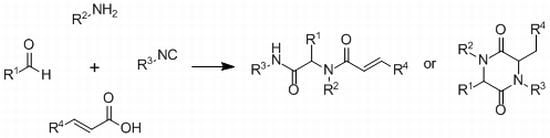
3.3. General Procedure for the Synthesis of Piperazine-2,5-diones 6a–j
To a solution of benzaldehyde derivative (1 eq.) in absolute methanol the corresponding amine (1 eq.) was added and stirred for 30 min at room temperature. Then p-toluenesulfonylmethyl isocyanide (1 eq.) and the corresponding benzoylacryclic acid derivative (1 eq.) were added. The solution was stirred until the reaction, controlled by means of TLC, was finished. If necessary, the reaction mixture was heated.
3-(2-Bromophenyl)-6-(2-oxo-2-phenylethyl)-4-(pyridin-3-ylmethyl)-1-(tosylmethyl)piperazine-2,5-dione (6a). Quantities: 2-bromobenzaldehyde (470 µL, 4.0 mmol), 3-(aminomethyl)pyridine (410 µL, 4.0 mmol), p-toluenesulfonylmethyl isocyanide (940 mg, 4.8 mmol), 3-benzoylacrylic acid (710 mg, 4.0 mmol). The solvent was evaporated in vacuo and the residue was purified by column chromatography (1st silica gel 60, n-hexane/ethyl acetate 1:20) to give the purified mixture of cis- and trans-product in a yield of 35%. A second purification step by column chromatography (2nd flash silica gel, dichloromethane/methanol 10:0.5) gave the cis- and trans-diastereomers separately. trans-6a: Colorless amorphous solid (341 mg, 13%): mp 111–114 °C; IR ν 1657, 1596, 1422, 1319, 1140, 1085, 762, 688 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.40 (dd, 4.8 Hz, 1.6 Hz, 1H), 8.06 (d, 1.8 Hz, 1H), 8.02 (dd, 7.8 Hz, 1.2 Hz, 2H), 7.71 (d, 8.1 Hz, 2H), 7.70 (tt, 7.8 Hz, 1.2 Hz, 1H), 7.58 (t, 7.8 Hz, 2H), 7.55 (dd, 7.7 Hz, 1.3 Hz, 1H), 7.37–7.29 (m, 2H), 7.35 (d, 8.1 Hz, 2H), 7.26 (td, 7.7 Hz, 1.7 Hz, 1H), 7.23 (dd, 7.8 Hz, 4.8 Hz, 1H), 7.13 (dd, 7.7 Hz, 1.7 Hz, 1H), 5.68 (s, 1H), 5.36 (d, 14.8 Hz, 1H), 4.97 (d, 14.8 Hz, 1H), 4.96–4.92 (m, 1H), 4.84 (d, 15.4 Hz, 1H), 4.33 (dd, 19.1 Hz, 1H), 3.92 (dd, 19.1 Hz, 1H), 3.74 (d, 15.4 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.6, 165.9, 164.5, 149.1, 148.4, 144.7, 135.8, 135.6, 135.2, 135.0, 133.7, 132.8, 130.9, 130.6, 130.3, 129.8, 128.6, 128.1 (2C), 128.0, 124.1, 123.1, 62.8, 62.4, 55.9, 45.0, 39.9, 21.0; HRMS (MS-TOF): [M+H]+ calcd. for C32H29BrN3O5S: 646.1006, found: 646.1001. cis-6a: Colorless amorphous solid (161 mg, 6%): mp 99–100 °C; IR ν 1657, 1595, 1422, 1323, 1140, 1084, 761, 688 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.48 (dd, 4.7 Hz, 1.7 Hz, 1H), 8.24 (d, 1.7 Hz, 1H), 8.10 (d, 9.3 Hz, 1.2 Hz, 2H), 7.84 (dd, 7.8 Hz, 1.3 Hz, 1H), 7.70 (tt, 7.4 Hz, 1.2 Hz, 1H), 7.63–7.56 (m, 4H), 7.56 (dd, 7.8 Hz, 1.0 Hz, 1H), 7.51 (dt, 7.9 Hz, 1.7 Hz, 1H), 7.45 (td, 7.8 Hz, 1.0 Hz, 1H), 7.34 (dd, 7.9 Hz, 4.7 Hz, 1H), 7.27 (td, 7.8 Hz, 1.3 Hz, 1H), 7.19 (d, 8.1 Hz, 2H), 5.24 (s, 1H), 5.06–5.00 (m, 1H), 5.12 (d, 14.7 Hz, 1H), 5.01 (d, 14.7 Hz, 1H), 4.87 (d, 15.5 Hz, 1H), 4.21 (dd, 18.7 Hz, 4.4 Hz, 1H), 3.99 (dd, 18.7 Hz, 4.1 Hz, 1H), 3.76 (d, 15.5 Hz, 1H), 2.26 (s, 3H); 13C NMR (100 M Hz, DMSO): δ 196.9, 165.2, 163.9, 149.1, 148.6, 144.7, 136.1, 135.6, 135.0, 134.1, 133.4, 132.7, 131.1, 130.4, 129.8, 129.6, 128.6, 128.4, 128.3, 128.1, 124.7, 123.4, 63.9, 61.4, 56.0, 44.9, 40.6, 21.0; HRMS (MS-TOF): [M+H]+ calcd. for C32H29BrN3O5S: 646.1006, found: 646.1008.
3-(2-Oxo-2-phenylethyl)-6-phenyl-1-(pyridin-3-ylmethyl)-4-(tosylmethyl)piperazine-2,5-dione (trans-6b). Quantities: benzaldehyde (900 µL, 8.9 mmol), 3-(aminomethyl)pyridine (910 µL, 8.9 mmol), p-toluenesulfonylmethyl isocyanide (1.74 g, 8.9 mmol), 3-benzoylacrylic acid (1.60 g, 8.9 mmol). The precipitate was filtered, dissolved in dichloromethane and crystallization initiated by adding diethyl ether to give pure trans-6b. Colorless solid (2.28 g, 45%): mp 204–205 °C; IR ν 2952, 1662, 1597, 1429, 1418, 1319, 1289, 1141, 1087, 757, 713, 691 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.42 (dd, 4.9 Hz, 1.6 Hz, 1H), 8.17 (d, 1.6 Hz, 1H) 8.00 (d, 7.5 Hz, 2H), 7.69 (t, 7.5 Hz, 1H), 7.63 (d, 8.3 Hz, 2H), 7.57 (t, 7.5 Hz, 2H), 7.41 (dt, 7.5 Hz, 1.6 Hz, 1H), 7.39–7.30 (m, 1H), 7.33 (d, 7.8 Hz, 2H), 7.27 (d, 8.3 Hz, 2H), 7.25 (dd, 7.5 Hz, 4.9 Hz, 1H ), 7.08 (dd, 7.8 Hz, 1.2 Hz, 2H), 5.48 (d, 14.9 Hz, 1H), 5.20 (s, 1H), 4.93 (dd, 4.4 Hz, 3.5 Hz, 1H), 4.88 (d, 14.9 Hz, 1H), 4.86 (d, 15.3 Hz, 1H), 4.23 (dd, 18.9 Hz, 4.4 Hz, 1H), 3.88 (dd, 18.9 Hz, 3.5 Hz, 1H), 3.79 (d, 15.3 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.3, 165.7, 165.5, 149.0, 148.3, 144.7, 135.9, 135.7, 135.4, 134.6, 133.6, 131.4, 129.8, 128.6, 128.5, 128.3, 128.03, 127.97, 127.90, 123.1, 63.4, 61.7, 55.4, 45.2, 37.9, 21.0; LCMS (ESI+): m/z 567.4 [M]+; Anal. calcd. for C32H29N3O5S: C 67.71, H 5.15, N 7.40, S 5.65; found: C 67.39, H 5.11, N 7.33, S 5.54.
3-(2-Chlorophenyl)-6-(2-oxo-2-phenylethyl)-4-(pyridin-3-ylmethyl)-1-(tosylmethyl)piperazine-2,5-dione (6c). Quantities: 2-chlorobenzaldehyde (230 µL, 2.0 mmol), 3-(aminomethyl)pyridine (200 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The solvent was evaporated in vacuo and the residue was purified by column chromatography (1st silica gel 60, dichloromethane/methanol 10:0.5) to give the purified mixture of cis- and trans-product in a yield of 35%. A second purification step by column chromatography (2nd flash silica gel, chloroform/ethanol 10:0.1) gave the cis- and trans-diastereomers separately. trans-6c: Slightly yellow solid (102 mg, 9%): mp 135–138 °C; IR ν 2940, 1652, 1596, 1433, 1324, 1141, 1086, 1029, 815, 753, 689 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.40 (dd, 4.7 Hz, 1.7 Hz, 1H), 8.05 (d, 1.7 Hz, 1H), 8.01 (dd, 7.7 Hz, 1.2 Hz, 2H), 7.71 (d, 8.3 Hz, 2H), 7.69 (tt, 7.7 Hz, 1.2 Hz, 1H), 7.57 (t, 7.7 Hz, 2H), 7.38–7.31 (m, 5H), 7.30–7.25 (m, 1H), 7.23 (dd, 7.8 Hz, 4.7 Hz, 1H), 7.17 (d, 7.0 Hz, 1H), 5.66 (s, 1H), 5.38 (d, 14.8 Hz, 1H), 4.97 (d, 14.8 Hz, 1H), 4.94 (dd, 4.2 Hz, 3.4 Hz, 1H), 4.83 (d, 15.4 Hz, 1H), 4.31 (dd, 19.1 Hz, 4.2 Hz, 1H), 3.92 (dd, 19.1 Hz, 3.4 Hz, 1H), 3.77 (d, 15.4 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.6, 165.9, 164.6, 149.1, 148.4, 144.7, 135.8, 135.5, 135.0, 133.7, 133.5, 133.4, 130.9, 130.7, 130.1, 129.8, 129.6, 128.6, 128.07, 128.05, 127.4, 123.1, 62.8, 60.4, 55.9, 45.0, 39.9, 21.0; HRMS (MS-TOF): [M+H]+ calcd. for C32H29ClN3O5S: 602.1511, found: 602.1510. cis-6c: slightly yellow solid (103 mg, 9%): mp 79–82 °C; IR ν 2934, 1657, 1596, 1423, 1326, 1141, 1085, 1028, 815, 752, 688 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.48 (dd, 4.7 Hz, 1.7 Hz, 1H), 8.22 (d, 1.7 Hz, 1H), 8.09 (d, 7.5 Hz, 2H), 7.83 (d, 7.8 Hz, 1H), 7.70 (tt, 7.5 Hz, 1.2 Hz, 1H), 7.60 (d, 8.0 Hz, 2H), 7.59 (t, 7.5 Hz, 2H), 7.51 (dt, 7.9 Hz, 1.7 Hz, 1H), 7.44–7.36 (m, 3H), 7.36–7.32 (m, 1H), 7.18 (d, 8.0 Hz, 2H), 5.23 (s, 1H), 5.12 (d, 14.5 Hz, 1H), 5.03 (dd, 4.4 Hz, 4.2 Hz, 1H), 5.00 (d, 14.5 Hz, 1H), 4.88 (d, 15.4 Hz, 1H), 4.19 (dd, 18.7 Hz, 4.4 Hz, 1H), 3.98 (dd, 18.7 Hz, 4.2 Hz, 1H), 3.77 (d, 15.4 Hz, 1H), 2.26 (s, 3H); 13C NMR (100 MHz, DMSO): δ 196.9, 165.3, 163.9, 149.1, 148.6, 144.7, 136.1, 135.6, 134.1, 133.8, 133.4, 133.2, 131.0, 130.2, 129.8, 129.6, 129.4, 128.6, 128.3, 128.1, 127.8, 123.3, 63.9, 58.9, 56.0, 45.0, 40.7, 20.9; HRMS (MS-TOF): [M+H]+ calcd. for C32H29ClN3O5S: 602.1511, found: 602.1513.
3-(2-Iodophenyl)-6-(2-oxo-2-phenylethyl)-4-(pyridin-3-ylmethyl)-1-(tosylmethyl)piperazine-2,5-dione (6d). Quantities: 2-iodobenzaldehyde (465 mg, 2.0 mmol), 3-(aminomethyl)pyridine (200 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The solvent was evaporated in vacuo and the residue was purified by column chromatography (1st silica gel 60, dichloromethane/methanol 10:0.5) to give the purified mixture of cis- and trans-product in a yield of 45%. A second purification step by column chromatography (2nd flash silica gel, chloroform/ethanol 10:0.2) gave the cis- and trans-diastereomers separately. trans-6d: Colorless amorphous solid (210 mg, 15%): mp 101–104 °C; IR ν 2929, 1657, 1596, 1580, 1422, 1318, 1140, 1085, 1013, 815, 759, 688 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.41 (dd, 4.8 Hz, 1.7 Hz, 1H), 8.08 (d, 1.7 Hz, 1H), 8.02 (dd, 7.9 Hz, 1.3 Hz, 2H), 7.81 (dd, 7.7 Hz, 1.4 Hz, 1H), 7.73–7.67 (m, 2H), 7.71 (d, 8.2 Hz, 2H), 7.58 (t, 7.9 Hz, 2H), 7.35 (d, 8.2 Hz, 2H), 7.32 (td, 7.7 Hz, 1.1 Hz, 1H), 7.25 (dd, 7.9 Hz, 4.8 Hz, 1H), 7.07 (td, 7.7 Hz, 1.4 Hz, 1H), 7.03 (dd, 7.7 Hz, 1.1 Hz, 1H), 5.61 (s, 1H), 5.39 (d, 14.7 Hz, 1H), 4.96–4.93 (m, 1H), 4.95 (d, 14.7 Hz, 1H), 4.86 (d, 15.5 Hz, 1H), 4.35 (dd, 19.1 Hz, 4.1 Hz, 1H), 3.91 (dd, 19.1 Hz, 3.2 Hz, 1H), 3.67 (d, 15.5 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.7, 165.9, 164.6, 149.1, 148.4, 144.7, 139.4, 138.4, 135.8, 135.6, 134.8, 133.7, 132.2, 130.8, 130.2, 129.8, 129.4, 128.6, 128.1 (3C), 101.7, 66.4, 62.5, 55.9, 44.9, 38.8, 21.0; HRMS (MS-TOF): [M+H]+ calcd. for C32H29IN3O5S: 694.0867, found: 694.0864. cis-6d: Colorless amorphous solid (75 mg, 5%): mp 115–118 °C; IR ν 2933, 1657, 1596, 1579, 1421, 1323, 1140, 1085, 1012, 814, 754, 688 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.47 (dd, 4.8 Hz, 1.9 Hz, 1H), 8.26 (d, 1.9 Hz, 1H), 8.10 (dd, 7.7 Hz, 1.2 Hz, 2H), 7.82 (dd, 7.8 Hz, 1.1 Hz, 1H), 7.78 (dd, 7.8 Hz, 1.3 Hz, 1H), 7.70 (tt, 7.7 Hz, 1.2 Hz, 1H), 7.62–7.57 (m, 4H), 7.52 (dt, 8.0 Hz, 1.9 Hz, 1H), 7.45 (td, 7.8 Hz, 1.1 Hz, 1H), 7.35 (dd, 8.0 Hz, 4.8 Hz, 1H), 7.20 (d, 8.0 Hz, 2H), 7.08 (td, 7.8 Hz, 1.3 Hz, 1H), 5.14 (s, 1H), 5.11 (d, 14.5 Hz, 1H), 5.04 (dd, 4.4 Hz, 3.9 Hz, 1H), 5.00 (d, 14.5 Hz, 1H), 4.85 (d, 15.4 Hz, 1H), 4.19 (dd, 18.9 Hz, 4.4 Hz, 1H), 3.99 (dd, 18.6 Hz, 3.9 Hz, 1H), 3.73 (d, 15.4 Hz, 1H), 2.27 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 196.9, 165.2, 164.0, 149.0, 148.5, 144.8, 139.4, 138.3, 136.1, 135.6, 134.0, 133.4, 131.1, 130.3, 129.7, 129.0, 128.8, 128.6, 128.3, 128.1, 123.4, 102.5, 66.0, 63.9, 55.9, 44.9, 40.8, 21.1; HRMS (MS-TOF): [M+H]+ calcd. for C32H29IN3O5S: 694.0867, found: 694.0868.
3-(4-Fluorophenyl)-6-(2-oxo-2-phenylethyl)-4-(pyridin-3-ylmethyl)-1-(tosylmethyl)piperazine-2,5-dione (trans-6e). Quantities: 4-fluorobenzaldehyde (220 µL, 2.0 mmol), 3-(aminomethyl)pyridine (200 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The precipitate was filtered and washed with cold methanol to give pure trans-6e. Colorless solid (494 mg, 42%): mp 174–175 °C; IR ν 2984, 1684, 1652, 1597, 1579, 1510, 1422, 1329, 1221, 1145, 1088, 812, 753, 687 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.41 (dd, 4.9 Hz, 1.8 Hz, 1H), 8.17 (d, 1.8 Hz, 1H), 7.98 (d, 7.7 Hz, 2H), 7.69 (tt, 7.7 Hz, 1.1 Hz, 1H), 7.65 (d, 8.3 Hz, 2H), 7.57 (t, 7.7 Hz, 2H), 7.41 (dt, 8.0 Hz, 1.8 Hz, 1H), 7.28 (d, 8.0 Hz, 2H), 7.25 (dd, 8.0 Hz, 4.9 Hz, 1H), 7.12 (d, 7.3 Hz, 4H), 5.49 (d, 14.9 Hz, 1H), 5.24 (s, 1H), 4.93 (dd, 4.2 Hz, 3.5 Hz, 1H), 4.88 (d, 14.9 Hz, 1H), 4.82 (d, 15.3 Hz, 1H), 4.25 (dd, 18.9 Hz, 4.2 Hz, 1H), 3.89 (dd, 18.9 Hz, 3.5 Hz, 1H), 3.84 (d, 15.3 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.4, 165.6, 165.3, 161.9 (d, 1J(C,F) = 244.9 Hz), 149.0, 148.3, 144.7, 135.9, 135.5, 134.5, 133.6, 132.1 (d, 4J(C,F) = 3.0 Hz), 131.4, 130.2 (d, 3J(C,F) = 8.6 Hz), 129.7, 128.6, 128.0 (2C), 123.1, 115.2 (d, 2J(C,F) = 21.9 Hz), 62.7, 61.5, 55.4, 45.2, 38.0, 20.9; LCMS (ESI+): m/z 586.0 [M+H]+; Anal. calcd. for C32H28FN3O5S: C 65.63, H 4.82, N 7.18, S 5.48; found: C 65.95, H 4.88, N 7.27, S 5.36.
1-Benzyl-3-(2-oxo-2-phenylethyl)-6-phenyl-4-(tosylmethyl)piperazine-2,5-dione (trans-6f). Quantities: benzaldehyde (200 µL, 2.0 mmol), benzylamine (220 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The precipitate was filtered and washed with cold methanol to give pure trans-6f. Colorless solid (628 mg, 55%): mp 201–203 °C; IR ν 2929, 1662, 1598, 1421, 1318, 1143, 1087, 814, 749, 691 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.01 (dd, 7.4 Hz, 1.5 Hz, 2H), 7.69 (tt, 7.4 Hz, 1.5 Hz, 1H), 7.62 (d, 8.3 Hz, 2H), 7.58 (t, 7.4 Hz, 2H), 7.41–7.31 (m, 3H), 7.27–7.22 (m, 5H), 7.05 (dd, 7.8 Hz, 1.4 Hz, 2H), 7.00 (dd, 6.6 Hz, 2.9 Hz, 2H), 5.48 (d, 14.9 Hz, 1H), 5.05 (d, 15.2 Hz, 1H), 5.04 (s, 1H), 4.92 (t, 3.7 Hz, 1H), 4.87 (d, 14.9 Hz, 1H), 4.20 (dd, 18.8 Hz, 3.7 Hz, 1H), 3.90 (dd, 18.8 Hz, 3.7 Hz, 1H), 3.57 (d, 15.2 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 MHz, DMSO): δ 197.2, 165.5, 165.4, 144.7, 136.0, 135.5, 135.4, 134.5, 133.6, 129.7, 128.6, 128.5, 128.3, 128.2, 128.02, 127.97, 127.7 (2C), 127.2, 62.9, 61.5, 55.4, 46.9, 37.7, 21.0; LCMS (ESI+): m/z 566.8 [M]+; Anal. calcd. for C33H30N2O5S: C 69.94, H 5.34, N 4.94, S 5.66; found: C 70.10, H 5.35, N 5.07, S 5.60.
3-(2-Oxo-2-phenylethyl)-1-phenethyl-6-phenyl-4-(tosylmethyl)piperazine-2,5-dione (trans-6g). Quantities: benzaldehyde (200 µL, 2.0 mmol), phenethylamine (250 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The precipitate was filtered, dissolved in dichloromethane and extracted with aqueous saturated solution of sodium hydrogen carbonate to give pure trans-6g. Colorless solid (135 mg, 12%): mp 198–201 °C; IR ν 2931, 1685, 1655, 1597, 1427, 1326, 1142, 1087, 813, 752, 689 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.02 (dd, 7.5 Hz, 1.3 Hz, 2H), 7.69 (tt, 7.5 Hz, 1.3 Hz, 1H), 7.65 (d, 8.2 Hz, 2H), 7.57 (t, 7.5 Hz, 2H), 7.39–7.34 (m, 3H), 7.31 (d, 8.2 Hz, 2H), 7.21 (t, 7.2 Hz, 2H), 7.17 (dd, 7.1 Hz, 2.2 Hz, 2H), 7.14 (tt, 7.2 Hz, 1.4 Hz, 1H), 7.00 (dd, 7.2 Hz, 1.4 Hz, 2H), 5.49 (d, 14.8 Hz, 1H), 5.36 (s, 1H), 4.88 (d, 14.8 Hz, 1H), 4.84 (dd, 4.4 Hz, 3.6 Hz, 1H), 4.15 (dd, 18.7 Hz, 4.4 Hz, 1H), 3.83 (dd, 18.7 Hz, 3.6 Hz, 1H), 3.71 (ddd, 13.3 Hz, 10.7 Hz, 5.3 Hz, 1H), 2.80 (ddd, 12.7 Hz, 10.5 Hz, 5.3 Hz, 1H), 2.65 (ddd, 13.3 Hz, 10.5 Hz, 5.6 Hz, 1H), 2.40 (ddd, 12.7 Hz, 10.7 Hz, 5.6 Hz, 1H), 2.36 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.1, 165.8, 164.9, 144.7, 138.5, 136.6, 135.9, 134.6, 133.6, 129.8, 128.6, 128.5, 128.33, 128.28, 128.2, 128.1, 128.0 (2C), 126.1, 63.2, 61.8, 55.4, 46.1, 38.1, 31.6, 21.0; Anal. calcd. for C34H32N2O5S: C 70.32, H 5.55, N 4.82, S 5.52; found: C 70.44, H 5.68, N 4.93, S 5.52.
1-(4-Methoxybenzyl)-3-(2-oxo-2-phenylethyl)-6-phenyl-4-(tosylmethyl)piperazine-2,5-dione (trans-6h). Quantities: benzaldehyde (200 µL, 2.0 mmol), 4-methoxybenzylamine (260 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-benzoylacrylic acid (350 mg, 2.0 mmol). The precipitate was filtered and washed with cold methanol to give pure trans-6h. Colorless solid (460 mg, 39%): mp 241–243 °C; IR ν 2930, 1659, 1611, 1598, 1611, 1598, 1510, 1432, 1318, 1247, 1178, 1144, 1088, 824, 754, 690 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.00 (dd, 7.6 Hz, 1.2 Hz, 2H), 7.69 (tt, 7.6 Hz, 1.2 Hz, 1H), 7.60 (d, 8.1 Hz, 2H), 7.58 (t, 7.6 Hz, 2H), 7.42–7.33 (m, 3H), 7.24 (d, 8.1 Hz, 2 H), 7.05 (dd, 7.7 Hz, 1.6 Hz, 2H), 6.92 (d, 8.7 Hz, 2H), 6.80 (d, 8.7 Hz, 2H), 5.47 (d, 14.9 Hz, 1H), 5.04 (d, 14.9 Hz, 1H), 4.98 (s, 1H), 4.90 (dd, 4.5 Hz, 3.7 Hz, 1H), 4.86 (d, 14.9 Hz, 1H), 4.18 (dd, 18.7 Hz, 4.5 Hz, 1H), 3.88 (dd, 18.7 Hz, 3.7 Hz, 1H), 3.72 (s, 3H), 3.44 (d, 14.9 Hz, 1H), 2.33 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 197.1, 165.6, 165.3, 158.5, 144.6, 136.0, 135.6, 134.5, 133.5, 129.7, 129.3, 128.6, 128.5, 128.2, 128.02, 127.96, 127.7, 127.1, 113.6, 62.6, 61.5, 55.4, 54.9, 46.2, 37.6, 21.0; LCMS (ESI+): m/z 596.4 [M]+; Anal. calcd. for C34H32N2O6S: C 68.44, H 5.41, N 4.69, S 5.37; found: C 68.47, H 5.43, N 4.86, S 5.40.
3-(2-(4-Chlorophenyl)-2-oxoethyl)-6-phenyl-1-(pyridin-3-ylmethyl)-4-(tosylmethyl)piperazine-2,5-dione (trans-6i). Quantities: benzaldehyde (200 µL, 2.0 mmol), 3-(aminomethyl)pyridine (200 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-(4-chlorobenzoyl)acrylic acid (420 mg, 2.0 mmol). The precipitate was filtered, dissolved in ethyl acetate and crystallization initiated by adding n-hexane to give pure trans-6i. Colorless solid (449 mg, 37%): mp 233–234 °C; IR ν 2940, 1686, 1651, 1591, 1576, 1435, 1325, 1142, 815, 754, 699 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.46 (dd, 4.7 Hz, 1.8 Hz, 1H), 8.17 (d, 1.8 Hz, 1H), 7.99 (ddd, 8.6 Hz, 2.4 Hz, 1.8 Hz, 2H), 7.64 (ddd, 8.8 Hz, 2.4 Hz, 1.8 Hz, 2H), 7.62 (d, 8.5 Hz, 2H), 7.41 (dt, 7.9 Hz, 1.8 Hz, 1H), 7.37–7.29 (m, 3H), 7.26 (d, 8.5 Hz, 2H), 7.26–7.23 (m, 1H), 7.07 (dd, 7.8 Hz, 1.1 Hz, 2H), 5.46 (d, 14.9 Hz, 1H), 5.18 (s, 1H), 4.93 (dd, 4.4 Hz, 3.6 Hz, 1H), 4.88 (d, 14.9 Hz, 1H), 4.86 (d, 15.3 Hz, 1H), 4.21 (dd, 18.9 Hz, 4.4 Hz, 1H), 3.88 (dd, 18.9 Hz, 3.6 Hz, 1H), 3.79 (d, 15.3 Hz, 1H), 2.34 (s, 3H); 13C-NMR (100 MHz, DMSO): δ 196.4, 165.6, 165.4, 149.0, 148.3, 144.7, 128.7, 138.5, 135.6, 135.4, 134.6, 134.5, 131.4, 129.9, 129.7, 128.5, 128.3, 127.98, 127.92, 123.2, 63.4, 61.7, 55.4, 45.3, 38.0, 21.0; LCMS (ESI+): m/z 602.0 [M]+; HRMS (MS-TOF): [M+H]+ calcd. for C32H29ClN3O5S: 602.1511, found: 602.1515.
3-(2-(4-Methoxyphenyl)-2-oxoethyl)-6-phenyl-1-(pyridin-3-ylmethyl)-4-(tosylmethyl)piperazine-2,5-dione (trans-6j). Quantities: benzaldehyde (200 µL, 2.0 mmol), 3-(aminomethyl)pyridine (200 µL, 2.0 mmol), p-toluenesulfonylmethyl isocyanide (390 mg, 2.0 mmol), 3-(4-methoxybenzoyl)acrylic acid (410 mg, 2.0 mmol). The precipitate was filtered and washed with cold methanol to give pure trans-6j. Colorless solid (434 mg, 36%): mp 250–252 °C; IR ν 2938, 1650, 1601, 1576, 1510, 1437, 1327, 1261, 1171, 1142, 1089, 825, 754, 699 cm−1; 1H-NMR (400 MHz, DMSO) δ 8.41 (dd, 4.8 Hz, 1.8 Hz, 1H), 8.15 (d, 1.8 Hz, 1H), 7.97 (d, 9.0 Hz, 2H), 7.64 (d, 8.2 Hz, 2H), 7.39 (dt, 7.9 Hz, 1. 8 Hz, 1H), 7.36-7.30 (m, 3H), 7.24 (dd, 7.9 Hz, 4.8 Hz, 1H), 7.28 (d, 8.2 Hz, 2H), 7.08 (d, 9.0 Hz, 2H), 7.06 (dd, 8.3 Hz, 1.4 Hz, 2H), 5.48 (d, 14.8 Hz, 1H), 5.17 (s, 1H), 4.89 (dd, 4.3 Hz, 3.2 Hz, 1H), 4.85 (d, 15.4 Hz, 1H), 4.84 (d, 14.8 Hz, 1H), 4.18 (18.7 Hz, 4.3 Hz, 1H), 3.87 (s, 3H), 3.81 (18.7 Hz, 3.2 Hz, 1H), 3.78 (d, 15.4 Hz, 1H), 2.34 (s, 3H); 13C NMR (100 M Hz, DMSO): δ 195.6, 165.8, 165.6, 163.5, 149.0, 148.3, 144.7, 136.0, 135.4, 134.6, 131.4, 130.4, 129.8, 128.9, 128.4, 128.3, 128.04, 128.00, 123.1, 113.8, 63.4, 61.6, 55.52, 55.50, 45.2, 37.5, 21.0; LCMS (ESI+): m/z 598.2 [M+H]+; HRMS (MS-TOF): [M+H]+ calcd. for C33H32N3O6S: 598.2006, found: 598.2009.