Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using "Click" Dendrimer-Stabilized Palladium Nanoparticles
Abstract
:1. Introduction
2. Palladium Nanoparticle (PdNP) Catalysts in Carbon-Carbon Coupling Reactions
3. Highly Efficient “Click”-Dendrimer-Encapsulated and Stabilized Pd Nanoparticle Pre-Catalysts
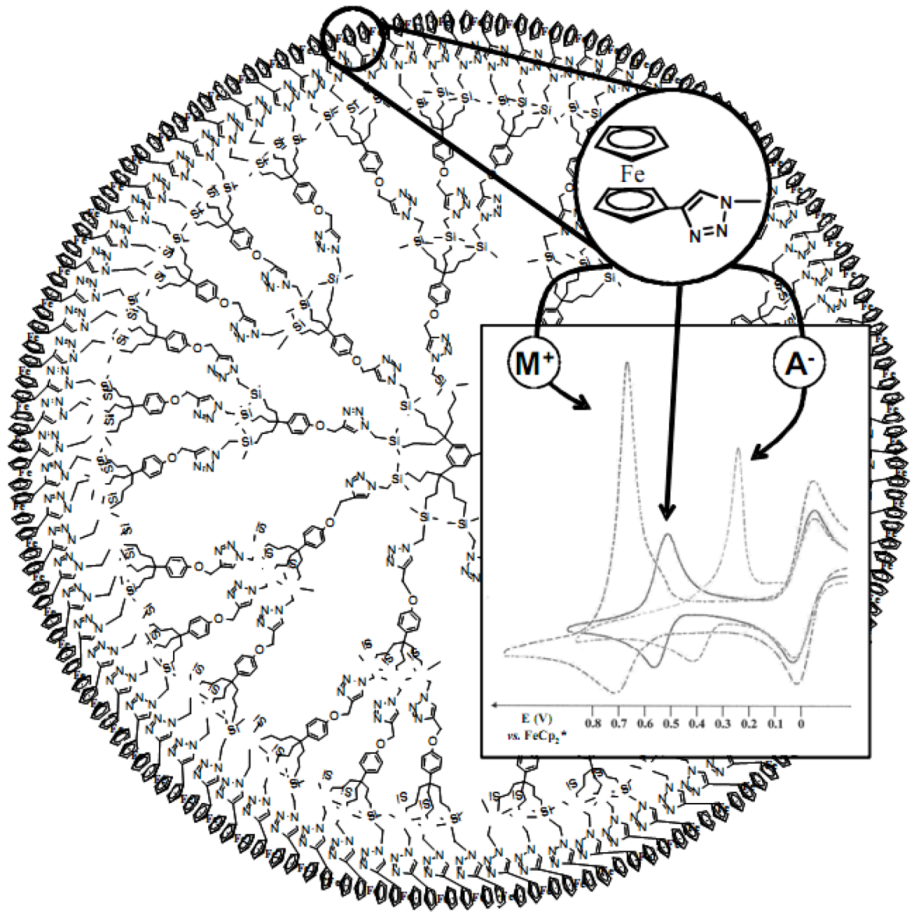


4. “Homeopathic” Catalysis of Miyaura-Suzuki C-C Coupling by “Click” Dendrimer-Stabilized PdNPs under Ambient Conditions


5. Conclusions and Prospects
Acknowledgements
References
- Kleij, R.A.; van Leeuwen, P.W.N.M.; van der Made, A.W. Catalyst compositions and polymerization process, hexakisphosphines and hexahalo compounds. EP0456317 1991. [Chem. Abstr. 1992, 116, 129870]. [Google Scholar]
- Oosterom, G.E.; Reek, J.N.H.; Kamer, P.C.J.; van Leeuwen, P.W.N.M. Transition Metal Catalysis Using Functionalized Dendrimers. Angew. Chem. Int. Ed. 2001, 40, 1828–1849. [Google Scholar] [CrossRef]
- Brunner, H.; Fürst, J.; Ziegler, J. Enantioselektive katalyse: LXXXI. Optisch aktive zweischalenphosphine. J. Organomet. Chem. 1993, 454, 87–94. [Google Scholar]
- Knapen, J.W.J.; van der Made, A.W.; de Wilde, J.C.; van Leeuwen, P.W.N.M.; Wijkens, P.; Grove, D.M.; van Koten, G. Homogeneous catalysts based on silane dendrimers functionalized with arylnickel(II) complexes. Nature 1994, 372, 659–663. [Google Scholar]
- Miedaner, A.; Curtis, C.J.; Barkley, R.M.; DuBois, D.L. Electrochemical Reduction of CO2 Catalyzed by Small Organophosphine Dendrimers Containing Palladium. Inorg. Chem. 1994, 33, 5482–5490. [Google Scholar] [CrossRef]
- Lee, J.-J.; Ford, W.T.; Moore, J.A.; Li, Y. Reactivity of Organic Anions Promoted by a Quaternary Ammonium Ion Dendrimer. Macromolecules 1994, 27, 4632–4634. [Google Scholar] [CrossRef]
- Brunner, H. Dendrizymes: Expanded ligands for enantioselective catalysis. J. Organomet. Chem. 1995, 500, 39–46. [Google Scholar]
- Ardoin, N.; Astruc, D. The Molecular Trees: From Syntheses towards Applications. Bull. Soc. Chim. Fr. 1995, 132, 875–909. [Google Scholar]
- Astruc, D.; Chardac, F. Dendritic Catalysts and Dendrimers in Catalysis. Chem. Rev. 2001, 101, 2991–3031. [Google Scholar] [CrossRef]
- van Heerbeek, R.; Kamer, P.C.J.; van Leeuwen, P.W.N.M.; Reek, J.N.H. Dendrimers as Support for Recoverable Catalysts and Reagents. Chem. Rev. 2002, 102, 3717–3756. [Google Scholar] [CrossRef]
- Gade, L. Dendrimer Catalysis; Springer: Heidelberg, Germany, 2006. [Google Scholar]
- Helms, B.; Fréchet, J.M.J. The Dendrimer Effect in Homogeneous Catalysis. Adv. Synth. Catal. 2006, 348, 1125–1148. [Google Scholar] [CrossRef]
- Méry, D; Astruc, D. Dendritic catalysis: Major concepts and recent progress. Coord. Chem. Rev. 2006, 250, 1965–1979. [Google Scholar] [CrossRef]
- Hwang, S.-H.; Shreiner, C.D.; Moorefield, C.N.; Newkome, C.N. Recent progress and applications for metallodendrimers. New J. Chem. 2007, 31, 1192–1217. [Google Scholar] [CrossRef]
- de Jesús, E.; Flores, J.C. Dendrimers: Solutions For Catalyst Separation and Recycling–A Review. Ind. Eng. Chem. Res. 2008, 47, 7968–7981. [Google Scholar] [CrossRef]
- Martinez-Olid, F.; Benito, J.M.; Flores, J.C.; de Jesus, E. Polymetallic Carbosilane Dendrimers Containing N,N'-Iminopyridine Chelating Ligands: Applications in Catalysis. Isr. J. Chem. 2009, 49, 99–108. [Google Scholar] [CrossRef]
- Astruc, D.; Boisselier, E.; Ornelas, C. Dendrimers Designed for Functions: From Physical, Photophysical, and Supramolecular Properties to Applications in Sensing, Catalysis, Molecular Electronics, Photonics, and Nanomedicine. Chem. Rev. 2010, 110, 1857–1959. [Google Scholar] [CrossRef]
- Tsuji, J. Modern Palladium Catalysis. Palladium Reagents and Catalysts: New Perspectives for the 21rst Century; Wiley: Chichester, UK, 2004. [Google Scholar]
- Andrés, R.; de Jesus, E.; Flores, J.C. Catalysts based on palladium dendrimers. New J. Chem. 2007, 31, 1161–1191. [Google Scholar] [CrossRef]
- Astruc, D. Palladium Catalysis Using Dendrimers: Molecular Catalysts versus Nanoparticles. Tetrahedron Asymmetry 2010, in press. [Google Scholar]
- Reetz, M.-T.; Lohmer, G.; Schwickardi, R. Systhesis and Catalytic Activity of Dendritic Diphosphane Metal Complexes. Angew. Chem. Int. Ed. Engl. 1997, 36, 1526–1529. [Google Scholar] [CrossRef]
- Dijskstra, H.P.; Kruihof, C.A.; Ronde, N.; van de Coevering, R.; Ramon, D.J.; Vogt, D.; van Klink, G.M.P.; van Koten, G. Shape-Persistent Nanosize Organometallic Complexes: Synthesis and Application in a Nanofiltration Membrane Reactor. J. Org. Chem. 2003, 68, 675–685. [Google Scholar]
- Dijskstra, H.P.; Ronde, N.; Ramon, D.J.; van Klink, G.M.P.; Vogt, D.; van Koten, G. Application of a Homogeneous Dodecakis(NCN-PdII) Catalyst in a Nanofiltration Membrane Reactor under Continuous Reaction Conditions. Adv. Synth. Catal. 2003, 345, 364–369. [Google Scholar] [CrossRef]
- Astruc, D.; Heuze, K.; Gatard, S.; Méry, D.; Nlate, S.; Plault, L. Metallodendritic Catalysis for Redox and Carbon-Carbon Bond Formation Reactions: A Step towards Green Chemistry. Advan. Syn. Catal. 2005, 347, 329–338. [Google Scholar] [CrossRef]
- Brienbauer, R.; Jacobsen, E.N. Cooperative Asymmetric Catalysis with Dendrimeric [Co(salen)] Complexes. Angew. Chem. Int. Ed. 2000, 39, 3604–3607. [Google Scholar] [CrossRef]
- Francavilla, C.; Drake, M.D.; Bright, F.V.; Detty, M.R. Dendrimeric Organochalcogen Catalysts for the Activation of Hydrogen Peroxide: Improved Catalytic Activity through Statistical Effects and Cooperativity in Successive Generations. J. Am. Chem. Soc. 2001, 123, 57–67. [Google Scholar] [CrossRef]
- Dahan, A.; Portnoy, M. Dendritic effect in polymer-supported catalysis of the intramolecular Pauson–Khand reaction. Chem. Commun. 2002, 2700–2701. [Google Scholar] [CrossRef]
- Gatard, S.; Nlate, S.; Cloutet, E.; Bravic, G.; Blais, J.C.; Astruc, D. Dendritic Stars by Ring-Opening-Metathesis Polymerization from Ruthenium-Carbene Initiators. Angew. Chem. Int. Ed. 2003, 42, 452–456. [Google Scholar] [CrossRef]
- Dahan, A.; Portnoy, M. Remarkable Dendritic Effect in the Polymer-Supported Catalysis of the Heck Arylation of Olefins. Org. Lett. 2003, 5, 1197–2000. [Google Scholar] [CrossRef]
- Gatard, S.; Kahlal, S.; Méry, D.; Nlate, S.; Cloutet, E.; Saillard, J.-Y.; Astruc, D. Synthesis, Chemistry, DFT Calculations, and ROMP Activity of Monomeric Benzylidene Complexes Containing a Chelating Diphosphine and of Four Generations of Metallodendritic Analogues. Positive and Negative Dendritic Effects and Formation of Dendritic Ruthenium−Polynorbornene Stars. Organometallics 2004, 23, 1313–1324. [Google Scholar]
- Fujihara, T.; Obora, Y.; Tokunaga, M.; Sato, H. Dendrimer N-heterocyclic carbene complexes with rhodium(I) at the core. Chem. Commun. 2005, 4526–4528. [Google Scholar]
- Xang, X.; Xu, H.; Dong, Z.; Wang, Y.; Liu, J. Highly Efficient Dendrimer-Based Mimic of Glutathione Peroxidase. J. Am. Chem. Soc. 2004, 126, 10556–10557. [Google Scholar] [CrossRef]
- Delors, E.; Darbre, T.; Reymond, J.L. A Strong Positive Dendritic Effect in a Peptide Dendrimer-Catalyzed Ester Hydrolysis Reaction. J. Am. Chem. Soc. 2004, 126, 15642–15643. [Google Scholar]
- Ouali, A.; Laurent, R.; Caminade, A.M.; Majoral, J.P.; Taillefer, M. Enhanced Catalytic Properties of Copper in O- and N-Arylation and Vinylation Reactions, Using Phosphorus Dendrimers as Ligands. J. Am. Chem. Soc. 2006, 128, 15990–15991. [Google Scholar] [CrossRef]
- Fujihara, T.; Yoshida, S.; Ohta, H.; Tsuji, Y. Triarylphosphanes with Dendritically Arranged Tetraethylene Glycol Moieties at the Periphery: An Efficient Ligand for the Palladium-Catalyzed Suzuki-Miyaura Coupling Reaction. Angew. Chem. Int. Ed. 2008, 47, 8310–8313. [Google Scholar] [CrossRef]
- Heuzé, K.; Méry, D.; Gauss, D.; Astruc, D. Copper-free, recoverable dendritic Pd catalysts for the Sonogashira reaction. Chem. Commun. 2003, 2274–2275. [Google Scholar]
- Heuzé, K.; Méry, D.; Gauss, D.; Blais, J.-C.; Astruc, D. Copper-Free Monomeric and Dendritic Palladium Catalysts for the Sonogashira Reaction: Substituent Effects, Synthetic Applications, and the Recovery and Re-Use of the Catalysts. Chem. Eur. J. 2004, 10, 3936–3944. [Google Scholar] [CrossRef]
- Astruc, D.; Blais, J.-C.; Daniel, M.-C.; Gatard, S.; Nlate, S.; Ruiz, J. C. R. Nano-scale Metallodendritic Complexes in Electron-Transfer Processes and Catalysis. Chimie 2003, 6, 1117–1127. [Google Scholar] [CrossRef]
- Lemo, J.; Heuzé, K.; Astruc, D. Efficient Dendritic Diphosphino Pd(II) Catalysts for the Suzuki Reaction of Choroarenes. Org. Letters 2005, 7, 2253–2256. [Google Scholar] [CrossRef]
- Zhao, M.; Sun, L.; Crooks, R.M. Preparation of Cu Nanoclusters within Dendrimer Templates. J. Am. Chem. Soc. 1998, 120, 4877–4878. [Google Scholar] [CrossRef]
- Balogh, L.; Tomalia, D.A. Poly(Amidoamine) Dendrimer-Templated Nanocomposites. 1. Synthesis of Zerovalent Copper Nanoclusters. J. Am. Chem. Soc. 1998, 120, 7355–7356. [Google Scholar] [CrossRef]
- Esumi, K.; Suzuki, A.; Aihara, N.; Usu, K. Torigoe, Preparation of Gold Colloids with UV Irradiation Using Dendrimers as Stabilizer K. Langmuir 1998, 14, 3157–3159. [Google Scholar]
- Crooks, R.M.; Zhao, M.; Sun, L.; Chechik, V.; Yeung, L.K. Dendrimer-Encapsulated Metal Nanoparticles: Synthesis, Characterization, and Applications to Catalysis. Acc. Chem. Res. 2001, 34, 181–190. [Google Scholar]
- Niu, Y.; Crooks, R.M. Dendrimer-encapsulated metal nanoparticles and their applications to catalysis. C. R. Chimie 2003, 8, 1049–1059. [Google Scholar]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Synthesis, Characterization, and Applications of Dendrimer-Encapsulated Nanoparticles. J. Phys. Chem. B 2005, 109, 692–704. [Google Scholar]
- Zhao, M.; Crooks, R.M. Homogeneous Hydrogenation Catalysis with Monodisperse, Dendrimer-Encapsulated Pd and Pt Nanoparticles. Angew. Chem. Int. Ed. 1999, 38, 364–366. [Google Scholar] [CrossRef]
- Yeung, L.K.; Crooks, R.M. Heck Heterocoupling within a Dendritic Nanoreactor. Nano Lett. 2001, 1, 14–17. [Google Scholar] [CrossRef]
- Yeung, L.K.; Lee, C.T.; Johnston, K.P.; Crooks, R.M. Catalysis in supercritical CO2 using dendrimer-encapsulated palladium nanoparticles. Chem. Commun. 2001, 2290. [Google Scholar]
- Scott, R.W.J.; Wilson, O.M.; Crooks, R.M. Titania-Supported Au and Pd Composites Synthesized from Dendrimer-Encapsulated Metal Nanoparticle Precursors. Chem. Mater. 2004, 16, 5682–5688. [Google Scholar]
- Scottt, R.W.J.; Datye, A.K.; Crooks, R.M. Bimetallic Palladium−Platinum Dendrimer-Encapsulated Catalysts. J. Am. Chem. Soc. 2003, 125, 3708–3709. [Google Scholar] [CrossRef]
- Wilson, O.M.; Scott, R.W.J.; Garcia-Martinez, J.C.; Crooks, R.M. Synthesis, Characterization, and Structure-Selective Extraction of 1−3-nm Diameter AuAg Dendrimer-Encapsulated Bimetallic Nanoparticles. J. Am. Chem. Soc. 2005, 127, 1015–1024. [Google Scholar]
- Scott, R.W.J.; Sivadiranarayana, C.; Wilson, O.M.; Yan, Z.; Goodman, D.W.; Crooks, R.M. Titania-Supported PdAu Bimetallic Catalysts Prepared from Dendrimer-Encapsulated Nanoparticle Precursors. J. Am. Chem. Soc. 2005, 127, 1380–1381. [Google Scholar]
- Garcia-Martinez, J.C.; Lezutekong, R.; Crooks, R.M. Dendrimer-Encapsulated Pd Nanoparticles as Aqueous, Room-Temperature Catalysts for the Stille Reaction. J. Am. Chem. Soc. 2005, 127, 5097–5098. [Google Scholar] [CrossRef]
- Feng, Z.V.; Lyon, J.L.; Croley, J.S.; Crooks, R.M.; Vanden Bout, D.A.; Stevenson, K.J. Synthesis and Catalytic Evaluation of Dendrimer-Encapsulated Cu Nanoparticles. An Undergraduate Experiment Exploring Catalytic Nanomaterials. J. Chem. Ed. 2009, 86, 368–372. [Google Scholar]
- Yamamoto, K.; Higushi, M.; Shiki, S.; Tsuruta, M.; Chiba, H. Stepwise radial complexation of imine groups in phenylazomethine dendrimers. Nature 2002, 415, 509–511. [Google Scholar] [CrossRef]
- Higushi, M.; Shiki, S.; Ariga, S.; Yamamoto, K. First Synthesis of Phenylazomethine Dendrimer Ligands and Structural Studies. J. Am. Chem. Soc. 2001, 123, 4414–4420. [Google Scholar]
- Satoh, N.; Nakashima, T.; Kamikura, K.; Yamamoto, K. Quantum size effect in TiO2 nanoparticles prepared by finely controlled metal assembly on dendrimer templates. Nature Nanotechnol. 2008, 2, 106–111. [Google Scholar]
- Nakamura, I.; Yamanoi, Y.; Yonezawa, T.; Imaoka, T.; Yamamoto, K.; Nishihara, H. Nanocage catalysts—rhodium nanoclusters encapsulated with dendrimers as accessible and stable catalysts for olefin and nitroarene hydrogenations. Chem. Commun. 2008, 5716–5718. [Google Scholar]
- Beller, M.; Lohmer, G.; Kühlein, K.; Reisinger, C.P.; Herrmann, W.A. First palladium-catalyzed Heck reactions with efficient colloidal catalyst systems. J. Organomet. Chem. 1996, 520, 257. [Google Scholar]
- Reetz, M.T.; Lohmer, G. Propylene carbonate stabilized nanostructured palladium clusters as catalysts in Heck reactions. Chem. Commun. 1996, 1921–1922. [Google Scholar] [CrossRef]
- Biffis, A.; Zecca, M.; Basato, M. Palladium metal catalysts in Heck C-C coupling reactions. J. Mol. Catal. A Chem. 2001, 173, 249–260. [Google Scholar] [CrossRef]
- Astruc, D.; Lu, F.; Ruiz, J. Nanoparticles as Recyclable Catalysts: The Fast-growing Frontier between Homogeneous and Heterogeneous Catalysts. Angew. Chem. Int. Ed. 2005, 44, 7852–7872. [Google Scholar] [CrossRef]
- Phan, N.T.S.; van der Sluis, M.; Jones, C.J. On the Nature of the Active Species in Palladium Catalyzed Mizoroki-Heck and Suzuki-Miyaura Couplings - Homogeneous or Heterogeneous Catalysis, A Critical Review. Adv. Syn. Catal. 2006, 348, 609–679. [Google Scholar] [CrossRef]
- de Vries, G.J. A unifying mechanism for all high-temperature Heck reactions. The role of palladium colloids and anionic species. Dalton. Trans. 2006, 421–429. [Google Scholar] [CrossRef]
- Astruc, D. Palladium Nanoparticles as Efficient Green Homogeneous and Heterogeneous Carbon-Carbon Coupling Pre-catalysts: A Unifying View. Inorg. Chem. 2007, 46, 1884–1894. [Google Scholar] [CrossRef]
- Djakovitch, L.; Köhler, K.; de Vries, J.G. The Role of Nanoparticles as Catalysts for Carbon-Carbon Coupling Reactions. In Nanoparticles and Catalysis; Astruc, D., Ed.; Wiley-VCH: Weinheim, Germany, 2007; Volume Chapter 10, pp. 303–348. [Google Scholar]
- He, J.-H.; Ichinose, I.; Kunitake, T.; Nakao, A.; Shiraishi, Y.; Toshima, N. Facile Fabrication of Ag−Pd Bimetallic Nanoparticles in Ultrathin TiO2-Gel Films: Nanoparticle Morphology and Catalytic Activity. J. Am. Chem. Soc. 2003, 125, 11034. [Google Scholar] [CrossRef]
- Newkome, G.R.; Moorefield, C.N.; Vögtle, F. Dendrimers and Dendrons. Concepts, Syntheses, Applications; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Tomalia, D.A.; Fréchet, J.M.J. Dendrimers and Other Dendritic Polymers; Wiley: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Astruc, D. Dendrimers and Nanosciences. C. R. Chimie 2003, 6, 709–1208. [Google Scholar] [CrossRef]
- Moulines, F.; Astruc, D. Tentacled Iron Sandwiches. Angew. Chem. Int. Ed. Engl. 1988, 27, 1347–1349. [Google Scholar] [CrossRef]
- Moulines, F.; Djakovitch, L.; Boese, R.; Gloaguen, B.; Thiel, W.; Fillaut, J.-L.; Delville, M.-H.; Astruc, D. Organometallic Molecular Trees as Multi-Electron and Proton Reservoirs: CpFe+ Induced Nona-Allylation of Mesitylene and Phase-Transfer Catalyzed Synthesis of a Redox Active Nona-Iron Complex. Angew. Chem. Int. Ed. Engl. 1993, 32, 1075–1077. [Google Scholar] [CrossRef]
- Sartor, V.; Djakovitch, L.; Fillaut, J.-L.; Moulines, F.; Neveu, F.; Marvaud, V; Guittard, J.; Blais, J.-C.; Astruc, D. Organoiron Routes to a New Dendron for Fast Dendritic Syntheses Using Divergent and Convergent Methods. J. Am. Chem. Soc. 1999, 121, 2929–2930. [Google Scholar]
- Ruiz, J.; Lafuente, G.; Marcen, S.; Ornelas, C.; Lazare, S.; Cloutet, E.; Blais, J.-C.; Astruc, D. Construction of Giant Dendrimers Using a Tripodal Buiding Block. J. Am. Chem. Soc. 2003, 125, 7250–7257. [Google Scholar]
- Astruc, D.; Ruiz, J. Organoiron-mediated dendrimer syntheses with 1→3 connectivity and applications. Tetrahedron Report N° 903. Tetrahedron 2010, 66, 1769–1785. [Google Scholar] [CrossRef]
- Astruc, D. Organo-iron complexes of aromatic compounds. Applications in synthesis. Tetrahedron Report n° 157. Tetrahedron 1983, 39, 4027–4095. [Google Scholar] [CrossRef]
- Catheline, D.; Astruc, D. The Use of Ferrocene in Organometallic Synthesis: a two Step Preparation of Cyclopentadienyliron Acetonitrile and Phosphine Cations via Photolysis of Cyclopentadienyliron Tricarbonyl or Arene Cations. J. Organometal. Chem. 1984, 272, 417–426. [Google Scholar] [CrossRef]
- Newkome, G.R.; Yao, Z.; Baker, G.R.; Gupta, V.K. Micelles. Part 1. Cascade molecules: A new approach to micelles. A [27]-arborol. J. Org. Chem. 1985, 50, 2003–2004. [Google Scholar] [CrossRef]
- Newkome, G.R.; Shreiner, C. The Syntheses of Dendrimers with 1→3 Connectivity. Chem. Rev. 2010, 110. in press. [Google Scholar]
- Ornelas, C.; Méry, D.; Cloutet, E.; Ruiz, J.; Astruc, D. Cross Olefin Metathesis for the Selective Functionalization, Ferrocenylation, and Solubilization in Water of Olefin-terminated Dendrimers, Polymers and Gold Nanoparticles and for a Divergent Dendrimer Construction. J. Am. Chem. Soc. 2008, 130, 1495–1506. [Google Scholar]
- Ornelas, C.; Ruiz, J.; Cloutet, E.; Alves, S.; Astruc, D. Click Assembly of 1,2,3-Triazole-Linked Dendrimers Including Ferrocenyl Dendrimers that Sense Both Oxo-anions and Metal Cations. Angew. Chem. Int. Ed. 2007, 46, 872–877. [Google Scholar] [CrossRef]
- Ornelas, C.; Salmon, L.; Ruiz, J.; Astruc, D. "Click" Dendrimers: Synthesis, Redox Sensing of Pd(OAc)2, and Remarkable Catalytic Hydrogenation Activity of Precise Pd Nanoparticles Stabilized by 1,2,3-Triazole-Containing Dendrimers. Chem. Eur. J. 2008, 14, 50–64. [Google Scholar] [CrossRef]
- Boisselier, E.; Diallo, A. K.; Salmon, L.; Ornelas, C.; Ruiz, J.; Astruc, D. Encapsulation and Stabilization of Gold Nanoparticles with “Click” Polyethyleneglycol Dendrimers. J. Am. Chem. Soc. 2010, 132, 2729–2742. [Google Scholar]
- Daniel, M.-C.; Ruiz, J.; Blais, J.-C.; Daro, N.; Astruc, D. Synthesis of Five Generations of Redox Stable Pentamethylamidoferrocenyl Dendrimers and Compared Use of Amidoferrocenyl- and Pentamethylamidoferrocenyl Dendrimers As Electrochemical Exoreceptors for the Selective Recognition of H2PO4-, HSO4- and Adenosyl-5’-Triphosphate (ATP) Anions. Stereoelectronic and Hydrophobic Roles of the Cp Permethylation. Chem. Eur. J. 2003, 9, 4371–4379. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Ferrocenyl-terminated Dendrimers: Design for Applications in Molecular Electronics, Molecular Recognition and Catalysis. J. Inorg. Organomet. Polym. Mater. 2008, 18, 4–17. [Google Scholar] [CrossRef]
- Astruc, D.; Ornelas, C.; Ruiz, J. Metallocenyl Dendrimers and their Applications in Molecular Electronics, Sensing and Catalysis. Acc. Chem. Res. 2008, 41, 841–856. [Google Scholar] [CrossRef]
- Badèche, S.; Daran, J.-C.; Ruiz, J.; Astruc, D. Synthesis and Coordination Chemistry of Ferrocenyl-1,2,3-triazolyl Ligands. Inorg. Chem. 2008, 47, 4903–4908. [Google Scholar] [CrossRef]
- Ornelas, C.; Salmon, L.; Ruiz, J.; Astruc, D. Catalytically Efficient Palladium Nanoparticles Stabilized by Click Ferrocenyl Dendrimers. Chem. Commun. 2007, 4946–4948. [Google Scholar]
- Suzuki, A. The Susuki Reaction with Arylboron Compounds in Arene Chemistry. In Modern Arene Chemistry; Astruc, D., Ed.; Wiley-VCH: Weinheim, Germany, 2002; pp. 53–106. [Google Scholar]
- Diallo, A.K.; Ornelas, C.; Salmon, L.; Ruiz, J.; Astruc, D. Catalytically Efficient Palladium Nanoparticles Stabilized by Click Ferrocenyl Dendrimers. Angew. Chem. Int. Ed. Engl. 2007, 46, 8644–8648. [Google Scholar] [CrossRef]
- Ornelas, C.; Ruiz, J.; Salmon, L.; Astruc, D. Sulfonated “Click” Dendrimer-Stabilized Palladium Nanoparticles as Highly Efficient Catalysts for Olefin Hydrogenation and Suzuki Coupling Reactions Under Ambient Conditions in Aqueous Media. Adv. Syn. Catal. 2008, 350, 837–845. [Google Scholar] [CrossRef]
- Ornelas, C.; Diallo, A.K.; Ruiz, J.; Astruc, D. “Click” polymer-supported palladium nanoparticles as highly efficient catalysts for olefin hydrogenation and Suzuki coupling reaction under ambient conditions. Adv. Synth. Catal. 2009, 351, 2147–2154. [Google Scholar]
- Lu, F.; Ruiz, J.; Astruc, D. Palladium-dodecanethiolate nanoparticles as stable and recyclable catalysts for the Suzuki-Miyaura reaction of aryl halides under ambient conditions. Tetrahedron Lett. 2004, 9443–9445. [Google Scholar]
- Oee, M.; Murata, M.; Mizugaki, T.; Ebitani, K.; Kaneda, K. Dendritic Nanoreactors Encapsulating Pd Particles for Substrate-Specific Hydrogenation of Olefins. Nano Lett. 2002, 2, 999. [Google Scholar] [CrossRef]
- Mizugaki, T.; Muratra, M.; Fukubayashi, S.; Mitsudome, T.; Jitsujkawa, K.; Kaneda, K. PAMAM dendron-stabilised palladium nanoparticles: Effect of generation and peripheral groups on particle size and hydrogenation activity. Chem. Commun. 2008, 241–243. [Google Scholar]
- Lemo, J.; Heuzé, K.; Astruc, D. Synthesis and Catalytic Activity of DAB-dendrimer Encapsulated Pd Nanoparticles for the Suzuki Coupling Reaction. Inorg. Chim. Acta 2006, 359, 4909–4911. [Google Scholar] [CrossRef]
- Chung, Y.; Rhree, H.K. Partial hydrogenation of 1,3-cyclooctadiene using dendrimer-encapsulated Pd–Rh bimetallic nanoparticles. J. Mol. Cat. A 2003, 206, 291–294. [Google Scholar] [CrossRef]
- Narayanan, R.; El-Sayed, M.A. Effect of Colloidal Catalysis on the Nanoparticle Size Distribution: Dendrimer−Pd vs PVP−Pd Nanoparticles Catalyzing the Suzuki Coupling Reaction. J. Phys. Chem. B 2004, 108, 8572–8577. [Google Scholar] [CrossRef]
- Rahim, E.H.; Kamounah, F.S.; Frederiksen, J.; Christensen, J.B. Heck Reactions Catalyzed by PAMAM-Dendrimer Encapsulated Pd(0) Nanoparticles. Nano Lett. 2001, 1, 499–502. [Google Scholar] [CrossRef]
- Bernechea, M.; de Jesus, E.; Lopez-Mardomingo, C.; Terreros, P. Dendrimer-Encapsulated Pd Nanoparticles versus Palladium Acetate as Catalytic Precursors in the Stille Reaction in Water. Inorg. Chem. 2009, 48, 4491–4496. [Google Scholar] [CrossRef]
- Sample Availability: Not Available.
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Astruc, D.; Ornelas, C.; Diallo, A.K.; Ruiz, J. Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using "Click" Dendrimer-Stabilized Palladium Nanoparticles. Molecules 2010, 15, 4947-4960. https://doi.org/10.3390/molecules15074947
Astruc D, Ornelas C, Diallo AK, Ruiz J. Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using "Click" Dendrimer-Stabilized Palladium Nanoparticles. Molecules. 2010; 15(7):4947-4960. https://doi.org/10.3390/molecules15074947
Chicago/Turabian StyleAstruc, Didier, Cátia Ornelas, Abdou K. Diallo, and Jaime Ruiz. 2010. "Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using "Click" Dendrimer-Stabilized Palladium Nanoparticles" Molecules 15, no. 7: 4947-4960. https://doi.org/10.3390/molecules15074947
APA StyleAstruc, D., Ornelas, C., Diallo, A. K., & Ruiz, J. (2010). Extremely Efficient Catalysis of Carbon-Carbon Bond Formation Using "Click" Dendrimer-Stabilized Palladium Nanoparticles. Molecules, 15(7), 4947-4960. https://doi.org/10.3390/molecules15074947



