Abstract
Cerium(III) nitrate hexahydrate efficiently catalyzes the three-component Biginelli reaction under solvent-free conditions of an aldehyde, a β-keto ester or β-diketone and urea or thiourea to afford the corresponding 3,4-dihydropyrimidin-2(1H)-ones or –thiones in excellent yields.
Introduction
3,4-Dihydropyrimidin-2(1H)-ones (DHPM) and their sulfur analogs have been reported to possess remarkable pharmacological properties. Some of which have antiviral, antitumor, antibacterial, antiinflammatory, and antihypertensive activities [1,2,3,4,5], some are calcium channel modulators [6], and α1a adrenoceptor-selective antagonists [7]. The structurally rather simple DHPM monastrol (Figure 1) specifically inhibits the mitotic kinesin Eg5 motor protein and can be considered as a new lead for the development of anticancer drugs [8]. The batzelladine alkaloids A and B (Figure 1) inhibit the binding of HIV envelope protein gp-120 to human CD4 cells and, therefore, are potential new leads for AIDS therapy [9]. As a result new synthetic methods for the efficient preparation of these heterocyclic compounds are of great importance. Very recently, several modified and improved procedures for the one-pot synthesis of dihydropyrimidinones have been reported [10], but many of reported methods have drawbacks such long reaction times, harsh reaction conditions, the use of stoichiometric reagents or of toxic and inflammable solvents, difficult work-ups or low yields of products and incompatibility with other functional groups in the molecules. Consequently, there is a need to develop new methods using less hazardous reagents and solvents, or even better, ones that can be carried out under solvent-free conditions.
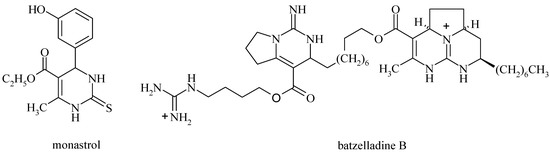
Figure 1.
Examples of biologically active DHPMs.
Figure 1.
Examples of biologically active DHPMs.
Results and Discussion
In recent years lanthanide salts have been used as catalysts in the Biginelli reaction, for example: LaCl3·7H2O in refluxing EtOH [11a] or Yb(OTf)3 under solvent-free conditions [11b]. Bose and co-workers have reported the CeCl3·7H2O-catalyzed synthesis of DHPMs in moderate to good yields (65-80%) under solvent-free conditions, but their procedure suffers from some drawbacks such as the very long reaction times needed (up to 10 h) and the use of fairly high amounts of the catalyst (25 mol %) [11c]. In this paper, we wish to report a simple and efficient method for the synthesis of DHPMs using cerium(III) nitrate under solvent-free conditions. Thus, the reactions of several activated and deactivated aromatic and aliphatic aldehydes with a β-keto ester (or β-diketone) and urea using a catalytic amount of Ce(NO3)3·6H2O produced a range of DHPMs in excellent yields under solvent-free conditions at 80 ºC (Scheme 1).
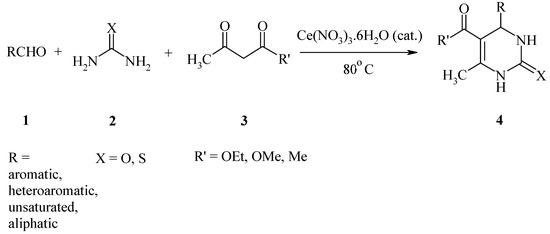
Scheme 1.
Scheme 1.
Thiourea was also used as one of the ingredients with similar success to provide the corresponding 3,4-dihydropyrimidin-2(1H)-thiones, which are also of interest for their biological activities [8]. In all cases, 3,4-dihydropyrimidin-2(1H)-ones (and -thiones) were the sole products and no by-products were observed. The experimental procedure is very simple and convenient, and under the reaction conditions used, can tolerate a variety of other functional groups such as methoxy, nitro, hydroxy, halides and olefins. The results are summarized in Table 1, which clearly indicates the generality and scope of the reaction with respect to various aromatic, heteroaromatic, unsaturated, and aliphatic aldehydes.
According to the mechanism suggested by Kappe [12], we propose a mechanism for the Ce(III)-catalyzed Biginelli reaction as shown in Scheme 2. The aldehyde may react with urea to form an acyl imine intermediate 5, which is activated by Ce(III). Subsequent nucleophilic addition of the activated β-dicarbonyl compound 6 followed by cyclization and dehydration affords the 3,4-dihydropyrimidin-2(1H)-one 4.
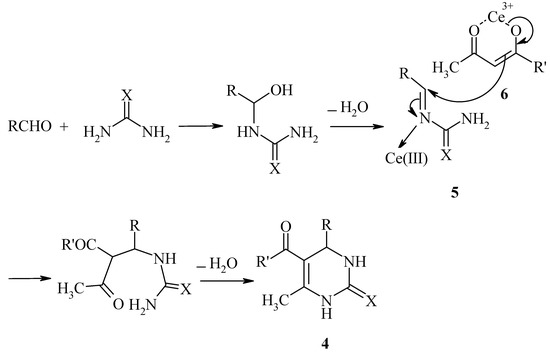
Scheme 2.
Scheme 2.
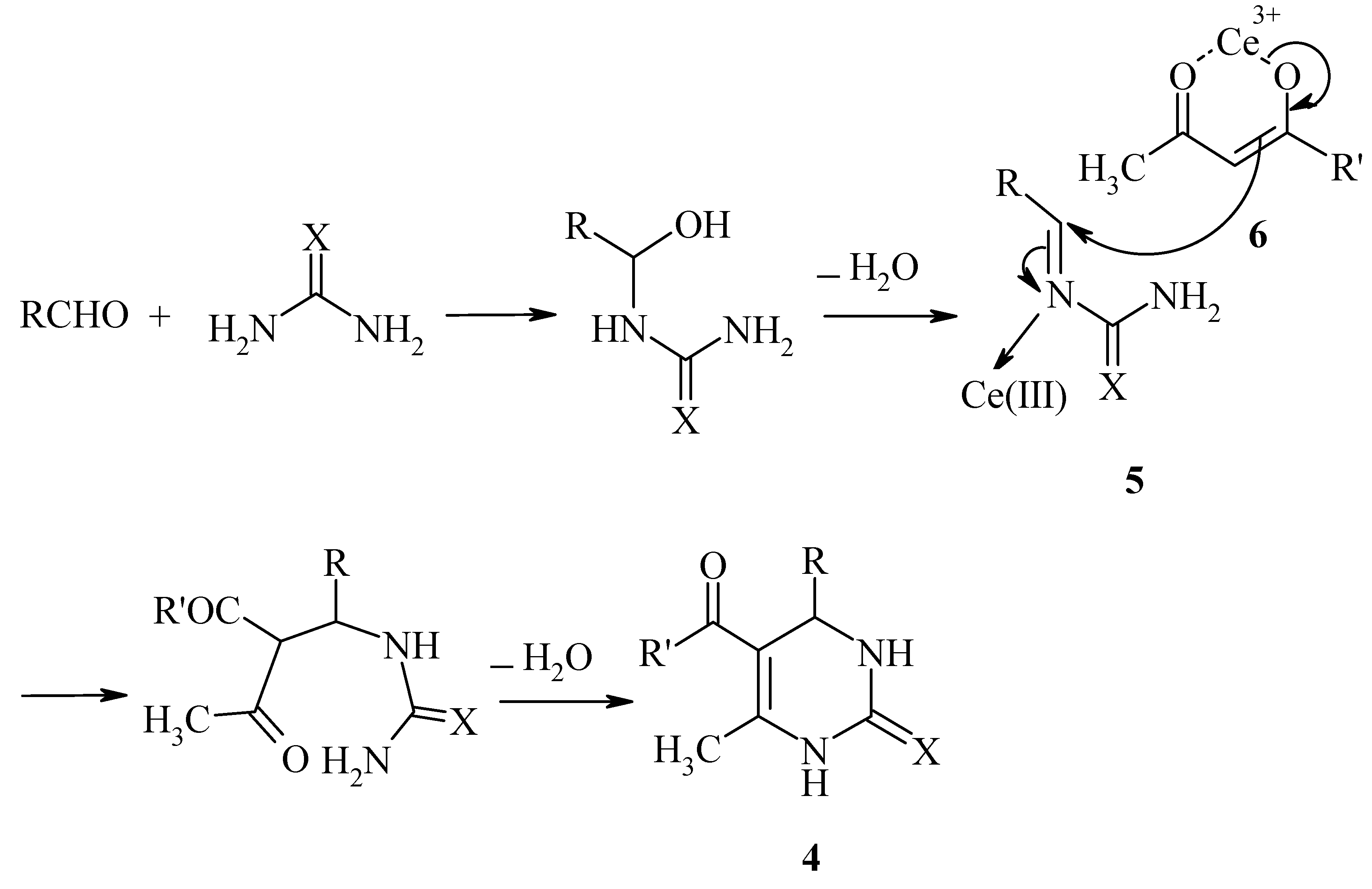

Table 1.
Ce(NO3)3·6H2O-Catalyzed Synthesis of Dihydropyrimidinones and Dihydropyrimidinethiones under Solvent-Free Conditions a.
| Entry | R | R' | X | time (min) | Yield (%)b |
|---|---|---|---|---|---|
| 1 | C6H5 | OEt | O | 10 | 98 |
| 2 | 2-MeC6H4 | OEt | O | 15 | 95 |
| 3 | 4-MeC6H4 | OEt | O | 20 | 93 |
| 4 | 2-MeOC6H4 | OEt | O | 15 | 97 |
| 5 | 3-MeOC6H4 | OEt | O | 10 | 97 |
| 6 | 4-MeOC6H4 | OEt | O | 10 | 98 |
| 7 | 2-ClC6H4 | OEt | O | 25 | 90 |
| 8 | 3-ClC6H4 | OEt | O | 20 | 92 |
| 9 | 4-ClC6H4 | OEt | O | 25 | 94 |
| 10 | 4-BrC6H4 | OEt | O | 25 | 93 |
| 11 | 4-FC6H4 | OEt | O | 25 | 95 |
| 12 | 3-O2NC6H4 | OEt | O | 25 | 89 |
| 13 | 4-O2NC6H4 | OEt | O | 25 | 91 |
| 14 | 2,4-(MeO)2C6H3 | OEt | O | 10 | 97 |
| 15 | 1-Naphthyl | OEt | O | 30 | 91 |
| 16 | 2-Furyl | OEt | O | 15 | 93 |
| 17 | PhCH=CH | OEt | O | 25 | 84 |
| 18 | n-Pentyl | OEt | O | 35 | 87 |
| 19 | n-Hexyl | OEt | O | 40 | 84 |
| 20 | C6H5 | OMe | O | 10 | 96 |
| 21 | 4-O2NC6H4 | OMe | O | 20 | 93 |
| 22 | 4-MeOC6H4 | OMe | O | 10 | 97 |
| 23 | C6H5 | Me | O | 10 | 96 |
| 24 | 4-O2NC6H4 | Me | O | 30 | 90 |
| 25 | 4-MeOC6H4 | Me | O | 10 | 97 |
| 26 | C6H5 | OEt | S | 10 | 96 |
| 27 | 4-O2NC6H4 | OEt | S | 10 | 90 |
| 28 | 4-MeOC6H4 | OEt | S | 10 | 97 |
| 29 | 3-HOC6H4 | OEt | S | 30 | 96 |
| 30 | C6H5 | OMe | S | 10 | 95 |
| 31 | 4-MeOC6H4 | OMe | S | 10 | 96 |
| 32 | C6H5 | Me | S | 10 | 94 |
a All products are known compounds and were characterized by comparison of their spectral data and m.p. values with those of authentic samples.b Isolated yields
Conclusions
In summary, we have developed a new and efficient synthetic methodology for the synthesis of 3,4-dihydropyrimidin-2(1H)-ones and their thio analogs under solvent-free conditions using a catalytic amount of Ce(III) nitrate. Moreover, the mild reaction conditions, short reaction times, high yields of the products, ease of work-up, compatibility with various functional groups, and the environmentally friendly nature of the procedure should make the present method a useful and important addition to the known methodologies for the Biginelli reaction.
Acknowledgements
This research was supported by the Research Council of University of Tehran as a research project (6102036/1/02).
Experimental
General
All chemicals were obtained from Merck (Germany) and were used without further purification. Melting points were measured on an Electrothermal 9100 apparatus, and are uncorrected. 1H-NMR spectra were recorded for deuterioacetone solutions using tetramethylsilane as the internal standard on a Bruker DRX-500 AVANCE spectrometer at 500.1 MHz operating at ambient temperature (δ in ppm and J in Hz).
Typical Procedure: 5-(Ethoxycarbonyl)-6-methyl-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (Entry 1)
A mixture of benzaldehyde (0.106 g, 1 mmol), ethyl acetoacetate (0.130 g, 1 mmol), urea (0.090 g, 1.5 mmol), and Ce(NO3)3·6H2O (5 mol%) was heated with stirring at 80°C for 10 min. The progress of the reaction was monitored by TLC. After completion, the reaction mixture was cooled to room temperature, and the precipitate was washed with cold water (3 × 5 mL). The pure product was obtained as colorless crystals by recrystallization from 95% EtOH, mp 200-201°C (Lit. [11b] 201-203°C); 1H-NMR (acetone-d6): TM 1.15 (3 H, t, J = 7.1 Hz, OCH2CH3), 2.39 (3 H, s, CH3), 4.05 (2 H, q, J = 7.1 Hz, OCH2CH3), 5.38 (1 H, d, J = 3.0 Hz, NHCH), 6.88 (1 H, br. s, NH), 7.24 (1 H, t, J = 7.3 Hz, CHpara), 7.30 (2 H, t, J = 7.5 Hz, 2 CHmeta), 7.36 (2 H, t, J = 7.5 Hz, 2 CHortho) and 8.29 (1 H, br. s, NH). The catalyst remaining in the aqueous phase can be recovered by removing the water by heating and then drying under vacuum at 100 °C for 2 h.
References and Notes
- Biginelli, P. Gazz. Chim. Ital. 1893, 23, 360.Kappe, C. O. Tetrahedron 1993, 49, 6937.
- Studer, A.; Jeger, P.; Wipf, P.; Curran, D. P. J. Org. Chem. 1997, 62, 2917.
- Kappe, C. O. Acc. Chem. Res. 2000, 33, 879. [CrossRef]
- Atwal, K. S.; Rovnyak, G. C.; O’Reilly, B. C.; Schwartz, J. J. Org. Chem. 1989, 54, 5898.
- Atwal, K. S.; Swanson, B. N.; Unger, S. E.; Floyd, D. M.; Moreland, S.; Hedberg, A.; O’Reilly, B. C. J. Med. Chem. 1991, 34, 806.Rovnyak, G. C.; Atwal, K. S.; Hedberg, A.; Kimball, S. D.; Moreland, S.; Gougoutas, J. Z.; O’Reilly, B. C.; Schwartz, J.; Malley, M. F. J. Med. Chem. 1992, 35, 3254.
- Rovnyak, G. C.; Kimbal, S. D.; Beyer, B.; Cucinotta, G.; Dimarco, J. D.; Gougoutas, J.; Hedberg, A.; Malley, M.; MaCarthy, J. P.; Zhang, R.; Mereland, S. J. Med. Chem. 1995, 38, 119.Kappe, C. O.; Fabian, W. M. F.; Semons, M. A. Tetrahedron 1997, 53, 2803.
- Barrow, J. C.; Nantermet, P. G.; Selnick, H. G.; Glass, K. L.; Rittle, K. E.; Gilbert, K. F.; Steele, T. G.; Homnick, C. F.; Freidinger, R. M.; Ransom, R. W.; Kling, P.; Reiss, D.; Broten, T. P.; Schorn, T. W.; Chang, R. S. L.; O’Malley, S. S.; Olah, T. V.; Ellis, J. D.; Barrish, A.; Kassahun, K.; Leppert, P.; Nagarathnam, D.; Forray, C. J. Med. Chem. 2000, 43, 2703.
- Mayer, T. U.; Kapoor, T. M.; Haggarty, S. J.; King, R. W.; Schreiber, S. L.; Mitchison, T. J. Science 1999, 286, 971.Haggarty, S. J.; Mayer, T. U.; Miyamoto, D. T.; Fathi, R.; King, R. W.; Mitchison, T. J.; Schreiber, S. L. Chem. Biol. 2000, 7, 275.
- Patil, A. D.; Kumar, N. V.; Kokke, W. C.; Bean, M. F.; Freyer, A. J.; De Brosse, C.; Mai, S.; Truneh, A.; Faulkner, D. J.; Carte, B.; Breen, A. L.; Hertzberg, R. P.; Johnson, R. K.; Westley, J. W.; Potts, B. C. M. J. Org. Chem. 1995, 60, 1182.
- (a) the use of water-based biphasic media, see: Bose, A. K.; Manhas, M. S.; Pednekar, S.; Ganguly, S. N.; Dang, H.; He, W.; Mandadi, A. Tetrahedron Lett. 2005, 46, 1901. (b) using Bi(NO3)3, see: Khodaei, M. M.; Khosropour, A. R.; Jowkar, M. Synthesis 2005, 1301. (c) using LiBr, see: Baruah, P. P.; Gadhwal, S.; Prajapati, D.; Sandhu, J. S. Chem. Lett. 2002, 1038. (d) using InBr3, see: Martins, M. A. P.; Teixeira, M. V. M.; Cunico, W.; Scapin, E.; Mayer, R.; Pereira, C. M. P.; Zanatta, N.; Bonacorso, H. G.; Peppe, C.; Yuan, Y.-F. Tetrahedron Lett. 2004, 45, 8991. (e) using I2, see: Bhosale, R. S.; Bhosale, S. V.; Bhosale, S. V.; Wang, T.; Zubaidha, P. K. Tetrahedron Lett. 2004, 45, 9111. (f) using FeCl3, see: Wang, Z.-T.; Xu, L.-W.; Xia, C.-G.; Wang, H.-Q. Tetrahedron Lett. 2004, 45, 7951. (g) using KHSO4, see: Tu, S.; Fang, F.; Zhu, S.; Li, T.; Zhang, X.; Zhuang, Q. Synlett 2004, 537. (h) using CdCl2, see: Narsaiah, A. V.; Basak, A. K.; Nagaiah, K. Synthesis 2004, 1253. (i) using ZnCl2 see: Sun, Q.; Wang, Y.-Q.; Ge, Z.-M.; Cheng, T.-M.; Li, R.-T. Synthesis 2004, 1047. (j) using Cu(OTf)2, see: Paraskar, A. S.; DewKar, G. K.; Sudalai, A. Tetrahedron Lett. 2003, 44, 3305. (k) using Me3SiI, see: Sabitha, G.; Reddy, G. S. K. K.; Reddy, C. S.; Yadav, J. S. Synlett 2003, 858. (l) using boric acid, see: Tu, S.; Fang, F.; Miao, C.; Jiang, H.; Feng, Y.; Shi, D.; Wang, X. Tetrahedron Lett. 2003, 44, 6153. (m) using ZrCl4, see: Reddy, C. V.; Mahesh, M.; Raju, P. V. K.; Romeshbabu, T.; Reddy, V. V. N. Tetrahedron Lett. 2002, 43, 2657.
- Lu, J.; Bai, Y.; Wang, Z.; Ma, B.; Yang, H. Tetrahedron Lett. 2000, 41, 9075.Ma, Y.; Qian, C.; Wang, L.; Yang, M. J. Org. Chem. 2000, 65, 3864.Bose, D. S.; Fatima, L.; Mereyala, H. B. J. Org. Chem. 2003, 68, 587.
- Kappe, C. O. J. Org. Chem. 1997, 62, 7201.
- Sample Availability: Samples are available from authors.
© 2006 by MDPI (http://www.mdpi.org). Reproduction is permitted for noncommercial purposes.
