Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters
Abstract
:1. Introduction
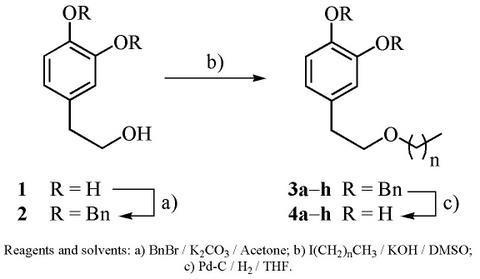
2. Results and Discussion
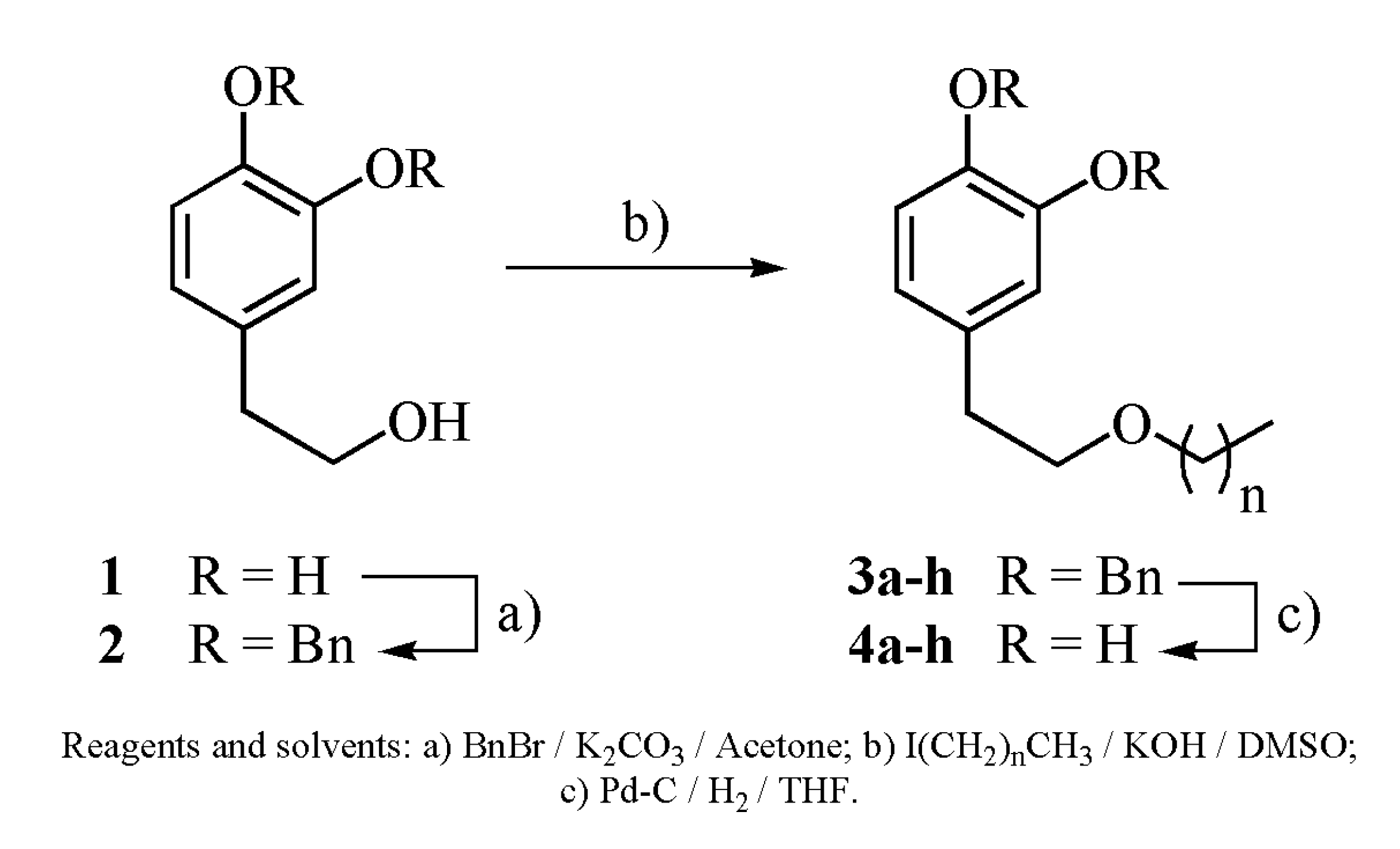
| entry | n | Alkylation Product (R = Bn) | Yield (%) | Deprotection Product (R = H) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 0 | 3a | 91 | 4a | 96 |
| 2 | 1 | 3b | 86 | 4b | 88 |
| 3 | 2 | 3c | 78 | 4c | 91 |
| 4 | 3 | 3d | 84 | 4d | 98 |
| 5 | 5 | 3e | 82 | 4e | 91 |
| 6 | 7 | 3f | 80 | 4f | 83 |
| 7 | 11 | 3g | 67 | 4g | 82 |
| 8 | 17 | 3h | 60 | 4h | 98 |

3. Experimental
3.1. General
3.2. Isolation and purification of hydroxytyrosol from olive oil waste waters (OOWW)
3.3. Synthetic procedures
3.3.1. General procedure for alkylation of hydroxytyrosol
3.3.2. General procedure for cleavage of Bn protective groups
3.4. Evaluation of oxidative stability of lipid matrices
4. Conclusions
Acknowledgements
- Samples Availability: Samples of compounds 3a–h and 4a–h are available from authors.
References and Notes
- Alburquerque, J.A.; Gonzalvez, J.; Garcıa, D.; Cegarra, J. Agrochemical characterisation of "alperujo", a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresource Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Arvanitoyannis, I.S.; Kassaveti, A.; Stefanatos, S. Olive oil waste treatment: a comparative and critical presentation of methods, advantages and disadvantages. Crit. Rev. Food Sci. Nutr. 2007, 47, 187–229. [Google Scholar] [CrossRef]
- Mateos, R.; Espartero, J.L.; Trujillo, M.; Ríos, J.J.; Leon, M.; Alcudia, F.; Cert, A. Determination of phenols, flavones, and lignans in virgin olive oils by solid-phase extraction and high-performance liquid chromatography with diode array ultraviolet detection. J. Agric. Food Chem. 2001, 49, 2185–2192. [Google Scholar] [CrossRef]
- Mateos, R.; Domínguez, M.M.; Espartero, J.L.; Cert, A. Antioxidant effect of phenolic compounds, α-tocopherol, and other minor components in virgin olive oil. J. Agric. Food Chem. 2001, 51, 7170–7175. [Google Scholar]
- Chimi, H.; Sadik, A.; Le Tutour, B.; Rahmani, M. Comparative study of antioxidant abilities of tyrosol, hydroxytyrosol, caffeic acid, oleuropein and BHT in olive oil. Laboratory note. Rev. Franc. Corps Gras 1988, 35, 339–344. [Google Scholar]
- Servili, M.; Montedoro, G.F. Recovery of polyphenols from olive vegetation waters and evaluation of their antioxidative capacities. Ind. Alimentari 1989, 28, 14–18. [Google Scholar]
- Capasso, R.; Evidente, A.; Visca, C. Production of hydroxytyrosol from olive oil vegetation waters. Agrochim. 1994, 38, 165–171. [Google Scholar]
- Fernandez-Bolanos, J.; Rodriguez, G.; Rodriguez, R.; Heredia, A.; Guillen, R.; Jimenez, A. Production in large quantities of highly purified hydroxytyrosol from liquid-solid waste of two-phase olive oil processing or "alperujo". J. Agric. Food Chem. 2002, 50, 6804–6811. [Google Scholar] [CrossRef]
- Allouche, N.; Fki, I.; Sayadi, S. Toward a high yield recovery of antioxidants and purified hydroxytyrosol from olive mill wastewater. J. Agric. Food Chem. 2004, 52, 267–273. [Google Scholar] [CrossRef]
- Fernandez-Bolanos, J.; Heredia, A.; Rodriguez, G.; Rodriguez, R.; Jimenez, A.; Guillen, R. Method for obtaining purified hydroxytyrosol from products and by-products derived from the olive tree. US 6849770 B2 2005. [Google Scholar]
- Agalias, A.; Magiatis, P.; Skaltsounis, A.L.; Mikros, E.; Tsarbopoulos, A.; Gikas, E.; Spanos, I.; Manios, T. A new process for the management of olive oil mill waste water and recovery of natural antioxidants. J. Agric. Food Chem. 2007, 55, 2671–2676. [Google Scholar] [CrossRef]
- Japon-Lujan, R.; Luque, M.D. Static-dynamic superheated liquid extraction of hydroxytyrosol and other biophenols from alperujo (a semisolid residue of the olive oil industry). J. Agric. Food Chem. 2007, 55, 3629–3634. [Google Scholar] [CrossRef]
- Garcia-Granados, A.; Parra, A. Method for the industrial recovery of tyrosol and hydroxytyrosol contained in the solid by-products of industrial olive crushing. PCT Int. Appl. WO 2007093659 2007. [Google Scholar]
- Visioli, F.; Galli, C.; Galli, G.; Caruso, D. Biological activities and metabolic fate of olive oil phenols. Eur. J. Lipid Sci. Technol. 2002, 104, 677–684. [Google Scholar] [CrossRef]
- Tuck, K.L.; Hayball, P.J. Major phenolic compounds in olive oil: metabolism and health effects. J. Nutr. Biochem. 2002, 13, 636–644. [Google Scholar] [CrossRef]
- Bianco, A.; Ramunno, A. The Chemistry of Olea europaea. In Studies in Natural Products Chemistry; Atta-ur-Rahman, Ed.; Elsevier: The Netherlands, 2006; Volume 33, pp. 859–903. [Google Scholar]
- Covas, M.I.; Ruiz-Gutiérrez, V.; De La Torre, R.; Kafatos, A.; Lamuela-Raventós, R.M.; Osada, J.; Owen, R.W.; Visioli, F. Minor components of olive oil: evidence to date of health benefits in humans. Nutr. Rev. 2006, 64, S20–S30. [Google Scholar] [CrossRef]
- Bendini, A.; Cerretani, L.; Carrasco-Pancorbo, A.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Fernandez-Gutierrez, A.; Lercker, G. Phenolic molecules in virgin olive oils: a survey of their sensory properties, health effects, antioxidant activity and analytical methods. An overview of the last decade. Molecules 2007, 12, 1679–1719. [Google Scholar] [CrossRef]
- Guiso, M.; Marra, C.; Cavarischia, C. Isochromans from 2-(3',4'-dihydroxy)phenylethanol. Tetrahedron Lett. 2001, 42, 6531–6534. [Google Scholar]
- Bianco, A.; Coccioli, F.; Guiso, M.; Marra, C. The occurrence in olive oil of a new class of phenolic compounds: hydroxy-isochromans. Food Chem. 2002, 77, 405–411. [Google Scholar] [CrossRef]
- Lorenz, P.; Zeh, M.; Martens-Lobenhoffer, J.; Schmidt, H.; Wolf, G.; Horn, T.F.W. Natural and newly synthesized hydroxy-1-aryl-isochromans: a class of potential antioxidants and radical scavengers. Free Rad. Res. 2005, 39, 535–545. [Google Scholar] [CrossRef]
- Gordon, M. H.; Paiva-Martins, F.; Almeida, M. Antioxidant activity of hydroxytyrosol acetate compared with that of other olive oil polyphenols. J. Agric. Food Chem. 2001, 49, 2480–2485. [Google Scholar] [CrossRef]
- 23. Alcudia, F.; Cert, A.; Espartero, J.L.; Mateos, R.; Trujillo, M. Process for the preparation of hydroxytyrosol esters for use as additives in food, cosmetics and pharmaceutical compositions. PCT Int. Appl. WO 2004005237 2004. [Google Scholar]
- Torregiani, E.; Seu, G.; Minassi, A.; Appendino, G. Cerium(III) chloride-promoted chemoselective esterification of phenolic alcohols. Tetrahedron Lett. 2005, 46, 2193–2196. [Google Scholar] [CrossRef]
- Torres, A.; Penalver, P.; Rondon, D.; Morales, J.C. Efficient lipase-catalyzed synthesis of new lipid antioxidants based on a catechol structure. Tetrahedron 2005, 61, 7654–7660. [Google Scholar] [CrossRef]
- Grasso, S.; Siracusa, L.; Spatafora, C.; Renis, M.; Tringali, C. Hydroxytyrosol lipophilic analogues: Enzymatic synthesis, radical scavenging activity and DNA oxidative damage protection. Bioorg. Chem. 2007, 35, 137–152. [Google Scholar] [CrossRef]
- Bernini, R.; Mincione, E.; Barontini, M.; Crisante, F. Convenient synthesis of hydroxytyrosol and its lipophilic derivatives from tyrosol or homovanillyl alcohol. J. Agric. Food Chem. 2008, 56, 8897–8904. [Google Scholar] [CrossRef]
- Trujillo, M.; Mateos, R.; Collantes, L.; Espartero, J.L.; Cert, R.; Jover, M.; Alcudia, F.; Bautista, J.; Cert, A.; Parrado, J. Lipophilic hydroxytyrosyl esters. Antioxidant activity in lipid matrices and biological systems. J. Agric. Food Chem. 2006, 54, 3779–3785. [Google Scholar]
- Hamada, A.; Yaden, E.L.; Horng, J.S.; Ruffolo, R.R.; Patil, P.N.; Miller, D.D. N-Substituted imidazolines and ethylenediamines and their action on α- and β-adrenergic receptors. J. Med. Chem. 1985, 28, 1269–1273. [Google Scholar] [CrossRef]
- Pereira-Caro, G.; Madrona, A.; Bravo, L.; Espartero, J.L.; Alcudia, F.; Cert, A. Mateos, R. Antioxidant activity evaluation of alkyl hydroxytyrosyl ethers, a new class of hydroxytyrosol derivatives. Food Chem. 2009, 115, 86–91. [Google Scholar] [CrossRef]
© 2009 by the authors; licensee Molecular Diversity Preservation International, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Madrona, A.; Pereira-Caro, G.; Mateos, R.; Rodríguez, G.; Trujillo, M.; Fernández-Bolaños, J.; Espartero, J.L. Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters. Molecules 2009, 14, 1762-1772. https://doi.org/10.3390/molecules14051762
Madrona A, Pereira-Caro G, Mateos R, Rodríguez G, Trujillo M, Fernández-Bolaños J, Espartero JL. Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters. Molecules. 2009; 14(5):1762-1772. https://doi.org/10.3390/molecules14051762
Chicago/Turabian StyleMadrona, Andrés, Gema Pereira-Caro, Raquel Mateos, Guillermo Rodríguez, Mariana Trujillo, Juan Fernández-Bolaños, and José L. Espartero. 2009. "Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters" Molecules 14, no. 5: 1762-1772. https://doi.org/10.3390/molecules14051762
APA StyleMadrona, A., Pereira-Caro, G., Mateos, R., Rodríguez, G., Trujillo, M., Fernández-Bolaños, J., & Espartero, J. L. (2009). Synthesis of Hydroxytyrosyl Alkyl Ethers from Olive Oil Waste Waters. Molecules, 14(5), 1762-1772. https://doi.org/10.3390/molecules14051762