Perinatal Environmental Health Education Intervention to Reduce Exposure to Endocrine Disruptors: The PREVED Project
Abstract
:1. Background
2. Methods
2.1. PREVED Project
2.2. RE-AIM Method
2.2.1. Reach, Adoption, and Implementation Assessment
2.2.2. Efficacy Assessment
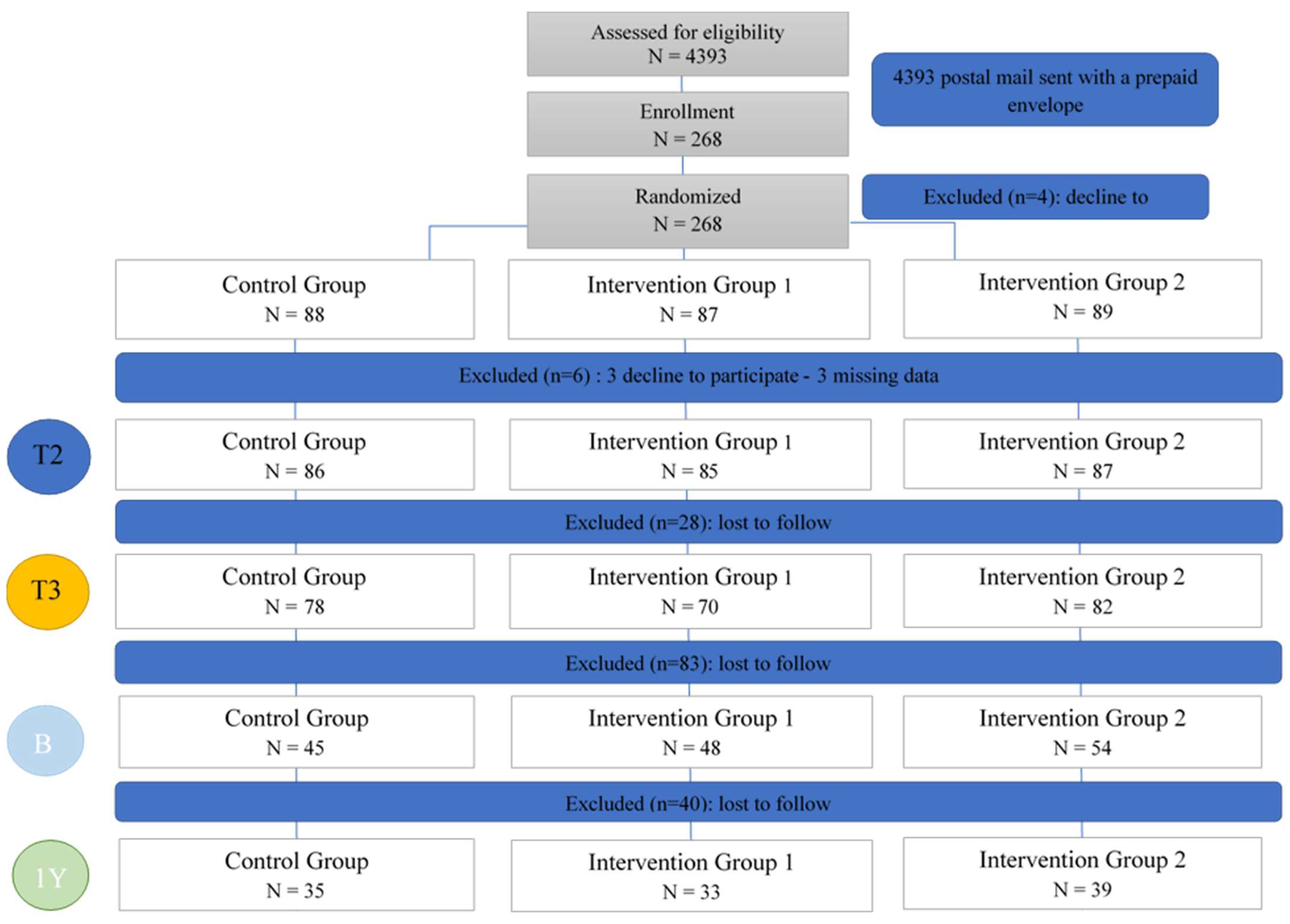
Efficacy Study Design
Efficacy Study Outcomes
2.3. Statistical Analysis
3. Results
3.1. Reach, Adoption, and Implementation
3.1.1. Reach
3.1.2. Program Adoption
Quantitative Analysis
Qualitative Analysis
3.1.3. Implementation
3.2. Efficacy: RCT Results
3.2.1. Intent-to-Treat (ITT) Analysis
3.2.2. Per-Protocol-Analysis
3.3. Maintenance
4. Discussion
4.1. Main Results
4.1.1. Reach and Adoption
4.1.2. Effectiveness = Efficacy ∗ Implementation
4.2. Strengths and Limits
4.2.1. Evaluation Model
4.2.2. Outcome Choice of the Randomized Control Trial
4.2.3. Construction
Consortium
Behavior Theory Model
Diagnosis with Pregnant Women
Public Heath Deployment
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Trial Registration Number
Abbreviations
| ANOVA | analysis of variance |
| BPA | Bisphenol A |
| BuPB | Butylparaben |
| ClxBPA | Chlorinated derivatives of Bisphenol A |
| DCBPA | Dichlorobisphenol A |
| DisProSe | Dispositif partenarial de recherche interventionnelle en Promotion de la Santé environnementale |
| DOHaD | Developmental Origins Hypothesis of Health and Diseases |
| EDDS cohort | Endocrine Disruptors Deux-Sèvres cohort |
| EDs | Endocrine disruptors |
| EPICES | Évaluation de la précarité et des inégalités de santé dans les Centres d’examens de santé |
| EtPB | Ethylparaben |
| GP | general practitioners |
| HBM | Health Belief Model |
| ITT analysis | Intent-to-treat analysis |
| KAP | Knowledge, attitudes, and practices |
| kNN-TN | Truncation k-Nearest Neighbor imputation |
| LC-MS/MS | Liquid Chromatography with tandem Mass Spectrometry |
| LoD | Limit of Detection |
| LoQ | Limit of Quantification |
| MCBPA | Monochlorobisphenol A |
| MePB | Methylparaben |
| PB | Paraben |
| PP analysis | Per-protocol analysis |
| PHIR | Population health intervention research |
| PMI | Protection Maternelle et Infantile |
| PREVED | PREgnancy preVention Endocrine Disruptors |
| PrPB | Propylparaben |
| Q1 | Consumption questionnaire |
| Q2 | Psychosocial questionnaire |
| QR | Quick Response |
| RCT | Randomized Controlled Trial |
| RE-AIM | Reach, Efficacy, Adoption, Implementation, and Maintenance |
| TCBPA | Trichlorobisphenol A |
| TIDieR | Template for Intervention Description and Replication |
| TTBPA | Tetrachlorobisphenol A |
| WHO | World Health Organization |
References
- Barouki, R.; Gluckman, P.D.; Grandjean, P.; Hanson, M.; Heindel, J.J. Developmental origins of non-communicable disease: Implications for research and public health. Environ. Health 2012, 11, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaudriault, P.; Mazaud-Guittot, S.; Lavoué, V.; Coiffec, I.; Lesné, L.; Dejucq-Rainsford, N.; Scholze, M.; Kortenkamp, A.; Jegou, B. Endocrine Disruption in Human Fetal Testis Explants by Individual and Combined Exposures to Selected Pharmaceuticals, Pesticides, and Environmental Pollutants. Environ. Health Perspect. 2017, 125, 087004. [Google Scholar] [CrossRef] [PubMed]
- Albouy-Llaty, M.; Dupuis, A.; Grignon, C.; Strezlec, S.; Pierre, F.; Rabouan, S.; Migeot, V. Estimating drinking-water ingestion and dermal contact with water in a French population of pregnant women: The EDDS cohort study. J. Expo. Sci. Environ. Epidemiol. 2014, 25, 308–316. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.-H.; Zhang, J.; Huang, R.-P.; Yin, H.; Dang, Z. Human exposure of bisphenol A and its analogues: Understandings from human urinary excretion data and wastewater-based epidemiology. Environ. Sci. Pollut. Res. 2019, 27, 3247–3256. [Google Scholar] [CrossRef] [PubMed]
- Plattard, N.; Dupuis, A.; Migeot, V.; Haddad, S.; Venisse, N. An overview of the literature on emerging pollutants: Chlorinated derivatives of Bisphenol A (ClxBPA). Environ. Int. 2021, 153, 106547. [Google Scholar] [CrossRef] [PubMed]
- Biesterbos, J.W.; Dudzina, T.; Delmaar, C.J.; Bakker, M.I.; Russel, F.; von Goetz, N.; Scheepers, P.T.; Roeleveld, N. Usage patterns of personal care products: Important factors for exposure assessment. Food Chem. Toxicol. 2013, 55, 8–17. [Google Scholar] [CrossRef]
- Marie, C.; Lémery, D.; Vendittelli, F.; Sauvant-Rochat, M.-P. Perception of Environmental Risks and Health Promotion Attitudes of French Perinatal Health Professionals. Int. J. Environ. Res. Public Health 2016, 13, 1255. [Google Scholar] [CrossRef] [Green Version]
- Hanson, M.; Gluckman, P. Developmental origins of noncommunicable disease: Population and public health implications. Am. J. Clin. Nutr. 2011, 94, 1754S–1758S. [Google Scholar] [CrossRef] [Green Version]
- Di Renzo, G.C.; Conry, J.A.; Blake, J.; DeFrancesco, M.S.; DeNicola, N.; Martin, J.N., Jr.; McCue, K.A.; Richmond, D.; Shah, A.; Sutton, P.; et al. International Federation of Gynecology and Obstetrics opinion on reproductive health impacts of exposure to toxic environmental chemicals. Int. J. Gynecol. Obstet. 2015, 131, 219–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellingham, M.; Sharpe, R.M. Chemical Exposures During Pregnancy: Dealing with Potential, but Unproven, Risks to Child Health. Available online: https://www.rcog.org.uk/en/guidelines-research-services/guidelines/sip37/ (accessed on 11 February 2021).
- Carwile, J.L.; Ye, X.; Zhou, X.; Calafat, A.M.; Michels, K.B. Canned Soup Consumption and Urinary Bisphenol A: A Randomized Crossover Trial. JAMA 2011, 306, 2218–2220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rudel, R.A.; Gray, J.M.; Engel, C.L.; Rawsthorne, T.W.; Dodson, R.E.; Ackerman, J.M.; Rizzo, J.; Nudelman, J.L.; Brody, J.G. Food Packaging and Bisphenol A and Bis(2-Ethyhexyl) Phthalate Exposure: Findings from a Dietary Intervention. Environ. Health Perspect. 2011, 119, 914–920. [Google Scholar] [CrossRef] [PubMed]
- Sathyanarayana, S.; Alcedo, G.; Saelens, B.; Zhou, C.; Dills, R.L.; Yu, J.; Lanphear, B.P. Unexpected results in a randomized dietary trial to reduce phthalate and bisphenol A exposures. J. Expo. Sci. Environ. Epidemiol. 2013, 23, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Glasgow, R.E.; Harden, S.M.; Gaglio, B.; Rabin, B.; Smith, M.L.; Porter, G.C.; Ory, M.G.; Estabrooks, P.A. RE-AIM Planning and Evaluation Framework: Adapting to New Science and Practice With a 20-Year Review. Front. Public Health 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Rouillon, S.; El Ouazzani, H.; Hardouin, J.-B.; Enjalbert, L.; Rabouan, S.; Migeot, V.; Albouy-Llaty, M. How to Educate Pregnant Women about Endocrine Disruptors? Int. J. Environ. Res. Public Health 2020, 17, 2156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ouazzani, H.E.L.; Rouillon, S.; Venisse, N.; Sifer-Rivière, L.; Dupuis, A.; Cambien, G.; Ayraud-Thevenot, S.; Gourgues, A.-S.; Pierre-Eugène, P.; Pierre, F.; et al. Impact of perinatal environmental health education intervention on exposure to endocrine disruptors during pregnancy—PREVED study: Study protocol for a randomized controlled trial. Trials 2021, 22, 1–12. [Google Scholar] [CrossRef]
- Minary, L.; Trompette, J.; Kivits, J.; Cambon, L.; Tarquinio, C.; Alla, F. Which design to evaluate complex interventions? Toward a methodological framework through a systematic review. BMC Med. Res. Methodol. 2019, 19, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Craig, P.; Dieppe, P.; Macintyre, S.; Michie, S.; Nazareth, I.; Petticrew, M. Developing and evaluating complex interventions: The new Medical Research Council guidance. Int. J. Nurs. Stud. 2012, 50, 587–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sass, C.; Moulin, J.-J.; Guéguen, R.; Abric, L.; Dauphinot, V.; Dupré, C.; Giordanella, J.; Girard, F.; Guenot, C.; Lobertreau, E.; et al. Le Score Epices: Un Score Individuel de Précarité. Construction Du Score et Mesure Des Relations Avec Des Données de Santé, Dans Une Population de 197 389 Personnes. BEH 2006, 14, 93–96. [Google Scholar]
- Rosenstock, I.M. Historical Origins of the Health Belief Model. In The Health Belief Model and Personal Health Behavior; Slack: Thorofare, NJ, USA, 1974; ISBN 978-0-913590-34-8. [Google Scholar]
- Gringnon, C.; Venisse, N.; Rouillon, S.; Brunet, B.; Bacle, A.; Thevenot, S.; Migeot, V.; Dupuis, A. Ultrasensitive determination of bisphenol A and its chlorinated derivatives in urine using a high-throughput UPLC-MS/MS method. Anal. Bioanal. Chem. 2016, 408, 2255–2263. [Google Scholar] [CrossRef]
- De Keizer, J.; Paul, J.; Albouy, M.; Dupuis, A.; Migeot, V.; Rabouan, S.; Venisse, N.; Gand, E. Simulation et imputation de plusieurs variables corrélées dans un contexte de données manquantes de façon non aléatoires (MNAR). Rev. D’épidémiologie St. Publique 2021, 69, S32–S33. [Google Scholar] [CrossRef]
- Rouillon, S.; El Ouazzani, H.; Rabouan, S.; Migeot, V.; Albouy-Llaty, M. Determinants of Risk Perception Related to Exposure to Endocrine Disruptors during Pregnancy: A Qualitative and Quantitative Study on French Women. Int. J. Environ. Res. Public Health 2018, 15, 2231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, M.N.; Fage-Butler, A.M. Antenatal group consultations: Facilitating patient-patient education. Patient Educ. Couns. 2016, 99, 1999–2004. [Google Scholar] [CrossRef] [PubMed]
- Petosa, R.L.; Smith, L.H. Peer Mentoring for Health Behavior Change: A Systematic Review. Am. J. Health Educ. 2014, 45, 351–357. [Google Scholar] [CrossRef]
- Novak, I. Effective home programme intervention for adults: A systematic review. Clin. Rehabil. 2011, 25, 1066–1085. [Google Scholar] [CrossRef]
- Mankikar, D.; Campbell, C.; Greenberg, R. Evaluation of a Home-Based Environmental and Educational Intervention to Improve Health in Vulnerable Households: Southeastern Pennsylvania Lead and Healthy Homes Program. Int. J. Environ. Res. Public Health 2016, 13, 900. [Google Scholar] [CrossRef] [Green Version]
- Dilani, A.; Morelli, A. Health Promotion by Design in Elderly Care. In The International Academy for Design and Health; Research Center Design and Health: Stockholm, Sweden, 2005; pp. 287–299. [Google Scholar]
- Monson, K.; Moeller-Saxone, K.; Humphreys, C.; Harvey, C.; Herrman, H. Promoting mental health in out of home care in Australia. Health Promot. Int. 2019, 35, 1026–1036. [Google Scholar] [CrossRef]
- Ferrer, R.A.; Klein, W.M. Risk perceptions and health behavior. Curr. Opin. Psychol. 2015, 5, 85–89. [Google Scholar] [CrossRef] [Green Version]
- Gaube, S.; Lermer, E.; Fischer, P. The Concept of Risk Perception in Health-Related Behavior Theory and Behavior Change. In Perceived Safety: A Multidisciplinary Perspective; Risk Engineering; Raue, M., Streicher, B., Lermer, E., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 101–118. ISBN 978-3-030-11456-5. [Google Scholar] [CrossRef]
- Waters, E.A.; McQueen, A.; Cameron, L.D. Perceived Risk and Its Relationship to Health-Related Decisions and Behavior. Available online: https://www.oxfordhandbooks.com/view/10.1093/oxfordhb/9780199795833.001.0001/oxfordhb-9780199795833-e-013 (accessed on 4 June 2021).
- Lawlor, E.R.; Cupples, M.E.; Donnelly, M.; Tully, M.A. Implementing community-based health promotion in socio-economically disadvantaged areas: A qualitative study. J. Public Health 2019, 42, 839–847. [Google Scholar] [CrossRef]
- Dereumeaux, C.; Saoudi, A.; Pecheux, M.; Berat, B.; de Crouy-Chanel, P.; Zaros, C.; Brunel, S.; Delamaire, C.; le Tertre, A.; Lefranc, A.; et al. Biomarkers of exposure to environmental contaminants in French pregnant women from the Elfe cohort in 2011. Environ. Int. 2016, 97, 56–67. [Google Scholar] [CrossRef]
- SPF Imprégnation De La Population Française Par Les Bisphénols A, S et F: Programme National De Biosurveillance, Esteban 2014–2016. Available online: https://www.santepubliquefrance.fr/import/impregnation-de-la-population-francaise-par-les-bisphenols-a-s-et-f-programme-national-de-biosurveillance-esteban-2014-2016 (accessed on 19 July 2021).
- Arbuckle, T.E.; Marro, L.; Davis, K.; Fisher, M.; Ayotte, P.; Belanger, P.; Dumas, P.; Leblanc, A.; Bérubé, R.; Gaudreau, E.; et al. Exposure to Free and Conjugated Forms of Bisphenol A and Triclosan among Pregnant Women in the MIREC Cohort. Environ. Health Perspect. 2015, 123, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Zimmers, S.M.; Browne, E.P.; O’Keefe, P.W.; Anderton, D.L.; Kramer, L.; Reckhow, D.A.; Arcaro, K.F. Determination of free Bisphenol A (BPA) concentrations in breast milk of U.S. women using a sensitive LC/MS/MS method. Chemosphere 2014, 104, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Shirai, S.; Suzuki, Y.; Yoshinaga, J.; Shiraishi, H.; Mizumoto, Y. Urinary excretion of parabens in pregnant Japanese women. Reprod. Toxicol. 2013, 35, 96–101. [Google Scholar] [CrossRef]
- Charnock, C.; Finsrud, T. Combining esters of para-hydroxy benzoic acid (parabens) to achieve increased antimicrobial activity. J. Clin. Pharm. Ther. 2007, 32, 567–572. [Google Scholar] [CrossRef]
- Peng, C.-Y.; Tsai, E.-M.; Kao, T.-H.; Lai, T.-C.; Liang, S.-S.; Chiu, C.-C.; Wang, T.-N. Canned food intake and urinary bisphenol a concentrations: A randomized crossover intervention study. Environ. Sci. Pollut. Res. 2019, 26, 27999–28009. [Google Scholar] [CrossRef]
- Barrett, E.S.; Velez, M.; Qiu, X.; Chen, S.-R. Reducing Prenatal Phthalate Exposure Through Maternal Dietary Changes: Results from a Pilot Study. Matern. Child Health J. 2015, 19, 1936–1942. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraudeau, B.; Alberti, C. Is randomized trial design adapted to population health intervention research? Glob. Health Promot. 2021, 28, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Villeval, M.; Bidault, E.; Shoveller, J.; Alias, F.; Basson, J.-C.; Frasse, C.; Génolini, J.-P.; Pons, E.; Verbiguié, D.; Grosclaude, P.; et al. Enabling the transferability of complex interventions: Exploring the combination of an intervention’s key functions and implementation. Int. J. Public Health 2016, 61, 1031–1038. [Google Scholar] [CrossRef]
- Glasgow, R.E.; Vogt, T.M.; Boles, S.M. Evaluating the public health impact of health promotion interventions: The RE-AIM framework. Am. J. Public Health 1999, 89, 1322–1327. [Google Scholar] [CrossRef] [Green Version]
- Nhim, K.; Gruss, S.M.; Porterfield, D.S.; Jacobs, S.; Elkins, W.; Luman, E.T.; Van Aacken, S.; Schumacher, P.; Albright, A. Using a RE-AIM framework to identify promising practices in National Diabetes Prevention Program implementation. Implement. Sci. 2019, 14, 1–15. [Google Scholar] [CrossRef]
- Braun, J.M.; Smith, K.W.; Williams, P.L.; Calafat, A.M.; Berry, K.; Ehrlich, S.; Hauser, R. Variability of Urinary Phthalate Metabolite and Bisphenol A Concentrations before and during Pregnancy. Environ. Health Perspect. 2012, 120, 739–745. [Google Scholar] [CrossRef]
- Fisher, M.; Arbuckle, T.E.; Mallick, R.; Leblanc, A.; Hauser, R.; Feeley, M.; Koniecki, D.; Ramsay, T.; Provencher, G.; Bérubé, R.; et al. Bisphenol A and phthalate metabolite urinary concentrations: Daily and across pregnancy variability. J. Expo. Sci. Environ. Epidemiol. 2014, 25, 231–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jusko, T.A.; Shaw, P.A.; Snijder, C.A.; Pierik, F.H.; Koch, H.M.; Hauser, R.; Jaddoe, V.W.; Burdorf, A.; Hofman, A.; Tiemeier, H.; et al. Reproducibility of urinary bisphenol A concentrations measured during pregnancy in the Generation R Study. J. Expo. Sci. Environ. Epidemiol. 2014, 24, 532–536. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.W.; Braun, J.M.; Williams, P.L.; Ehrlich, S.; Correia, K.; Calafat, A.M.; Ye, X.; Ford, J.; Keller, M.; Meeker, J.; et al. Predictors and Variability of Urinary Paraben Concentrations in Men and Women, Including before and during Pregnancy. Environ. Health Perspect. 2012, 120, 1538–1543. [Google Scholar] [CrossRef]
- Neville, M.C.; Morton, J.; Umemura, S. Lactogenesis: The Transition from Pregnancy to Lactation. Pediatr. Clin. N. Am. 2001, 48, 35–52. [Google Scholar] [CrossRef]
- Affeltranger, B. Recherche Interventionnelle En Santé Des Populations: L’expérience de l’INCa. 2013. Available online: https://bdsp-ehesp.inist.fr/vibad/index.php?action=getRecordDetail&idt=476159 (accessed on 1 December 2021).
- Terral, P.; Ferron, C.; Potvin, L. Leçons d’un colloque: Les enjeux épistémiques et politiques de la recherche interventionnelle en santé des populations. Glob. Health Promot. 2021, 28, 62–65. [Google Scholar] [CrossRef]
- Gaborit, E.; Terral, P.; Génolini, J.-P. Étudier de près les modes de coordination pour construire un partenariat visant à réduire les inégalités épistémiques. Glob. Health Promot. 2021, 28, 39–46. [Google Scholar] [CrossRef]
- Michie, S.; Richardson, M.; Johnston, M.; Abraham, C.; Francis, J.; Hardeman, W.; Eccles, M.P.; Cane, J.; Wood, C.E. The Behavior Change Technique Taxonomy (v1) of 93 Hierarchically Clustered Techniques: Building an International Consensus for the Reporting of Behavior Change Interventions. Ann. Behav. Med. 2013, 46, 81–95. [Google Scholar] [CrossRef]
- Davis, R.; Campbell, R.; Hildon, Z.; Hobbs, L.; Michie, S. Theories of behaviour and behaviour change across the social and behavioural sciences: A scoping review. Health Psychol. Rev. 2014, 9, 323–344. [Google Scholar] [CrossRef]
- Potvin, L.; Gendron, S.; Bilodeau, A.; Chabot, P. Integrating Social Theory into Public Health Practice. Am. J. Public Health 2005, 95, 591–595. [Google Scholar] [CrossRef]
- Sumner, J.A.; Carey, R.N.; Michie, S.; Johnston, M.; Edmondson, D.; Davidson, K. Using rigorous methods to advance behaviour change science. Nat. Hum. Behav. 2018, 2, 797–799. [Google Scholar] [CrossRef]
- Roche, J.; Bell, L.; Galvão, C.; Golumbic, Y.N.; Kloetzer, L.; Knoben, N.; Laakso, M.; Lorke, J.; Mannion, G.; Massetti, L.; et al. Citizen Science, Education, and Learning: Challenges and Opportunities. Front. Sociol. 2020, 5, 613814. [Google Scholar] [CrossRef]
- Juneau, C.-E.; Jones, C.M.; McQueen, D.V.; Potvin, L. Evidence-based health promotion: An emerging field. Glob. Health Promot. 2011, 18, 79–89. [Google Scholar] [CrossRef]
- Rouillon, S.; Deshayes-Morgand, C.; Enjalbert, L.; Rabouan, S.; Hardouin, J.-B.; Migeot, V.; Albouy-Llaty, M.; Group DisProSE. Endocrine Disruptors and Pregnancy: Knowledge, Attitudes and Prevention Behaviors of French Women. Int. J. Environ. Res. Public Health 2017, 14, 1021. [Google Scholar] [CrossRef] [Green Version]
- Albouy-Llaty, M.; Rouillon, S.; El Ouazzani, H.; Group Dis-ProSE; Rabouan, S.; Migeot, V. Environmental Health Knowledge, Attitudes, and Practices of French Prenatal Professionals Working with a Socially Underprivileged Population: A Qualitative Study. Int. J. Environ. Res. Public Health 2019, 16, 2544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rafidison, D. Evaluation de l’utilisation du Questionnaire PREVED (Pregnancy Prevention Endocrine Disruptor) Par Les Médecins Généralistes. Ph.D. Thesis, University of Poitiers, Poitiers, France, 2020. [Google Scholar]
- Villeval, M. Do the Key Functions of an Intervention Designed from the Same Specifications Vary According to Context? Investigating the Transferability of a Public Health Intervention in France. Available online: https://implementationscience-biomedcentral-com.proxy.insermbiblio.inist.fr/track/pdf/10.1186/s13012-019-0880-8.pdf (accessed on 7 June 2021).
- Squires, J.E.; Graham, I.; Bashir, K.; Nadalin-Penno, L.; Lavis, J.; Francis, J.; Curran, J.; Grimshaw, J.M.; Brehaut, J.; Ivers, N.; et al. Understanding context: A concept analysis. J. Adv. Nurs. 2019, 75, 3448–3470. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 78) | Intervention Group (n = 152) | |||
|---|---|---|---|---|
| n1 | % | n2 | % | |
| Maternal age (years) | ||||
| <30 | 14 | 17.9 | 29 | 19.1 |
| 30–34 | 35 | 44.9 | 73 | 40.0 |
| ≥35 | 29 | 37.2 | 50 | 32.9 |
| Mean; ±SD | 33.1 | ±4.3 | 32.8 | ±4.0 |
| Marital status | ||||
| Married/Cohabiting/Civil partnership | 78 | 100.0 | 152 | 100.0 |
| Parity | ||||
| Nulliparous | 19 | 24.4 | 50 | 32.9 |
| 1 child | 25 | 32.1 | 60 | 39.5 |
| ≥2 children | 34 | 43.6 | 42 | 27.6 |
| Women’s BMI (kg/m²) | ||||
| <18.5 | 4 | 5.1 | 13 | 8.6 |
| 18.5–25 | 59 | 75.6 | 112 | 73.7 |
| 25–30 | 13 | 16.7 | 21 | 13.8 |
| >30 | 2 | 2.6 | 6 | 3.9 |
| Mean; ±SD | 22.4 | ±3.2 | 22.6 | ±3.7 |
| Did you finish your studies? | ||||
| No | 11 | 14.1 | 8 | 5.3 |
| Yes | 67 | 85.9 | 141 | 92.7 |
| Women’s educational level | ||||
| None | 1 | 1.3 | 0 | 0.0 |
| Primary, secondary school (<12th grade) | 1 | 1.3 | 2 | 1.3 |
| Certificate of Professional Competence | 0 | 0.0 | 1 | 0.7 |
| French baccalaureate (12th grade) | 3 | 3.8 | 9 | 5.9 |
| French baccalaureate +2 (12–14th grade) | 10 | 12.8 | 22 | 14.5 |
| Higher education (>14th grade) | 59 | 75.6 | 112 | 73.7 |
| Other | 4 | 5.2 | 6 | 3.9 |
| Country of birth | ||||
| Metropolitan France | 70 | 89.7 | 144 | 94.7 |
| Overseas France | 2 | 2.6 | 0 | 0.0 |
| Other | 6 | 7.7 | 8 | 5.3 |
| EPICES score | ||||
| Precarious situation (≥30.17) | 8 | 10.3 | 22 | 14.5 |
| No precarious situation (<30.17) | 70 | 89.7 | 130 | 85.5 |
| Mean; ±SD | 8.3 | ±11.1 | 9.3 | ±12.2 |
| Control Group (n = 78) | Temporal p-Value (First-Second Visit) | Intervention Group (n = 152) | Temporal p-Value (First-Second Visit) | |||
|---|---|---|---|---|---|---|
| n1 | % | n2 | % | |||
| Smoking | ||||||
| Three months before conception | ||||||
| No | 64 | 82.1 | 0.26 | 113 | 74.3 | 0.02 |
| Yes | 14 | 17.9 | 39 | 25.7 | ||
| During the first trimester | ||||||
| No | 69 | 88.5 | 130 | 85.5 | ||
| Yes | 9 | 11.5 | 22 | 14.5 | ||
| Alcohol consumption * | ||||||
| Before conception | ||||||
| No | 4 | 5.1 | <0.001 | 25 | 16.4 | <0.001 |
| Yes | 74 | 94.9 | 127 | 83.6 | ||
| Drinking more than 4 glasses during the 1st trimester * (n = 73 for control group; 147 for intervention group) | ||||||
| Never | 69 | 94.5 | 124 | 84.4 | ||
| Very rarely | 4 | 5.5 | 20 | 13.6 | ||
| Once a month | 0 | 0.0 | 1 | 0.6 | ||
| Two or three times a month | 0 | 0.0 | 2 | 1.4 | ||
| Consumption of canned tuna (number/week) | ||||||
| At the first visit | ||||||
| No | 24 | 30.8 | <0.001 | 43 | 28.3 | <0.001 |
| Yes | 54 | 69.2 | 109 | 71.7 | ||
| Mean; ±SD * (n1 = 54; n2 = 108) | 1.8 | ±1.7 | 1.8 | ±1.1 | ||
| At the second visit | ||||||
| No | 46 | 59.0 | 84 | 55.3 | ||
| Yes | 32 | 41.0 | 68 | 44.7 | ||
| Mean; ±SD * (n1 = 32; n2 = 68) | 1.5 | ±0.8 | 1.7 | ±1.0 | ||
| Consumption of preserved sweetcorn (number/week) | ||||||
| At the first visit | ||||||
| No | 39 | 50.0 | 0.002 | 66 | 43.4 | 0.003 |
| Yes | 39 | 50.0 | 86 | 56.6 | ||
| Mean; ±SD * (n1 = 39; n2 = 86) | 1.8 | ±1.9 | 2.2 | ±1.7 | ||
| At the second visit | ||||||
| No | 58 | 74.4 | 92 | 60.5 | ||
| Yes | 20 | 25.6 | 60 | 39.5 | ||
| Mean; ±SD * (n1 = 21; n2 = 60) | 1.3 | ±0.6 | 1.8 | ±1.4 | ||
| Consumption of other canned food (number/week) | ||||||
| At the first visit | ||||||
| No | 18 | 23.1 | 0.004 | 28 | 18.4 | <0.001 |
| Yes | 60 | 76.9 | 124 | 81.6 | ||
| Mean; ±SD * (n1 = 59; n2 = 123) | 2.9 | ±2.9 | 2.3 | ±2.2 | ||
| At the second visit | ||||||
| No | 35 | 44.9 | 55 | 36.2 | ||
| Yes | 43 | 55.1 | 97 | 63.8 | ||
| Mean; ±SD * (n1 = 43; n2 = 93) | 2.2 | ±1.9 | 2.0 | ±1.4 | ||
| Total canned food consumption (number/week) | ||||||
| At the first visit | 0.02 | |||||
| Mean; ±SD * (n1 = 71; n2 = 142) | 4.7 | ±4.7 | 4.7 | ±3.8 | 0.04 | |
| At the second visit | ||||||
| Mean; ±SD * (n1 = 59; n2 = 119) | 2.9 | ±2.3 | 3.4 | ±2.4 | ||
| Consumption of canned drink products (/day) | ||||||
| At the first visit | ||||||
| No | 66 | 84.6 | 0.97 | 124 | 81.6 | 0.37 |
| Yes | 12 | 15.4 | 28 | 18.4 | ||
| Mean; ±SD * (n1 = 13; n2 = 28) | 1.7 | ±1.7 | 1.5 | ±1.3 | ||
| At the second visit (n1 = 77; n2 = 151) | ||||||
| No | 65 | 84.4 | 129 | 85.4 | ||
| Yes | 12 | 15.6 | 22 | 14.6 | ||
| Mean; ±SD * (n1 = 12; n2 = 22) | 1.4 | ±0.7 | 1.7 | ±1.4 | ||
| Consumption of consumption of plastic drink bottles (/week) | ||||||
| At the first visit | ||||||
| No | 45 | 57.7 | 0.52 | 79 | 52.0 | 0.68 |
| Yes | 33 | 42.3 | 73 | 48.0 | ||
| Mean; ±SD * (n1 = 33; n2 = 72) | 1.4 | ±1.1 | 1.4 | ±0.9 | ||
| At the second visit * (n1 = 78; n2 = 151) | ||||||
| No | 41 | 52.6 | 82 | 54.3 | ||
| Yes | 37 | 47.4 | 69 | 54.7 | ||
| Mean; ±SD * (n1 = 37; n2 = 68) | 1.4 | ±1.1 | 1.5 | ±1.1 | ||
| Consumption of fresh fruit and vegetables (number/day) | ||||||
| Mean; ±SD | 4.0 | ±1.5 | 0.48 | 3.8 | ±1.3 | 0.31 |
| At the second visit | ||||||
| Mean; ±SD | 4.3 | ±1.7 | 4.1 | ±1.3 | ||
| Consumption of organic fruit and vegetables | ||||||
| At the first visit | ||||||
| No | 14 | 17.9 | 0.67 | 33 | 21.7 | 0.57 |
| Yes | 64 | 82.1 | 119 | 78.3 | ||
| At the second visit | ||||||
| No | 12 | 15.4 | 29 | 19.1 | ||
| Yes | 66 | 84.6 | 123 | 80.1 | ||
| Consumption of fast-food (/month) | ||||||
| At the first visit | ||||||
| No | 38 | 48.7 | 0.63 | 70 | 46.1 | 0.08 |
| Yes | 40 | 51.3 | 82 | 53.9 | ||
| Mean; ±SD * (n1 = 40; n2 = 82) | 1.6 | ±1.0 | 1.6 | ±0.9 | ||
| At the second visit | ||||||
| No | 35 | 44.9 | 55 | 36.2 | ||
| Yes | 43 | 55.1 | 97 | 63.8 | ||
| Mean; ±SD * (n1 = 30; n2 = 73) | 1.53 | ±0.8 | 1.6 | ±1.3 | ||
| Consumption of ready-made meals (number/week) | ||||||
| At the first visit | ||||||
| No | 38 | 48.7 | <0.001 | 70 | 46.1 | <0.001 |
| Yes | 40 | 51.3 | 82 | 53.9 | ||
| Mean; ±SD * (n1 = 28; n2 = 39) | 1.5 | ±0.9 | 1.5 | ±1.2 | ||
| At the second visit | ||||||
| No * (n1 = 40; n2 = 91) | 37 | 92.5 | 82 | 90.1 | ||
| Yes | 3 | 7.5 | 9 | 9.9 | ||
| Mean; ±SD * (n1 = 21; n2 = 23) | 1.5 | ±1.4 | 1.3 | ±0.8 | ||
| Workshop1 Indoor Air Quality | Workshop2 Food | Workshop3 Care Product | Total (n Workshops) | |
|---|---|---|---|---|
| Total (n Workshops) | 148 | 145 | 143 | 436 |
| Neutral location | 69 | 69 | 66 | 204 |
| Contextualized location | 79 | 76 | 77 | 232 |
| 2017 | 95 | 95 | 97 | 287 |
| 2018 | 36 | 35 | 32 | 103 |
| 2019 | 17 | 15 | 14 | 46 |
| Control Group (n = 78) | Intervention Group (n = 152) | p | |||
|---|---|---|---|---|---|
| n1 | % | n2 | % | ||
| Consumption of canned tuna (number/week) * (N = 169) | |||||
| Decrease | 34 | 57.6 | 72 | 65.4 | 0.47 |
| Stable | 9 | 27.1 | 21 | 19.1 | |
| Increase | 16 | 15.3 | 17 | 15.5 | |
| Consumption of canned tuna (number/week) | |||||
| No consumption in first visit and second visit | 19 | 24.4 | 41 | 27.0 | 0.10 |
| Consumption in first visit and no consumption in second visit | 27 | 34.6 | 43 | 28.3 | |
| Consumption in first visit and second visit | 27 | 34.6 | 66 | 43.4 | |
| No consumption in first visit and consumption in second visit | 5 | 6.4 | 2 | 1.3 | |
| Consumption of preserved sweetcorn (number/week) * (N = 137) | |||||
| Decrease | 28 | 62.2 | 56 | 60.9 | 0.84 |
| Stable | 94 | 20.0 | 22 | 23.9 | |
| Increase | 8 | 17.8 | 14 | 15.2 | |
| Consumption of preserved sweetcorn (number/week) | |||||
| No consumption in first visit and second visit | 33 | 42.3 | 60 | 39.5 | 0.02 |
| Consumption in first visit and no consumption in second visit | 25 | 32.1 | 32 | 21.1 | |
| Consumption in first visit and second visit | 14 | 17.9 | 54 | 35.5 | |
| No consumption in first visit and consumption in second visit | 6 | 7.7 | 6 | 3.9 | |
| Consumption of other canned food (number/week) * (N = 198) | |||||
| Decrease | 38 | 56.7 | 66 | 50.4 | 0.69 |
| Stable | 15 | 22.4 | 35 | 26.7 | |
| Increase | 14 | 20.9 | 30 | 22.9 | |
| Consumption of other canned food (number/week) | |||||
| No consumption in first visit and second visit | 10 | 12.8 | 20 | 13.1 | 0.15 |
| Consumption in first visit and no consumption in second visit | 25 | 32.0 | 35 | 23.0 | |
| Consumption in first visit and second visit | 35 | 44.9 | 89 | 58.6 | |
| No consumption in first visit and consumption in second visit | 8 | 10.3 | 8 | 5.3 | |
| Total canned food consumption (number/week) * (N = 227) | |||||
| Decrease | 51 | 68.0 | 92 | 63.9 | 0.41 |
| Stable | 8 | 10.7 | 25 | 17.4 | |
| Increase | 16 | 21.3 | 27 | 18.7 | |
| Consumption of canned drink products (number/day) * (N = 55) | |||||
| Decrease | 7 | 43.7 | 21 | 53.8 | 0.66 |
| Stable | 2 | 12.5 | 6 | 15.4 | |
| Increase | 7 | 43.7 | 12 | 30.8 | |
| Consumption of canned drink products (number/day) * (N = 228) | |||||
| No consumption in first visit and second visit | 61 | 79.2 | 113 | 74.8 | 0.43 |
| Consumption in first visit and no consumption in second visit | 4 | 5.2 | 16 | 10.6 | |
| Consumption in first visit and second visit | 8 | 10.4 | 11 | 7.3 | |
| No consumption in first visit and consumption in second visit | 4 | 5.2 | 11 | 7.3 | |
| Consumption of consumption of plastic drink bottles (number/week) * (N = 136) | |||||
| Decrease | 11 | 23.9 | 27 | 30.0 | 0.76 |
| Stable | 20 | 43.5 | 36 | 40.0 | |
| Increase | 15 | 32.6 | 27 | 30.0 | |
| Consumption of consumption of plastic drink bottles (number/week) * (N = 229) | |||||
| No consumption in first visit and second visit | 32 | 41.0 | 60 | 39.7 | 0.71 |
| Consumption in first visit and no consumption in second visit | 9 | 11.5 | 22 | 14.6 | |
| Consumption in first visit and second visit | 24 | 30.8 | 51 | 33.8 | |
| No consumption in first visit and consumption in second visit | 13 | 16.7 | 18 | 11.9 | |
| Consumption of fresh fruit and vegetables (number/day) | |||||
| Decrease | 18 | 23.1 | 35 | 23.0 | 0.73 |
| Stable | 27 | 34.6 | 60 | 39.5 | |
| Increase | 33 | 42.3 | 57 | 37.5 | |
| Consumption of organic fruit and vegetables (number/day) | |||||
| No consumption in first visit and second visit | 7 | 9.0 | 21 | 13.8 | 0.75 |
| Consumption in first visit and no consumption in second visit | 5 | 6.4 | 8 | 5.3 | |
| Consumption in first visit and second visit | 59 | 75.6 | 111 | 73.0 | |
| No consumption in first visit and consumption in second visit | 7 | 9.0 | 12 | 7.9 | |
| Consumption of fast-food (number/month) * (N = 134) | |||||
| Decrease | 17 | 40.5 | 32 | 34.8 | 0.09 |
| Stable | 22 | 52.4 | 39 | 42.4 | |
| Increase | 3 | 7.1 | 21 | 22.8 | |
| Consumption of fast-food (number/month) | 0.7 | ||||
| No consumption in first visit and second visit | 35 | 44.9 | 61 | 40.1 | |
| Consumption in first visit and no consumption in second visit | 12 | 15.4 | 19 | 12.5 | |
| Consumption in first visit IT and second visit | 28 | 35.9 | 63 | 41.4 | |
| No consumption in first visit and consumption in second visit | 3 | 3.9 | 9 | 5.9 | |
| Consumption of ready-made meals (number/week) * (N = 80) | |||||
| Decrease | 13 | 43.3 | 30 | 60.0 | 0.35 |
| Stable | 11 | 36.7 | 8 | 16.0 | |
| Increase | 6 | 20.0 | 12 | 24.0 | |
| Consumption of ready-made meals (number/week) * (N = 228) | |||||
| No consumption in first visit and second visit | 47 | 61.0 | 101 | 66.9 | 0.01 |
| Consumption in first visit and no consumption in second visit | 2 | 2.6 | 11 | 2.3 | |
| Consumption in first visit and second visit | 19 | 24.7 | 14 | 9.3 | |
| No consumption in first visit and consumption in second visit | 2 | 2.6 | 11 | 7.3 | |
| Control Group (n1 = 78) | Intervention Group (n2 = 152) | p | |||
|---|---|---|---|---|---|
| Δm * | IC 95% | Δm * | IC 95% | ||
| 1-Endocrine-disrupting chemicals knowledge (score/40.5) | +3.55 | [+2.68; +4.42] | +3.40 | [+2.88; +3.90] | 0.75 |
| Sources of exposure to endocrine-disrupting chemicals (score/22) | +2.15 | [+1.48; +2.82] | +1.97 | [+1.54; +2.40] | 0.64 |
| Definition of endocrine disruptors (score/7) | +0.44 | [+0.16; +0.71] | +0.36 | [+0.20; +0.52] | 0.63 |
| Ability to name molecules (score/6.5) | +0.77 | [+0.54; +1.00] | +0.83 | [+0.66; +0.99] | 0.69 |
| Pathways of exposure to endocrine Disrupting chemicals (score/5) | +0.19 | [+0.07; +0.31] | +0.23 | [+0.15; +0.31] | 0.59 |
| 2-Perceived ability to avoid chemical exposure (/100) | +7.09 | [+2.33; +11.85] | +5.11 | [+1.80; +8.42] | 0.50 |
| 3-Risk from endocrine disrupting chemicals (perceived severity score/100) to: | +20.21 | [+16.19; +24.23] | +19.20 | [+15.77; +22.63] | 0.72 |
| The health of pregnant women | +7.16 | [+1.40; +12.91] | +7.61 | [+3.28; +11.94] | 0.90 |
| The newborn health | +8.09 | [+2.28; +8.54] | +5.41 | [+2.28; +8.54] | 0.31 |
| The adolescent health | +7.56 | [+3.50; +11.63] | +4.73 | [+2.05; +7.41] | 0.24 |
| The adult health | +6.06 | [+1.38; +10.74] | +4.47 | [+0.87; +8.08] | 0.60 |
| 4-Risk assessment of endocrine-disrupting chemicals: | |||||
| Perceived vulnerability score/1400 | +275.60 | [+229.20; +322.00] | +256.40 | [+228.60; +284.20] | 0.46 |
| Perceived vulnerability score/100 | +19.69 | [+16.37; +23.00] | +18.31 | [+16.33; +20.30] | 0.46 |
| Risk perception score (3+4)/100 | +19.50 | [+17.12; +22.78] | +18.76 | [+16.68; +20.84] | 0.51 |
| Global score (1+2+3+4): Score/100 | +15.63 | [+13.21; +18.05] | +14.45 | [+13.00; +15.91] | 0.38 |
| Subjective knowledge on endocrine Disrupting chemicals (score/100) | +28.24 | [+24.23; +32.26] | +19.77 | [+16.51; +23.03] | 0.002 |
| The information received on endocrine-disrupting chemicals were (score/100) | |||||
| Understandable * (n = 74; 142) | −1.65 | [−8.33; +5.03] | −1.35 | [−6.14; +3.45] | 0.99 |
| Scientific * (n = 74; 142) | −2.39 | [−7.39; +2.61] | +2.15 | [−0.87; +5.18] | 0.10 |
| Realistic * (n = 74; 142) | +3.96 | [−1.01; +8.93] | −0.36 | [−3.85; +3.12] | 0.15 |
| Stressful * (n = 74; 142) | −1.91 | [−6.05; +2.24] | −3.34 | [−5.80; −0.88] | 0.53 |
| Complete * (n = 73; 142) | +6.30 | [−0.18; +12.78] | +11.70 | [+7.72; +15.68] | 0.14 |
| Events during the pregnancy you are daily concerned with (score/100) | |||||
| Pregnancy pain | −7.13 | [−13.09; −1.17] | −0.93 | [−5.07; +3.22] | 0.09 |
| The consequences of consumption of toxic substances for the child * (n = 77; 151) | +1.64 | [−7.16; +10.43] | +1.18 | [−4.61; +6.97] | 0.93 |
| Infectious diseases * (n = 76; 152) | −8.25 | [−14.79; −1.71] | −6.80 | [−11.53; −2.07] | 0.72 |
| Genetic diseases | −5.5 | [−11.60; +0.50] | −5.48 | [−9.63; −1.33] | 0.98 |
| Child illnesses linked to chemical exposure | −1.73 | [−7.31; +3.85] | +0.22 | [−3.06; +3.50] | 0.52 |
| Concept of a healthy baby | |||||
| Healthy birth weight* (n = 78; 151) | +5.21 | [−0.63; +11.04] | −3.25 | [−7.75; +1.25] | 0.03 |
| Full-term birth | +3.05 | [−3.05; +9.15] | −1.47 | [−5.51; +2.56] | 0.21 |
| Normal intelligence quotient (IQ) | +6.08 | [−0.60; +12.75] | +6.19 | [+1.76; +10.63] | 0.98 |
| Ability to have children | +7.50 | [+1.18; +13.82] | +1.91 | [−1.70; +5.51] | 0.13 |
| Normal puberty | +4.73 | [+0.19; +9.27] | +1.64 | [−2.15; +5.44] | 0.33 |
| Normal weight (no obesity; no overweight) | +1.24 | [−2.80; +5.28] | +0.37 | [−3.51; +4.24] | 0.76 |
| No asthma | +3.36 | [−0.91; +7.63] | +0.96 | [−3.06; +4.98] | 0.42 |
| No behavior disorders | +3.69 | [−1.50; +8.89] | +4.64 | −[+0.41; +8.87] | 0.79 |
| Can play like all the other children | −1.14 | [−6.57; +4.28] | −0.21 | [−4.47; +4.05] | 0.80 |
| Not get sick so often | +3.03 | [−2.16; +8.22] | +0.05 | [−4.18; +4.28] | 0.40 |
| Able to make friends and fit in | −0.99 | [−6.32; +4.34] | +5.54 | [+1.54; +9.53] | 0.06 |
| Successful professional life | +0.53 | [−5.12; +6.17] | +5.96 | [+1.94; +9.98] | 0.12 |
| Successful emotional life | −1.35 | [−7.03; +4.33] | +5.32 | [+1.59; 9.05] | <0.05 |
| Efforts towards avoiding chemical exposure | |||||
| Financially | −2.30 | [−7.25; +2.63] | +0.40 | [−2.76; +3.57] | 0.34 |
| In terms of time * (n = 77; 152) | +6.36 | [+0.98; +11.75] | +3.11 | [−0.40; +6.62] | 0.30 |
| In terms of comfort * (n = 77; 152) | −1.00 | [−8.12; +6.12] | −2.05 | [−6.12; +2.03] | 0.79 |
| Locus of control | |||||
| Internal locus of control (score/6) | +0.02 | [−0.03; +0.08] | −0.03 | [−0.07; +0.01] | 0.11 |
| External locus of control: chance (score/3) | −0.01 | [−0.10; +0.08] | +0.01 | [−0.05; +0.06] | 0.83 |
| External locus of control: medical personnel (score/4) | +0.06 | [−0.02; +0.14] | −0.03 | [−0.08; +0.02] | 0.06 |
| Sense of coherence | |||||
| Comprehensive (score/5) | −0.06 | [−0.24; +0.13] | +0.06 | [−0.07; +0.19] | 0.28 |
| Meaningful (score/4) | −0.03 | [−0.18; +0.13] | +0.02 | [−0.11; +0.14] | 0.67 |
| Manageable (score/4) | −0.16 | [−0.32; +0.01] | −0.09 | [−0.20; +0.03] | 0.46 |
| Rosenberg self-esteem scale (score/40) | +0.56 | [−0.05; +1.18] | +0.49 | [+0.01; 0.96] | 0.85 |
| Assessment of anxiety in general (score/100) | −3.74 | [−7,65; +0.16] | −1.05 | [−3.66; +1.56] | 0.25 |
| Anxiety evolution: before and after the questionnaire (score/100) | −6.65 | [−12.35; −0.96] | −4.76 | [−8.93; −0.60] | 0.60 |
| Important risk taking about | |||||
| Professional life | −2.62 | [−7.40; +2.17] | −1.63 | [−5.19; +1.94] | 0.75 |
| Sport activities | +3.81 | [+0.42; +7.20] | −0.09 | [−3.02; +2.83] | 0.11 |
| Sexual practices | +1.26 | [−1.34; +3.85] | +0.97 | [−1.25; +3.20] | 0.88 |
| Road traffic | −0.81 | [−3.84; +2.22] | +2.24 | [−0.57; +5.05] | 0.15 |
| Use of substance | +0.82 | [−2.10; +3.74] | −0.15 | [−2.56; +2.26] | 0.63 |
| Control Group (n = 80) | Intervention Group (n = 145) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Bisphenol A (BPA) | |||||
| Rising indicator | 25 | 31 | 47 | 32 | |
| Same indicator | 34 | 43 | 63 | 44 | 0.94 |
| Decline indicator | 21 | 26 | 35 | 24 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Rising indicator | 15 | 19 | 34 | 23 | |
| Same indicator | 48 | 60 | 67 | 46 | 0.13 |
| Decline indicator | 17 | 21 | 44 | 31 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Rising indicator | 22 | 27 | 37 | 26 | |
| Same indicator | 39 | 49 | 75 | 52 | 0.91 |
| Decline indicator | 19 | 24 | 33 | 22 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Rising indicator | 13 | 16 | 22 | 15 | |
| Same indicator | 55 | 69 | 99 | 68 | 0.94 |
| Decline indicator | 12 | 15 | 24 | 17 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Rising indicator | 14 | 17 | 22 | 15 | |
| Same indicator | 47 | 59 | 88 | 61 | 0.90 |
| Decline indicator | 19 | 24 | 35 | 24 | |
| MethylParaben (MePB) | |||||
| Rising indicator | 15 | 19 | 28 | 19 | |
| Same indicator | 50 | 62 | 71 | 49 | 0.08 |
| Decline indicator | 15 | 19 | 46 | 32 | |
| EthylParaben (EtPB) | |||||
| Rising indicator | 22 | 27 | 29 | 20 | |
| Same indicator | 35 | 44 | 59 | 41 | 0.22 |
| Decline indicator | 23 | 29 | 57 | 39 | |
| PropylParaben (PrPB) | |||||
| Rising indicator | 8 | 10 | 22 | 15 | |
| Same indicator | 62 | 78 | 99 | 68 | 0.33 |
| Decline indicator | 10 | 12 | 24 | 17 | |
| ButylParaben (BuPB) | |||||
| Rising indicator | 2 | 3 | 2 | 1 | |
| Same indicator | 77 | 96 | 139 | 96 | 0.64 |
| Decline indicator | 1 | 1 | 4 | 3 | |
| Control Group (n = 93) | Intervention Group (n = 132) | p | |||
|---|---|---|---|---|---|
| Mean (ng/mL) | n | Mean (ng/mL) | n | ||
| LoQ divides by two (LoQ/2) | |||||
| MethylParaben (MePB) | |||||
| Second trimester (urine) | 15.8 | 86 | 13.6 | 171 | 0.85 |
| Third trimester (urine) | 7 | 80 | 14.8 | 146 | 0.69 |
| Birth (colostrum) | 0.28 | 39 | 0.21 | 88 | 0.27 |
| One Year (urine) | 3 | 35 | 0.4 | 72 | 0.27 |
| Bisphenol A (BPA) | |||||
| Birth (colostrum) | 1.16 | 45 | 1 | 102 | 0.99 |
| Truncation k-Nearest Neighbor (kNN-TN) | |||||
| MethylParaben (MePB) | |||||
| Second trimester (urine) | 16 | 86 | 13.8 | 171 | 0.86 |
| Third trimester (urine) | 6.8 | 80 | 14.3 | 146 | 0.08 |
| Birth (colostrum) | 0.3 | 39 | 0.27 | 88 | 0.38 |
| One year (urine) | 1.4 | 35 | 0.4 | 72 | 0.29 |
| Bisphenol A (BPA) | |||||
| Birth (colostrum) | 1.53 | 45 | 1.21 | 102 | 0.048 |
| Control Group (n1 = 81) | Intervention Group (n2 = 149) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Consumption Questionnaire | |||||
| Consumption of canned tuna (number/week) * (N = 169) | |||||
| Decrease | 40 | 65.6 | 66 | 61.1 | 0.83 |
| Stable | 12 | 19.7 | 25 | 23.2 | |
| Increase | 9 | 14.7 | 26 | 15.7 | |
| Consumption of preserved sweetcorn (number/week) * (N = 137) | |||||
| Decrease | 27 | 60.0 | 57 | 62.0 | 0.94 |
| Stable | 11 | 24.4 | 20 | 21.7 | |
| Increase | 7 | 15.6 | 15 | 16.3 | |
| Consumption of other canned food (number/week) * (N = 198) | |||||
| Decrease | 41 | 58.6 | 63 | 49.2 | 0.44 |
| Stable | 15 | 21.4 | 35 | 27.4 | |
| Increase | 14 | 20.00 | 30 | 23.4 | |
| Total canned food consumption (number/week) * (N = 227) | |||||
| Decrease | 50 | 66.8 | 93 | 65.0 | 0.94 |
| Stable | 12 | 15.8 | 21 | 14.7 | |
| Increase | 14 | 18.4 | 29 | 20.3 | |
| Consumption of canned drink products (number/day) * (N = 55) | |||||
| Decrease | 13 | 61.9 | 15 | 44.1 | 0.05 |
| Stable | 0 | 0.0 | 8 | 23.5 | |
| Increase | 8 | 38.1 | 11 | 32.4 | |
| Consumption of consumption of plastic drink bottles (number/week) * (N = 136) | |||||
| Decrease | 15 | 30.6 | 23 | 26.4 | 0.85 |
| Stable | 20 | 40.8 | 36 | 41.4 | |
| Increase | 14 | 28.6 | 28 | 32.2 | |
| Consumption of fresh fruit and vegetables number (/day) | |||||
| Decrease | 21 | 25.9 | 32 | 21.5 | 0.67 |
| Stable | 31 | 38.3 | 56 | 37.6 | |
| Increase | 29 | 35.8 | 61 | 40.9 | |
| Consumption of fast-food (number/month) * (N = 134) | |||||
| Decrease | 16 | 33.3 | 33 | 38.4 | 0.12 |
| Stable | 19 | 39.6 | 42 | 48.8 | |
| Increase | 13 | 27.1 | 11 | 12.8 | |
| Consumption of ready-made meals (number/week) * (N = 80) | |||||
| Decrease | 15 | 57.7 | 28 | 51.8 | 0.45 |
| Stable | 4 | 15.4 | 15 | 27.8 | |
| Increase | 7 | 26.9 | 11 | 20.4 | |
| Psychosocial questionnaire | |||||
| 1-Endocrine-disrupting chemicals knowledge (score/40.5) ** | +3.22 | [+2.52; +3.93] | +3.57 | [+2.99; +4.14] | 0.47 |
| 2-Perceived ability to avoid chemical exposure (/100) ** | +7.09 | [+2.33; +11.85] | +5.11 | [+1.80; +8.42] | 0.50 |
| 3-Risk from endocrine-disrupting chemicals (severity score/100) ** | +15.61 | [+10.64; 20.58] | +21.69 | [+18.67; +24.70] | 0.03 |
| 4-Risk assessment of endocrine-disrupting chemicals (vulnerability score/1400) ** | +222.00 | [+187.00; +257.10] | +285.10 | [+253.60; +316.6] | 0.008 |
| Risk perception score (3+4)/100 ** | +15.73 | [+12.96; +18.51] | +21.03 | [+18.99; +23.07] | 0.003 |
| Global Score (1+2+3+4)/100 ** | +12.39 | [+10.57; +14.21] | +16.2 | [+14.55; +17.83] | 0.002 |
| 0 or 1 Workshop (n1 = 92) | 2 or 3 Workshops in Neutral Location (n2 = 60) | 2 or 3 Workshops in Contextualized Location (n3 = 75) | p | ||||
|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | ||
| Consumption Questionnaire | |||||||
| Consumption of canned tuna (number/week) * | |||||||
| Decrease | 42 | 64.6 | 29 | 65.9 | 33 | 57.9 | 0.90 |
| Stable | 14 | 21.5 | 8 | 18.2 | 14 | 24.6 | |
| Increase | 9 | 13.9 | 7 | 15.9 | 10 | 17.5 | |
| Consumption of preserved sweetcorn (number/week) * | |||||||
| Decrease | 28 | 58.3 | 26 | 59.1 | 29 | 67.4 | 0.63 |
| Stable | 13 | 27.1 | 11 | 25.0 | 6 | 14.0 | |
| Increase | 7 | 14.6 | 7 | 15.9 | 8 | 18.6 | |
| Consumption of other canned food (number/week) * | |||||||
| Decrease | 46 | 56.8 | 20 | 38.5 | 36 | 57.1 | 0.16 |
| Stable | 16 | 19.7 | 19 | 36.5 | 15 | 23.8 | |
| Increase | 19 | 23.5 | 13 | 25.0 | 12 | 19.1 | |
| Total canned food consumption (number/week) * | |||||||
| Decrease | 55 | 63.2 | 37 | 63.8 | 48 | 67.6 | 0.60 |
| Stable | 13 | 14.9 | 12 | 29.7 | 8 | 11.3 | |
| Increase | 19 | 21.8 | 9 | 15.5 | 15 | 21.1 | |
| Consumption of canned drink products (number/day) * | |||||||
| Decrease | 13 | 59.1 | 6 | 37.5 | 8 | 50.0 | 0.03 |
| Stable | 0 | 0.0 | 6 | 37.5 | 2 | 12.5 | |
| Increase | 9 | 40.9 | 4 | 25.0 | 6 | 37.5 | |
| Consumption of consumption of plastic drink bottles (number/week) * | |||||||
| Decrease | 16 | 29.1 | 9 | 26.5 | 12 | 27.3 | 0.94 |
| Stable | 21 | 38.2 | 16 | 47.1 | 18 | 40.9 | |
| Increase | 18 | 32.7 | 9 | 26.5 | 14 | 31.8 | |
| Consumption of fresh fruit and vegetables (number/day) | |||||||
| Decrease | 25 | 27.2 | 11 | 18.3 | 16 | 21.3 | 0.68 |
| Stable | 35 | 38.0 | 24 | 40.0 | 27 | 36.0 | |
| Increase | 32 | 34.8 | 25 | 41.7 | 32 | 42.7 | |
| Consumption of fastfood (number/month) * | |||||||
| Decrease | 19 | 34.6 | 15 | 39.5 | 13 | 34.2 | 0.004 |
| Stable | 18 | 32.7 | 19 | 50.0 | 23 | 60.5 | |
| Increase | 18 | 32.7 | 4 | 10.5 | 2 | 5.3 | |
| Consumption of ready-made meals (number/week) * | |||||||
| Decrease | 19 | 59.4 | 13 | 59.1 | 11 | 44.0 | 0.07 |
| Stable | 4 | 12.5 | 4 | 18.2 | 11 | 44.0 | |
| Increase | 9 | 28.1 | 5 | 22.7 | 3 | 12.0 | |
| Psychosocial questionnaire | |||||||
| Risk perception score/100 ** | +16.18 | [−24.23; +58.45] | +20.27 | [−16.00; +48.37] | +21.41 | [−9.15; +56.52] | 0.019 |
| Global score/100 ** | +12.7 | [−14.3; +42.6] | +16.0 | [−6.9; +38.0] | +16.4 | [−13.1; +45.5] | 0.026 |
| Control Group (n BPA = 56) (n Parabens = 48) | Intervention Group (n BPA = 91) (n Parabens = 79) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Bisphenol A (BPA) | |||||
| Superior to LoD | 42 | 75 | 71 | 78 | 0.67 |
| Inferior to LoD | 14 | 25 | 20 | 22 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Superior to LoD | 23 | 41 | 44 | 48 | 0.39 |
| Inferior to LoD | 33 | 59 | 47 | 52 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Superior to LoD | 26 | 46 | 43 | 47 | 0.92 |
| Inferior to LoD | 30 | 54 | 48 | 53 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Superior to LoD | 39 | 70 | 56 | 62 | 0.32 |
| Inferior to LoD | 17 | 30 | 35 | 38 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Superior to LoD | 29 | 52 | 46 | 51 | 0.88 |
| Inferior to LoD | 27 | 48 | 45 | 49 | |
| MethylParaben (MePB) | |||||
| Superior to LoD | 44 | 92 | 68 | 86 | 0.34 |
| Inferior to LoD | 4 | 8 | 11 | 14 | |
| EthylParaben (EtPB) | |||||
| Superior to LoD | 31 | 65 | 45 | 57 | 0.40 |
| Inferior to LoD | 17 | 35 | 34 | 43 | |
| PropylParaben (PrPB) | |||||
| Superior to LoD | 14 | 29 | 25 | 32 | 0.77 |
| Inferior to LoD | 34 | 71 | 54 | 68 | |
| ButylParaben (BuPB) | |||||
| Superior to LoD | 6 | 13 | 1 | 1 | 0.01 |
| Inferior to LoD | 42 | 87 | 78 | 99 | |
| Control Group (n = 88) | Intervention Group (n = 137) | p | |||
|---|---|---|---|---|---|
| n | % | n | % | ||
| Bisphenol A (BPA) | |||||
| Rising indicator | 28 | 32 | 44 | 32 | |
| Same indicator | 38 | 43 | 59 | 43 | 0.76 |
| Decline indicator | 22 | 25 | 34 | 25 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Rising indicator | 16 | 18 | 33 | 24 | |
| Same indicator | 51 | 58 | 64 | 47 | 0.25 |
| Decline indicator | 21 | 24 | 40 | 29 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Rising indicator | 22 | 25 | 37 | 27 | |
| Same indicator | 44 | 50 | 70 | 51 | 0.85 |
| Decline indicator | 22 | 25 | 30 | 22 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Rising indicator | 15 | 17 | 20 | 14 | |
| Same indicator | 60 | 68 | 94 | 69 | 0.84 |
| Decline indicator | 13 | 15 | 23 | 17 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Rising indicator | 15 | 17 | 21 | 15 | |
| Same indicator | 51 | 58 | 84 | 61 | 0.88 |
| Decline indicator | 22 | 25 | 32 | 24 | |
| MethylParaben (MePB) | |||||
| Rising indicator | 17 | 19 | 26 | 19 | |
| Same indicator | 55 | 63 | 66 | 48 | 0.04 |
| Decline indicator | 16 | 18 | 45 | 33 | |
| EthylParaben (EtPB) | |||||
| Rising indicator | 22 | 25 | 29 | 22 | |
| Same indicator | 40 | 45 | 54 | 39 | 0.32 |
| Decline indicator | 26 | 30 | 54 | 39 | |
| PropylParaben (PrPB) | |||||
| Rising indicator | 10 | 11 | 20 | 15 | |
| Same indicator | 69 | 79 | 92 | 67 | 0.16 |
| Decline indicator | 9 | 10 | 25 | 18 | |
| ButylParaben (BuPB) | |||||
| Rising indicator | 2 | 2 | 2 | 1 | |
| Same indicator | 85 | 97 | 131 | 96 | 0.66 |
| Decline indicator | 1 | 1 | 4 | 3 | |
| Control Group (n BPA = 51) (n Parabens = 44) | Intervention Group (n BPA = 96) (n Parabens = 83) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Bisphenol A (BPA) | |||||
| Superior to LoD | 39 | 76 | 74 | 77 | 0.93 |
| Inferior to LoD | 12 | 24 | 22 | 23 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Superior to LoD | 21 | 41 | 46 | 48 | 0.43 |
| Inferior to LoD | 30 | 59 | 50 | 52 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Superior to LoD | 25 | 49 | 44 | 46 | 0.71 |
| Inferior to LoD | 26 | 51 | 52 | 54 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Superior to LoD | 34 | 67 | 61 | 64 | 0.71 |
| Inferior to LoD | 17 | 33 | 35 | 36 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Superior to LoD | 25 | 49 | 50 | 52 | 0.72 |
| Inferior to LoD | 26 | 51 | 46 | 48 | |
| MethylParaben (MePB) | |||||
| Superior to LoD | 41 | 93 | 71 | 86 | 0.32 |
| Inferior to LoD | 3 | 7 | 12 | 14 | |
| EthylParaben (EtPB) | |||||
| Superior to LoD | 30 | 68 | 46 | 55 | 0.16 |
| Inferior to LoD | 14 | 32 | 37 | 45 | |
| PropylParaben (PrPB) | |||||
| Superior to LoD | 13 | 30 | 26 | 31 | 0.84 |
| Inferior to LoD | 31 | 70 | 57 | 69 | |
| ButylParaben (BuPB) | |||||
| Superior to LoD | 6 | 14 | 1 | 1 | 0.007 |
| Inferior to LoD | 38 | 86 | 82 | 99 | |
| Control Group (n = 35) | Intervention Group (n = 72) | ||||
|---|---|---|---|---|---|
| N | % | N | % | p | |
| Bisphenol A (BPA) | |||||
| Rising indicator | 11 | 32 | 14 | 19 | |
| Same indicator | 11 | 32 | 36 | 50 | 0.16 |
| Decline indicator | 13 | 36 | 22 | 31 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Rising indicator | 5 | 14 | 11 | 15 | |
| Same indicator | 18 | 52 | 23 | 32 | 0.14 |
| Decline indicator | 12 | 34 | 38 | 53 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Rising indicator | 2 | 6 | 7 | 9 | |
| Same indicator | 21 | 60 | 40 | 56 | 0.77 |
| Decline indicator | 12 | 34 | 25 | 35 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Rising indicator | 3 | 9 | 2 | 3 | |
| Same indicator | 25 | 71 | 56 | 78 | 0.40 |
| Decline indicator | 7 | 20 | 14 | 19 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Rising indicator | 9 | 26 | 9 | 13 | |
| Same indicator | 21 | 60 | 44 | 61 | 0.14 |
| Decline indicator | 5 | 14 | 19 | 26 | |
| MethylParaben (MePB) | |||||
| Rising indicator | 12 | 34 | 17 | 24 | |
| Same indicator | 17 | 49 | 23 | 32 | 0.02 |
| Decline indicator | 6 | 17 | 32 | 44 | |
| EthylParaben (EtPB) | |||||
| Rising indicator | 15 | 43 | 20 | 28 | |
| Same indicator | 11 | 31 | 37 | 51 | 0.14 |
| Decline indicator | 9 | 26 | 15 | 21 | |
| PropylParaben (PrPB) | |||||
| Rising indicator | 4 | 12 | 15 | 21 | |
| Same indicator | 25 | 71 | 47 | 65 | 0.48 |
| Decline indicator | 6 | 17 | 10 | 14 | |
| ButylParaben (BuPB) | |||||
| Rising indicator | 2 | 5 | 3 | 4 | |
| Same indicator | 32 | 92 | 66 | 92 | 0.89 |
| Decline indicator | 1 | 3 | 3 | 4 | |
| Control Group (n = 43) | Intervention Group (n = 64) | p | |||
|---|---|---|---|---|---|
| N | % | N | % | ||
| Bisphenol A (BPA) | |||||
| Rising indicator | 12 | 28 | 13 | 20 | |
| Same indicator | 16 | 37 | 31 | 49 | 0.48 |
| Decline indicator | 15 | 35 | 20 | 31 | |
| BPA Mono-Chlorinated (MCBPA) | |||||
| Rising indicator | 6 | 14 | 10 | 16 | |
| Same indicator | 20 | 46 | 21 | 33 | 0.35 |
| Decline indicator | 17 | 40 | 33 | 51 | |
| BPA Di-Chlorinated (DCBPA) | |||||
| Rising indicator | 3 | 7 | 6 | 10 | |
| Same indicator | 25 | 58 | 36 | 56 | 0.91 |
| Decline indicator | 15 | 35 | 22 | 34 | |
| BPA Tri-Chlorinated (TCBPA) | |||||
| Rising indicator | 3 | 7 | 2 | 3 | |
| Same indicator | 32 | 74 | 49 | 77 | 0.65 |
| Decline indicator | 8 | 19 | 13 | 20 | |
| BPA Tetra-Chlorinated (TTBPA) | |||||
| Rising indicator | 9 | 21 | 9 | 14 | |
| Same indicator | 27 | 63 | 38 | 59 | 0.37 |
| Decline indicator | 7 | 16 | 17 | 27 | |
| MethylParaben (MePB) | |||||
| Rising indicator | 14 | 33 | 15 | 23 | |
| Same indicator | 19 | 44 | 21 | 33 | 0.09 |
| Decline indicator | 10 | 23 | 28 | 44 | |
| EthylParaben (EtPB) | |||||
| Rising indicator | 19 | 44 | 16 | 25 | |
| Same indicator | 13 | 30 | 35 | 55 | 0.04 |
| Decline indicator | 11 | 26 | 13 | 20 | |
| PropylParaben (PrPB) | |||||
| Rising indicator | 4 | 10 | 15 | 23 | |
| Same indicator | 32 | 74 | 40 | 63 | 0.17 |
| Decline indicator | 7 | 16 | 9 | 14 | |
| ButylParaben (BuPB) | |||||
| Rising indicator | 2 | 4 | 3 | 5 | |
| Same indicator | 40 | 94 | 58 | 90 | 0.82 |
| Decline indicator | 1 | 2 | 3 | 5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Ouazzani, H.; Fortin, S.; Venisse, N.; Dupuis, A.; Rouillon, S.; Cambien, G.; Gourgues, A.-S.; Pierre-Eugène, P.; Rabouan, S.; Migeot, V.; et al. Perinatal Environmental Health Education Intervention to Reduce Exposure to Endocrine Disruptors: The PREVED Project. Int. J. Environ. Res. Public Health 2022, 19, 70. https://doi.org/10.3390/ijerph19010070
El Ouazzani H, Fortin S, Venisse N, Dupuis A, Rouillon S, Cambien G, Gourgues A-S, Pierre-Eugène P, Rabouan S, Migeot V, et al. Perinatal Environmental Health Education Intervention to Reduce Exposure to Endocrine Disruptors: The PREVED Project. International Journal of Environmental Research and Public Health. 2022; 19(1):70. https://doi.org/10.3390/ijerph19010070
Chicago/Turabian StyleEl Ouazzani, Houria, Simon Fortin, Nicolas Venisse, Antoine Dupuis, Steeve Rouillon, Guillaume Cambien, Anne-Sophie Gourgues, Pascale Pierre-Eugène, Sylvie Rabouan, Virginie Migeot, and et al. 2022. "Perinatal Environmental Health Education Intervention to Reduce Exposure to Endocrine Disruptors: The PREVED Project" International Journal of Environmental Research and Public Health 19, no. 1: 70. https://doi.org/10.3390/ijerph19010070
APA StyleEl Ouazzani, H., Fortin, S., Venisse, N., Dupuis, A., Rouillon, S., Cambien, G., Gourgues, A.-S., Pierre-Eugène, P., Rabouan, S., Migeot, V., & Albouy-Llaty, M. (2022). Perinatal Environmental Health Education Intervention to Reduce Exposure to Endocrine Disruptors: The PREVED Project. International Journal of Environmental Research and Public Health, 19(1), 70. https://doi.org/10.3390/ijerph19010070







