Abstract
Bi(OTf)3 and SiO2-Bi(OTf)3 are found to effectively catalyze the Ferrier rearrangement of tri-O-acetyl glycals with different alcohols providing an effective route to 2,3-unsaturated O-glycosides with good anomeric selectivity and good to excellent yields after short reaction times.
Introduction
The Lewis acid catalyzed allylic rearrangement of glycals in the presence of alcohols is known as the Ferrier rearrangement [1]. This rearrangement leads to the formation of alkyl and aryl 2,3- unsaturated-O-glycosides, which are versatile chiral intermediates in the synthesis of several biologically active natural products [2]. 2,3-Unsaturated-O-glycosides are also important building blocks in the synthesis of some antibiotics [3]. The Ferrier rearrangement involves the intermediacy of an allylic oxycarbenium ion to which the nucleophile adds preferentially in a quasi-axial orientation. The Lewis acid catalysts used for this rearrangement include BF3·OEt2 [4], SnCl4 [5] and FeCl3 [6]. Other reagents such as DDQ [7], NIS [8], I2 [9], acidic Montmorillonite K-10 [10], BiCl3 [11] and InCl3 [12] are also known to bring about the Ferrier rearrangement under different conditions. In recent years Sc(OTf)3 [13] and Yb(OTf)3 [14] have also been employed for the Ferrier rearrangement. However, many of these procedures have limitations in terms of yields, stereoselectivities, reaction temperatures, reaction times and amounts of reagent or catalyst used. For example, 1.6 equivalents of NIS [8], or varied amounts of BF3·OEt2 [4] are needed to bring about the transformation. Reagents such as Sc(OTf)3 and Yb(OTf)3 are relatively expensive and while using Yb(OTf)3 for this transformation, the reaction time is somewhat longer in some cases (see Table 2). Therefore, there is a need for the introduction of convenient and inexpensive reagents which are cheaper, require shorter reaction times and offer good anomeric selectivity.
To this end bismuth(III) trifluoromethane sufonate (bismuth triflate) [Bi(OTf)3], has drawn our attention for use in the Ferrier rearrangement as it is cheap, easy to prepare [15], and has low toxicity. Bismuth triflate has been used as a catalyst for the Friedel-Crafts acylation [16], sulfonylation of arenes [17], the Diels-Alder reaction [18], aza Diels-Alder reaction [19], acylation of alcohols [20], epoxide rearrangements [21] and acylal synthesis [22].
Results and Discussion
Herein, we wish to report that Bi(OTf)3 acts as a mild and highly efficient reagent for the O-glycosylation of 3,4,6-tri-O-acetylglucal (1) with diverse alcohols (Scheme 1). The glycosylation of tri-O-acetyl glucal with primary, secondary, benzyl, allyl and propargyl alcohols proceeded smoothly at ambient temperature to afford, after short reaction times, the corresponding alkyl 2,3-unsaturated glycosides 2 in good to high yields, with the α-anomer being the major or the exclusive product. Our results are summarized in Table 1. As shown there, these glycosylation reactions proceed smoothly in the presence of 2 mol % of Bi(OTf)3 or 2 mol% of the catalyst supported on 250 mg of SiO2 in dichloromethane per 100 mg of the 3,4,6-tri-O-acetylglucal substrate. In all the given examples, except for entry 12 (β-naphthol), the corresponding O-glycosylation products are obtained. In the case of entry 12 the corresponding 1-C-glucoside was obtained, presumably via rearrangement of the corresponding O-glucoside. Formation of the C-glucoside was confirmed by its acetylation followed by the characterization of the corresponding acetylated product by 1H-NMR, 13C-NMR, IR and mass spectral data (cf. Experimental section).
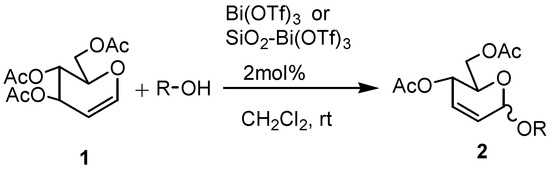
Scheme 1.
Scheme 1.
Further, we have also found that bismuth triflate supported on silica gel also acts as an efficient reagent for the above transformation. The use of SiO2-supported Bi(OTf)3 reagent had in some cases interesting effects in terms of yield (entries 9,10,14), reaction time (entries 1, 3, 13) and selectivity (entries 1, 2, 5), as can be seen in Table 1. Therefore it appears that increasing the surface of the catalyst is advantageous in certain cases, although it reduces the reactivity. It is therefore clear that either of the reagents could be more useful, depending on the glycosyl acceptor.

Table 1.
Bi(OTf)3 catalyzed glycosylation of alcohols.
| S. No. | Alcohol | Bi(OTf)3 | Bi(OTf)3 – SiO2 | ||||
|---|---|---|---|---|---|---|---|
| Reaction time | Yield (%) a | Anomeric ratio(a:b) b | Reaction time | Yield (%) a | Anomeric ratio(a:b)b | ||
| 1. | CH3CH2OH | 1 h | 78 | 5.5 : 1 | 40 min | 72 | 12 : 1 |
| 2. | CH3(CH2)2OH | 45 min | 72 | 6 : 1 | 1 h | 70 | 10 : 1 |
| 3. | CH3OH | 6 h | 56 | 6.6 : 1 | 75 min | 81 | 6.9 : 1 |
| 4. | 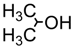 | 20 min | 70 | 5.5 : 1 | 45 min | 60 | 5.3 : 1 |
| 5. | CH3(CH2)7OH | 50 min | 84 | 4.5 : 1 | 1 h | 83 | 8.9 :1 |
| 6. | 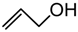 | 3 min | 75 | α | 2 h | 51 | α |
| 7. | 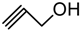 | 5 min | 73 | α | 2.5 h | 76 | 7.8 : 1 |
| 8. | 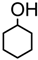 | 30 min | 82 | α | 2 h | 80 | 3 : 1 |
| 9. | 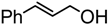 | 25 min | 53 | 6 : 1 | 40 min | 81 | 8.7 : 1 |
| 10. |  | 3 min | 69 | 4 : 1 | 15 min | 90 | 2.2 : 1 |
| 11. | Cholesterol | 2 h | 70 | α | 3 h | 74 | 15 : 1c |
| 12. | 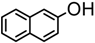 | 10 min | 66 | 6 : 1 | 20 min | 58 | 3.2 : 1d |
| 13. | 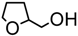 | 12 min | 73 | 16 : 1 | 5 min | 61 | 12 : 1 |
| 14. |  | 5 min | 79 | 6 : 1 | 20 min | 83 | 6 : 1 |
- (a)
- Isolated yields
- (b)
- Anomeric ratio is determined by 1H-NMR (400MHz) spectroscopy.
- (c)
- 4 mol % of the catalyst was used and reaction warmed to 40oC.
- (d)
- Only C-Ferrier product is observed.
A comparison of the literature methods with the new Bi(OTf)3 and Bi(OTf)3-SiO2 reagents is presented in Table 2. Although BiCl3 is known [11] to bring about the Ferrier rearrangement (cf. entries 1, 3, 4 and 7, Table 2), it is clear from the data that with alcohols such as benzyl alcohol (entry 1), propargyl alcohol (entry 3), and allyl alcohol (entry 4) the time required using bismuth chloride is between 1 to 1.5 h, in comparison to 3 min required using Bi(OTf)3.

Table 2.
Comparison of results of Bi(OTf)3 with other catalysts
| S.No. | Alcohol | Catalyst | Time | Yield (%) | (α :β) ratio | Amount of catalyst used |
|---|---|---|---|---|---|---|
| 1. | 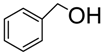 | CAN | 3 h | 90 | 7 : 1 | 10 mol % |
| Sc(OTf)3 | 3.5 h | 85 | 5 : 1 | 5 mol % | ||
| Yb(OTf)3 | 3 h | 94 | 9 : 1 | 10 mol% | ||
| BiCl3 | 1 h | 94 | 10 : 1 | 5 mol% | ||
| InCl3 | 10 min | 86 | 6.3 : 1 | 20 mol % | ||
| Bi(OTf)3-SiO2 | 15 min | 90 | 2.2 : 1 | 2 mol % | ||
| Bi(OTf)3 | 3 min | 69 | 4 : 1 | 2 mol % | ||
| 2. | 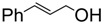 | CAN | 2.5 h | 89 | 9 : 1 | 10 mol % |
| Sc(OTf)3 | 2.5 h | 92 | 9 : 1 | 5 mol % | ||
| Bi(OTf)3-SiO2 | 40 min | 81 | 8.7 : 1 | 2 mol % | ||
| Bi(OTf)3 | 25 min | 53 | 6 : 1 | 2 mol % | ||
| 3. |  | CAN | 6 h | 80 | 4 : 1 | 10 mol % |
| Sc(OTf)3 | 1.5 h | 93 | 10 : 1 | 5 mol % | ||
| Yb(OTf)3 | 4 h | 91 | 10 : 1 | 10 mol % | ||
| BiCl3 | 1.5 h | 95 | 10 : 1 | 5 mol % | ||
| Bi(OTf)3-SiO2 | 2.5 h | 76 | 7.8 : 1 | 2 mol % | ||
| Bi(OTf)3 | 5 min | 73 | α | 2 mol % | ||
| 4. |  | CANSc | 3 h | 90 | 4 : 1 | 10 mol % |
| (OTf)3 | 1.5 h | 95 | 7 : 1 | 5 mol % | ||
| BiCl3 | 1.5 h | 95 | 11 : 1 | 5 mol % | ||
| Bi(OTf)3-SiO2 | 2 h | 51 | α | 2 mol % | ||
| Bi(OTf)3 | 3 min | 75 | α | 2 mol % | ||
| I2 | 1 h | 88 | 7 : 1 | 20 mol % | ||
| 5. | 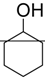 | CAN | 4.5 h | 80 | 14 : 1 | 10 mol% |
| Sc(OTf)3 | 3 h | 83 | 7 : 1 | 5 mol% | ||
| Yb(OTf)3 | 18 h | 89 | 11 : 1 | 10 mol % | ||
| InCl3 | 30 min | 90 | 9 : 1 | 20 mol % | ||
| Bi(OTf)3 | 30 min | 82 | α | 2 mol % | ||
| Bi(OTf)3-SiO2 | 2 h | 80 | 3 : 1 | 2 mol % | ||
| 6. | 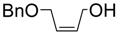 | CAN | 4 h | 87 | 7 : 1 | 10 mol % |
| Sc(OTf)3 | 2 h | 92 | 9 : 1 | 5 mol % | ||
| Bi(OTf)3 | 5 min | 79 | 6 : 1 | 2 mol % | ||
| Bi(OTf)3-SiO2 | 20 min | 83 | 6 : 1 | 2 mol % | ||
| 7. | Cholesterol | CAN | 5 h | 78 | 10 : 1 | 10 mol % |
| BiCl3 | 2 h | 90 | 4 : 1 | 5 mol % | ||
| Bi(OTf)3 | 2 h | 70 | α | 2 mol % | ||
| Bi(OTf)3-SiO2 | 3 h | 74 | 15:1 | 2 mol% |
Likewise, with allyl alcohol, propargyl alcohol and cholesterol (entry 7) the Ferrier product is a mixture of α and β anomers using bismuth chloride whereas with Bi(OTf)3 only the α anomer is obtained. In general, the amount of the BiCl3 uesd is 5 mol%, whereas Bi(OTf)3 is used in only 2 mol% indicating that it is more reactive than BiCl3. Thus, overall it appears that Bi(OTf)3 has distinct advantages over BiCl3 in terms of reaction time, selectivity, and amount of the catalyst required.
Furanoid skeletons (Scheme 2) are important components [23] of many biologically important natural products. Certain monosaccharides under acidic conditions lead to furans [24]. An optically active furandiol such as 2-(D-glycero-1,2-dihydroxyethyl)furan (4) is a potential chiral building block in organic synthesis [25]. The transformation of D-glucal 3 to furandiol 4 with the HgSO4-dioxane system was first reported by Gonzalez et al. [26]. Following this report, Hayashi et al. [27] screened several catalysts such as Pd(OAc)2, RuCl2(PPh)3, Sm(OTf)3 and Yb(OTf)3 for the transformation of 3 into 4, reporting yields ranging from 44% to 70% and requiring 30-165 minutes at 80-100oC, but some of these reagents are expensive and HgSO4 is toxic. Some improved procedures for this transformation can be found in the literature. Recently, Balasubramanian et al. [28] used InCl3·3H2O for this purpose. The reaction requires 10 mol % of the catalyst and is completed in 2.5 h to give 4 in 82% yield. More recently, in our laboratory [29], HClO4-SiO2 has been found to be an efficient acidic catalyst for the formation of 4 in 89% yield. It therefore seemed logical to focus our attention on the use of the present reagent system for the formation of 4. Thus, use of 1 mol% of Bi(OTf)3 transformed D-glucal into 4 in after 1 h, albeit in only 59% yield. Increasing the amount of the catalyst from 1 mol% to 3 mol% reduced the reaction time to 15 minutes, but the yield also dropped to 47%. Reaction of galactal, on the other hand, required 2 mol% of Bi(OTf)3 and it took 5 minutes for the reaction to complete, resulting in a 47% yield of the diol 4.
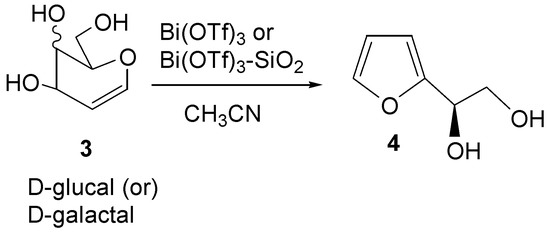
Scheme 2.
Scheme 2.
Conclusions
We have developed a highly stereoselective, Bi(OTf)3 and SiO2-Bi(OTf)3 catalyzed Ferrier glycosylation to produce 2,3-unsaturated glycosides. Compared to other methods, our method appears to have advantages such as easy preparation of the inexpensive catalyst, shorter reaction times, good yields, high anomeric selectivity, mild reaction conditions and low catalyst loadings. We believe that it should find use in organic synthesis.
Experimental:
General
All the alcohols used for the reactions are commercially available and were purchased from different chemical companies. All liquid alcohols were freshly distilled before use and solid alcohols were recrystallized. CH3CN was dried over P2O5 followed by over CaH2. After distillation it was stored over 4Å molecular sieves and used directly for the reactions. Dichloromethane was dried over anhydrous CaCl2 and freshly distilled over CaH2 prior to use. All reactions were carried out under an inert nitrogen atmosphere. All products were purified by silica gel column chromatography (100-200 mesh) using pet. ether and ethyl acetate as the eluents. Proton NMR spectra were recorded on a Jeol 400 MHz NMR spectrometer using CDCl3 as the solvent. The yields reported are after purification. All compounds are known and the structures were confirmed by 1H-NMR and 13C-NMR spectra and comparison of physical properties with the available literature data.
Preparation of the silica gel supported catalyst
Bismuth triflate (40 mg) was dissolved in dry CH3CN (4 mL). To this solution was added activated silica gel (2g, 100-200 mesh) and a slurry was prepared by evaporating the solvent under vacuum. The slurry is kept under a nitrogen atmosphere and 250 mg of this reagent, which contains 2 mol % (5mg ) of bismuth triflate, is used for each reaction .
General synthetic procedure
To a stirred mixture of tri-O-acetyl D-glucal (100 mg, 0.3676 mmol) and alcohol (1.1 eq) in dichloromethane (3 mL) under nitrogen was added 2 mol% of Bi(OTf)3 (5 mg) at ambient temperature and the reaction was monitored by TLC. After the reaction was over, the reaction mixture was quenched with 20% aqueous NaHCO3 solution and the crude product was extracted with dichloromethane (3 x 10 mL). The combined organic layers were washed with water, brine, and finally dried over anhydrous Na2SO4. Pure compound was obtained by column chromatography on SiO2 (100- 200 mesh).
Spectral data for the 1-C-glucoside corresponding to entry 12: 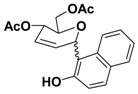
1H-NMR (400 MHz, CDCl3) (α-anomer) δ: 2.13 (s, 3H), 2.15 (s, 3H), 4.08-4.11 (dt, J = 2.92, 5.84, 9.28 Hz, 1H), 4.32-4.39 (m, 2H), 5.65 (dd, J = 1.96, 9.28 Hz, 1H), 5.87-5.90 (m, 1H), 5.97-6.00 (dd, 1H, J = 4.4, 11.84 Hz), 6.31 (brs, 1H), 7.09-7.79 (m, 6H), 8.53 (s, 1H). (β-anomer) δ: 8.34 (s, 1H), 6.11(brs, 1H); 13C-NMR (100 MHz, CDCl3) (α-anomer) δ: 20.7, 20.9, 62.4, 64.2, 75.3, 75.3, 119.9, 120.6, 112.6-154.0 (10 aromatic carbons), 170.1, 170.8; IR(CH2Cl2) ν: 3362, 1742, 1622 1264, 1230 cm-1; MSES+: 379 [M + Na]+, 295 [(M+2)-86+Na]+, 237 [M-143+Na] +. Acetate of 1-C-glucoside: 1H-NMR (400 MHz, CDCl3) (α-anomer) δ: 2.06 (s, 3H), 2.14 (s, 3H), 2.38 (s, 3H), 4.06-4.12 (m, 1H), 4.21-4.34 (m, 2H), 5.67-5.69 (m, 1H), 5.89-6.02 (m, 3H); 13C-NMR (100 MHz, CDCl3) (α-anomer) δ: 20.8, 21.0, 21.1, 63.5, 65.2, 71.6, 75.4, 121.4-146.7 (10 aromatic and 2 olefinic carbons), 169.6, 170.4, 171.0. IR (CH2Cl2) ν: 3055, 1740, 1232, 739 cm-1. MSES+: 819 [2M+Na]+, 421 [M+Na]+.
Acknowledgements
We thank the Department of Science and Technology, New Delhi for financial support through a project SP/S1/G-21/2001.
References
- Ferrier, R.J.; Prasad, N. Synthesis of 2,3-Dideoxy-α-D-erythro-hex-2-enopyranosides from Tri-O-acetyl-D-glucal. J. Chem. Soc. C 1969, 570. [Google Scholar] [CrossRef]
- Dorgan, B.J.; Jackson, R.F.W. Synthesis of C-Linked Glycosyl Amino Acid Derivatives using Organozinc Reagents. Synlett 1996, 859. [Google Scholar] Fraser-Reid, B. Some progeny of 2,3- unsaturated sugars - they little resemble grandfather glucose: ten years later. Acc. Chem. Res 1985, 18, 347. [Google Scholar] Ferrier, R.J. Unsaturated sugars. Adv. Carbohyd. Chem. Biochem 1969, 24, 199. [Google Scholar]
- Williams, N.R.; Wander, J.D. The Carbohydrates: Chemistry and Biochemistry; Academic Press: New York, 1980; p. 761. [Google Scholar]
- Descotes, G.; Martin, J.C. Sur1’ isomerisation du 1,5-anhydro-3,4,6-tri-O-benzyl-1,2-didesoxy-D-arabino-hex-1-enitol en presence d’acides de Lewis. Carbohydr. Res. 1977, 56, 168. [Google Scholar] Klaffke, W.; Pudlo, P.; Springer, D.; Thiem, J. Artificial deoxy glycosides of antracyclines. Liebigs Ann. Chem 1991, 509. [Google Scholar]
- Grynkiewicz, G.; Priebe, W.; Zamojski, A. Synthesis of alkyl 4,6-di-o-acetyl-2,3-dideoxy-α- D-threo-hex-2-enopyranosides from 3,4,6-tri-o-acetyl-1,5-anhydro-2-deoxy-D-lyxo-hex-1-enitol (3,4,6-tri-O-acetyl- -galactal). Carbohydrate Res. 1979, 68, 33. [Google Scholar] Bhate, P.; Horton, D.; Priebe, W. Allylic rearrangement of 6-deoxyglycals having practical utility. Carbohydr. Res. 1985, 144, 331. [Google Scholar]
- Masson, C.; Soto, J.; Bessodes, M. Ferric Chloride: A new and very efficient catalyst for the Ferrier glycosylation reaction. Synlett 2000, 1281. [Google Scholar]
- Toshima, K.; Ishizuka, T.; Matsuo, G.; Nakata, M.; Konoshita, M. Glycosidation of Glycals by 2,3-Dichloro-5,6-dicyano-p-benzoquinone (DDQ) as a catalytic promoter. J. Chem. Soc., Chem. Commun. 1993, 704. [Google Scholar] [CrossRef]
- Fraser-Reid, B.; Madsen, R. Ferrier rearrangement under nonacidic conditions based on iodonium-induced rearrangements of allylic n-pentenyl esters, n-pentenyl glycosides, and phenyl thioglycosides. J. Org. Chem. 1995, 60, 3851. [Google Scholar]
- Koreeda, M.; Houston, T.A.; Shull, B.K.; Klemke, E.; Tuinman, R.J. Iodine catalyzed Ferrier reaction. A mild and highly versatile glycosylation of hydroxyl and phenolic groups. Synlett 1995, 90. [Google Scholar] [CrossRef]
- Toshima, K.; Ishizuka, T.; Matsuo, G.; Nakata, M. Practical glycosidation method of glycols using Montmorillonite K-10 as an environmentally acceptable and inexpensive industrial catalyst. Synlett 1995, 306. [Google Scholar] [CrossRef]
- Swamy, N.R.; Venkateswarulu, Y. An efficient method for the synthesis of 2,3-unsaturated glycopyranosides catalyzed by bismuth trichloride in Ferrier rearrangement. Synthesis 2002, 598. [Google Scholar] [CrossRef]
- Babu, B.S.; Balasubramanian, K.K. Indium trichloride catalyzed glycosidation. An expeditious synthesis of 2,3-unsaturated glycopyranosides. Tetrahedron Lett 2000, 41, 1271. [Google Scholar] [CrossRef]
- Yadav, J.S.; Subba Reddy, B.V.; Murthy, C.V.S.R.; Mahesh Kumar, G. Scandium Triflate catalyzed Ferrier rearrangement: An efficient synthesis of 2,3- unsaturated glycopyranosides. Synlett 2000, 1450. [Google Scholar] [CrossRef]
- Takhi, M.; Adel, A-H.; Rehman, A.; Schimdt, R.R. Highly stereoselective synthesis of pseudoglycals via Yb(OTf)3 catalyzed Ferrier glycosylation. Synlett 2001, 427. [Google Scholar] [CrossRef]
- Repichet, S.; Zwick., A.; Vendier, L.; LeRoux, C.; Dubac, J. A practical, cheap and environmentally friendly preparation of bismuth(III)trifluoromethanesulfonate. Tetrahedron Lett. 2002, 43, 993. [Google Scholar] Labrouillere, M.; LeRoux, C.; Gaspard, H.; Laporteric, A.; Dubac, J. An efficient method for the preparation of Bismuthe (III) trifluoromethanesulfonate. Tetrahedron Lett. 1999, 40, 285. [Google Scholar]
- Labrouillere, M.; LeRoux, C.; Gaspard, H.; Laporteric, A.; Dubac, J.R. Surprising catalytic activity of bismuth (III) triflate in the Friedel-Craft’s acylation reaction. Tetrahedron Lett. 1997, 38, 8871. [Google Scholar] Repichet, S.; Le Roux, C.; Dubac, J.; Desmure, J.R. Bismuth (III) Trifluoromethanesulfonate: A chameleon catalyst for the Friedel-Crafts acylation. Eur. J. Org. Chem. 1998, 2743. [Google Scholar]
- Repichet, S.; Le Roux, C.; Hernandez, P.; Dubac, J. Bismuth (III) trifluromethanesulfonate: An efficient catalyst for sulfonylation of arenes. J. Org. Chem. 1999, 64, 6479. [Google Scholar] [CrossRef]
- Garrigues, B.; Gonjanga, F.; Robert, H.; Garrigues, B.; Dubac, J. Bismuth (III) chloride or triflate catalyzed dienophilic activity of α-ethylenic aldehydes and ketones. J. Org. Chem 1997, 62, 4880. [Google Scholar] Robert, H.; Garrigues, B.; Dubac, J. The carbonyl-Diels-Alder reaction catalyzed by bismuth (III) chloride. Tetrahedron Lett. 1998, 39, 1161. [Google Scholar] [CrossRef]
- Laurent-Robert, H.; Garrigues, B.; Dubac, J. Bismuth (III) chloride and triflate: New efficient catalyst for the aza-Diels-Alder reaction. Synlett 2000, 1160. [Google Scholar]
- Orita, A.; Tanahashi, C.; Kakuda, A.; Otera, J. Highly efficient and versatile acylation of alcohols with Bi(OTf)3 as a catalyst. Angew. Chem. Int. Ed. 2000, 39, 2877. [Google Scholar] [CrossRef]
- Bhatia, K.A.; Leonard, N.M.; Oswald, M.C.; Eash, K.J.; Mohan, R.S. A facile and efficient method for the rearrangement of aryl substituted epoxides to aldehydes and ketones using bismuth triflate. Tetrahedron Lett. 2001, 42, 8129. [Google Scholar] [CrossRef]
- Carrigan, M.D.; Eash, K.J.; Oswald, M.C.; Mohan, R.S. An efficient method for chemoselective synthesis of acylals from aromatic adehydes using bismuth triflate. Tetrahedron Lett. 2001, 42, 8133. [Google Scholar] [CrossRef]
- Westly, J.W. Polyether Antibiotics Naturally Occuring Acid Ionophores; Marcel Dekker: NewYork, 1982; vol. 1 and 2. [Google Scholar] Hanessian, S. Approaches to the total Synthesis of natural products using “Chiral Templates” derived from carbohydrates. Acc. Chem. Res. 1979, 12, 159. [Google Scholar]
- Bosshard, P.; Eugster, C.H. Advances in Heterocyclic Chemistry; Katritzky, A.R., Boulton, A.J., Eds.; Academic Press: New York, 1966; Vol.7, p. 377. [Google Scholar] Harris, J.M.; O’Doherty, G.A. Enantioselective synthesis of 5-substituted-α,β-unsaturated-δ-lactones: Application to the synthesis of styryllactones. Tetrahedron Lett. 2000, 41, 183. [Google Scholar]
- Babu, R.S.; Zhou, M.; O’Doherty, G.A. De Novo synthesis of oligosaccharides using palladium-catalyzed glycosylation reaction. J. Am. Chem. Soc. 2004, 126, 3428. [Google Scholar] Saeed, M.; Ilg, T.; Schick, M.; Abbas, M.; Voelter, W. Total synthesis and anti-leishmanial activity of R-(-)- argentilactone. Tetrahedron Lett. 2001, 47, 7401. [Google Scholar] Harris, J.M.; Keranen, M.D.; Nguyen, H.; Young, V.G.; O’Doherty, G.A. Syntheses of four D- and L-hexoses via diastereoselective and enantioselective dihydroxylation reactions. Carbohydr. Res. 2000, 328, 17. [Google Scholar]
- Gonzalez, F.; Lesage, S.; Perlin, A.S. Catalysis by mercuric ion of reactions of glycals with water. Carbohydr. Res. 1975, 42, 267. [Google Scholar] [CrossRef]
- Hayashi, M.; Kawabata, H.; Yamada, K. Metal catalyzed transformation of D-glucal to optically active furandiol. Chem. Commun. 1999, 965. [Google Scholar] [CrossRef]
- Babu, B.S.; Balasubramanian, K.K. A facile synthesis of chiral furan diol from glycals catalyzed by indium trichloride. J. Org. Chem. 2000, 65, 4198. [Google Scholar] [CrossRef]
- Agarwal, A.; Rani, S.; Vankar, Y.D. Protic acid (HClO4 supported silica gel) mediated synthesis of 2,3-unsaturated-O-glycosides and chiral furan diol from 2,3-glycals. J. Org. Chem. 2004, 69, 6137. [Google Scholar] [CrossRef] [PubMed]
- Sample availability: Contact the authors.
© 2005 by MDPI (http://www.mdpi.org). Reproduction is permitted for non commercial purposes.
