Abstract
Following our previous results on an environmentally benign one-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides under aqueous conditions, we investigate herein the Suzuki-Miyaura cross-coupling reactions of aryl and heteroaryl derivatives at the C5 position of unprotected 2'-deoxyuridine in the same media with a common catalyst system avoiding exotic ligands, since palladium acetate and triphenylphosphine afforded the expected products in moderate to good yields.
1. Introduction
Nucleosides attract attention due to the central role they play in all living systems. Therefore synthesis of unnatural nucleosides arises continuous interest because of their wide biological potential. For instance, 5-substituted 2'-deoxyuridines have been reported as efficient candidates in DNA labeling, modification, and other studies [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17], and they also exhibit significant antiviral [18,19,20,21,22,23], antibacterial [24], and anticancer activities [25,26,27]. Due to the importance of modified nucleosides, all major classes of palladium-catalyzed reactions have been extensively developed to introduce various substituents [28,29,30,31,32,33,34,35,36,37,38]. Among them, the Suzuki-Miyaura reaction is a powerful and widely used method for carbon-carbon cross coupling reactions. Until lately, this reaction would be carried out in lipophilic media that required working with protected nucleosides. However protection/deprotection sequences induce generally a loss of material and increase the waste production. Recently, Suzuki-Miyaura reactions on unprotected 2'-deoxyuridines in an aqueous-organic solvent system were described, where tetrahydrofuran, acetonitrile, methanol or dimethylformamide was used as co-solvent [1,2,3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,39,40]. To the best of our knowledge, only two examples in the 5-iodouridine [41,42] and one in the 2'-deoxy-uridine [43] series are reported in the literature where the experimental conditions required either tris(3-sulfonatophenyl)phosphine trisodium salt (TPPTS) as a specific ligand or palladium supported on reverse phase glass beads. This induced us to disclose herein a similar straightforward method as a natural extension of the current available methods. Based on our interest in environmentally sound processes [44,45], we investigated the development of a Suzuki-Miyaura reaction with 2'-deoxyuridines in a completely aqueous medium using a readily available and inexpensive catalyst/ligand system.
2. Results and Discussion
The conditions of the reaction were optimized using the unprotected 5-iodo-2'-deoxyuridine 1 (5-IdU) and 4-methoxyphenylboronic acid. We started by using a mixture of water and acetonitrile in presence of palladium acetate (3 mol %), triphenylphosphine (5 mol %), and sodium carbonate (1.5 equiv) at 80 °C. After 4 hours under these conditions, the starting material 1 was completely consumed and the expected product was isolated in 62% yield. Then, we were pleased to observe that a complete aqueous medium did not prevent the reaction from proceeding but even slightly improved the yield (Table 1, entry 2). An increase in the catalyst loading induced no noticeable change. However the concentration of the reaction mixture appeared to be significant, since 2b was obtained in 75% yield (entry 4). Further optimizations showed that increasing the amount of boronic acid or replacing the ligand by tri(4,6-dimethyl-3-sulfonatophenyl)phosphine trisodium (TXTPS) [46,47], CataXCium F. Sulf. [48] or tris[bis(N-2-hydroxyethyl)aminomethyl]phosphine [49], which are well-known to be highly hydrophilic, did not improve the reaction outcome (entries 4–7) [50].

Table 1.
Suzuki-Miyaura cross coupling optimization. 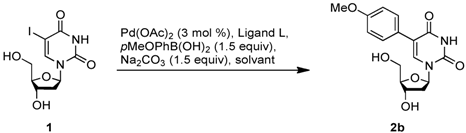

| Entry | Ligand (L) a | Solvent | Conditions | Yield (%) b |
|---|---|---|---|---|
| 1 | PPh3 | H2O:CH3CN 2:1 | 80 °C, 4 h | 62 |
| 2 | PPh3 | H2O (5 mL) | 80 °C, 4 h | 67 |
| 3 | PPh3c | H2O (5 mL) | 80 °C, 4 h | 69 |
| 4 | PPh3 | H2O (2.5 mL) | 80 °C, 4 h | 75 (74) d |
| 5 | TXPTS | H2O (2.5 mL) | 80 °C, 4 h | 71 |
| 6 | CataCXium F sulf | H2O (2.5 mL) | 80 °C, 4 h | traces |
| 7 | P(CH2N(C2H4OH)2)3 | H2O (2.5 mL) | 80 °C, 4 h | 70 |
| 8 | PPh3 | H2O (2.5 mL) | 120 °C, 10 min MW | 75 (66) e |
| 9 | PPh3 | H2O (2.5 mL) | 120 °C, 10 min MW | 70 f |
a Ratio Pd/L: 1/1.8; b Isolated yield; c Pd(OAc)2 (10 mol %) and PPh3 (25 mol %); d with 2 equiv. of R-B(OH)2; e with 1 mL H2O; f with Na2PdCl4.
Then, under the best conditions, the use of microwave irradiation significantly reduced the reaction time with the same yield. Concentrating the media and changing the catalyst induced no positive changes (entries 8 and 9).
To probe the scope of the reaction, the use of different arylboronic acids was examined (Table 2). The expected products were cleanly obtained in good yields with substrates that contained electron withdrawing and donating groups in the para and meta positions (entries 1 and 2). It is worth noting that the use of potassium trifluoroborate is compatible with these experimental conditions as a strict stoichiometric amount of potassium phenyltrifluoroborate gave 2a with the same range of yields (entry 1).

Table 2.
Substrates scope. 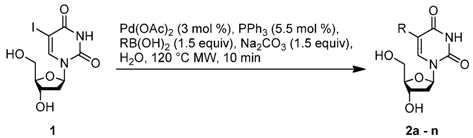

| Entry | RB(OH)2 | Products | Yield (%) a | |
|---|---|---|---|---|
| 1 |  |  | 2a: R1 = H | 70 (62) b |
| 2b: R1 = OMe | 75 | |||
| 2c: R1 = Ac | 72 | |||
| 2d: R1 = CHO | 79 | |||
| 2e: R1 = F | 74 | |||
| 2f: R1 = NO2 | 68 | |||
| 2 |  | 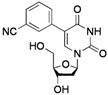 | 2g | 70 |
| 3 | 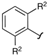 | 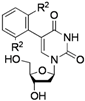 | 2h: R2 = Me | - |
| 2i: R2 = OMe | - | |||
| 4 | 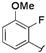 | 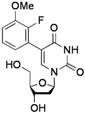 | 2j | 53 c |
| 5 | 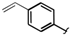 | 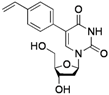 | 2k | 30 |
| 6 | 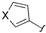 | 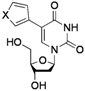 | 2l: X = O | 67 (73) d |
| 2m: X = S | 81 | |||
| 7 |  | 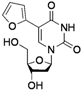 | 2n | 44c |
a Isolated yield; b with 1 equiv. Ph-BF3K; c with 3 equiv. of R-B(OH)2; d with 2 equiv. of R-B(OH)2.
The effects of steric hindrance were also tested with mono- and di-ortho-substituted boronic acids showing a limitation of the method by causing a moderate and drastic loss of yield, respectively (entries 3 and 4). Then the challenging styrene-4-boronic acid was also tried to compare the reaction with the competitive Heck cross-coupling. In this case, the desired product was isolated in 30% yield with no trace of the alkenyl compound [51]. To expend the range of applicable substrates, 5-iodo-2'-deoxyuridine 1 was coupled with a variety of heteroarylboronic acids (Table 2, entries 6 and 7). Moderate to good yields were observed with thiophene-3-, furan-2-, and furan-3-boronic acids requiring occasionally a larger excess of the boronic moiety (entries 6 and 7). Unfortunately, with pyridin-2- and pyridin-3-boronic acids no reaction was observed, and the same result was obtained with the more stable potassium pyridin-3-trifluoroborate.
3. Experimental
3.1. General
Solvents and reagents were purchased from commercial suppliers and used without further purification. 1H-NMR and 13C-NMR were recorded on a Bruker Avance DPX 250 or 400 MHz spectrometers. High-resolution mass spectra (HRMS) were recorded with a TOF spectrometer in the electrospray ionisation (ESI) mode or with a Finnigan MAT 95 XL in the chemical ionisation (CI) mode at the Regional Center of Physical Measurement University Blaise Pascal. All commercial solvents were used without further purification. Column chromatography was carried out using Silica gel 60N (spherical, neutral, 40–63 µm, Merck). Melting point was measure on Thermo Scientific 9200. Intfrared (IR) spectra were obtained on FT-IR Thermo Scientific Nicolet iS10. Thin layer chromatography (TLC) was carried out on Merck silica gel 60F254 precoated plates. Visualization was made with ultraviolet light.
3.2. General procedure
Under nitrogen, 5-IdU 1 (100 mg, 0.282 mmol), PPh3 (4.1 mg, 0.016 mmol), sodium carbonate (44.8 mg, 0.423 mmol), and ary/hetarylboronic acid (0.423 mmol) were dissolved in water (2.5 mL). Then Pd(OAc)2 (2.5 mg, 0.011 mmol) was added to the mixture before sealing the vial. Then the mixture was irradiated for 10 min at 120 °C. After completion, water was added (5 mL) and the pH was adjusted to 7 using aqueous HCl 10%. The solution was concentrated under reduced pressure and the residue was finally purified by silica gel chromatography to afford the desired product.
5-Phenyl-2'-deoxyuridine (2a). DCM/MeOH (96/4), spectroscopic data conformed to the literature [40].
5-(4-Methoxyphenyl)-2'-deoxyuridine (2b). DCM/MeOH (96/4), spectroscopic data conformed to the literature [40].
5-(4-Acetylphenyl)-2'-deoxyuridine (2c). DCM/MeOH (96/4), white solid (72% yield); mp >250 °C (slow degradation); 1H-NMR (250 MHz, DMSO-d6) δ 11.60 (bs, 1H), 8.41 (s, 1H), 7.96 (d, J = 8.5 Hz, 2H), 7.75 (d, J = 8.5 Hz, 2H), 6.24 (t, 1H, J = 6.5 Hz), 5.28 (d, 1H, J = 2.7 Hz), 5.19 (t, 1H, J = 3.2 Hz), 4.37–4.26 (m, 1H), 3.88–4.81 (m, 1H), 3.69–3.60 (m, 2H), 2.59 (s, 3H), 2.35–2.12 (m, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 197.8, 162.3, 150.2, 139.7, 138.5, 135.6, 128.5, 128.1, 112.6, 88.0, 85.2, 70.5, 61.3, 27.1 (one peak under the DMSO-d6 signal); IR (neat): 3421, 3162, 3054, 2921, 2831, 1671, 1657, 1598, 1558, 958 cm−1; HRMS (ESI) calcd. for C17H18N2O6Na (M+Na) 369.1063, Found 369.1077.
5-(4-Formylphenyl)-2'-deoxyuridine (2d). DCM/MeOH (96/4), spectroscopic data conformed to the literature [7].
5-(4-Fluorophenyl)-2'-deoxyuridine (2e). DCM/MeOH (96/4), spectroscopic data conformed to the literature [40].
5-(4-Nitrosophenyl)-2'-deoxyuridine (2f). DCM/MeOH (96/4), spectroscopic data conformed to the literature [52].
5-(3-Cyanophenyl)-2'-deoxyuridine (2g). DCM/MeOH (96/4), white solid (70% yield); mp 179.2–180.5 °C; 1H-NMR (250 MHz, DMSO-d6) δ 11.63 (bs, 1H), 8.34 (s, 1H), 8.01 (s, 1H), 7.89 (d, J = 7.9 Hz, 1H), 7.76 (d, J = 7.9 Hz, 1H), 7.58 (t, J = 7.9 Hz, 1H), 6.22 (t, J = 6.5 Hz, 1H), 5.26 (d, J = 3.9 Hz, 1H), 5.17 (t, J = 4.7 Hz, 1H), 4.35–4.24 (m, 1H), 3.81 (q, J = 3.9 Hz, 1H), 3.65 (dd, J = 8.6, 4.7 Hz, 1H), 3.58 (dd, J = 8.6, 3.9 Hz, 1H), 2.35–2.12 (m, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 162.3, 150.2, 139.7, 135.0, 133.0, 131.6, 131.1, 129.8, 119.3, 111.8, 111.8, 88.0, 85.1, 70.3, 61.2 (one peak under DMSO d6 signal); IR (neat): 3468, 3336, 3207, 3071, 2236, 1682, 1263, 1086, 945, 817 cm−1; HRMS (ESI) calcd. for C16H15N3O5Na (M+Na) 352.0909, Found 352.0906.
5-(2-Fluoro-3-methoxyphenyl)-2'-deoxyuridine (2j). DCM/MeOH (96/4), white solid (53% yield); mp 173.2–174.2 °C; 1H-NMR (250 MHz, DMSO-d6) δ 11.52 (bs, 1H), 8.05 (s, 1H), 7.13 (m, 2H), 6.91 (dt, J = 6.3, 2.8 Hz, 1H), 6.22 (t, J = 6.7 Hz, 1H), 5.25 (d, J = 3.6 Hz, 1H), 4.97 (t, J = 4.7 Hz, 1H), 4.23–4.25 (m, 1H), 3.84 (s, 3H), 3.81–3.75 (m, 1H), 3.60–3.45 (m, 2H), 2.21–2.12 (m, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 161.8, 150.5, 148.6, 147.7, 140.1, 124.3, 123.1, 121.9, 113.7, 109.3, 88.0, 84.9, 70.8, 61.5, 56.5 (one peak under the DMSO-d6 signal); IR (neat): 3411, 3040, 2943, 2837, 1697, 1663, 1480, 1271, 1096, 1043, 1021, 790 cm−1; HRMS (ESI) calcd. for C16H17N2O6FNa (M+Na) 375.0968, Found 375.0971.
5-(4-Vinylphenyl)-2'-deoxyuridine (2k). DCM/MeOH (96/4), pale yellow gel (30% yield); 1H-NMR (400 MHz, DMSO-d6) δ 11.57 (bs, 1H), 8.31 (s, 1H), 7.62 (d, J = 8.3 Hz, 2H), 7.53 (d, J = 8.3 Hz, 2H), 6.80 (dd, J = 17.7, 10.8 Hz, 1H), 6.30 (t, J = 6.5 Hz, 1H), 5.91 (d, J = 17.7 Hz, 1H), 5.33 (d, J = 10.8 Hz, 1H), 5.32 (d, J = 3.6 Hz, 1H), 5.19 (t, J = 4.8 Hz, 1H), 4.38–4.34 (m, 1H), 3.91–3.82 (m, 1H), 3.71–3.59 (m, 2H), 2.37–2.14 (m, 2H); 13C-NMR (125 MHz, DMSO-d6) δ 162.5, 150.3, 138.4, 136.7, 136.4, 128.4, 126.3, 114.7, 113.4, 88.0, 85.0, 70.6, 61.4 (one peak under the DMSO-d6 signal); IR (neat): 3395, 3056, 2961, 1667, 1262, 1088, 1047, 1027, 792 cm−1; HRMS (ESI) calcd. for C17H18N2O5Na (M+Na) 353.1113, Found 353.1112.
5-(Furan-3-yl)-2'-deoxyuridine (2l). DCM/MeOH (96/4), spectroscopic data conformed to the literature [53].
5-(Thiophen-3-yl)-2'-deoxyuridine (2m). DCM/MeOH (96/4), spectroscopic data conformed to the literature [53].
5-(Furan-2-yl)-2'-deoxyuridine (2n). DCM/MeOH (96/4), spectroscopic data conformed to the literature [54].
4. Conclusions
In summary, we disclose herein a successful Suzuki-Miyaura reaction with 5-iodo-2'-deoxyuridine in a completely aqueous medium. The inexpensive and common triphenylphosphine combined with palladium acetate gave rise to the expected products in moderate to good yields. Furthermore, the variety of aryl and heteroaryl derivatives introduced demonstrates the generality of this method.
- Sample Availability: Samples of the compounds 2a–g, j, l, m are available from the authors.
References
- Amann, N.; Pandurski, E.; Fiebig, T.; Wagenknecht, H.-A. Electron Injection into DNA: Synthesis and Spectroscopic Properties of Pyrenyl-Modified Oligonucleotides. Chem. Eur. J. 2002, 8, 4877–4883. [Google Scholar] [CrossRef]
- Amann, N.; Pandurski, E.; Fiebig, T.; Wagenknecht, H.-A. A model nucleoside for electron injection into DNA: 5-Pyrenyl-2-deoxyribose. Angew. Chem. Int. Ed. Engl. 2002, 41, 2978–2980. [Google Scholar] [CrossRef]
- Okamoto, A.; Inasaki, T.; Saito, I. Synthesis and ESR studies of nitronyl nitroxide-tethered oligodeoxynucleotides. Tetrahedron Lett. 2005, 46, 791–795. [Google Scholar]
- Wagner, C.; Wagenknecht, H.-A. Reductive Electron Transfer in Phenothiazine-Modified DNA Is Dependent on the Base Sequence. Chem. Eur. J. 2005, 11, 1871–1876. [Google Scholar] [CrossRef]
- Roy, V.; Zerrouki, R.; Krausz, P. New dinucleoside analogues via cross-coupling metathesis. Nucleos. Nucleot. Nucl. Acid. 2005, 24, 289–301. [Google Scholar]
- Okamoto, A.; Tainaka, K.; Unzai, T.; Saito, I. Synthesis and fluorescence properties of dimethylaminonaphthalene-deoxyuridine conjugates as micropolarity-sensitive probes. Tetrahedron 2007, 63, 3465–3470. [Google Scholar] [CrossRef]
- Capek, P.; Cahová, H.; Pohl, R.; Hocek, M.; Gloeckner, C.; Marx, A. An Efficient Method for the Construction of Functionalized DNA Bearing Amino Acid Groups through Cross-Coupling Reactions of Nucleoside Triphosphates Followed by Primer Extension or PCR. Chem. Eur. J. 2007, 13, 6196–6203. [Google Scholar]
- Ehrenschwender, T.; Wagenknecht, H.-A. Synthesis and Spectroscopic Characterization of BODIPY-Modified Uridines as Potential Fluorescent Probes for Nucleic Acids. Synthesis 2008, 2008, 3657–3662. [Google Scholar] [CrossRef]
- Wanninger-Weiß, C.; Wagenknecht, H.-A. Synthesis of 5-(2-pyrenyl)-2'-deoxyuridine as a DNA Modification for Electron-Transfer Studies: The Critical Role of the Position of the Chromophore Attachment. Eur. J. Org. Chem. 2008, 2008, 64–71. [Google Scholar] [CrossRef]
- Colombeau, L.; Teste, K.; Hadj-Bouazza, A.; Chaleix, V.; Zerrouki, R.; Kreamer, M.; Sainte Catherine, O. Synthesis and biological activity of chloroethyl pyrimidine nucleosides. Nucleos. Nucleot. Nucl. Acid. 2008, 27, 110–120. [Google Scholar] [CrossRef]
- Jacobsen, M.F.; Ferapontova, E.E.; Gothelf, K.V. Synthesis and electrochemical studies of an anthraquinone-conjugated nucleoside and derived oligonucleotides. Org. Biomol. Chem. 2009, 7, 905–908. [Google Scholar] [CrossRef]
- Kalachova, L.; Pohl, R.; Hocék, M. Synthesis of 2'-Deoxyuridineand 2'-Deoxycytidine Nucleosides Bearing Bipyridine and Terpyridine Ligands at Position 5. Synthesis 2009, 2009, 105–112. [Google Scholar] [CrossRef]
- Raindlová, V.; Pohl, R.; Šanda, M.; Hocek, M. Direct Polymerase Synthesis of Reactive Aldehyde-Functionalized DNA and Its Conjugation and Staining with Hydrazines. Angew. Chem. Int. Ed. Engl. 2010, 49, 1064–1066. [Google Scholar]
- Macíčková-Cahová, H.; Pohl, R.; Horáková, P.; Havran, L.; Špaček, J.; Fojta, M.; Hocek, M. Alkylsulfanylphenyl Derivatives of Cytosine and 7-Deazaadenine Nucleosides, Nucleotides and Nucleoside Triphosphates: Synthesis, Polymerase Incorporation to DNA and Electrochemical Study. Chem. Eur. J. 2011, 17, 5833–5841. [Google Scholar]
- Segal, M.; Fischer, B. Analogues of uracil nucleosides with intrinsic fluorescence (NIF-analogues): Synthesis and photophysical properties. Org. Biomol. Chem. 2012, 10, 1571–1580. [Google Scholar] [CrossRef]
- Riedl, J.; Pohl, R.; Ruíšek, L.; Hocek, M. Synthesis and Photophysical Properties of Biaryl-Substituted Nucleos(t)ides. Polymerase Synthesis of DNA Probes Bearing Solvatochromic and pH-Sensitive Dual Fluorescent and 19F-NMR Labels. J. Org. Chem. 2012, 77, 1026–1044. [Google Scholar] [CrossRef]
- Peyrat, S.; Xie, J. Synthesis of Thymidine Dimers from 5'-O-Aminothymidine. Synthesis 2012, 2012, 1718–1724. [Google Scholar]
- De Clercq, E. Antiviral and Antitumor Activities of 5-Substituted 2'-Deoxyuridines. Meth. Find. Exp. Clin. Pharmacol. 1980, 2, 253–267. [Google Scholar]
- De Clercq, E. Biochemical aspects of the selective antiherpes activity of Nucleoside Analogues. Biochem. Pharmacol. 1984, 33, 2159–2169. [Google Scholar] [CrossRef]
- De Winter, H.; Herdewijn, P. Understanding the Binding of 5-Substituted 2'-Deoxyuridine Substrates to Thymidine Kinase of Herpes Simplex Virus Type-1. J.Med. Chem. 1996, 39, 4727–4737. [Google Scholar] [CrossRef]
- Wigerinck, P.; Snoeck, R.; Claes, P.; de Clercq, E.; Herdewijn, P. Synthesis and Antiviral Activity of 5-Heteroaryl-Substituted 2'-Deoxyuridines. J. Med. Chem. 1991, 34, 1767–1772. [Google Scholar] [CrossRef]
- Wigerinck, P.; Pannecouque, C.; Snoeck, R.; Claes, P.; de Clercq, E.; Herdewijn, P. 5-(5-Bromothien-2-yl)-2'-deoxyuridine and 5-(5-chlorothien-2-yl)-2'-deoxyuridine are equipotent to (E)-5-(2-bromovinyl)-2'-deoxyuridine in the inhibition of herpes simplex virus type I replication. J. Med. Chem. 1991, 34, 2383–2389. [Google Scholar] [CrossRef]
- Herdewijn, P. 5-Substituted-2'-deoxyuridines as anti-HSV agents: Synthesis and structure activity relationship. Antivir. Chem. Chemother. 1994, 5, 131–146. [Google Scholar]
- Kögler, M.; Vanderhoydonck, B.; de Jonghe, S.; Rozenski, J.; Van Belle, K.; Herman, J.; Louat, T.; Parchina, A.; Sibley, C.; Lescrinier, E.; Herdewijn, P. Synthesis and Evaluation of 5-Substituted 2'-deoxyuridine Monophosphate Analogues As Inhibitors of Flavin-Dependent Thymidylate Synthase in Mycobacterium tuberculosis. J. Med. Chem. 2011, 54, 4847–4862. [Google Scholar] [CrossRef]
- De Clercq, E.; Descamp, J.; DeSomer, P.; Barr, P.J.; Jones, A.S.; Walker, R.T. (E)-5-(2-bromovinyl)-2'-Deoxyuridine, A potent ans Selective Antiherpes Agent. Proc. Natl. Acad. Sci. USA 1979, 76, 2947–2951. [Google Scholar] [CrossRef]
- De Clercq, E.; Descamps, J.; Verhelst, G.; Walker, R.T.; Jones, A.S.; Torrence, P.F.; Shugar, D. Comparative Efficacy of Antiherpes Drugs Against Different Strains of Herpes Simplex Virus. J. Infect. Dis. 1980, 141, 563–574. [Google Scholar] [CrossRef]
- Kundu, N.G.; Dasgupta, S.K. Synthesis of 5-(acylethynyl)uracils and their corresponding 2'-Deoxyribonucleosides through Palladium-Catalyzed Reactions. J. Chem. Soc. Perkin Trans. I 1993, 21, 2657–2663. [Google Scholar]
- Lakshman, M.K. Palladium-catalyzed C-N and C-C cross-coupling as versatile, new avenues for modification of purine 2'-deoxynucleosides. J. Organomet. Chem. 2002, 653, 234–251. [Google Scholar] [CrossRef]
- Agrofoglio, L.A.; Gillaizeau, I.; Saito, Y. Palladium-assisted routes to nucleosides. Chem. Rev. 2003, 103, 1875–1916. [Google Scholar] [CrossRef]
- Lakshman, M.K. Synthesis of biologically important nucleoside analogs by palladium-catalyzed C-N bond formation. Curr. Org. Synth. 2005, 2, 83–112. [Google Scholar] [CrossRef]
- Thoresen, L.H.; Jiao, G.-S.; Haaland, W.C.; Metzker, M.L.; Burgess, K. Rigid, Conjugated, Fluoresceinated Thymidine Triphosphates: Syntheses and Polymerase Mediated Incorporation into DNA Analogues. Chem. Eur. J. 2003, 9, 4603–4610. [Google Scholar] [CrossRef]
- Ogino, M.; Yoshimura, Y.; Nakazawa, A.; Saito, I.; Fujimoto, K. Template-directed DNA photoligation via a 5-cyanovinyldeoxyuridine. Org. Lett. 2005, 7, 2853–2856. [Google Scholar]
- Takeda, S.; Tsukiji, S.; Nagamune, T. A cysteine-appended deoxyuridine for the postsynthetic DNA modification using native chemical ligation. Tetrahedron Lett. 2005, 46, 2235–2238. [Google Scholar] [CrossRef]
- Ding, H.; Greenberg, M.M. Hole Migration is the Major Pathway Involved in Alkali-Labile Lesion Formation in DNA by the Direct Effect of Ionizing Radiation. J. Am. Chem. Soc. 2007, 129, 772–773. [Google Scholar] [CrossRef]
- Ikonen, S.; Macíčková-Cahová, H.; Pohl, R.; Šanda, M.; Hocek, M. Synthesis of nucleoside and nucleotide conjugates of bile acids, and polymerase construction of bile acid-functionalized DNA. Org. Biomol. Chem. 2010, 8, 1194–1201. [Google Scholar]
- Cho, J.H.; Prickett, C.D.; Shaughnessy, K.H. Efficient Sonogashira Coupling of Unprotected halonucleosides in Aqueous Solvents Using Water-Soluble Palladium Catalysts. Eur. J. Org. Chem. 2010, 2010, 3678–3683. [Google Scholar] [CrossRef]
- Kielkowski, P.; Pohl, R.; Hocek, M. Synthesis of Acetylene Linked Double-Nucleobase Nucleos(t)ide Building Blocks and Polymerase Construction of DNA Containing Cytosines in the Major Groove. J. Org. Chem. 2011, 76, 3457–3462. [Google Scholar] [CrossRef]
- Cho, J.H.; Shaughnessy, K.H. Aqueous-Phase Heck Coupling of 5-Iodouridine and Alkenes under Phosphine-Free Conditions. Synlett 2011, 2011, 2963–2960. [Google Scholar] [CrossRef]
- Casalnuovo, A.L.; Calabrese, J.C. Palladium-catalyzed alkylations in aqueous media. J. Am. Chem. Soc. 1990, 112, 4324–4326. [Google Scholar] [CrossRef]
- Western, E.C.; Daft, J.R.; Johnson, E.M.; Gannett, P.M.; Shaughnessy, K.H. Efficient One-Step Suzuki Arylation of Unprotected Halonucleosides using Water-Soluble Palladium Catalysts. J. Org. Chem. 2003, 68, 6767–6774. [Google Scholar] [CrossRef]
- Daku, K.M.L.; Newton, R.F.; Pearce, S.P.; Vile, J.; Williams, J.M.J. Suzuki cross-coupling reactions using reverse-phase glass beads in aqueous media. Tetrahedron Lett. 2003, 44, 5095–5098. [Google Scholar]
- Pesnot, T.; Wagner, G.K. Novel derivatives of UDP-glucose: Concise synthesis and fluorescent properties. Org. Biomol. Chem. 2008, 6, 2884–2891. [Google Scholar] [CrossRef]
- Sartori, G.; Enderlin, G.; Hervé, G.; Len, C. Highly Effective Synthesis of C-5-Substituted 2'-Deoxyuridine Using Suzuki-Miyaura Cross-Coupling in Water. Synthesis 2012, 2012, 767–772. [Google Scholar]
- El Kazzouli, S.; Berteina-Raboin, S.; Agrofoglio, L.A. Supported synthesis and functionnalization of 2"-deoxyuridine by suzuki-miyaura cross-coupling. Nucleos. Nucleot. Nucl. Acid. 2007, 26, 1395–1398. [Google Scholar] [CrossRef]
- Fresneau, N.; Hiebel, M.-A.; Agrofoglio, L.A.; Berteina-Raboin, S. One-pot Sonogashira-cyclization protocol to obtain substituted furopyrimidine nucleosides in aqueous conditions. Tetrahedron Lett. 2012, 53, 1760–1763. [Google Scholar] [CrossRef]
- Huang, R.; Shaughnessy, K.H. Water-Soluble Palladacycles as Precursors to Highly Recyclable Catalysts for the Suzuki Coupling of Aryl Bromides in Aqueous Solvents. Organometallics 2006, 25, 4105–4112. [Google Scholar] [CrossRef]
- Moore, L.R.; Western, E.C.; Craciun, R.; Spruell, J.M.; Dixon, D.A.; O’Halloran, K.P.; Shaughnessy, K.H. Sterically Demanding, Sulfonated, Triarylphosphines: Application to Palladium-Catalyzed Cross-Coupling, Steric and Electronic Properties, and Coordination Chemistry. Organometallics 2008, 27, 576–593. [Google Scholar] [CrossRef]
- Fleckenstein, C.A.; Plenio, H. Aqueous cross-coupling: Highly efficient Suzuki-Miyaura coupling of N-heteroaryl halides and N-heteroarylboronic acids. Green Chem. 2007, 9, 1287–1291. [Google Scholar] [CrossRef]
- Krauter, J.G.E.; Beller, M. An Easy and Practical Synthetic Route to Electron Rich Water Soluble Ligands: α-Aminomethylation of Trishydroxymethylphosphine. Tetrahedron 2000, 56, 771–774. [Google Scholar] [CrossRef]
- Shaughnessy, K.H. Hydrophilic Ligands and Their Application in Aqueous-Phase Metal-Catalyzed Reactions. Chem. Rev. 2009, 109, 643–710. [Google Scholar] [CrossRef]
- Zayas, H.A.; Bowyer, M.C.; Gordon, C.P.; Holdsworth, C.I.; Mc Cluskey, A. Synthesis of biaryl-styrene monomers by microwave-assisted Suzuki coupling. Tetrahedron Lett. 2009, 50, 5894–5895. [Google Scholar]
- Chang, G.; Mertes, M.P. Linear free energy relationships studies of 5-substituted 2,4-dioxopyrimidine nucleosides. J. Org. Chem. 1987, 52, 3625–3631. [Google Scholar] [CrossRef]
- Wigerinck, P.; Kerremans, L.; Cleas, P.; Snoeck, R.; Maudgal, P.; de Clercq, E.; Herdewijn, P. Synthesis and antiviral activity of 5-thien-2-yl-2'-deoxyuridine analogs. J. Med. Chem. 1993, 36, 538–543. [Google Scholar] [CrossRef]
- Aucagne, V.; Berteina-Raboin, S.; Guenot, P.; Agrofoglio, L.A. Palladium-catalyzed parallel solid-phase synthesis of nucleosides. J. Comb. Chem. 2004, 6, 717–723. [Google Scholar] [CrossRef]
© 2012 by the authors; licensee MDPI, Basel, Switzerland. This article is an open-access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
