1. Introduction
The genus
Calophyllum (Clusiaceae/Guttiferae) comprises an extensive group of tropical trees with approximately 180–200 species restricted to the hot and humid tropics [
1]. The genus includes various trees, shrubs, lianas and herbs of economical interest for the production of fruits, timber, chemical compounds with pharmaceutical properties and paints [
2].
Calophyllum brasiliense Cambess, popularly known as
guanandi [
3], is a rich source of bioactive compounds such as coumarins, xanthones, steroids, triterpenes and bioflavonoids [
4,
5,
6,
7,
8]. Ethnopharmacological studies have already reported the use of this species against bronchitis, gastritis, hepatitis [
9], pain [
10], inflammations, diabetes, hypertension [
11], diarrhea [
12] and herpes [
13]. It is one of the most studied species due to its biological activities, with special attention to the antibacterial [
14,
15,
16], antifungal [
14], cytotoxic [
16], tumor inhibitory [
17], and HIV-1 IIIb/LAV replication inhibitory, which are attributed to the leaves, stems and roots extracts [
18]. Extracts and fractions of its leaves have demonstrated leishmanicidal effects against promastigotes and amastigotes of
Leishmania amazonensis [
19,
20], as well as antiviral activity [
21].
Traditionally, the extraction of bioactive compounds from herbs has been performed by steam distillation or by the use of organic solvent-based methods such as the maceration, percolation and Soxhlet techniques. An alternative method is the use of the supercritical fluid technology that employs gases above their critical pressures and temperatures as solvents to selectively extract soluble components from raw materials [
22]. Carbon dioxide (CO
2) has gained the best acceptance since it offers many advantages, such as mild supercritical conditions, low cost, easy manufacture, non-toxic and non-flammable properties, ready availability and easy removal from the extracted products [
23]. Beside this, the use of carbon dioxide provides the advantage of being suitable for extracting thermo- labile compounds due the fact that excessive solvent heating is not necessary [
24,
25]. Nowadays, supercritical fluid extraction, which was developed in 1960, is used in a wide variety of areas, including the ood, pharmacy and environmental engineering industries [
26].
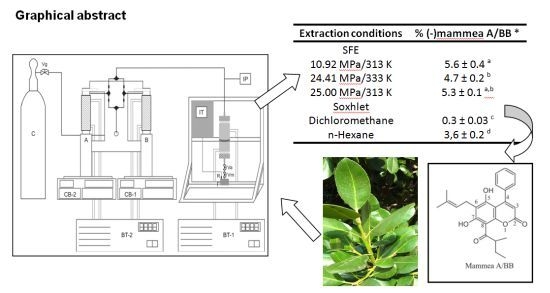
Within this context, the objective of the present work was to study the chemical composition and the biological activity of leaf extracts from
Calophyllum brasiliense Cambess, obtained by conventional and SFE methods. The experiments with supercritical CO
2 were carried out in a laboratory scale unit at different temperature and pressures, but at a constant solvent flow rate. Two different granulometries of the vegetal samples were also considered (30 and 50 mesh). Selected extracts obtained by conventional and SFE methods were further subjected to antioxidant activities and phenolic compounds assays. These extracts were also analyzed by high performance liquid chromatography (HPLC) for their (−)-mammea A/BB contents since it has important biological activity, mainly against protozoans and tumors [
27], high cytotoxic activity against some tumor cell lines [
16,
28], molluscicidal activity against the
Biomphalaria glabratas nail [
29] antileishmanial activity against
L. amazonensis [
19,
20] and trypanocidal effects in vitro against
Trypanosoma cruz [
30]. The kinetic curves of the extraction were correlated by a second-order empirical model.
3. Experimental
3.1. Pre-treatment of the Vegetal Matrix
The leaves of Calophyllum brasiliense Cambess were collected on Cardoso Island in the state of São Paulo, Brazil, in December 2010, and the exsiccate was deposited in the Herbarium of the Botanic Institute of São Paulo as number SP363818. The botanical material was dried in a circulating air oven (Quimis Q-31) at 313 K temperature. After 72 h, the leaves were milled in a home processor (WALITA RI7625). Tyler sieves (W. S. Tyler, Mentor, OH, USA) were used to classify the samples according to particle size. The leaves trapped in the 30 and 50 mesh sieves were chosen for further extraction steps.
3.4. Quantification of (−)-Mammea A/BB
The quantification of (-) mammea A/BB in the extracts was based on the methodology described by Brenzan
et al. [
38] using a High Performance Liquid Chromatography (HPLC) device. The equipment consisted of a Varian 920 LC with a DAD (diode array) detector, equipped with a quaternary pump and auto sampler injector, controlled by Galaxie Software, reverse phase column Metasil ODS 150 × 4.6 mm with a 5 μm particle (METACHEM), and a column temperature controlled at 303 K.
The qualitative and quantitative analysis were performed by using a gradient elution protocol constituted by acetonitrile (J.T. Baker, 99.99% purity)-water as mobile phase in the following proportions: 5:95 to 55:45 v/v (0–10 min.), 55:45 to 80:20 v/v (10–20 min.), 80:20 to 100:0 v/v (20–30 min.) and 100% acetonitrile (30–40 min.), with a flow rate of 0.6 mL/ min.
The calibration curve was established by the external standard method using (−)-mammea A/BB coumarin isolated from the leaves of
Calophyllum brasiliense, according to Brenzan
et al. [
19]. All measurements were undertaken in triplicate.
3.5. Total Phenol Contents
To determine the total phenolic contents, the method described by Meda
et al. [
39] was employed with modifications, using the Folin-Denis (Sigma-Aldrich, 100% purity) instead of the Folin-Ciocalteau reagent. The color of the solution is expected to change from green to blue in positive reactions.
Extracts were prepared at a concentration of 1 mg/mL in methanol (FMaia, 99.8% purity). Next, 2.5 mL of 10% Folin-Denis reagent solution (10 mL of the reagent in 100 mL of ultra-pure water) was added in a 0.5 mL extract solution. Finally, 2.0 mL of 14% sodium carbonate solution (Nuclear, 99.9% purity, 14 g of the reagent in 100 mL of ultra-pure water) was added after 5 minutes. The mixture was kept in the dark for 2 h. The absorbance was measured at 760 nm in a spectrophotometer (Shimadzu, UV-1203). For the negative control, a mixture of 0.5 mL methanol, 2.5 mL of 10% Folin-Denis reagent and 2.0 mL of sodium carbonate solution was used.
Gallic acid (Vetec), recognized as an antioxidant agent, was used as standard to construct the calibration curve. Concentrations ranging from 0.8 µg/mL to 7 µg/mL were applied and the preparation of these solutions followed the description above. The total phenolic contents was determined by the intersection of the absorbance of the samples across the calibration curve (R2 = 0.9991). Total phenolic content was expressed as mg of gallic acid equivalents (GAE) per g of extract.
3.6. Antioxidant Activity
The antioxidant activity of the extracts was evaluated according to the methodology proposed by Blois [
40] and Brand-Williams
et al. [
41]. This method measures the sequestering activity of the free radical 2,2-diphenyl-1-picryl hydrazyl (DPPH
●), purple colored, since it is reduced by antioxidant molecules forming yellow colored diphenylpicryl hydrazine.
The extracts were diluted in methanol up to concentrations that varied from 25 to 350 μg/mL. Next, 2,850 μL of the DPPH solution (0.6 mM) were added to150 μL of each tested sample. For the blank control, the volume of the samples was substituted by distilled water. The reaction was kept for 1 h at room temperature, in the dark, and the absorbance was measured at 515 nm.
The antioxidant activity (AA%) is expressed as a percentage of DPPH radical elimination, calculated according to the following equation:
Where A.blank is the absorbance of the blank and A.sample is the absorbance of the extract solution. The concentration of the extracts resulting in 50% of inhibition (IC
50) was calculated from the inhibition percentage plotting graph. All tests were run in triplicate, and the average value was calculated.
3.7. Mathematical Modeling
The kinetic curves using CO
2 extraction of
C. brasiliense were modeled using a second-order empirical model proposed by Corso
et al. [
42] and De Souza
et al. [
43], that does not require knowledge of the axial concentration profile of the desired chemical species throughout the extraction bed. The equation of mass balance of concentration of extract in the fluid phase results in the following differential equation:
where C is the concentration of extract in the solvent (g/mL), q is the bioactive compounds concentration in the solid matrix (g
extract/g
solid), ρ
bed is the density in the bed (g/mL), u is the interstitial velocity (cm/min), t is the extraction time (min), ε is the bed porosity, z is the coordinate in the axial direction of the bed (cm). The next equation assumes that the extraction rate is proportional to the product of extraction capacity of the solvent in fluid phase (C
eq. –C) and the oil concentration in solid matrix (q):
in which, k is the kinetic constant (mL/g min), C
eq is the equilibrium concentration of extract in the solvent (g/mL).
This equation represents the analytical solution of model:
where t
r = L/u is the residence time (min), L is the length (cm) of column, A = (z/u)β, B = (-tu + z)β/αu, β = kc
eqα and α = ρ
bedq
0/εC
eq.
The extracted mass as a function of time was calculated by the equation:
in which Q
f is the flow rate of the solvent and C
out is the concentration of extract in the fluid phase at the extractor outlet. Constant k was determined by minimizing the target function defined by the equation:
in which
![Molecules 18 06215 i011]()
is the calculated extracted mass,
![Molecules 18 06215 i012]()
is the mass experimentally obtained, n exp is the number of experimental data of the kinetic curve.









 is the calculated extracted mass,
is the calculated extracted mass,  is the mass experimentally obtained, n exp is the number of experimental data of the kinetic curve.
is the mass experimentally obtained, n exp is the number of experimental data of the kinetic curve.