Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. 'Budrovka': A Comparative Study with L. angustifolia Mill.
Abstract
:1. Introduction
2. Results and Discussion
2.1. Contents of polyphenols and HPTLC of phenolic acids
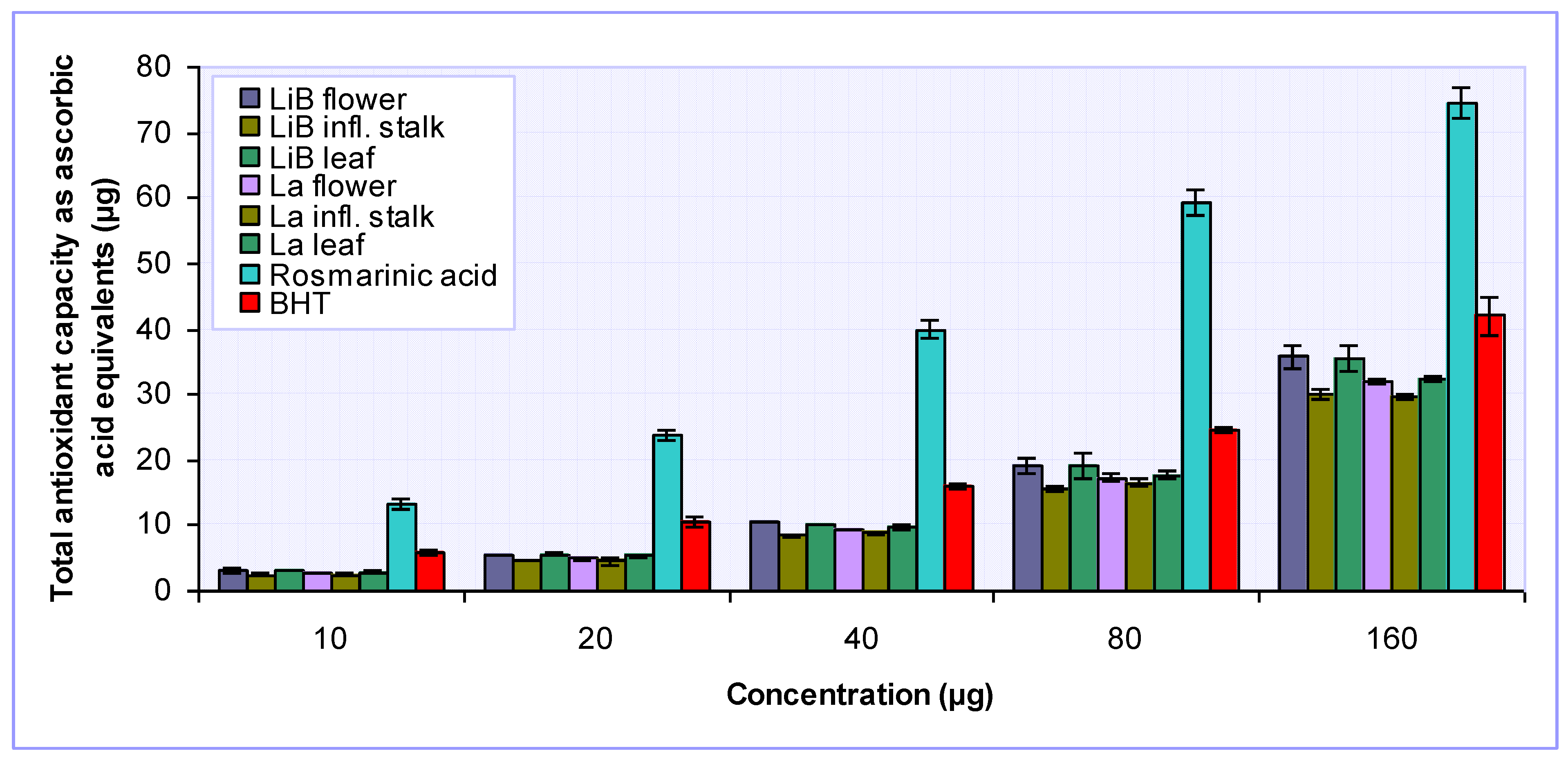
2.2. Antioxidant activity of Lavandula x intermedia ‘Budrovka’ and L. angustifolia extracts
2.3. Correlations between polyphenols and antioxidant activity
3. Experimental
3.1. Plant material and extraction procedure
3.2. Chemicals
3.3. Phytochemical analyses of polyphenols
3.4. Evaluation of antioxidant activity
3.4.1. Determination of DPPH free radical-scavenging activity
3.4.2. Determination of iron chelating activity
3.4.3. Determination of reducing power
3.4.4. Determination of inhibition of lipid peroxidation
3.4.5. Determination of total antioxidant capacity
3.5. Statistical analysis
4. Conclusions
Acknowledgements
References and Notes
- Tsang, A.H.; Chung, K.K. Oxidative and nitrosative stress in Parkinson’s disease. BBA-Mol. Basis Dis. 2009, 1792, 643–650. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Rhodes, C.; Moncol, J.; Izakovic, M.; Mazur, M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem.-Biol. Interact. 2006, 160, 1–40. [Google Scholar] [CrossRef] [PubMed]
- Sohal, R.S. Role of oxidative stress and protein oxidation in the aging process. Free Radical Biol. Med. 2002, 33, 37–44. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Moure, A.; Cruz, J.M.; Franco, D.; Domínguez, J.M.; Sineiro, J.; Domínguez, H.; José Núñez, M.; Parajó, J.C. Natural antioxidants from residual sources. Food Chem. 2001, 72, 145–171. [Google Scholar] [CrossRef]
- Riceevans, C.; Miller, N.; Paganga, G. Antioxidant properties of phenolic compounds. Trends Plant Sci. 1997, 2, 152–159. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Soobrattee, M.; Neergheen, V.; Luximon-Ramma, A.; Aruoma, O.; Bahorun, T. Phenolics as potential antioxidant therapeutic agents: Mechanism and actions. Mutat. Res. Fundam. Mol. Mech. Mutag. 2005, 579, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Perron, N.; Brumaghim, J. A review of the antioxidant mechanisms of polyphenol compounds related to iron binding. Cell Biochem. Biophys. 2009, 53, 75–100. [Google Scholar] [CrossRef] [PubMed]
- Kustrak, D.; Besic, J. Aetheroleum Lavandulae and Aetheroleum Lavandulae hybridae in Pharmacopoeia Jugoslavia III. Pharm. Acta Helv. 1975, 50, 373–378. [Google Scholar] [PubMed]
- Ramic, S.; Murko, D.; Delic, F. Investigation of chemical composition of natural and synthetic lavender essential oils by means of thin-layer chromatography. Herba Polonica 1982, 28, 15–20. [Google Scholar]
- Torras-Claveria, L.; Jauregui, O.; Bastida, J.; Codina, C.; Viladomat, F. Antioxidant activity and phenolic composition of lavandin (Lavandula x intermedia Emeric ex Loiseleur) waste. J. Agric. Food Chem. 2007, 55, 8436–8443. [Google Scholar] [CrossRef] [PubMed]
- Miliauskas, G.; Venskutonis, P.R.; van Beek, T.A. Screening of radical scavenging activity of some medicinal and aromatic plant extracts. Food Chem. 2004, 85, 231–237. [Google Scholar] [CrossRef]
- Ivanova, D.; Gerova, D.; Chervenkov, T.; Yankova, T. Polyphenols and antioxidant capacity of Bulgarian medicinal plants. J. Ethnopharmacol. 2005, 96, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, A.; Proença, C.; Serralheiro, M.; Araújo, M. The in vitro screening for acetylcholinesterase inhibition and antioxidant activity of medicinal plants from Portugal. J. Ethnopharmacol. 2006, 108, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bouayed, J.; Piri, K.; Rammal, H.; Dicko, A.; Desor, F.; Younos, C.; Soulimani, R. Comparative evaluation of the antioxidant potential of some Iranian medicinal plants. Food Chem. 2007, 104, 364–368. [Google Scholar] [CrossRef]
- Tsai, T.; Tsai, T.; Chien, Y.; Lee, C.; Tsai, P. In vitro antimicrobial activities against cariogenic streptococci and their antioxidant capacities: A comparative study of green tea versus different herbs. Food Chem. 2008, 110, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Thring, T.; Hili, P.; Naughton, D. Anti-collagenase, anti-elastase and anti-oxidant activities of extracts from 21 plants. BMC Complement. Altern. Med. 2009, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Sariri, R.; Seifzadeh, S.; Sajedi, R.H. Anti-tyrosinase and antioxidant activity of Lavandula sp. extracts. Pharmacologyonline 2009, 2, 413–420. [Google Scholar]
- Gordon, M.H. The mechanism of antioxidant action in vitro. In Food Antioxidants; Hudson, B.J.F., Ed.; Elsevier Applied Science: London, UK, 1990; pp. 1–18. [Google Scholar]
- Tepe, B. Antioxidant potentials and rosmarinic acid levels of the methanolic extracts of Salvia virgata (Jacq), Salvia staminea (Montbret & Aucher ex Bentham) and Salvia verbenaca (L.) from Turkey. Bioresource Technol. 2008, 99, 1584–1588. [Google Scholar]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.; Noryati, I.; Sulaiman, S.; Rosma, A. In vitro antibacterial and antioxidant activities of Orthosiphon stamineus Benth. extracts against food-borne bacteria. Food Chem. 2010, 122, 1168–1172. [Google Scholar] [CrossRef]
- Welch, K.D.; Davis, T.Z.; Van Eden, M.E.; Aust, S.D. Deleterious iron-mediated oxidation of biomolecules. Free Radical Biol. Med. 2002, 32, 577–583. [Google Scholar] [CrossRef]
- Gülçin, I. Antioxidant and antiradical activities of l-carnitine. Life Sci. 2006, 78, 803–811. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, T.; Thomas, P.; Geraldine, P. In-vitro antioxidant activities of an ethanolic extract of the oyster mushroom, Pleurotus ostreatus. Innov. Food Sci. Emerg. Technol. 2009, 10, 228–234. [Google Scholar] [CrossRef]
- Niki, E. Lipid peroxidation products as oxidative stress biomarkers. BioFactors 2008, 34, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Houghton, P.J.; Zarka, R.; de las Heras, B.; Hoult, J.R. Fixed oil of Nigella sativa and derived thymoquinone inhibit eicosanoid generation in leukocytes and membrane lipid peroxidation. Planta Med. 1995, 61, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Prieto, P.; Pineda, M.; Aguilar, M. Spectrophotometric quantitation of antioxidant capacity through the formation of a phosphomolybdenum complex: specific application to the determination of vitamin E. Anal. Biochem. 1999, 269, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, X.; Huang, L. Correlation between antioxidant activities and phenolic contents of Radix angelicae sinensis (Danggui). Molecules 2009, 14, 5349–5361. [Google Scholar] [CrossRef] [PubMed]
- Osman, H.; Rahim, A.A.; Isa, N.M.; Bakhir, N.M. Antioxidant activity and phenolic content of Paederia foetida and Syzygium aqueum. Molecules 2009, 14, 970–978. [Google Scholar] [CrossRef] [PubMed]
- Moein, M.R.; Moein, S.; Ahmadizadeh, S. Radical scavenging and reducing power of Salvia mirzayanii subfractions. Molecules 2008, 13, 2804–2813. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines and Health Care (EDQM). European Pharmacopoeia, 4th ed.; Council of Europe: Strasbourg, France, 2004. [Google Scholar]
- Christ, B.; Müller, K.H. Zur serienmäßigen Bestimmung des Gehaltes an Flavonol-Derivaten in Drogen. Arch. Pharm. 1960, 293, 1033–1042. [Google Scholar] [CrossRef]
- Stahl, E.; Schild, W. Isolierung und Characterisierung von Naturstoffen; Gustav Fischer Verlag: New York, NY, USA, 1986; p. 147. [Google Scholar]
- Blois, M.S. Antioxidant determinations by the use of a stable free radical. Nature 1958, 181, 1199–1200. [Google Scholar] [CrossRef]
Sample Availability: Samples available from the authors. |





| Extracts | Contents (%) | ||||||
|---|---|---|---|---|---|---|---|
| Phenolic acids | Flavonoids | Anthocyanins | Procyanidins | Total tannins | Total polyphenols | ||
| LiB | flower | 3.42 ± 0.09 | 0.10 ± 0.01 | 0.02 ± 0.00 | 1.13 ± 0.07 | 2.02 ± 0.01 | 6.65 ± 0.14 |
| stalk | 1.62 ± 0.12 | 0.22 ± 0.01 | - | 0.86 ± 0.02 | 1.01 ± 0.04 | 3.09 ± 0.11 | |
| leaf | 3.80 ± 0.04 | 0.26 ± 0.01 | - | 1.30 ± 0.05 | 2.21 ± 0.03 | 7.05 ± 0.15 | |
| La | flower | 5.00 ± 0.11 | 0.09 ± 0.01 | 0.03 ± 0.00 | 1.32 ± 0.08 | 2.77 ± 0.05 | 8.46 ± 0.05 |
| stalk | 2.41 ± 0.06 | 0.19 ± 0.02 | - | 1.02 ± 0.03 | 1.38 ± 0.19 | 4.54 ± 0.22 | |
| leaf | 5.32 ± 0.14 | 0.25 ± 0.01 | - | 1.44 ± 0.02 | 3.18 ± 0.22 | 9.20 ± 0.17 | |
| Extracts | IC50* (µg/mL) | Total antioxidant capacity (mg AAE/g) | ||||
|---|---|---|---|---|---|---|
| DPPH· scavenging activity | Iron chelating activity | Reducing power | Inhibition of lipid peroxidation | |||
| LiB | flower | 17.17 ± 0.33 | 397.71 ± 10.26 | 33.78 ± 2.34 | 116.54 ± 9.96 | 294.00 ± 13.17 |
| infl. stalk | 45.25 ± 0.10 | 294.08 ± 12.86 | 66.92 ± 3.75 | 283.54 ± 5.02 | 240.75 ± 13.08 | |
| leaf | 15.06 ± 0.74 | 383.59 ± 15.55 | 28.73 ± 1.61 | 74.56 ± 4.71 | 290.75 ± 8.99 | |
| La | flower | 11.37 ± 0.69 | 319.21 ± 21.96 | 25.17 ± 0.16 | 89.36 ± 5.00 | 261.50 ± 7.07 |
| infl. stalk | 33.95 ± 1.42 | 236.92 ± 10.19 | 55.22 ± 1.10 | 240.48 ± 11.00 | 238.08 ± 8.38 | |
| leaf | 10.62 ± 0.02 | 302.79 ± 7.61 | 24.26 ± 0.76 | 54.57 ± 4.42 | 274.18 ± 5.07 | |
| Rosmarinic acid | 1.51 ± 0.07 | NA | 1.26 ± 0.14 | 9.18 ± 0.01 | 1064.47 ± 53.52 | |
| BHT | 6.45 ± 0.53 | NA | 4.64 ± 0.31 | - | 414.74 ± 36.81 | |
| EDTA | - | 13.37 ± 0.91 | - | - | ||
| Fisetin | - | - | - | 5.42 ± 0.60 | ||
| DPPH·scavenging activity | Iron chelating activity | Reducing power | Inhibition of lipid peroxidation | Total antioxidant capacity | |
|---|---|---|---|---|---|
| Phenolic acids | 0.9936** | −0.3425ns | 0.9804** | 0.9017* | 0.4643ns |
| Flavonoids | −0.1176ns | 0.1701ns | −0.0947ns | 0.2621ns | −0.0456ns |
| Procyanidins | 0.9638** | −0.3703ns | 0.9732** | 0.9536** | 0.5574ns |
| Total tannins | 0.9923** | −0.3548ns | 0.9772** | 0.9307** | 0.4886ns |
| Total polyphenols | 0.9933** | −0.4129ns | 0.9866** | 0.9132* | 0.5625ns |
© 2010 by the authors; licensee MDPI, Basel, Switzerland. This article is an Open Access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Blažeković, B.; Vladimir-Knežević, S.; Brantner, A.; Štefan, M.B. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. 'Budrovka': A Comparative Study with L. angustifolia Mill. Molecules 2010, 15, 5971-5987. https://doi.org/10.3390/molecules15095971
Blažeković B, Vladimir-Knežević S, Brantner A, Štefan MB. Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. 'Budrovka': A Comparative Study with L. angustifolia Mill. Molecules. 2010; 15(9):5971-5987. https://doi.org/10.3390/molecules15095971
Chicago/Turabian StyleBlažeković, Biljana, Sanda Vladimir-Knežević, Adelheid Brantner, and Maja Bival Štefan. 2010. "Evaluation of Antioxidant Potential of Lavandula x intermedia Emeric ex Loisel. 'Budrovka': A Comparative Study with L. angustifolia Mill." Molecules 15, no. 9: 5971-5987. https://doi.org/10.3390/molecules15095971




