Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva
Abstract
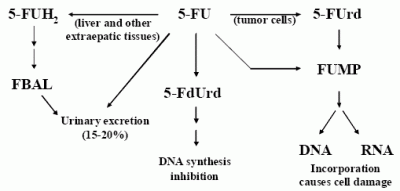
:Introduction

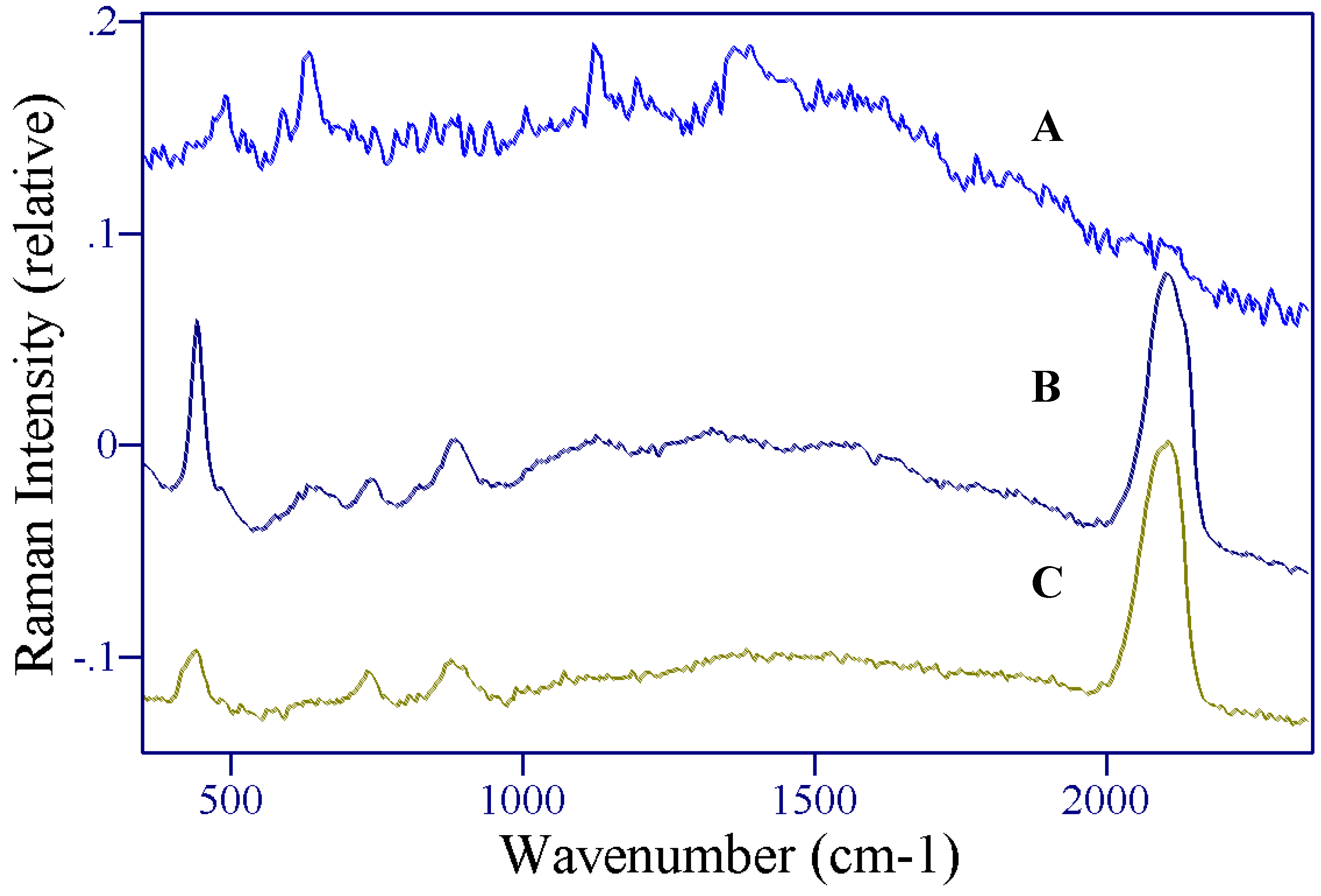
Results and Discussion




 |








Conclusions
Experimental
General
Solutions and measurements
Acknowledgements
References and Notes
- Malet-Martino, M.; Martino, R. Clinical studies of three oral prodrugs of 5-fluorouracil. T. Oncologist 2002, 1, 288–323. [Google Scholar] [CrossRef]
- Baker, S. D.; Verweij, J.; Rowinsky, E. K.; Donehower, R. C.; Schellens, J. H.; Grochow, L. B.; Sparreboom, A. Role of body surface area in dosing of investigational anticancer agents in adults, 1991-2001. J. Nat. Cancer Inst. 2002, 94, 1883–1888. [Google Scholar] [CrossRef]
- Diasio, R. B.; Harris, B. E. Clinical Pharmacology of 5-fluorouracil. Clin. Pharmacokinet. 1989, 16, 215. [Google Scholar] [CrossRef]
- Malet-Martino, M.; Martino, R. T. Clinical Studies of Three Oral Prodrugs of 5-Fluorouracil (Capecitabine, UFT, S-1): A Revie. Oncologist 2002, 7, 288. [Google Scholar] [CrossRef]
- Casale, F.; Canaparo, R.; Serpe, L.; Muntoni, E.; Pepa, C. D.; Costa, M.; Mairone, L.; Zara, G. P.; Fornari, G.; Eandi, M. Plasma concentrations of 5-fluorouracil and its metabolites in colon cancer patients. Pharmacol. Res. 2004, 50, 173–179. [Google Scholar] [CrossRef]
- Diasio, R. B.; Beavers, T. L.; Carpenter, J. T. Familial deficiency of dihydropyrimidine dehydrogenase. J. Clin. Invest. 1988, 81, 47–51. [Google Scholar] [CrossRef]
- Fleming, G. F.; Vokes, E. E.; Buse, J. B.; Mick, R.; Dushay, J.; Levitan, D.; Dolan, M. E. Peripheral blood mononuclear cell dihydropyrimidine dehydrogenase activity in volunteers with and without diabetes mellitus. Cancer Chemother. Pharmacol. 1996, 37, 569–573. [Google Scholar] [CrossRef]
- Dawling, S.; Essex, E. G.; Ward, N.; Widdop, B. Gas Chromatographic Measurement of Cocaine in Serum, Plasma and Whole Blood. Ann. Clin. Biochem.Sept. 1990, 27, 478–481. [Google Scholar] [CrossRef]
- Helbock, H. J.; Beckman, K. B.; Shigenaga, M. K.; Walter, P.; Woodall, A. A.; Yeo, H. C.; Ames, B. N. DNA oxidation matters: The HPLC-EC assay of 8-oxo-deoxyguanosine and 8-oxo-guanine. Proc. Natl. Acad. Sci. USA 1998, 95, 288–293. [Google Scholar] [CrossRef]
- Jankowiak, R.; Small, G. J. Fluorescence Line Narrowing: A High Resolution Window on DNA and Protein Damage from Chemical Carcinogens. (invited article). Chem. Res. Toxicol. 1991, 4, 256. [Google Scholar] [CrossRef]
- Burton, L. C.; James, C. A. Rapid Method for the Determination of Ifosfamide and Cyclophosphamide in Plasma by High-Performance Liquid Chromatography with Solid-Phase Extraction. J. Chromatogr. 1988, 431, 450–454. [Google Scholar] [CrossRef]
- McKinney, P. E.; Phillips, S.; Gomez, H. F.; Brent, J.; MacIntyre, M. Vitreous Humor Cocaine and Metabolite Concentrations: Do Postmortem Specimens Reflect Blood Levels at the Time of Death. J. Forensic Sci.Jan. 1995, 40, 102–107. [Google Scholar]
- Goodman, M.; Riley, M. B. Chemotherapy: Principles of administration. In Cancer Nursing: Principles and Practice, 4th Ed.; Groenwald S., L., Frogge M., H., Goodman, G., Yarbro C., H., Eds.; Jones and Bartlett: Boston, MA, USA, 1997; pp. 31–386. [Google Scholar]
- Cone, E. T.; Jenkins, A. J. Handbook of Analytical Therapeutic Drug Monitoring and Toxicology; Wong, S. H. Y., Sunshine, I., Eds.; CRC Press: New York, USA, 1997; Chapter 18. [Google Scholar]
- de Jonge, M. J.; Verwiej, J. V.; Loos, W. J.; Dallaire, B. K.; Sparreboom, A. Clinical pharmacokinetics of encapsulated oral 9-aminocamptothecin in plasma and saliva. Clin. Pharmacol. Ther. 1999, 65, 491–499. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, T.; Fujiwara, Y.; Sumiyoshi, H.; Isobe, T.; Yamaoka, N.; Yamakido, M. Salivary drug monitoring of irinotecan and its active metabolite in cancer patients. Cancer Chemothe.r Pharmacol. 1997, 40, 449–52. [Google Scholar] [CrossRef]
- van Warmerdam, L. J.; van Tellingen, O.; ten Bokkel Huinink, W. W.; Rodenhuis, S.; Maes, R. A.; Bijnen, J. H. Monitoring carboplatin concentrations in saliva: a replacement for plasma ultrafiltrate measurements? Ther. Drug Monit. 1995, 17:5, 465–470. [Google Scholar]
- Joulia, J. M.; Pinguet, F.; Ychou, M.; Duffour, J.; Astre, C.; Bressolle, F. Plasma and salivary pharmacokinetics of 5-fluorouracil (5FU) in patients with metastatic colorectal cancer receiving 5FU bolus plus continuous infusion with high-dose folinic acid. Eur. J. Cancer 1999, 35:2, 296–301. [Google Scholar]
- Chamberlain, J. The Analysis of Drugs in Biological Fluids, 2nd Ed. ed; CRC Press: France, 1995. [Google Scholar]
- Kneipp, K.; Wang, Y.; Dasari, R. R.; Feld, M. S. Approach to Single-Molecule Detection Using Surface-Enhanced Resonance Raman Scattering (SERRS): A Study Using Rhodamine 6G on Colloidal Silver. Appl. Spectrosc. 1995, 49, 780–784. [Google Scholar] [CrossRef]
- Nie, S.; Emory, S. R. Probing Single Molecules and Single Nanoparticles by Surface-Enhanced Raman Scattering. Science 1997, 275, 1102. [Google Scholar] [CrossRef]
- Garrel, R. L. Surface-Enhanced Raman Spectroscopy. Anal. Chem. 1989, 61, 401A–411A. [Google Scholar] [CrossRef]
- Storey, J. M. E.; Barber, T. E.; Shelton, R. D.; Wachter, E. A.; Carron, K. T.; Jiang, Y. Applications of Surface-Enhanced Raman Scattering (SERS) to Chemical Detection. Spectroscopy 1995, 10, 20–25. [Google Scholar]
- Sutherland, W. S.; Laserna, J. J.; Angebranndt, M. J.; Winefordner, J. D. Surface-Enhanced Raman Analysis of Sulfa Drugs on Colloidal Silver Dispersion. Anal. Chem. 1990, 62, 689–693. [Google Scholar] [CrossRef]
- Carter, J. C.; Brewer, W. E.; Angel, S. M. Raman spectroscopy for the in situ identification of cocaine and selected adulterants. Appl. Spectrosc. 2000, 54, 1876–1881. [Google Scholar] [CrossRef]
- Perez, R.; Ruperez, A.; Laserna, J. J. Evaluation of silver substrates for surface-enhanced Raman detection of drugs banned in sport practices. Anal. Chim. Acta 1998, 3, 76(2):255. [Google Scholar]
- Cîntă Pînzaru, S.; Pavel, I.; Leopold, N.; Kiefer, W. Identification and characterization of pharmaceuticals using Raman and surface-enhanced Raman Scattering. J. Raman Spectrosc. 2004, 35, 338–346. [Google Scholar] [CrossRef]
- Nabiev, I. R.; Morjani, H.; Manfait, M. Selective analysis of antitumor drug interaction with living cells as probed by surface-enhanced Raman spectroscopy. Eur. Biophys. J. 1991, 19, 311–316. [Google Scholar]
- Beljebbar, A.; Sockalingum, G.; Morjani, H.; Manfait, M. Raman and SERS micro-spectroscopy on living cells: a promising tool towards cellular-drug response and medical diagnosis. Proc. SPIE 1999, 3608, 175–184. [Google Scholar]
- Nabiev, I.; Baranov, A.; Chourpa, I.; Beljebbar, A.; Sockalingum, G.; Manfait, M. Does Adsorption on the Surface of Silver Colloid Perturb Drug/DNA Interactions? Comparative SERS, FT-SERS, and Resonance Raman Study of Mitoxantrone and Its Derivatives. J. Phys. Chem. 1995, 99, 1608–1613. [Google Scholar]
- Rivas, L.; Murza, A.; Sanchez-Cortes, S.; Garcia-Ramos, J. V. Adsorption of acridine drugs on silver: surface-enhanced resonance Raman evidence of the existence of different adsorption sites. Vib. Spectros. 2001, 25, 19–28. [Google Scholar] [CrossRef]
- Fabriciova, G.; Sanchez-Cortez, S.; Garcia-Ramos, J. V.; Miskovsky, P. Joint application of micro-Raman and surface-enhanced Raman spectroscopy to the interaction study of the antitumoral anthraquinone drugs danthron and quinzarin with albumins. J Raman Spectrosc. 2004, 35, 384–389. [Google Scholar] [CrossRef]
- Gift, A. D.; Shende, C.; Inscore, F. E.; Maksymiuk, P.; Farquharson, S. Five minute analysis of chemotherapy drugs and metabolites in saliva: Evaluating Dosage. Proc. SPIE 2004, 5261, 135–141. [Google Scholar]
- Farquharson, S.; Shende, C.; Inscore, F. E.; Maksymiuk, P.; Gift, A. D. Analysis of 5-fluorouracil in saliva using surface-enhanced Raman spectroscopy. J. Raman Spectrosc. 2005, 36, 208–212. [Google Scholar] [CrossRef]
- Gift, A. D.; Shende, C.; Inscore, F. E.; Farquharson, S. Analysis of the chemotherapy drug 5-fluorouracil and its metabolites by surface-enhanced Raman spectroscopy. Proc. SPIE 2005, 5588, 70–77. [Google Scholar]
- Farquharson, S.; Gift, A.; Shende, C.; Maksymiuk, P.; Inscore, F; Murren, J. Detection of 5-fluorouracil in saliva using surface-enhanced Raman spectroscopy. Vib. Spectrosc. 2005, 38, 79–84. [Google Scholar] [CrossRef]
- Lord, R. C.; Thomas, G. J., Jr. Raman spectral studies of nucleic acids and related molecules - I Ribonucleic acid derivatives. Spectrochim. Acta 1967, 23A, 2551–2591. [Google Scholar] [CrossRef]
- Tsuboi, M.; Takahashi, S.; Harada, I. Infrared and Raman spectra of nucleic acids-vibrations in the base residues. In Physico-chemial properties of nucleic acids; Academic Press: New York, USA, 1973; Vol. 2, pp. 91–145. [Google Scholar]
- Rastogi, V. K.; Mital, H. P.; Sharma., S. N. Laser Raman and IR Spectra of 5-Fluorouracil. In XIth International Conference on Raman Spectroscopy; London, UK, 1988. [Google Scholar]
- Rastogi, V. K.; Jain, V.; Yadav, R. A.; Singh, C.; Palafox, M. A. Fourier transform Raman spectrum and ab initio and density functional computations of the vibrational spectrum, molecular geometry, atomic charges and some molecular properties of anticarcinogenic drug 5-fluorouracil. J. Raman Spectrosc. 2000, 31, 595–603. [Google Scholar] [CrossRef]
- Otto, C.; van den Tureel, T. J. J.; de Mul, F. F. M.; Greve, J. Surface-enhanced Raman spectroscopy of DNA bases. J. Raman Spectrosc. 1986, 17, 289–298. [Google Scholar] [CrossRef]
- Torres, E.; Winefordner, J. D. Trace determination of nitrogen-containing drugs by surface enhanced raman scattering spectrometry on silver colloids. Anal. Chem. 1987, 59, 1626–1632. [Google Scholar] [CrossRef]
- Suh, J. S.; Moskovits, M. Surface-Enhanced Raman Spectroscopy of amino acids and nucleotide bases adsorbed on silver. J. Am. Chem. Soc. 1986, 108, 4711–4718. [Google Scholar] [CrossRef]
- Farquharson, S.; Lay, P. A.; Weaver, M. J. Surface-Enhanced Raman Spectroscopy of Pentaammineosmium (III)/(II) and Petaammineruthenium (II) Containing Pyridine, Pyrazine or 4,4’-Bipyridine Ligands at Silver Electrodes: Vibrational Assignments. Spectrochim. Acta 1984, 40A, 907–921. [Google Scholar] [CrossRef]
- Farquharson, S.; Gift, A.; Shende, C.; Inscore, F.; Murren, J. Detection of chemotherapy drugs in saliva by surface-enhanced Raman spectroscopy. In Applications of Surface-Enhanced Raman Spectroscopy; Farquharson, S., Ed.; CRC Press: Boca Raton, FL, accepted.
- Carmona, P.; Rodriguez-Casado, A.; Molina, M.; Escobar, R. Raman Spectroscopic study of uridine nucleotides: Structural information and conformational features. J. Raman Spectrosc. 1999, 30, 631–636. [Google Scholar] [CrossRef]
- Jang, Y. H.; Sowers, L. C.; Gagin, T.; Goddard, W. A., III. First Principles calculation of pKa Values for 5-Substituted Uracils. J. Phys. Chem. A 2001, 105, 274–280. [Google Scholar] [CrossRef]
- Farquharson, S.; Gift, A.; Maksymiuk, P.; Inscore, F.; Smith, W. pH dependence of methyl phosphonic acid, dipicolinic acid, and cyanide by surface-enhanced Raman spectroscopy. Proc. SPIE 2004, 5269, 117–125. [Google Scholar]
- Tadayyoni, M. A.; Farquharson, S.; Li, T. T. -T.; Weaver, M. J. Surface-Enhanced Raman Spectroscopy of Electrochemically Characterized Interfaces. Transition-Metal Isothiocyanate Adsorbates at Silver Electrodes. J. Phys. Chem. 1984, 88, 4701–4706. [Google Scholar] [CrossRef]
- Ermans, A. M.; Delange, F.; Van Der Velden, M.; Kinthaert, J. Possible role of cyanide and thiocyanate in the etiology of endemic cretinism. In Human Development and the Thyroid Gland. Relation to Endemic Cretinism; Stanbury, J. B., Kroc, R. L., Eds.; Plenum Press: New York, USA, 1972; p. 455. [Google Scholar]
- Farquharson, S.; Maksymiuk, P. Simultaneous chemical separation and surface-enhancement Raman spectral detection using silver-doped sol-gels. Appl. Spectrosc. 2003, 57, 479–482. [Google Scholar] [CrossRef]
- Farquharson, S.; Lee, Y. H.; Nelson, C. Material for SERS and SERS sensors and method for preparing the same. U.S. Pat. 6,623,977, 2003. [Google Scholar]
- Farquharson, S.; Maksymiuk, P. Simultaneous chemical separation and surface-enhanced Raman spectral detection using metal-doped sol-gels. U.S. Pat. 6,943,031, 2005. [Google Scholar]
- Farquharson, S.; Maksymiuk, P. Chemical separation and plural point, surface-enhanced Raman spectral detection using metal-doped sol-gels. U.S. Pat. 6,943,032, 2005. [Google Scholar]
- Jeanmaire, D. L.; van Duyne, R. P. Surface Raman Spectroelectrochemistry. J. Electroanal. Chem. 1977, 84, 1–20. [Google Scholar] [CrossRef]
- Dou, X.; Jung, Y. M. Surface-Enhanced Raman Scattering of Biological Molecules on Metal Colloid II. Appl. Spectrosc. 1999, 53, 1440. [Google Scholar] [CrossRef]
- Farquharson, S.; Smith, W. W.; Lee, Y. H.; Elliott, S.; Sperry, J. F. Detection of biological signatures: A comparison of electrolytic and metal-doped sol-gel surface-enhanced Raman media. Proc. SPIE 2002, 4575, 62–72. [Google Scholar] [CrossRef]
- Sample Availability: All samples used in this study are commercially available (e.g. Sigma-Aldrich). Contact the corresponding author regarding SERS sample systems.
© 2008 by the authors. Licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Farquharson, S.; Gift, A.; Shende, C.; Inscore, F.; Ordway, B.; Farquharson, C.; Murren, J. Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva. Molecules 2008, 13, 2608-2627. https://doi.org/10.3390/molecules13102608
Farquharson S, Gift A, Shende C, Inscore F, Ordway B, Farquharson C, Murren J. Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva. Molecules. 2008; 13(10):2608-2627. https://doi.org/10.3390/molecules13102608
Chicago/Turabian StyleFarquharson, Stuart, Alan Gift, Chetan Shende, Frank Inscore, Beth Ordway, Carl Farquharson, and John Murren. 2008. "Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva" Molecules 13, no. 10: 2608-2627. https://doi.org/10.3390/molecules13102608
APA StyleFarquharson, S., Gift, A., Shende, C., Inscore, F., Ordway, B., Farquharson, C., & Murren, J. (2008). Surface-enhanced Raman Spectral Measurements of 5-Fluorouracil in Saliva. Molecules, 13(10), 2608-2627. https://doi.org/10.3390/molecules13102608