One-Pot and Efficient Synthesis of Triazolo[1,2-a]indazole-triones via Reaction of Arylaldehydes with Urazole and Dimedone Catalyzed by Silica Nanoparticles Prepared from Rice Husk
Abstract
:1. Introduction
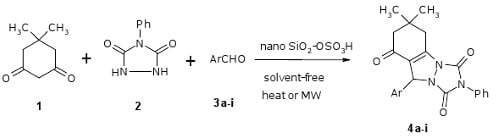
2. Results and Discussion



| Entry | Catalyst (mg) | Time (min) | Temperature (°C) | Yield (%) |
|---|---|---|---|---|
| 1 | Silica nanoparticles (100) | 30 | 80 | - |
| 2 | Nanosilicasulfuric acid (80) | 30 | 80 | 56 |
| 3 | Nanosilicasulfuric acid (100) | 30 | 80 | 67 |
| 4 | Nanosilicasulfuric acid (125) | 30 | 80 | 80 |
| 5 | Nanosilicasulfuric acid (150) | 30 | 80 | 80 |
| 6 | Nanosilicasulfuric acid (125) | 30 | 70 | 61 |
| 7 | Nanosilicasulfuric acid (125) | 30 | 90 | 80 |
| 8 | Nanosilicasulfuric acid (125) | 30 | 100 | 80 |
| 9 | Nanosilicasulfuric acid (125) | 25 | 80 | 64 |
| 10 | Nanosilicasulfuric acid (125) | 40 | 80 | 80 |
| Entry | Catalyst (mg) | Time (min) | Yield a (%) |
|---|---|---|---|
| 1 | Silica nanoparticles (125) | 5 | - |
| 2 | Nanosilicasulfuric acid (125) | 3 | 52 |
| 3 | Nanosilicasulfuric acid (125) | 4 | 74 |
| 4 | Nanosilicasulfuric acid (125) | 5 | 92 |
| 5 | Nanosilicasulfuric acid (125) | 6 | 81 |
| Entry | Aldehyde | Product | Method A | Method B | M.P.(°C) [19] | ||
|---|---|---|---|---|---|---|---|
| Time (min) | Yield a (%) | Time (min) | Yield a (%) b | ||||
| 1 |  |  | 30 | 80 | 10 | 92 [78] * | 189–190 [188–190] * |
| 2 |  |  | 45 | 75 | 10 | 91 [79] * | 161–163 [160–162] * |
| 3 |  |  | 25 | 87 | 10 | 94 [83] * | 125–126 [126–128] * |
| 4 |  |  | 20 | 90 | 10 | 94 [81] * | 173–175 [175–177] * |
| 5 |  |  | 25 | 86 | 10 | 94 [88] * | 169–171 [166–168] * |
| 6 |  |  | 35 | 80 | 10 | 93 [81] * | 175–177 [174–176] * |
| 7 |  |  | 25 | 84 | 10 | 92 [79] * | 171–172 [173–175] * |
| 8 |  |  | 25 | 90 | 10 | 96 [90] * | 105–106 [102–104] * |
| 9 |  |  | 35 | 86 | 10 | 95 [80] * | 185–186 [184–186] * |
| Run | Yield a (%) | |
|---|---|---|
| Method A | Method B | |
| 1 | 96 | 98 |
| 2 | 94 | 96 |
| 3 | 92 | 95 |
| 4 | 90 | 93 |
| 5 | 89 | 91 |
| 6 | 86 | 90 |
3. Experimental Section
3.1. General
3.2. General Procedure (Method A)
3.3. General Procedure (Method B)
4. Conclusions
Acknowledgments
References and Notes
- Bansal, V.; Ahmad, A.; Sastry, M. Fungus-mediated biotransformation of amorphous silica in rice husk to nanocrystaline silica. J. Am. Chem. Soc. 2006, 128, 14059–14066. [Google Scholar] [CrossRef]
- Witoon, T.; Chareonpanich, M. Limtrakul, synthesis of bimodal porous silica from rice husk via sol-gel process using chitosan as template. J. Mater. Lett. 2008, 62, 1476–1479. [Google Scholar] [CrossRef]
- Pijarn, N.; Jaroenworaluck, A.; Sunsaneeyametha, W.; Stevens, R. Synthesis and characterization of nanosized-silica gels formed under controlled conditions. Powder Technol. 2010, 203, 462–468. [Google Scholar] [CrossRef]
- Motaung, T.E.; Luyt, A.S. Effect of maleic anhydride grafting and the presence of oxidized wax on the thermal and mechanical behavior of LDPE/silica nanocomposites. Mater. Sci. Eng. A 2010, 527, 761–768. [Google Scholar] [CrossRef]
- Morpurgo, M.; Teoli, D.; Pignatto, M.; Attrezzi, M.; Spadaro, F.; Realdon, N. The effect of NaCO3, NaF and NH4OH on the stability and release behavior of sol-gel derived silica xerogels embedded with bioactive compounds. Acta Biomater. 2010, 6, 2246–2253. [Google Scholar] [CrossRef]
- Ge, J.; Huynh, T.; Hu, Y.; Yinhttp, Y. Hierarchical magnetite/silica nanoassemblies as magnetically recoverable catalyst-supports. Nano Lett. 2008, 8, 931–934. [Google Scholar] [CrossRef]
- Banerjee, S.; Sereda, G. One-step, three-component synthesis of highly substituted pyridines using silica nanoparticle as reusable catalyst. Tetrahedron Lett. 2009, 50, 6959–6962. [Google Scholar] [CrossRef]
- Banerjee, S.; Horn, A.; Khatri, H.; Sereda, G. A green one-pot multicomponent synthesis of 4H-pyrans and polysubstituted aniline derivatives of biological, pharmacological and optical applications using silica nanoparticles as reusable catalyst. Tetrahedron Lett. 2011, 52, 1878–1881. [Google Scholar] [CrossRef]
- Bagley, M.C.; Davis, T.; Dix, M.C.; Rokicki, M.J.; Kipling, D. Rapid synthesis of VX-745:p38 MAP kinase inhibition in Werner syndrome cells. Bioorg. Med. Chem. Lett. 2007, 17, 5107–5110. [Google Scholar] [CrossRef]
- Boatman, P.D.; Urban, J.; Nguyen, M.; Qabar, M.; Kahn, M. High-throughput synthesis and optimization of thrombin inhibitors via urazole α-addition and Micheal addition. Bioorg. Med. Chem. Lett. 2003, 13, 1445–1449. [Google Scholar] [CrossRef]
- Izydore, R.A.; Bernal-Ramirez, J.A.; Singh, P. Reaction of 4,4-diethyl-3,5-pyrazolidinedione with carboxylic acid anhydrides. N-acylation vs. O-acylation. J. Org. Chem. 1990, 55, 3761–3767. [Google Scholar] [CrossRef]
- Boldi, A.M.; Johnson, C.R.; Eissa, H.O. Solid-phase library synthesis of triazolopyridazines via [4+2] cycloadditions. Tetrahedron Lett. 1999, 40, 619–622. [Google Scholar] [CrossRef]
- Tanaka, S.; Seguchi, K.; Itoh, K.; Sera, A. Formation of tetracyclic oxazolidinones from cycloadducts of benzylidene ketones with 4-phenyl-4,5-dihydro-3H-1,2,4-triazole-3,5-dione(PTAD) by base-promoted backbone participation and rearrangement. J. Chem. Soc. Perkin Trans. 1994, 1, 2335–2339. [Google Scholar]
- Arroya, Y.; Rodriguez, J.F.; Santos, M.; Sanz Tejedor, M.A.; Vaco, I.; Garcia Ruano, J.L. Asymmetric synthesis of (3S,4R,5R)-4,5-dihydroxy-3-methyl-2,3,4,5-tetrahydropyridazine: A formal synthesis of 1-azagulfamine analogues. Tetrahedron: Asymmetry 2004, 15, 1059–1063. [Google Scholar] [CrossRef]
- Deghati, P.Y.F.; Wanner, M.J.; Koomen, G.J. An efficient hetero Diels-Alder approach to imidazo[4,5-c]pyridazines as purine analogues. Tetrahedron Lett. 1998, 39, 4561–4564. [Google Scholar] [CrossRef]
- Meehan, S.; Little, R.D. A new synthesis of diazenes (azoalkanes) using 4-(S,S-dimethylsulfoximino)-1,2,4-triazoline-3,5-dione. The construction of diazenes from amino nitrenes via base-induced sulfoximine cleavage. J. Org. Chem. 1997, 62, 3779–3781. [Google Scholar] [CrossRef]
- Menard, C.; Doris, E.; Mioskowski, C. ph3BiCO3: A mild reagent for in situ oxidation of urazoles to triazolinediones. Tetrahedron Lett. 2003, 44, 6591–6593. [Google Scholar] [CrossRef]
- Zolfigol, M.A.; Madrakian, E.; Ghaemi, E. Silica sulfuric acid/NaNO2 as a novel heterogeneous system for the nitration of phenols under mild conditions. Molecules 2002, 57, 734–742. [Google Scholar]
- Bazgir, A.; Seyyedhamzeh, M.; Yasaei, Z.; Mirzaei, P. A novel three-component method for the synthesis of triazolo[1,2-a]indazole-triones. Tetrahedron Lett. 2007, 48, 8790–8794. [Google Scholar] [CrossRef]
- Sample Availability: Samples of compounds 4a–i are available from the authors.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Hamidian, H.; Fozooni, S.; Hassankhani, A.; Mohammadi, S.Z. One-Pot and Efficient Synthesis of Triazolo[1,2-a]indazole-triones via Reaction of Arylaldehydes with Urazole and Dimedone Catalyzed by Silica Nanoparticles Prepared from Rice Husk. Molecules 2011, 16, 9041-9048. https://doi.org/10.3390/molecules16119041
Hamidian H, Fozooni S, Hassankhani A, Mohammadi SZ. One-Pot and Efficient Synthesis of Triazolo[1,2-a]indazole-triones via Reaction of Arylaldehydes with Urazole and Dimedone Catalyzed by Silica Nanoparticles Prepared from Rice Husk. Molecules. 2011; 16(11):9041-9048. https://doi.org/10.3390/molecules16119041
Chicago/Turabian StyleHamidian, Hooshang, Samieh Fozooni, Asadollah Hassankhani, and Sayed Zia Mohammadi. 2011. "One-Pot and Efficient Synthesis of Triazolo[1,2-a]indazole-triones via Reaction of Arylaldehydes with Urazole and Dimedone Catalyzed by Silica Nanoparticles Prepared from Rice Husk" Molecules 16, no. 11: 9041-9048. https://doi.org/10.3390/molecules16119041
APA StyleHamidian, H., Fozooni, S., Hassankhani, A., & Mohammadi, S. Z. (2011). One-Pot and Efficient Synthesis of Triazolo[1,2-a]indazole-triones via Reaction of Arylaldehydes with Urazole and Dimedone Catalyzed by Silica Nanoparticles Prepared from Rice Husk. Molecules, 16(11), 9041-9048. https://doi.org/10.3390/molecules16119041