pubescence. Aust. J. Basic Appl. Sci. 2012, 6, 515–518. [Google Scholar]
- Lai, A.; Cianciolo, V.; Chiavarini, S.; Sonnino, A. Effects of glandular trichomes on the development of Phytophthora infestans infection in potato (S. tuberosum). Euphytica 2000, 114, 165–174. [Google Scholar] [CrossRef]
- Gao, Y.; He, C.; Zhang, D.; Liu, X.; Xu, Z.; Tian, Y.; Liu, X.; Zang, S.; Pauly, M.; Zhou, Y.; et al. Two trichome birefringence-like proteins mediate xylan acetylation, which is essential for leaf blight resistance in rice. Plant Physiol. 2017, 173, 470–481. [Google Scholar] [CrossRef] [Green Version]
- Calo, L.; Carcia, I.; Gotor, C.; Romero, L.C. Leaf hairs influence phytopathogenic fungus infection and confer an increased resistance when expressing a Trichoderma α-1,3-glucanase. J. Exp. Bot. 2006, 57, 3911–3920. [Google Scholar] [CrossRef] [Green Version]
- Pshenichnikova, T.A.; Doroshkov, A.V.; Osipova, S.V.; Permyakov, A.V.; Permyakova, M.D.; Efimov, V.M.; Afonnikov, D.A. Quantitative characteristics of pubescence in wheat (Triticum aestivum L.) are associated with photosynthetic parameters under conditions of normal and limited water supply. Planta 2019, 249, 839–847. [Google Scholar] [CrossRef]
- Osipova, S.V.; Rudikvskii, A.V.; Permyakov, A.V.; Rudikovskaya, E.G.; Permyakova, M.D.; Verkhoturov, V.V.; Pshenichnikova, T.A. Physiological responses to water deficiency in bread wheat (Triticum aestivum L.) lines with genetically different leaf pubescence. Vavilov, J. Genet. Breed. 2020, 24, 813–820. [Google Scholar] [CrossRef]
- Skelton, R.P.; Midgley, J.J.; Nyaga, J.M.; Johnson, S.D.; Cramer, M.D. Is leaf pubescence of Cape Proteaceae a xeromorphic or radiation-protective trait? Aust. J. Bot. 2012, 60, 104–113. [Google Scholar] [CrossRef] [Green Version]
- Moles, A.T.; Laffan, S.W.; Keighery, M.; Dalrymple, R.L.; Tindall, M.L.; Chen, S.C. A hairy situation: Plant species in warm, sunny places are more likely to have pubescent leaves. J. Biogeogr. 2020, 47, 1–11. [Google Scholar] [CrossRef]
- Wang, X.; Shen, C.; Meng, P.; Tan, G.; Lv, L. Analysis and review of trichomes in plants. BMC Plant Biol. 2021, 21, 70. [Google Scholar]
- Grammatikopoulos, G.; Manetas, Y. Direct absorption of water by hairy leaves of phlomis fruticose and tis contribution to drought avoidance. Rev. Can. Bot. 1994, 72, 1805–1811. [Google Scholar] [CrossRef]
- Tao, Y.; Zhang, Y.M. Effects of leaf hair points of a desert moss on water retention and dew formation: Implications for desiccation tolerance. J. Plant. Res. 2012, 125, 351–360. [Google Scholar] [CrossRef] [PubMed]
- Konrad, W.; Burkhardt, J.; Ebner, M.; Roth-Nebelsick, R. Leaf pubescence as a possibility to increase water use efficiency by promoting condensation. Ecohydrology 2015, 8, 480–492. [Google Scholar] [CrossRef]
- Du, W.J.; Fu, S.X.; Yu, D.Y. Genetic analysis for the leaf pubescence density and water status traits in soybean [Glycine max (L.) Merr.]. Plant Breed. 2009, 128, 259–265. [Google Scholar] [CrossRef]
- Hu, B.; Wan, Y.; Li, X.; Zhang, F.; Yan, W.; Xie, J. Phenotypic characterization and genetic analysis of rice with pubescent leaves and glabrous hulls (PLgh). Crop Sci. 2013, 53, 1878–1886. [Google Scholar] [CrossRef]
- Gormus, O.; Kurt, F.; Sabagh, A.E. Impact of defoliation timings and leaf pubescence on yield and fiber quality of cotton. J. Agric. Sci. Technol. 2017, 19, 903–915. [Google Scholar]
- Blanco, A.; Bellomo, M.P.; Cenci, A.; Giovanni, C.D.; Ovidio, R.D.; Lacono, E.; Laddomada, B.; Pagnotta, M.A.; Porceddu, E.; Sciancalepore, A.; et al. A genetic linkage map of durum wheat. Theor. Appl. Genet. 1998, 97, 721–728. [Google Scholar] [CrossRef]
- Wu, P.; Yang, L.; Guo, G.; Hu, J.; Qiu, D.; Li, Y.; Shi, X.; Zhang, H.; Liu, H.; Zhao, J.; et al. Molecular mapping and identification of a candidate gene for new locus Hg2 conferring hairy glume in wheat. Plant Sci. 2021, 307, 110879. [Google Scholar] [CrossRef]
- Luo, W.; Liu, J.; Ding, P.; Li, C.; Liu, H.; Mu, Y.; Tang, H.; Jiang, Q.; Liu, Y.; Chen, G.; et al. Transcriptome analysis of near-isogenic lines for glume hairiness of wheat. Gene 2020, 739, 144517. [Google Scholar] [CrossRef]
- Wan, H.; Yang, Y.; Li, J.; Zhang, Z.; Yang, W. Mapping a major QTL for hairy leaf sheath introgressed from Aegilops tauschii and its association with enhanced grain yield in bread wheat. Euphytica 2015, 205, 275–285. [Google Scholar] [CrossRef]
- Dobrovolskaya, O.B.; Pshenichnikova, T.A.; Arbzzova, V.S.; Lohwasser, U.; Roder, M.S.; Borner, A. Molecular mapping of genes determining hairy leaf character in common wheat with respect to other species of the Triticeae. Euphytica 2007, 155, 285–293. [Google Scholar] [CrossRef]
- Maystrenko, O.I. Identification and location of genes controlling leaf hairing in young plants of common wheat. Genetika (Moscow) 1976, 12, 5–15. [Google Scholar]
- Taketa, S.; Chang, C.L.; Ishii, M.; Takeda, K. Chromosome arm location of the gene controlling leaf pubescence of a Chinese local wheat cultivar ‘Hong-mang-mai’. Euphytica 2002, 125, 141–147. [Google Scholar] [CrossRef]
- Doroshkov, A.V.; Afonnikov, D.A.; Dobrovolskaya, O.B.; Pshenichnikova, T.A. Interactions between leaf pubescence genes in bread wheat as assessed by high throughput phenotyping. Euphytica 2015, 207, 1–10. [Google Scholar] [CrossRef]
- Pshenichnikova, T.A.; Lapochkina, I.F.; Shchulina, L.V. The inheritance of morphological and biochemical traits introgressed into common wheat (Triticum aestivum L.) from Aegilops speltoides Tausch. Genet. Resour. Crop Evol. 2007, 54, 287–293. [Google Scholar] [CrossRef]
- Doroshkov, A.V.; Afonnikov, D.A.; Pshenichnikova, T.A. Genetic analysis of leaf pubescence in isogenic lines of bread wheat Novasibirskaya 67. Russ. J. Genet. 2014, 50, 172–180. [Google Scholar] [CrossRef]
- Simonov, A.V.; Smirnova, O.G.; Genaev, M.A.; Pshenichnikova, T.A. The identification of a new gene for leaf pubescence introgressed into bread wheat from Triticum timopheevii Zhuk. and its manifestation in a different genotypic background. Plant Genet. Resour. 2021, 19, 238–244. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Wan, H.; Yang, F.; Wei, H.; Xu, Z.; Ji, H.; Xia, X.; Li, J.; Yang, W. QTL mapping for adult-plant resistance to powdery mildew in Chinese elite common wheat Chuanmai104. Cereal Res. Commun. 2021, 49, 99–108. [Google Scholar] [CrossRef]
- Zhu, T.; Wang, L.; Rimbert, H.; Rodriguez, J.C.; Deal, K.R.; Oliveira, R.D.; Choulet, F.; Keeble-Gagnere, G.; Tibbits, J.; Rogers, J.; et al. Optical maps refine the bread wheat Triticum aestivum cv. Chinese Srping genome assembly. Plant J. 2021, 107, 303–314. [Google Scholar] [CrossRef]
- Shahinnia, F.; Leroy, J.; Saba, M.; Okamoto, M.; Zhong-Hua, C.; Langridge, P.; Fleury, D. Identification of quantitative trait loci for leaf stomatal and epidermal cell traits in wheat (Triticum aestivum L.). In Proceedings of the 12th International Wheat Genetic Symposium, Pacifico Yokohama, Japan, 8–14 September 2013; p. 132. [Google Scholar]
- Chen, J.; Jing, Y.; Zhang, X.; Li, L.; Wang, P.; Zhang, S.; Zhou, H.; Wu, J. Evolutionary and expression analysis provides evidence for the plant glutamate-like receptors family is involved in woody growth-related function. Sci. Rep. 2016, 6, 32013. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forde, B.G.; Roberts, M. Glutamate receptor-like channels in plants: A role as amino acid sensors in plant defence? F1000Prime Rep. 2014, 6, 37. [Google Scholar] [CrossRef]
- Li, Z.; Ye, X.; Qiu, X. Glutamate signaling enhances the heat tolerance of maize seedlings by plant glutamate receptor-like channels-mediated calcium signaling. Protoplasma 2019, 256, 1165–1169. [Google Scholar] [CrossRef]
- Ullah, I.; Akhtar, N.; Mehmood, N.; Shan, I.A.; Noor, M. Effect of mannitol induced drought stress on seedling traits and protein profile of two wheat cultivars. J. Anim. Plant Sci. 2014, 24, 1246–1251. [Google Scholar]
- Hadi, F.; Ayaz, M.; Ali, S.; Shafiq, M.; Ullah, R.; Jan, A.U. Comparative effect of polyethylene glycol and mannitol induced drought on growth (in vitor) of canola (Brassica napus), cauliflower (Brassica oleracea) and tomato (Lycopersicon esculentum) seedlings. Int. J. Biosci. 2014, 4, 34–41. [Google Scholar]
- Patel, T.K.; Williamson, J.D. Mannitol in plants, fungi, and plant-fungal interactions. Trends Plant Sci. 2016, 21, 486–497. [Google Scholar] [CrossRef]
- Langdon, T.; Seago, C.; Mende, M.; Leggett, M.; Thomas, H.; Forster, J.W.; Thomas, H.; Jones, R.N.; Jenkins, G. Retrotransposon evolution in diverse plant genomes. Genetics 2000, 156, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bennetzen, J.L. Plant Retrotransposons. Annu. Rev. Genet. 1999, 33, 479–532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).