Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages
Abstract
:1. Introduction
2. Results and Discussion
2.1. Protocols
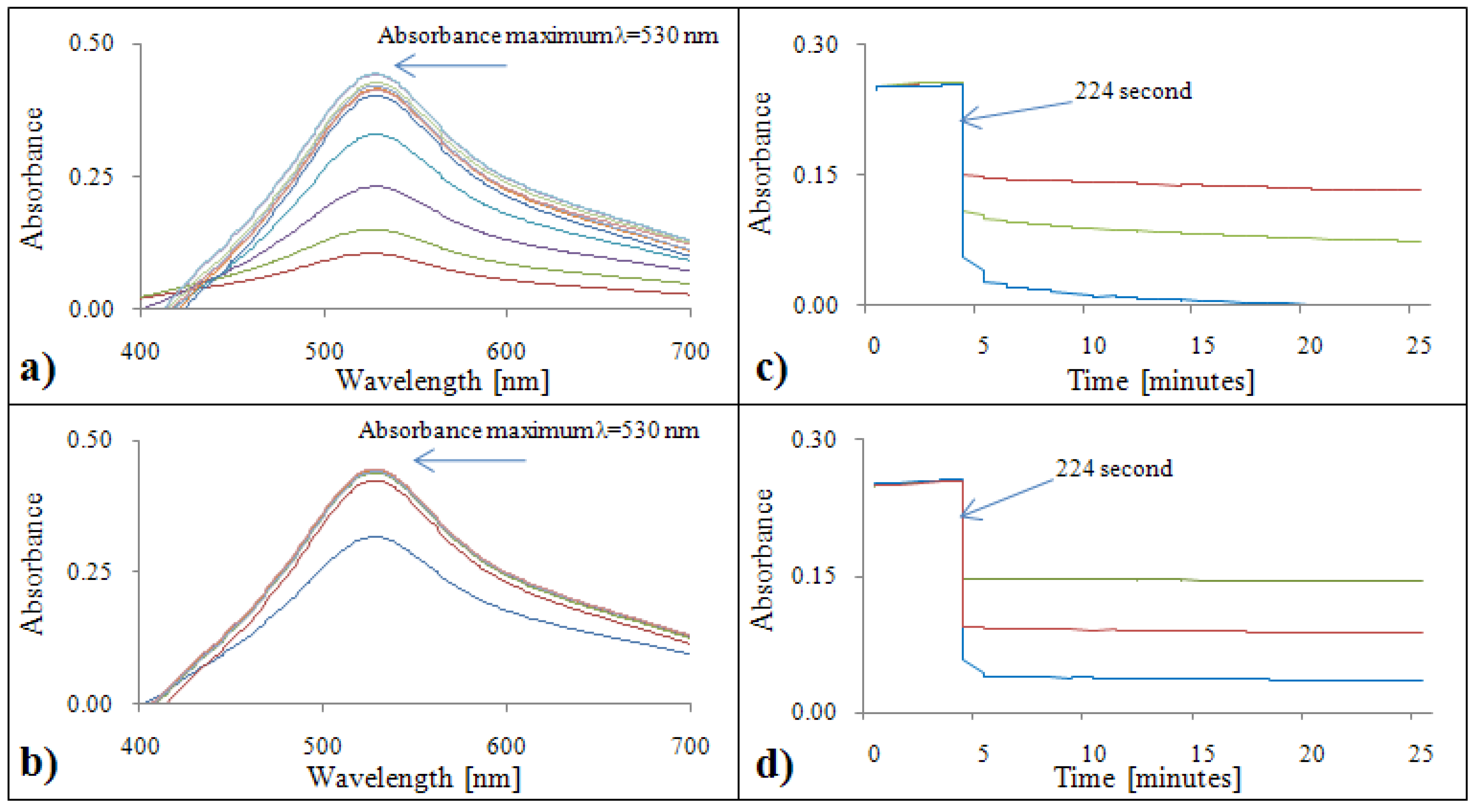
2.1.1. Determination of antioxidant activity by the DPPH• test
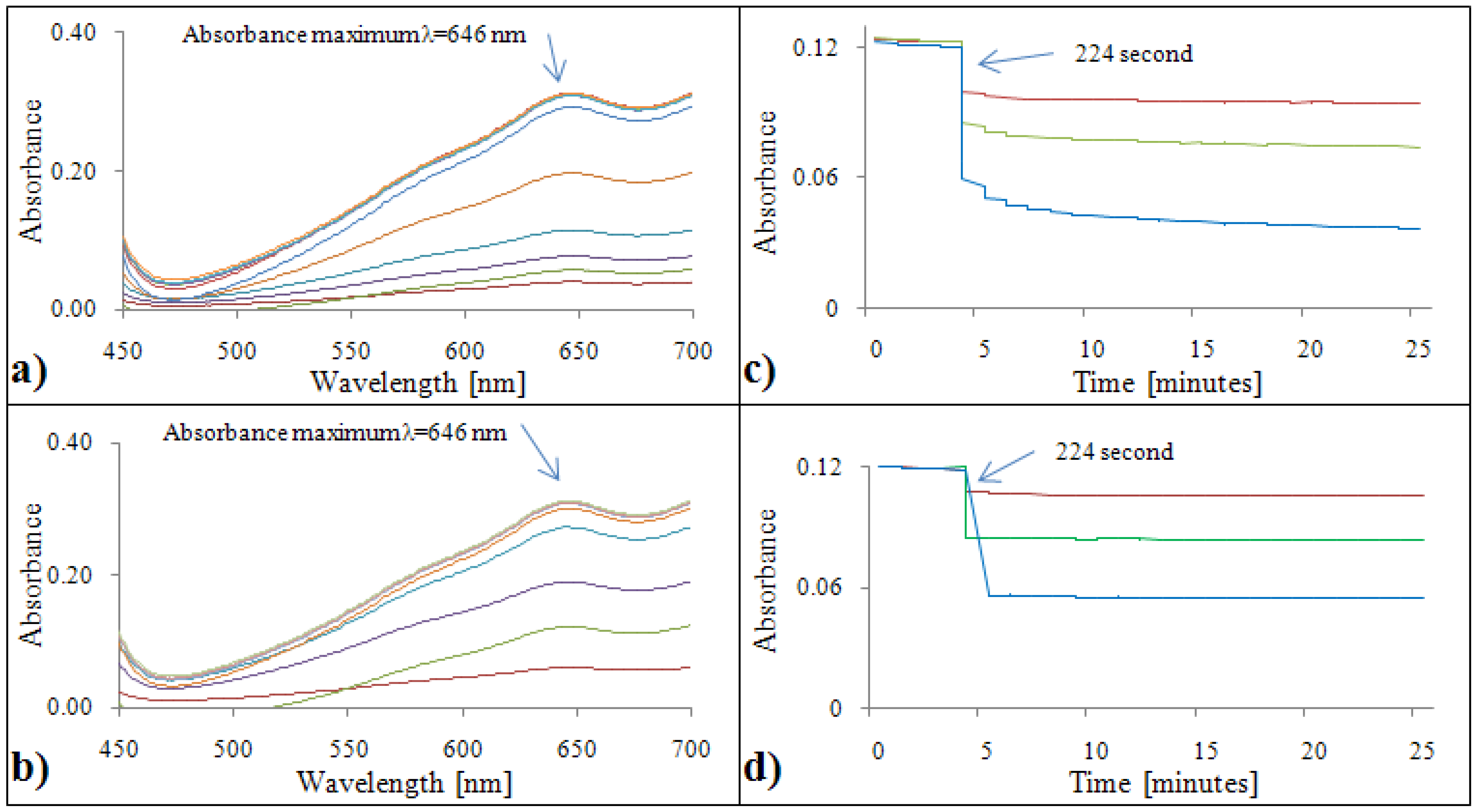
2.1.2. Determination of antioxidant activity by the ABTS test
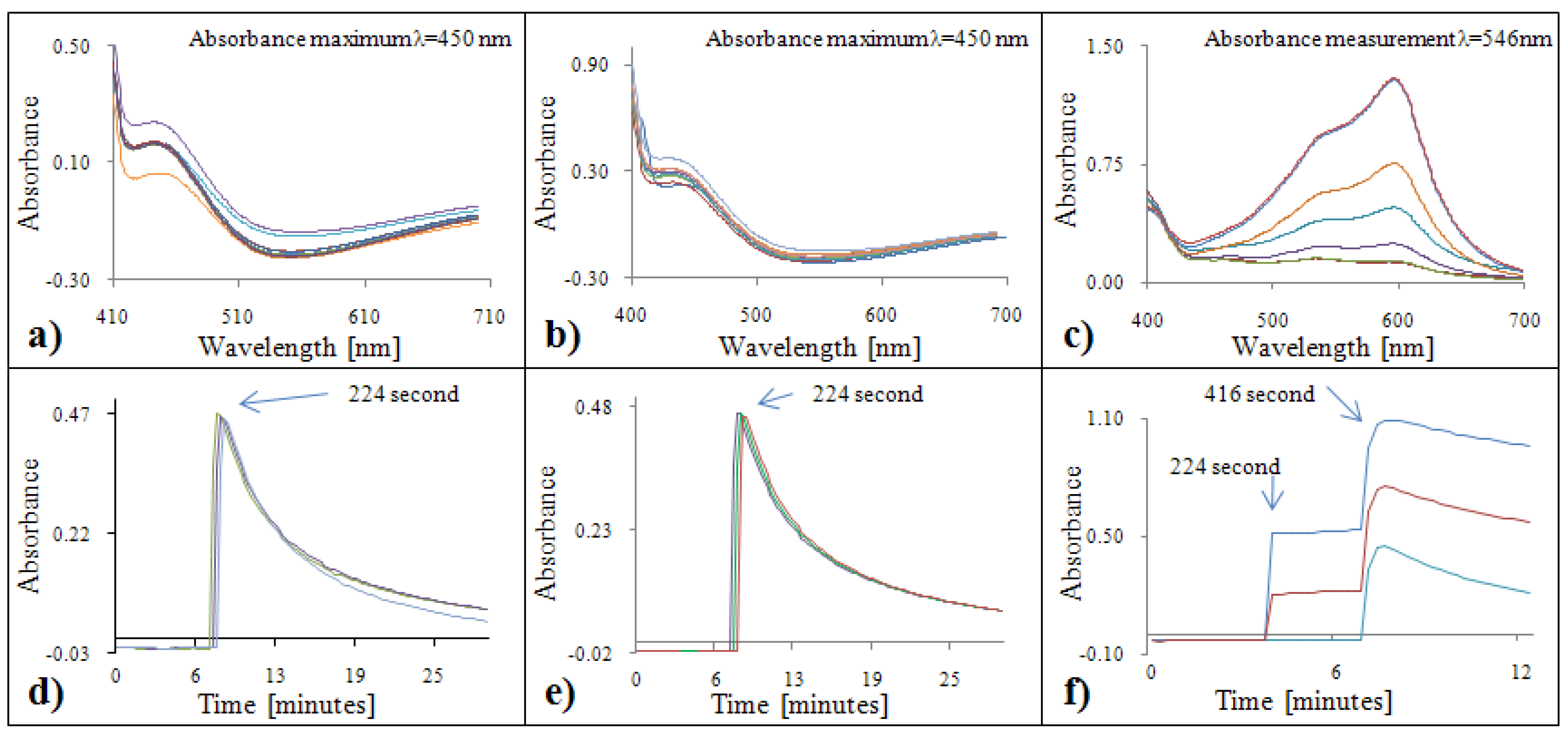
2.1.3. Determination of antioxidant activity by the FRAP method
2.1.4. Determination of antioxidant activity by the DMPD method
2.1.5. Determination of antioxidant activity by the Free Radicals method
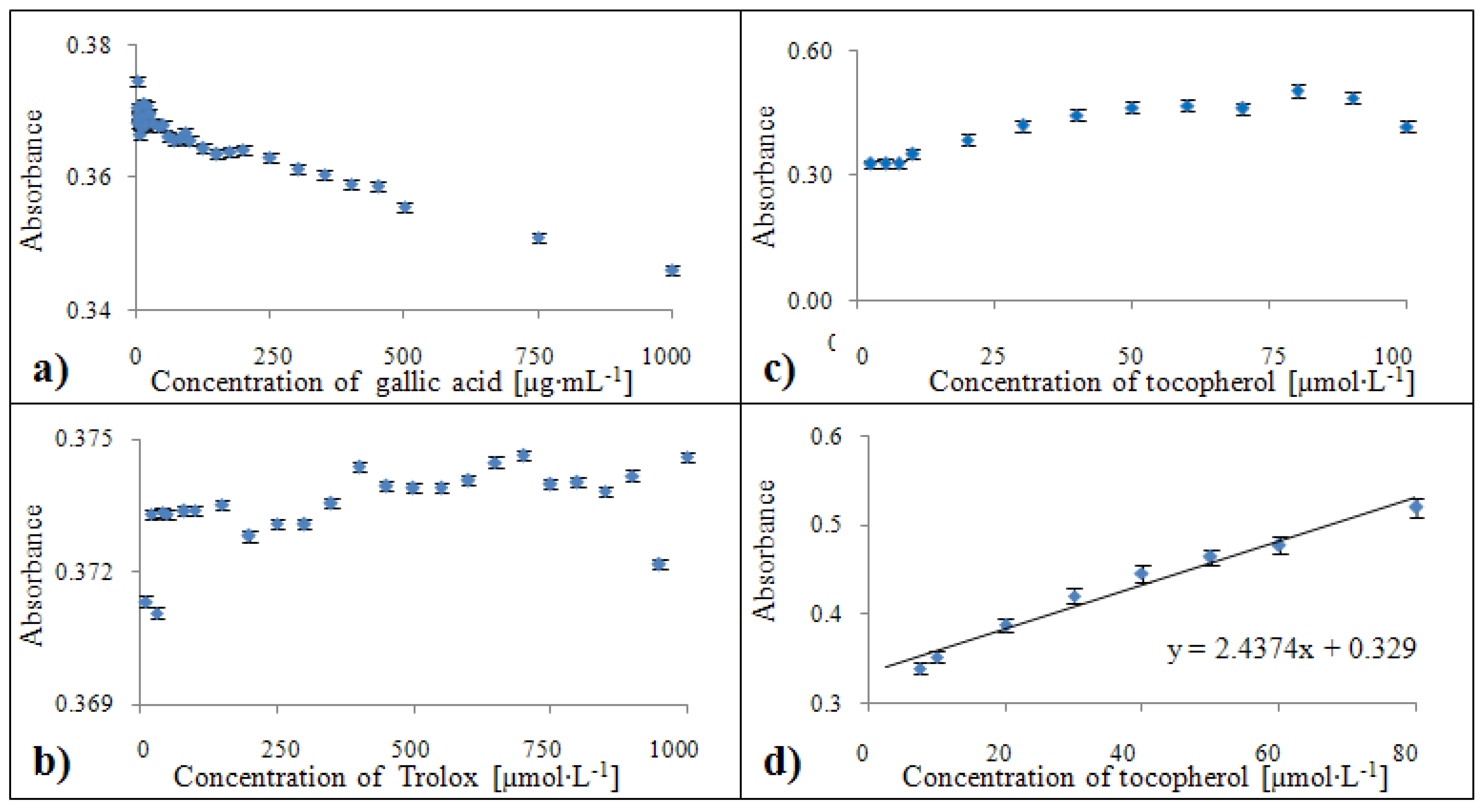
2.1.6. Determination of antioxidant activity by the Blue CrO5method
2.2. Analytical evaluation
2.2.1. Analytical evaluation of the DPPH• test
2.2.2. Analytical evaluation of the ABTS test
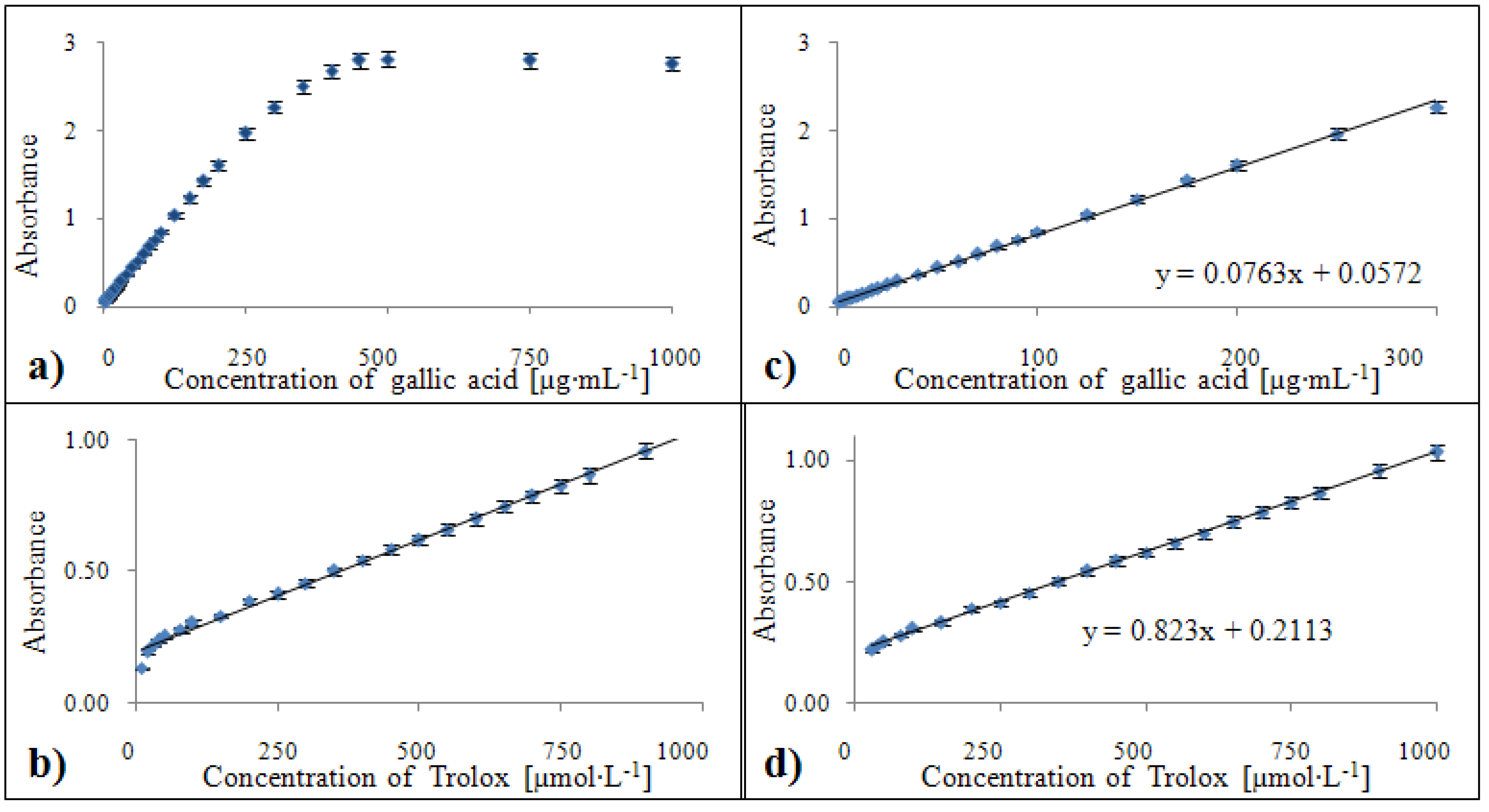
2.2.3. Analytical evaluation of the FRAP method
2.2.4. Analytical evaluation of the DMPD method
2.2.5. Analytical evaluation of the Free Radicals method
2.2.6. Analytical evaluation of the Blue CrO5 method
2.3. Calibration
2.3.1. Calibration of the DPPH• test
2.3.2. Calibration of the ABTS method
2.3.3. Calibration of the FRAP method
2.3.4. Calibration of the DMPD method
2.3.5. Calibration of the Free Radical method
2.3.6. Calibration of the Blue CrO5 method
3. Experimental
3.1. Apparatus
3.2. Chemicals
3.3. Standards
3.4. UV-Vis spectrometric protocols
3.4.1. DPPH
3.4.2. ABTS
3.4.3. FRAP
3.4.4. DMPB
3.4.5. Free radicals
3.4.6. Blue CrO5
3.5. Descriptive statistics
4. Conclusions
Acknowledgements
Abbreviations
References and Notes
- Beklova, M.; Zitka, O.; Gazdik, Z.; Adam, V.; Hodek, P.; Stiborova, M.; Horna, A.; Kizek, R. Electroanalytical techniques for determination of flavonoids. Toxicol. Lett. 2008, 180, S230. [Google Scholar] [CrossRef]
- Ling, L.T.; Radhakrishnan, A.K.; Subramaniam, T.; Cheng, H.M.; Palanisamy, U.D. Assessment of Antioxidant Capacity and Cytotoxicity of Selected Malaysian Plants. Molecules 2010, 15, 2139–2151. [Google Scholar] [CrossRef] [PubMed]
- RiceEvans, C.A.; Miller, N.J.; Paganga, G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic. Biol. Med. 1996, 20, 933–956. [Google Scholar] [CrossRef]
- Blazekovic, B.; Vladimir-Knezevic, S.; Brantner, A.; Bival Stefan, M. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. ‘Budrovka’: A Comparative Study with L. angustifolia Mill. Molecules 2010, 15, 5971–5987. [Google Scholar] [CrossRef] [PubMed]
- Gan, R.Y.; Kuang, L.; Xu, X.R.; Zhang, Y.A.; Xia, E.Q.; Song, F.L.; Li, H.B. Screening of Natural Antioxidants from Traditional Chinese Medicinal Plants Associated with Treatment of Rheumatic Disease. Molecules 2010, 15, 5988–5997. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Krska, B.; Adam, V.; Saloun, J.; Pokorna, T.; Reznicek, V.; Horna, A.; Kizek, R. Electrochemical Determination of the Antioxidant Potential of Some Less Common Fruit Species. Sensors 2008, 8, 7564–7570. [Google Scholar] [CrossRef] [PubMed]
- Gursoy, N.; Tepe, B.; Sokmen, M. Evaluacion of the chemical composition and antioxidant activity of the peel oil of citrus nobilis. Int. J. Food Prop. 2010, 13, 983–991. [Google Scholar] [CrossRef]
- Diopan, V.; Babula, P.; Shestivska, V.; Adam, V.; Zemlicka, M.; Dvorska, M.; Hubalek, J.; Trnkova, L.; Havel, L.; Kizek, R. Electrochemical and spectrometric study of antioxidant activity of pomiferin, isopomiferin, osajin and catalposide. J. Pharm. Biomed. Anal. 2008, 48, 127–133. [Google Scholar] [CrossRef] [PubMed]
- Romero, M.; Rojano, B.; Mella-Raipan, J.; Pessoa-Mahana, C.D.; Lissi, E.; Lopez-Alarcon, C. Antioxidant Capacity of Pure Compounds and Complex Mixtures Evaluated by the ORAC-Pyrogallol Red Assay in the Presence of Triton X-100 Micelles. Molecules 2010, 15, 6152–6167. [Google Scholar] [CrossRef] [PubMed]
- Schlesier, K.; Harwat, M.; Bohm, V.; Bitsch, R. Assessment of antioxidant activity by using different in vitro methods. Free Radic. Res. 2002, 36, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Sulc, M.; Lachman, J.; Hamouz, K.; Dvorak, P. Impact of phenolic content on antioxidant activity in yellow and purple-fleshed potatoes grown in the Czech Republic. Biol. Agric. Hortic. 2008, 26, 45–54. [Google Scholar] [CrossRef]
- Wondrak, G.; Villeneuve, N.F.; Lamore, S.D.; Bause, A.S.; Jiang, T.; Zhang, D.D. The Cinnamon-Derived Dietary Factor Cinnamic Aldehyde Activates the Nrf2-Dependent Antioxidant Response in Human Epithelial Colon Cells. Molecules 2010, 15, 3338–3355. [Google Scholar] [CrossRef] [PubMed]
- Zima, A.; Hosek, J.; Treml, J.; Muselik, J.; Suchy, P.; Prazanova, G.; Lopes, A.; Zemlicka, M. Antiradical and Cytoprotective Activities of Several C-Geranyl-substituted Flavanones from Paulownia tomentosa Fruit. Molecules 2010, 15, 6035–6049. [Google Scholar] [CrossRef] [PubMed]
- Antolovich, M.; Prenzler, P.D.; Patsalides, E.; McDonald, S.; Robards, K. Methods for testing antioxidant activity. Analyst 2002, 127, 183–198. [Google Scholar] [CrossRef] [PubMed]
- De Diego-Otero, Y.; Romero-Zerbo, Y.; el Bekay, R.; Decara, J.; Sanchez, L.; Rodriguez-de Fonseca, F.; del Arco-Herrera, I. alpha-Tocopherol Protects Against Oxidative Stress in the Fragile X Knockout Mouse: an Experimental Therapeutic Approach for the Fmr1 Deficiency. Neuropsychopharmacology 2009, 34, 1011–1026. [Google Scholar] [CrossRef] [PubMed]
- Ou, B.X.; Huang, D.J.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of antioxidant activities of common vegetables employing oxygen radical absorbance capacity (ORAC) and ferric reducing antioxidant power (FRAP) assays: A comparative study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Shimoda, K.; Hamada, H. Synthesis of β-Maltooligosaccharides of Glycitein and Daidzein and their Anti-Oxidant and Anti-Allergic Activities. Molecules 2010, 15, 5153–5161. [Google Scholar] [CrossRef] [PubMed]
- Karimi, E.; Oskoueian, E.; Hendra, R.; Jaafar, H.Z.E. Evaluation of Crocus sativus L. Stigma Phenolic and Flavonoid Compounds and Its Antioxidant Activity. Molecules 2010, 15, 2644–2656. [Google Scholar] [CrossRef] [PubMed]
- Kaurinovic, B.; Vlaisavljevic, S.; Popovic, M.; Vastag, D.; Djurendic-Brenesel, M. Antioxidant Properties of Marrubium peregrinum L. (Lamiaceae) Essential Oil. Molecules 2010, 15, 5943–5955. [Google Scholar] [CrossRef]
- Klejdus, B.; Mikelova, R.; Petrlova, J.; Potesil, D.; Adam, V.; Stiborova, M.; Hodek, P.; Vacek, J.; Kizek, R.; Kuban, V. Determination of isoflavones in soy bits by fast column high-performance liquid chromatography coupled with UV-visible diode-array detection. J. Chromatogr. A. 2005, 1084, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Huska, D.; Adam, V.; Horna, A.; Hubalek, J.; Beklova, M.; Kizek, R. Liquid chromatography with electrochemical detection as a tool for study of oxidative stress in organisms. Toxicol. Lett. 2009, 189, S126. [Google Scholar] [CrossRef]
- Parr, A.J.; Bolwell, G.P. Phenols in the plant and in man. The potential for possible nutritional enhancement of the diet by modifying the phenols content or profile. J. Sci. Food Agric. 2000, 80, 985–1012. [Google Scholar] [CrossRef]
- Record, I.R.; Dreosti, I.E.; McInerney, J.K. Changes in plasma antioxidant status following consumption of diets high or low in fruit and vegetables or following dietary supplementation with an antioxidant mixture. Br. J. Nutr. 2001, 85, 459–464. [Google Scholar] [CrossRef] [PubMed]
- Kolarovic, J.; Popovic, M.; Zlinska, J.; Trivic, S.; Vojnovic, M. Antioxidant Activities of Celery and Parsley Juices in Rats Treated with Doxorubicin. Molecules 2010, 15, 6193–6204. [Google Scholar] [CrossRef] [PubMed]
- Rop, O.; Reznicek, V.; Valsikova, M.; Jurikova, T.; Mlcek, J.; Kramarova, D. Antioxidant Properties of European Cranberrybush Fruit (Viburnum opulus var. edule). Molecules 2010, 15, 4467–4477. [Google Scholar] [CrossRef] [PubMed]
- Sochor, J.; Salas, P.; Zehnalek, J.; Krska, B.; Adam, V.; Havel, L.; Kizek, R. An assay for spectrometric determination of antioxidant activity of a biological extract. Listy Cukrov. Reparske. 2010, 126, 408–409. [Google Scholar]
- Sochor, J.; Zitka, O.; Skutkova, H.; Pavlik, D.; Babula, P.; Krska, B.; Horna, A.; Adam, V.; Provaznik, I.; Kizek, R. Content of phenolic compounds and antioxidant capacity in fruits of selected genotypes of apricot with resistance against Plum pox virus. Molecules 2010, 15, 6285–6305. [Google Scholar] [CrossRef] [PubMed]
- Tavares, L.; Carrilho, D.; Tyagi, M.; Barata, D.; Serra, A.T.; Duarte, C.M.M.; Duarte, R.O.; Feliciano, R.P.; Bronze, M.R.; Chicau, P.; Esprito-Santo, M.D.; Ferreira, R.B.; dos Santos, C.N. Antioxidant Capacity of Macaronesian Traditional Medicinal Plants. Molecules 2010, 15, 2576–2592. [Google Scholar] [CrossRef] [PubMed]
- Corona-Bustamante, A.; Viveros-Paredes, J.M.; Flores-Parra, A.; Peraza-Campos, A.L.; Martinez-Martinez, F.J.; Sumaya-Martinez, M.T.; Ramos-Organillo, A. Antioxidant Activity of Butyl- and Phenylstannoxanes Derivedfrom 2-, 3- and 4-Pyridinecarboxylic Acids. Molecules 2010, 15, 5445–5459. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Reznicek, V.; Adam, V.; Zitka, O.; Jurikova, T.; Krska, B.; Matuskovic, J.; Plsek, J.; Saloun, J.; Horna, A.; Kizek, R. Use of Liquid Chromatography with Electrochemical Detection for the Determination of Antioxidants in Less Common Fruits. Molecules 2008, 13, 2823–2836. [Google Scholar] [CrossRef] [PubMed]
- Gazdik, Z.; Zitka, O.; Petrlova, J.; Adam, V.; Zehnalek, J.; Horna, A.; Reznicek, V.; Beklova, M.; Kizek, R. Determination of Vitamin C (Ascorbic Acid) Using High Performance Liquid Chromatography Coupled with Electrochemical Detection. Sensors 2008, 8, 7097–7112. [Google Scholar] [CrossRef] [PubMed]
- Hodek, P.; Hanustiak, P.; Krizkova, J.; Mikelova, R.; Krizkova, S.; Stiborova, M.; Trnkova, L.; Horna, A.; Beklova, M.; Kizek, R. Toxicological aspects of flavonoid interaction with biomacromolecules. Neuroendocrinol. Lett. 2006, 27, 14–17. [Google Scholar] [PubMed]
- Jerkovic, I.; Marijanovic, Z. Oak (Quercus frainetto Ten.) Honeydew Honey-Approach to Screening of Volatile Organic Composition and Antioxidant Capacity (DPPH and FRAP Assay). Molecules 2010, 15, 3744–3756. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Lu, F.; Suo, X.; Nan, H.; Li, B. Antioxidant Properties of Cap and Stipe from Coprinus comatus. Molecules 2010, 15, 1473–1486. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Tomas-Barberan, F.A.; Hess-Pierce, B.; Holcroft, D.M.; Kader, A.A. Antioxidant activity of pomegranate juice and its relationship with phenolic composition and processing. J. Agric. Food Chem. 2000, 48, 4581–4589. [Google Scholar] [CrossRef] [PubMed]
- Howell, W.E.; Eastwell, K.C.; Li, T.S.C. Heat treatment, chemo-therapy and hydroponic culture for obtaining virus-free trees of sweet cherry. In Proceedings of the 18th International Symposium on Virus & Virus-Like Diseases of Temperate Fruit Crops - Top Fruit Diseases, Vols 1 and 2, International Society Horticultural Science, Leuven 1, 2001; Clark, M.F., Ed.; pp. 455–457.
- Huska, D.; Krystofova, O.; Adam, V.; Soukupova, I.; Beklova, M.; Trnkova, L.; Kizek, R. Comparison of electrochemical and spectrometric detection of-SH moieties. Amino Acids 2009, 37, 34–35. [Google Scholar]
- Parejo, L.; Codina, C.; Petrakis, C.; Kefalas, P. Evaluation of scavenging activity assessed by Co(II)/EDTA-induced luminol chemiluminescence and DPPH center dot (2,2-diphenyl-1-picrylhydrazyl) free radical assay. J. Pharmacol. Toxicol. Methods 2000, 44, 507–512. [Google Scholar] [CrossRef]
- Sulc, M.; Lachman, J.; Hamouz, K.; Orsak, M.; Dvorak, P.; Horackova, V. Selection and evaluation of methods for determination of antioxidant activity of purple- and red-fleshed potato varieties. Chem. Listy 2007, 101, 584–591. [Google Scholar]
- Adam, V.; Mikelova, R.; Hubalek, J.; Hanustiak, P.; Beklova, M.; Hodek, P.; Horna, A.; Trnkova, L.; Stiborova, M.; Zeman, L.; Kizek, R. Utilizing of square wave voltammetry to detect flavonoids in the presence of human urine. Sensors 2007, 7, 2402–2418. [Google Scholar] [CrossRef] [PubMed]
- Mikelova, R.; Hodek, P.; Hanustiak, P.; Adam, V.; Krizkova, S.; Havel, L.; Stiborova, M.; Horna, A.; Beklova, M.; Trnkova, L.; Kizek, R. Determination of isoflavones using liquid chromatography with electrochemical detection. Acta Chim. Slov. 2007, 54, 92–97. [Google Scholar]
- Klejdus, B.; Mikelova, R.; Adam, V.; Zehnalek, J.; Vacek, J.; Kizek, R.; Kuban, V. Liquid chromatographic-mass spectrometric determination of genistin and daidzin in soybean food samples after accelerated solvent extraction with modified content of extraction cell. Anal. Chim. Acta 2004, 517, 1–11. [Google Scholar] [CrossRef]
- Klejdus, B.; Vacek, J.; Adam, V.; Zehnalek, J.; Kizek, R.; Trnkova, L.; Kuban, V. Determination of isoflavones in soybean food and human urine using liquid chromatography with electrochemical detection. J. Chromatogr. B. 2004, 806, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Klejdus, B.; Mikelova, R.; Petrlova, J.; Potesil, D.; Adam, V.; Stiborova, M.; Hodek, P.; Vacek, J.; Kizek, R.; Kuban, V. Evaluation of isoflavones distribution in soy plants and soybeans by fast column high-performance liquid chromatography coupled with diode-array detector. J. Agr. Food Chem. 2005, 53, 5848–5852. [Google Scholar] [CrossRef] [PubMed]
- Gulcin, I.; Bursal, E.; Sehitoglu, M.H.; Bilsel, M.; Goren, A.C. Polyphenol contents and antioxidant activity of lyophilized aqueous extract of propolis from Erzurum, Turkey. Food Chem. Toxicol. 2010, 48, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Jagtap, U.B.; Panaskar, S.N.; Bapat, V.A. Evaluation of Antioxidant Capacity and Phenol Content in Jackfruit (Artocarpus heterophyllus Lam.) Fruit Pulp. Plant Food Hum. Nutr. 2010, 65, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Votruba, M.; Stopka, P.; Hroudova, J.; Vesely, K.; Nejedlova, L. A simple method for quantitative estimation of free radicals in serum. Klin. Biochem. Met. 1999, 7, 96–101. [Google Scholar]
- Charalampidis, P.S.; Veltsistas, P.; Karkabounas, S.; Evangelou, A. Blue CrO5 assay: A novel spectrophotometric method for the evaluation of the antioxidant and oxidant capacity of various biological substances. Eur. J. Med. Chem. 2009, 44, 4162–4168. [Google Scholar] [CrossRef] [PubMed]
- Grampp, G.; Landgraf, S.; Wesierskia, T.; Jankowska, B.; Kalisz, E.; Sabou, D.M.; Mladenova, B. Kinetics of the formation of the blue complex CrO(O-2)(2) formed by dichromate and H2O2 in acid solutions. A stopped-flow investigation using rapid-scan UV-VIS detection. Mon. Chem. 2002, 133, 1363–1372. [Google Scholar] [CrossRef]
Sample Availability: Samples of the compounds of interest are available from the authors. |







| Method | Wavelength [nm] | Interaction Time [sec.] | Measuring range [µg·mL-1] | Calibration equation | Confidence coefficient [R2] | Standard deviation [%] |
|---|---|---|---|---|---|---|
| DPPH | 510 | 1296 | 1 – 10 | y = -0.103ln(x) - 0.0931 | 0.996 | 1.80 |
| ABTS | 670 | 1296 | 1 – 20 | y = -0.0111x - 0.0196 | 0.996 | 2.10 |
| FRAP | 578/630 | 1296 | 1 – 300 | y = 0.076x + 0.057 | 0.999 | 1.50 |
| DMPD | 510 | 1296 | 1 – 25 | y = -0.060x + 0.180 | 0.999 | 2.30 |
| FR | 450 | 560 | 1 – 1000 | y = 0.035x + 0.267 | 0.996 | 1.50 |
| Method | Wavelength [nm] | Interaction Time [sec.] | Measuring range [µmol·L-1] | Calibration equation | Confidence coefficient [R2] | Standard deviation [%] |
|---|---|---|---|---|---|---|
| DPPH | 510 | 1296 | 10 – 150 | y = -0.0015x - 0.0516 | 0.996 | 2.40 |
| ABTS | 670 | 1296 | 10 – 550 | y = -0.0005x - 0.0129 | 0.999 | 1.50 |
| FRAP | 578/630 | 1296 | 30 – 1000 | y = 0.829x + 0.210 | 0.996 | 1.50 |
| DMPD | 510 | 1296 | 50 – 650 | y = -0.084x + 0.257 | 0.997 | 1.90 |
| FR | 450 | 560 | — | — | — | — |
| Method | Wavelength [nm] | Interaction Time [sec.] | Measuring range [µmol·L-1] | Calibration equation | Confidence coefficient [R2] | Standard deviation [%] |
|---|---|---|---|---|---|---|
| CrO5 | 546 | 224 | 5 - 80 | y = 2.530x + 0.3300 | 0.973 | 2.50 |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Sochor, J.; Ryvolova, M.; Krystofova, O.; Salas, P.; Hubalek, J.; Adam, V.; Trnkova, L.; Havel, L.; Beklova, M.; Zehnalek, J.; et al. Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages. Molecules 2010, 15, 8618-8640. https://doi.org/10.3390/molecules15128618
Sochor J, Ryvolova M, Krystofova O, Salas P, Hubalek J, Adam V, Trnkova L, Havel L, Beklova M, Zehnalek J, et al. Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages. Molecules. 2010; 15(12):8618-8640. https://doi.org/10.3390/molecules15128618
Chicago/Turabian StyleSochor, Jiri, Marketa Ryvolova, Olga Krystofova, Petr Salas, Jaromir Hubalek, Vojtech Adam, Libuse Trnkova, Ladislav Havel, Miroslava Beklova, Josef Zehnalek, and et al. 2010. "Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages" Molecules 15, no. 12: 8618-8640. https://doi.org/10.3390/molecules15128618
APA StyleSochor, J., Ryvolova, M., Krystofova, O., Salas, P., Hubalek, J., Adam, V., Trnkova, L., Havel, L., Beklova, M., Zehnalek, J., Provaznik, I., & Kizek, R. (2010). Fully Automated Spectrometric Protocols for Determination of Antioxidant Activity: Advantages and Disadvantages. Molecules, 15(12), 8618-8640. https://doi.org/10.3390/molecules15128618




