Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles
Abstract
:1. Introduction
2. Macrocyclic Drugs




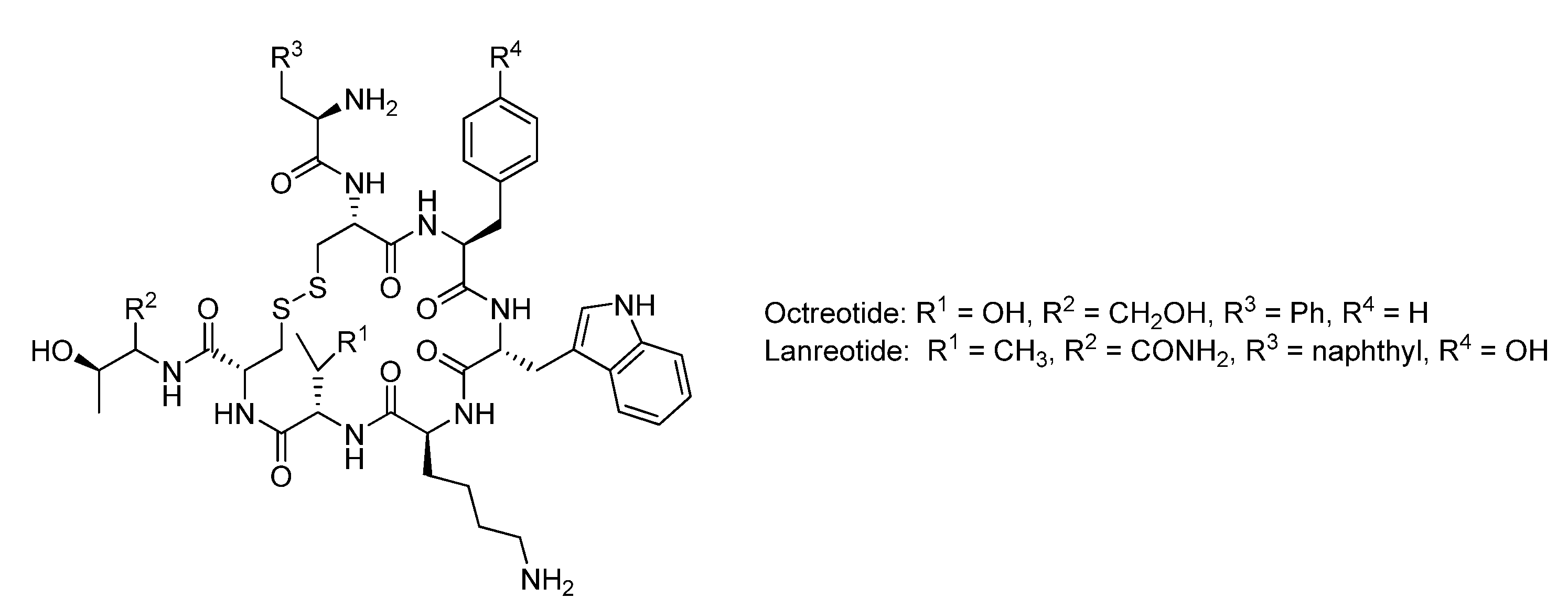
3. Chemical Methodologies for the Construction of Macrocycles
3.1. Macrolactonization and Macrolactamization






3.2. C-C, C-O, and C-N Coupling Reactions
3.2.1. C-C Bond Formation







3.2.2. C-O Bond Formation

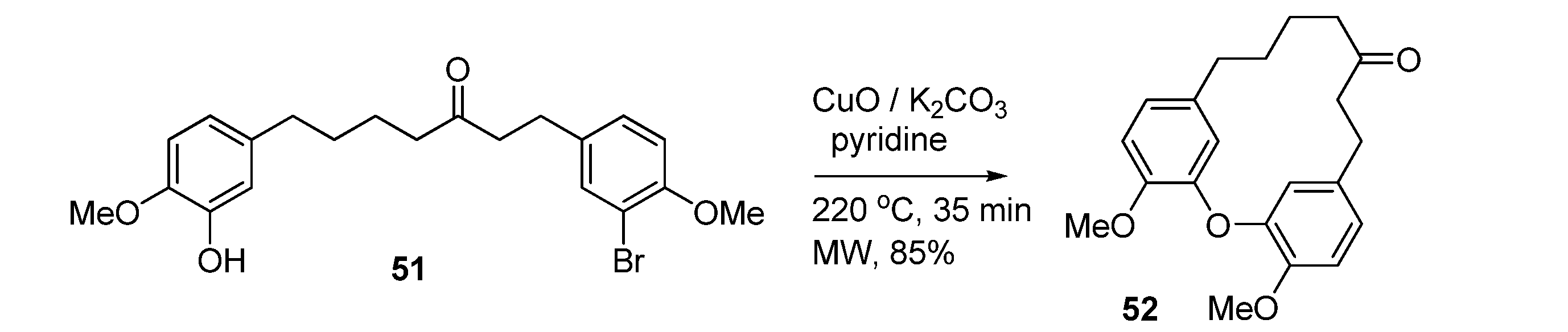
3.2.3. C-N Bond Formation

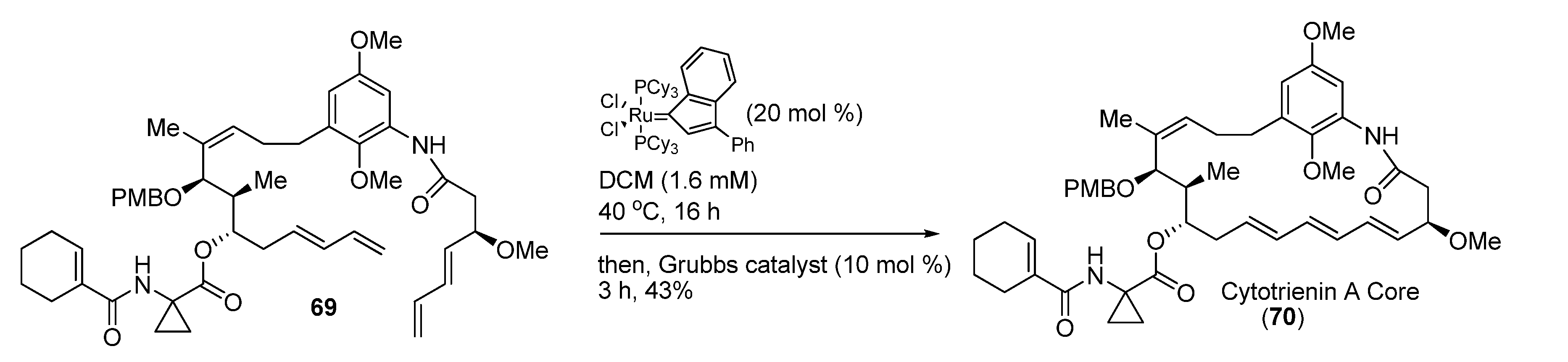
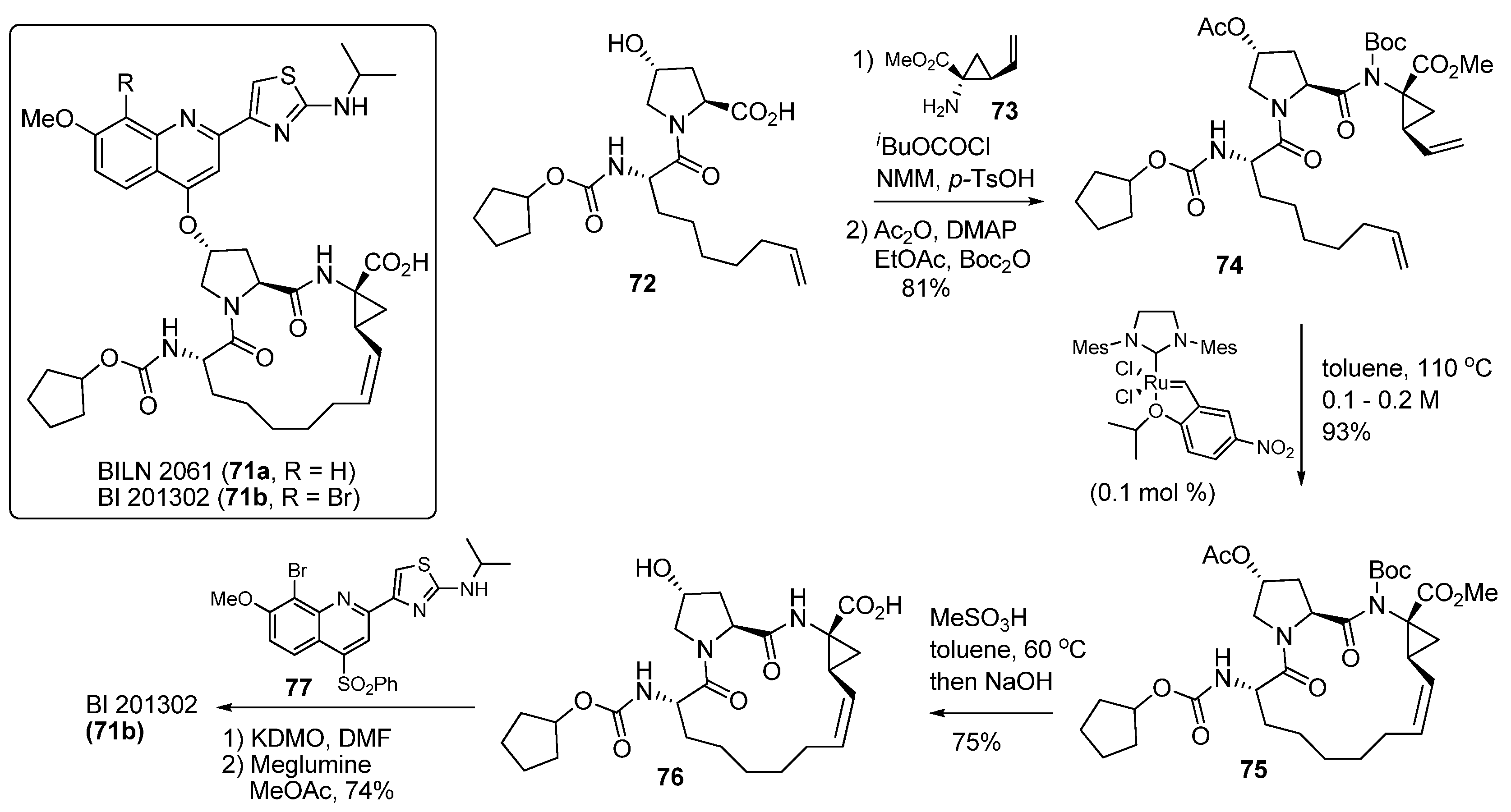
3.3. Ring-Closing Metathesis (RCM) Reaction










3.4. Click reaction





4. Conclusions
Acknowledgments
Conflicts of Interest
References
- Driggers, E.M.; Hale, S.P.; Lee, J.; Terrett, N.K. The exploration of macrocycles for drug discovery—An underexploited structural class. Nat. Rev. Drug Discov. 2008, 7, 608–624. [Google Scholar] [CrossRef]
- Mallinson, J.; Collins, I. Macrocycles in new drug discovery. Fut. Med. Chem. 2012, 4, 1409–1438. [Google Scholar] [CrossRef]
- Krahn, D.; Ottmann, C.; Kaiser, M. Macrocyclic proteasome inhibitors. Curr. Med. Chem. 2011, 18, 5052–5060. [Google Scholar] [CrossRef]
- Marsault, E.; Peterson, M.L. Macrocycles Are Great Cycles: Applications, Opportunities, and Challenges of Synthetic Macrocycles in Drug Discovery. J. Med. Chem. 2011, 54, 1961–2004. [Google Scholar] [CrossRef]
- Erb, W.; Zhu, J. From natural product to marketed drug: The tiacumicin odyssey. Nat. Prod. Rep. 2013, 30, 161–174. [Google Scholar] [CrossRef]
- Wessjohann, L.A.; Ruijter, E.; Garcia-Rivera, D.; Brandt, W. What can a chemist learn from nature's macrocycles?—A brief, conceptual view. Mol. Divers. 2005, 9, 171–186. [Google Scholar] [CrossRef]
- Ruan, B.F.; Zhu, H.L. The chemistry and biology of the bryostatins: Potential PKC inhibitors in clinical development. Curr. Med. Chem. 2012, 19, 2652–2664. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001, 46, 3–26. [Google Scholar] [CrossRef]
- Rybak, M.; Lomaestro, B.; Rotschafer, J.C.; Moellering, R.; Craig, W.; Billeter, M.; Dalovisio, J.R.; Levine, D.P. Therapeutic monitoring of vancomycin in adult patients: A consensus review of the American Society of Health-System Pharmacists, the Infectious Diseases Society of America, and the Society of Infectious Diseases Pharmacists. Am. J. Health Syst. Pharm. 2009, 66, 82–98. [Google Scholar] [CrossRef]
- Lexi-Comp OnlineTM; Medical database. Lexi-Comp, Inc.: Hudson, OH, USA, 2013.
- Dunbar, L.M.; Tang, D.M.; Manausa, R.M. A review of telavancin in the treatment of complicated skin and skin structure infections (cSSSI). Ther. Clin. Risk Manag. 2008, 4, 235–244. [Google Scholar]
- Saravolatz, L.D.; Stein, G.E.; Johnson, L.B. Telavancin: A Novel Lipoglycopeptide. Clin. Infect. Dis. 2009, 49, 1908–1914. [Google Scholar] [CrossRef]
- Higgins, D.L.; Chang, R.; Debabov, D.V.; Leung, J.; Wu, T.; Krause, K.M.; Sandvik, E.; Hubbard, J.M.; Kaniga, K.; Schmidt, D.E.; et al. Telavancin, a Multifunctional Lipoglycopeptide, Disrupts both Cell Wall Synthesis and Cell Membrane Integrity in Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2005, 49, 1127–1134. [Google Scholar] [CrossRef]
- Lunde, C.S.; Hartouni, S.R.; Janc, J.W.; Mammen, M.; Humphrey, P.P.; Benton, B.M. Telavancin Disrupts the Functional Integrity of the Bacterial Membrane through Targeted Interaction with the Cell Wall Precursor Lipid II. Antimicrob. Agents Chemother. 2009, 53, 3375–3383. [Google Scholar] [CrossRef]
- Jones, R.N.; Barry, A.L. Antimicrobial activity and spectrum of LY146032, a lipopeptide antibiotic, including susceptibility testing recommendations. Antimicrob. Agents Chemother. 1987, 31, 625–629. [Google Scholar] [CrossRef]
- Steenbergen, J.N.; Alder, J.; Thorne, G.M.; Tally, F.P. Daptomycin: A lipopeptide antibiotic for the treatment of serious Gram-positive infections. J. Antimicrob. Chemother. 2005, 55, 283–288. [Google Scholar] [CrossRef]
- Hurdle, J.G.; O'Neill, A.J.; Chopra, I.; Lee, R.E. Targeting bacterial membrane function: An underexploited mechanism for treating persistent infections. Nat. Rev. Microbiol. 2011, 9, 62–75. [Google Scholar] [CrossRef]
- Whitman, C.B.; Czosnowski, Q.A. Fidaxomicin for the treatment of Clostridium difficile infections. Ann. Pharmacother. 2012, 46, 219–228. [Google Scholar] [CrossRef]
- Hardesty, J.S.; Juang, P. Fidaxomicin: A macrocyclic antibiotic for the treatment of Clostridium difficile infection. Pharmacotherapy 2011, 31, 877–886. [Google Scholar] [CrossRef]
- Gerber, M.; Ackermann, G. OPT-80, a macrocyclic antimicrobial agent for the treatment of Clostridium difficile infections: A review. Expert Opin. Investig. Drugs 2008, 17, 547–553. [Google Scholar] [CrossRef]
- Johnson, B.A.; Anker, H.; Meleney, F.L. Bacitracin: A new antibiotic produced by a member of the B. subtilis group. Science 1945, 102, 376–377. [Google Scholar]
- Ohki, R.; Tateno, K.; Okada, Y.; Okajima, H.; Asai, K.; Sadaie, Y.; Murata, M.; Aiso, T. A Bacitracin-Resistant Bacillus subtilis Gene Encodes a Homologue of the Membrane-Spanning Subunit of the Bacillus licheniformis ABC Transporter. J. Bacteriol. 2003, 185, 51–59. [Google Scholar] [CrossRef]
- Stone, K.J.; Strominger, J.L. Mechanism of Action of Bacitracin: Complexation with Metal Ion and C55-Isoprenyl Pyrophosphate. Proc. Natl. Acad. Sci. USA 1971, 68, 3223–3227. [Google Scholar] [CrossRef]
- Falagas, M.E.; Kasiakou, S.K.; Saravolatz, L.D. Colistin: The Revival of Polymyxins for the Management of Multidrug-Resistant Gram-Negative Bacterial Infections. Clin. Infect. Dis. 2005, 40, 1333–1341. [Google Scholar] [CrossRef]
- Michalopoulos, A.; Falagas, M.E. Colistin and Polymyxin B in Critical Care. Crit. Care Clin. 2008, 24, 377–391. [Google Scholar] [CrossRef]
- Evans, M.E.; Feola, D.J.; Rapp, R.P. Polymyxin B sulfate and colistin: Old antibiotics for emerging multiresistant gram-negative bacteria. Ann. Pharmacother. 1999, 33, 960–967. [Google Scholar] [CrossRef]
- McDaniel, R.; Welch, M.; Hutchinson, C.R. Genetic Approaches to Polyketide Antibiotics. 1. Chem. Rev. 2005, 105, 543–558. [Google Scholar] [CrossRef]
- Ma, X.; Ma, S. Significant breakthroughs in search for anti-infectious agents derived from erythromycin A. Curr. Med. Chem. 2011, 18, 1993–2015. [Google Scholar] [CrossRef]
- Alvarez-Elcoro, S.; Enzler, M.J. The Macrolides: Erythromycin, Clarithromycin, and Azithromycin. Mayo Clin. Proc. 1999, 74, 613–634. [Google Scholar] [CrossRef]
- Zuckerman, J.M.; Qamar, F.; Bono, B.R. Review of Macrolides (Azithromycin, Clarithromycin), Ketolids (Telithromycin) and Glycylcyclines (Tigecycline). Med. Clin. N. Am. 2011, 95, 761–791. [Google Scholar] [CrossRef]
- Ackermann, G.; Rodloff, A.C. Drugs of the 21st century: Telithromycin (HMR 3647)-the first ketolide. J. Antimicrob. Chemother. 2003, 51, 497–511. [Google Scholar] [CrossRef]
- Zhanel, G.; Dueck, M.; Hoban, D.; Vercaigne, L.; Embil, J.; Gin, A.; Karlowsky, J. Review of Macrolides and Ketolides. Drugs 2001, 61, 443–498. [Google Scholar] [CrossRef]
- Rubinstein, E.; Keller, N. Spiramycin renaissance. J. Antimicrob. Chemother. 1998, 42, 572–576. [Google Scholar] [CrossRef]
- Mukhtar, T.A.; Wright, G.D. Streptogramins, Oxazolidinones, and Other Inhibitors of Bacterial Protein Synthesis. Chem. Rev. 2005, 105, 529–542. [Google Scholar] [CrossRef]
- Allington, D.R.; Rivey, M.P. Quinupristin/dalfopristin: A therapeutic review. Clin. Ther. 2001, 23, 24–44. [Google Scholar]
- Chopra, I. Bacterial RNA polymerase: A promising target for the discovery of new antimicrobial agents. Curr. Opin. Investig. Drugs 2007, 8, 600–607. [Google Scholar]
- Johansen, S.K.; Maus, C.E.; Plikaytis, B.B.; Douthwaite, S. Capreomycin Binds across the Ribosomal Subunit Interface Using tlyA-Encoded 22-O-Methylations in 16S and 23S rRNAs. Mol. Cell 2006, 23, 173–182. [Google Scholar] [CrossRef]
- Kathiravan, M.K.; Salake, A.B.; Chothe, A.S.; Dudhe, P.B.; Watode, R.P.; Mukta, M.S.; Gadhwe, S. The biology and chemistry of antifungal agents: A review. Bioorg. Med. Chem. 2012, 20, 5678–5698. [Google Scholar]
- Odds, F.C.; Brown, A.J.P.; Gow, N.A.R. Antifungal agents: Mechanisms of action. Trends Microbiol. 2003, 11, 272–279. [Google Scholar] [CrossRef]
- Chen, S.C.A.; Slavin, M.A.; Sorrell, T.C. Echinocandin antifungal drugs in fungal infections: A comparison. Drugs 2011, 71, 11–41. [Google Scholar]
- Holt, S.L.; Drew, R.H. Echinocandins: Addressing outstanding questions surrounding treatment of invasive fungal infections. Am. J. Health Syst. Pharm. 2011, 68, 1207–1220. [Google Scholar] [CrossRef]
- Wiederhold, N.P.; Lewis, R.E. The echinocandin antifungals: An overview of the pharmacology, spectrum and clinical efficacy. Expert Opin. Investig. Drugs 2003, 12, 1313–1333. [Google Scholar] [CrossRef]
- Campbell, W.C.; Fisher, M.H.; Stapley, E.O.; Albers-Schönberg, G.; Jacob, T.A. Ivermectin: A potent new antiparasitic agent. Science 1983, 221, 823–828. [Google Scholar]
- González, P.; González, F.A.; Ueno, K. Ivermectin in human medicine, an overview of the current status of its clinical applications. Curr. Pharm. Biotechnol. 2012, 13, 1103–1109. [Google Scholar] [CrossRef]
- Hollstein, U. Actinomycin. Chemistry and mechanism of action. Chem. Rev. 1974, 74, 625–652. [Google Scholar] [CrossRef]
- Bollag, D.M.; McQueney, P.A.; Zhu, J.; Hensens, O.; Koupal, L.; Liesch, J.; Goetz, M.; Lazarides, E.; Woods, C.M. Epothilones, a New Class of Microtubule-stabilizing Agents with a Taxol-like Mechanism of Action. Cancer Res. 1995, 55, 2325–2333. [Google Scholar]
- Lee, F.Y.F.; Borzilleri, R.; Fairchild, C.R.; Kim, S.-H.; Long, B.H.; Reventos-Suarez, C.; Vite, G.D.; Rose, W.C.; Kramer, R.A. BMS-247550: A Novel Epothilone Analog with a Mode of Action Similar to Paclitaxel but Possessing Superior Antitumor Efficacy. Clin. Cancer Res. 2001, 7, 1429–1437. [Google Scholar]
- Piekarz, R.L.; Robey, R.; Sandor, V.; Bakke, S.; Wilson, W.H.; Dahmoush, L.; Kingma, D.M.; Turner, M.L.; Altemus, R.; Bates, S.E. Inhibitor of histone deacetylation, depsipeptide (FR901228), in the treatment of peripheral and cutaneous T-cell lymphoma: A case report. Blood 2001, 98, 2865–2868. [Google Scholar] [CrossRef]
- Ho, S.; Clipstone, N.; Timmermann, L.; Northrop, J.; Graef, I.; Fiorentino, D.; Nourse, J.; Crabtree, G.R. The Mechanism of Action of Cyclosporin A and FK506. Clin. Immunol. Immunopathol. 1996, 80, S40–S45. [Google Scholar] [CrossRef]
- Groth, C.G.; Bäckman, L.; Morales, J.M.; Calne, R.; Kreis, H.; Lang, P.; Touraine, J.L.; Claesson, K.; Campistol, J.M.; Durand, D.; Wramner, L.; Brattström, C.; Charpentier, B. Sirolimus (rapamycin)-based therapy in human renal transplantation: Similar efficacy and different toxicity compared with cyclosporine. Transplantation 1999, 67, 1036–1042. [Google Scholar] [CrossRef]
- Graziani, E.I. Recent advances in the chemistry, biosynthesis and pharmacology of rapamycin analogs. Nat. Prod. Rep. 2009, 26, 602–609. [Google Scholar] [CrossRef]
- Escudier, B. Temsirolimus in the treatment of advanced renal cell carcinoma. Oncol. Rev. 2007, 1, 73–80. [Google Scholar] [CrossRef]
- Pawlikowski, M.; Mełeń-Mucha, G. Somatostatin analogs—From new molecules to new applications. Curr. Opin. Pharmacol. 2004, 4, 608–613. [Google Scholar] [CrossRef]
- Racine, M.S.; Barkan, A.L. Somatostatin analogs in medical treatment of acromegaly. Endocrine 2003, 20, 271–278. [Google Scholar] [CrossRef]
- Feelders, R.A.; Hofland, L.J.; Van Aken, M.O.; Neggers, S.J.; Lamberts, S.W.J.; de Herder, W.W.; van der Lely, A.J. Medical therapy of acromegaly: Efficacy and safety of somatostatin analogues. Drugs 2009, 69, 2207–2226. [Google Scholar] [CrossRef]
- Collins, J.C.; James, K. Emac—A comparative index for the assessment of macrocyclization efficiency. Med. Chem. Commun. 2012, 3, 1489–1495. [Google Scholar] [CrossRef]
- Harrowven, D.C.; Kostiuk, S.L. Macrocylic bisbibenzyl natural products and their chemical synthesis. Nat. Prod. Rep. 2012, 29, 223–242. [Google Scholar] [CrossRef]
- Terrett, N.K. Methods for the synthesis of macrocycle libraries for drug discovery. Drug Discov. Today Technol. 2010, 7, e97–e104. [Google Scholar] [CrossRef]
- Wessjohann, L.; Ruijter, E. Strategies for Total and Diversity-Oriented Synthesis of Natural Product(-Like) Macrocycles. In Natural Product Synthesis I; Springer Berlin Heidelberg: Berlin, Germany, 2005; Volume 243, pp. 137–184. [Google Scholar]
- Horton, A.E.; May, O.S.; Elsegood, M.R.J.; Kimber, M.C. Total synthesis of the marine-derived cyclic depsipeptide alternaramide. Synlett 2011, 22, 797–800. [Google Scholar]
- Parenty, A.; Moreau, X.; Niel, G.; Campagne, J.M. Update 1 of: Macrolactonizations in the Total Synthesis of Natural Products. Chem. Rev. 2013, 113, PR1–PR40. [Google Scholar] [CrossRef]
- Corey, E.J.; Nicolaou, K.C. Efficient and mild lactonization method for the synthesis of macrolides. J. Am. Chem. Soc. 1974, 96, 5614–5616. [Google Scholar] [CrossRef]
- Palomo, C.; Oiarbide, M.; García, J.M.; González, A.; Pazos, R.; Odriozola, J.M.; Bañuelos, P.; Tello, M.; Linden, A. A Practical Total Synthesis of Hapalosin, a 12-Membered Cyclic Depsipeptide with Multidrug Resistance-Reversing Activity, by Employing Improved Segment Coupling and Macrolactonization. J. Org. Chem. 2004, 69, 4126–4134. [Google Scholar] [CrossRef]
- Corey, E.J.; Clark, D.A. A new method for the synthesis of 2-pyridinethiol carboxylic esters. Tetrahedron Lett. 1979, 20, 2875–2878. [Google Scholar] [CrossRef]
- Corey, E.J.; Brunelle, D.J. New reagents for the conversion of hydroxy acids to macrolactones by the double activation method. Tetrahedron Lett. 1976, 17, 3409–3412. [Google Scholar] [CrossRef]
- Schnorrenberg, G.; Steglich, W. Economic Synthesis of Activated N-tert-Butyloxycarbonyl Amino Acid Esters. Angew. Chem. Int. Ed. Engl. 1979, 18, 307–308. [Google Scholar] [CrossRef]
- Nimitz, J.S.; Wollenberg, R.H. Macrolide ring closure. Silver ion promoted lactonization of ω-hydroxy thiolesters of 2-amino-4-mercapto-6-methylpyrimidine. Tetrahedron Lett. 1978, 19, 3523–3526. [Google Scholar] [CrossRef]
- Mukaiyama, T.; Usui, M.; Saigo, K. The facile synthesis of lactones. Chem. Lett. 1976, 5, 49–50. [Google Scholar] [CrossRef]
- Venkataraman, K.; Wagle, D.R. Cyanuric chloride, a useful reagent for macrocyclic lactonization. Tetrahedron Lett. 1980, 21, 1893–1896. [Google Scholar] [CrossRef]
- Inanaga, J.; Hirata, K.; Saeki, H.; Katsuki, T.; Yamaguchi, M. A Rapid Esterification by Means of Mixed Anhydride and Its Application to Large-ring Lactonization. Bull. Chem. Soc. Jpn. 1979, 52, 1989–1993. [Google Scholar] [CrossRef]
- Hikota, M.; Tone, H.; Horita, K.; Yonemitsu, O. Chiral synthesis of polyketide-derived natural products. 27. Stereoselective synthesis of erythronolide A via an extremely efficient macrolactonization by the modified Yamaguchi method. J. Org. Chem. 1990, 55, 7–9. [Google Scholar] [CrossRef]
- Tatsuta, K. Total synthesis of the big four antibiotics and related antibiotics. J. Antibiot. 2013, 66, 107–129. [Google Scholar] [CrossRef]
- Xie, L.; Zhu, S.-Y.; Shen, X.-Q.; He, L.-L.; Yang, J.-S. Total Synthesis of Batatoside L. J. Org. Chem. 2010, 75, 5764–5767. [Google Scholar] [CrossRef]
- Yin, Y.; Li, Y.; Kong, L. Pentasaccharide Glycosides from the Tubers of Sweet Potato (Ipomoea batatas). J. Agric. Food Chem. 2008, 56, 2363–2368. [Google Scholar] [CrossRef]
- Yin, Y.-Q.; Wang, J.-S.; Luo, J.-G.; Kong, L.-Y. Novel acylated lipo-oligosaccharides from the tubers of Ipomoea batatas. Carbohydr. Res. 2009, 344, 466–473. [Google Scholar] [CrossRef]
- García-Fortanet, J.; Murga, J.; Carda, M.; Marco, J.A.; Matesanz, R.; Díaz, J.F.; Barasoain, I. The total synthesis and biological properties of the cytotoxic macrolide FD-891 and its non-natural (Z)-C12 isomer. Chem. Eur. J. 2007, 13, 5060–5074. [Google Scholar] [CrossRef]
- García-Fortanet, J.; Murga, J.; Carda, M.; Marco, J.A. Stereoselective synthesis of the cytotoxic macrolide FD-891. Org. Lett. 2006, 8, 2695–2698. [Google Scholar] [CrossRef]
- Crimmins, M.T.; Caussanel, F. Enantioselective total synthesis of FD-891. J. Am. Chem. Soc. 2006, 128, 3128–3129. [Google Scholar] [CrossRef]
- Seki-Asano, M.; Tsuchida, Y.; Hanada, K.; Mizoue, K. Structures of new 18-membered macrolides FD-891 and FD-892. J. Antibiot. 1994, 47, 1234–1241. [Google Scholar] [CrossRef]
- Eguchi, T.; Yamamoto, K.; Mizoue, K.; Kakinuma, K. Structure revision of FD-891, a 16-membered macrolide antibiotic. J. Antibiot. 2004, 57, 156–157. [Google Scholar] [CrossRef]
- Yadav, J.S.; Das, S.K.; Sabitha, G. Stereoselective Total Synthesis of FD-891. J. Org. Chem. 2012, 77, 11109–11118. [Google Scholar] [CrossRef]
- Woodward, R.B.; Bader, F.E.; Bickel, H.; Frey, A.J.; Kierstead, R.W. The total synthesis of reserpine. Tetrahedron 1958, 2, 1–57. [Google Scholar] [CrossRef]
- Boden, E.P.; Keck, G.E. Proton-transfer steps in Steglich esterification: A very practical new method for macrolactonization. J. Org. Chem. 1985, 50, 2394–2395. [Google Scholar] [CrossRef]
- Lee, E.; Jeong, E.J.; Kang, E.J.; Sung, L.T.; Hong, S.K. Total Synthesis of Pamamycin-607. J. Am. Chem. Soc. 2001, 123, 10131–10132. [Google Scholar] [CrossRef]
- Swamy, K.C.K.; Kumar, N.N.B.; Balaraman, E.; Kumar, K.V.P.P. Mitsunobu and Related Reactions: Advances and Applications. Chem. Rev. 2009, 109, 2551–2651. [Google Scholar] [CrossRef]
- Ehrlich, G.; Hassfeld, J.; Eggert, U.; Kalesse, M. The Total Synthesis of (+)-Tedanolide. J. Am. Chem. Soc. 2006, 128, 14038–14039. [Google Scholar] [CrossRef]
- Xu, Y.; Chen, L.; Duan, X.; Meng, Y.; Jiang, L.; Li, M.; Zhao, G.; Li, Y. Total synthesis of hirsutellide A. Tetrahedron Lett. 2005, 46, 4377–4379. [Google Scholar] [CrossRef]
- Kunishima, M.; Kawachi, C.; Hioki, K.; Terao, K.; Tani, S. Formation of carboxamides by direct condensation of carboxylic acids and amines in alcohols using a new alcohol- and water-soluble condensing agent: DMT-MM. Tetrahedron 2001, 57, 1551–1558. [Google Scholar] [CrossRef]
- McCauley, J.A.; McIntyre, C.J.; Rudd, M.T.; Nguyen, K.T.; Romano, J.J.; Butcher, J.W.; Gilbert, K.F.; Bush, K.J.; Holloway, M.K.; Swestock, J.; et al. Discovery of Vaniprevir (MK-7009), a Macrocyclic Hepatitis C Virus NS3/4a Protease Inhibitor. J. Med. Chem. 2010, 53, 2443–2463. [Google Scholar] [CrossRef]
- Song, Z.J.; Tellers, D.M.; Journet, M.; Kuethe, J.T.; Lieberman, D.; Humphrey, G.; Zhang, F.; Peng, Z.; Waters, M.S.; Zewge, D.; et al. Synthesis of Vaniprevir (MK-7009): Lactamization To Prepare a 22-Membered Macrocycle. J. Org. Chem. 2011, 76, 7804–7815. [Google Scholar] [CrossRef]
- Zhang, W.; Moore, J.S. Shape-persistent macrocycles: Structures and synthetic approaches from arylene and ethynylene building blocks. Angew. Chem. Int. Ed. 2006, 45, 4416–4439. [Google Scholar] [CrossRef]
- Sasse, F.; Steinmetz, H.; Hofle, G.; Reichenbach, H. Rhizopodin, a new compound from Myxococcus stipitatus (myxobacteria) causes formation of rhizopodia-like structures in animal cell cultures: Production, isolation, physico-chemical and biological properties. J. Antibiot. 1993, 46, 741–748. [Google Scholar] [CrossRef]
- Jansen, R.; Steinmetz, H.; Sasse, F.; Schubert, W.-D.; Hagelüken, G.; Albrecht, S.C.; Müller, R. Isolation and structure revision of the actin-binding macrolide rhizopodin from Myxococcus stipitatus (Myxobacteria). Tetrahedron Lett. 2008, 49, 5796–5799. [Google Scholar] [CrossRef]
- Dieckmann, M.; Rudolph, S.; Dreisigacker, S.; Menche, D. Concise synthesis of the macrocyclic core of rhizopodin by a heck macrocyclization strategy. J. Org. Chem. 2012, 77, 10782–10788. [Google Scholar] [CrossRef]
- Dieckmann, M.; Kretschmer, M.; Li, P.; Rudolph, S.; Herkommer, D.; Menche, D. Total synthesis of rhizopodin. Angew. Chem. Int. Ed. 2012, 51, 5667–5670. [Google Scholar] [CrossRef]
- Pulukuri, K.K.; Chakraborty, T.K. Stereoselective synthesis of the monomeric unit of actin binding macrolide rhizopodin. Org. Lett. 2012, 14, 2858–2861. [Google Scholar] [CrossRef]
- Kretschmer, M.; Menche, D. Stereocontrolled synthesis of the C8-C22 fragment of rhizopodin. Org. Lett. 2012, 14, 382–385. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Sreekanth, M.; Pulukuri, K.K. Synthetic studies toward potent cytostatic macrolide rhizopodin: Stereoselective synthesis of the C16-C28 fragment. Tetrahedron Lett. 2011, 52, 59–61. [Google Scholar] [CrossRef]
- Chakraborty, T.K.; Pulukuri, K.K.; Sreekanth, M. Studies directed toward the synthesis of rhizopodin: Stereoselective synthesis of the C1-C15 fragment. Tetrahedron Lett. 2010, 51, 6444–6446. [Google Scholar] [CrossRef]
- Chen, Z.; Song, L.; Xu, Z.; Ye, T. Synthesis of the C9-C23 (C9′-C23′) fragment of the dimeric natural product rhizopodin. Org. Lett. 2010, 12, 2036–2039. [Google Scholar] [CrossRef]
- Hagelueken, G.; Albrecht, S.C.; Steinmetz, H.; Jansen, R.; Heinz, D.W.; Kalesse, M.; Schubert, W.D. The absolute configuration of rhizopodin and its inhibition of actin polymerization by dimerization. Angew. Chem. Int. Ed. 2009, 48, 595–598. [Google Scholar] [CrossRef]
- Velvadapu, V.; Paul, T.; Wagh, B.; Glassford, I.; DeBrosse, C.; Andrade, R.B. Total Synthesis of (−)-4,8,10-Tridesmethyl Telithromycin. J. Org. Chem. 2011, 76, 7516–7527. [Google Scholar] [CrossRef]
- Ezaki, M.; Iwami, M.; Yamashita, M.; Hashimoto, S.; Komori, T.; Umehara, K.; Mine, Y.; Kohsaka, M.; Aoki, H.; Imanaka, H. Biphenomycins A and B, novel peptide antibiotics. I. Taxonomy, fermentation, isolation and characterization. J. Antibiot. 1985, 38, 1453–1461. [Google Scholar] [CrossRef]
- Uchida, I.; Ezaki, M.; Shigematsu, N.; Hashimoto, M. Structure of WS-43708A, a novel cyclic peptide antibiotic. J. Org. Chem. 1985, 50, 1341–1342. [Google Scholar] [CrossRef]
- Lépine, R.; Zhu, J. Microwave-Assisted Intramolecular Suzuki-Miyaura Reaction to Macrocycle, a Concise Asymmetric Total Synthesis of Biphenomycin B. Org. Lett. 2005, 7, 2981–2984. [Google Scholar] [CrossRef]
- Unsworth, W.P.; Gallagher, K.A.; Jean, M.; Schmidt, J.P.; Diorazio, L.J.; Taylor, R.J.K. Direct Imine Acylation: Synthesis of the Proposed Structures of ‘Upenamide. Org. Lett. 2013, 15, 262–265. [Google Scholar] [CrossRef]
- Abe, H.; Chida, Y.; Kurokawa, H.; Inouye, M. Selective Binding of D2h-Symmetrical, Acetylene-Linked Pyridine/Pyridone Macrocycles to Maltoside. J. Org. Chem. 2011, 76, 3366–3371. [Google Scholar] [CrossRef]
- Yamasaki, R.; Shigeto, A.; Saito, S. Preparation of Shape-Persistent Macrocycles with a Single Pyridine Unit by Double Cross-Coupling Reactions of Aryl Bromides and Alkynes. J. Org. Chem. 2011, 76, 10299–10305. [Google Scholar] [CrossRef]
- Hassan, J.; Sévignon, M.; Gozzi, C.; Schulz, E.; Lemaire, M. Aryl-Aryl Bond Formation One Century after the Discovery of the Ullmann Reaction. Chem. Rev. 2002, 102, 1359–1470. [Google Scholar] [CrossRef]
- Isaka, M.; Rugseree, N.; Maithip, P.; Kongsaeree, P.; Prabpai, S.; Thebtaranonth, Y. Hirsutellones A–E, antimycobacterial alkaloids from the insect pathogenic fungus Hirsutella nivea BCC 2594. Tetrahedron 2005, 61, 5577–5583. [Google Scholar] [CrossRef]
- Halvorsen, G.T.; Roush, W.R. Stereoselective synthesis of the decahydrofluorene core of the hirsutellones. Tetrahedron Lett. 2011, 52, 2072–2075. [Google Scholar] [CrossRef]
- Huang, M.; Huang, C.; Liu, B. Studies toward the total synthesis of the hirsutellones. Tetrahedron Lett. 2009, 50, 2797–2800. [Google Scholar] [CrossRef]
- Huang, M.; Song, L.; Liu, B. Construction of the Cyclophane Core of the Hirsutellones via a RCM Strategy. Org. Lett. 2010, 12, 2504–2507. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Sarlah, D.; Wu, T.R.; Zhan, W. Total Synthesis of Hirsutellone B. Angew. Chem. Int. Ed. 2009, 48, 6870–6874. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Sun, Y.-P.; Sarlah, D.; Zhan, W.; Wu, T.R. Bioinspired Synthesis of Hirsutellones A, B, and C. Org. Lett. 2011, 13, 5708–5710. [Google Scholar] [CrossRef]
- Tilley, S.D.; Reber, K.P.; Sorensen, E.J. A Rapid, Asymmetric Synthesis of the Decahydrofluorene Core of the Hirsutellones. Org. Lett. 2009, 11, 701–703. [Google Scholar] [CrossRef]
- Uchiro, H.; Kato, R.; Arai, Y.; Hasegawa, M.; Kobayakawa, Y. Total Synthesis of Hirsutellone B via Ullmann-Type Direct 13-Membered Macrocyclization. Org. Lett. 2011, 13, 6268–6271. [Google Scholar] [CrossRef]
- Sharma, A.; Appukkuttan, P.; van der Eycken, E. Microwave-assisted synthesis of medium-sized heterocycles. Chem. Commun. 2012, 48, 1623–1637. [Google Scholar] [CrossRef]
- Su, Q.; Beeler, A.B.; Lobkovsky, E.; Porco, J.A.; Panek, J.S. Stereochemical Diversity through Cyclodimerization: Synthesis of Polyketide-like Macrodiolides. Org. Lett. 2003, 5, 2149–2152. [Google Scholar] [CrossRef]
- Pérez-Balado, C.; Nebbioso, A.; Rodríguez-Graña, P.; Minichiello, A.; Miceli, M.; Altucci, L.; de Lera, Á.R. Bispyridinium Dienes: Histone Deacetylase Inhibitors with Selective Activities. J. Med. Chem. 2007, 50, 2497–2505. [Google Scholar] [CrossRef]
- Afonso, A.; Feliu, L.; Planas, M. Solid-phase synthesis of biaryl cyclic peptides by borylation and microwave-assisted intramolecular Suzuki-Miyaura reaction. Tetrahedron 2011, 67, 2238–2245. [Google Scholar] [CrossRef]
- Nnanabu, E.; Burgess, K. Cyclic Semipeptoids: Peptoid-Organic Hybrid Macrocycles. Org. Lett. 2006, 8, 1259–1262. [Google Scholar] [CrossRef]
- Bedard, A.-C.; Collins, S.K. Microwave accelerated Glaser-Hay macrocyclizations at high concentrations. Chem. Commun. 2012, 48, 6420–6422. [Google Scholar] [CrossRef]
- Dong, H.; Limberakis, C.; Liras, S.; Price, D.; James, K. Peptidic macrocyclization via palladium-catalyzed chemoselective indole C-2 arylation. Chem. Commun. 2012, 48, 11644–11646. [Google Scholar] [CrossRef]
- Shen, L.; Simmons, C.J.; Sun, D. Microwave-assisted synthesis of macrocycles via intramolecular and/or bimolecular Ullmann coupling. Tetrahedron Lett. 2012, 53, 4173–4178. [Google Scholar] [CrossRef]
- Shen, L.; Sun, D. Total synthesis and structural revision of engelhardione. Tetrahedron Lett. 2011, 52, 4570–4574. [Google Scholar] [CrossRef]
- Shen;, L.; Maddox, M.M.; Adhikari, S.; Bruhn, D.F.; Kumar, M.; Lee, R.E.; Hurdle, J.G.; Lee, R.E.; Sun, D. Syntheses and evaluation of macrocyclic engelhardione analogs as antitubercular and antibacterial agents. J. Antibiot. 2013, in press. [Google Scholar] [CrossRef]
- Lundgren, R.J.; Stradiotto, M. Recent advances in the buchwald-hartwig amination reaction enabled by the application of sterically demanding phosphine ancillary ligands. Aldrichimica Acta 2012, 45, 59–62. [Google Scholar]
- Tsuchiya, K. Synthesis of macrocyclic aromatic amines via C-N coupling reaction. Yuki Gosei Kagaku Kyokaishi 2011, 69, 169–170. [Google Scholar] [CrossRef]
- Huang, K.H.; Veal, J.M.; Fadden, R.P.; Rice, J.W.; Eaves, J.; Strachan, J.-P.; Barabasz, A.F.; Foley, B.E.; Barta, T.E.; Ma, W.; et al. Discovery of Novel 2-Aminobenzamide Inhibitors of Heat Shock Protein 90 as Potent, Selective and Orally Active Antitumor Agents. J. Med. Chem. 2009, 52, 4288–4305. [Google Scholar] [CrossRef]
- Zapf, C.W.; Bloom, J.D.; McBean, J.L.; Dushin, R.G.; Nittoli, T.; Ingalls, C.; Sutherland, A.G.; Sonye, J.P.; Eid, C.N.; Golas, J.; et al. Design and SAR of macrocyclic Hsp90 inhibitors with increased metabolic stability and potent cell-proliferation activity. Bioorg. Med. Chem. Lett. 2011, 21, 2278–2282. [Google Scholar] [CrossRef]
- Zapf, C.W.; Bloom, J.D.; McBean, J.L.; Dushin, R.G.; Nittoli, T.; Otteng, M.; Ingalls, C.; Golas, J.M.; Liu, H.; Lucas, J.; et al. Macrocyclic lactams as potent Hsp90 inhibitors with excellent tumor exposure and extended biomarker activity. Bioorg. Med. Chem. Lett. 2011, 21, 3411–3416. [Google Scholar] [CrossRef]
- Zapf, C.W.; Bloom, J.D.; McBean, J.L.; Dushin, R.G.; Golas, J.M.; Liu, H.; Lucas, J.; Boschelli, F.; Vogan, E.; Levin, J.I. Discovery of a macrocyclic o-aminobenzamide Hsp90 inhibitor with heterocyclic tether that shows extended biomarker activity and in vivo efficacy in a mouse xenograft model. Bioorg. Med. Chem. Lett. 2011, 21, 3627–3631. [Google Scholar] [CrossRef]
- Grubbs, R.H.; Miller, S.J.; Fu, G.C. Ring-Closing Metathesis and Related Processes in Organic Synthesis. Acc. Chem. Res. 1995, 28, 446–452. [Google Scholar] [CrossRef]
- Fürstner, A. Olefin Metathesis and Beyond. Angew. Chem. Int. Ed. 2000, 39, 3012–3043. [Google Scholar] [CrossRef]
- Schrock, R.R.; Hoveyda, A.H. Molybdenum and Tungsten Imido Alkylidene Complexes as Efficient Olefin-Metathesis Catalysts. Angew. Chem. Int. Ed. 2003, 42, 4592–4633. [Google Scholar] [CrossRef]
- Connon, S. J.; Blechert, S. Recent Developments in Olefin Cross-Metathesis. Angew. Chem. Int. Ed. 2003, 42, 1900–1923. [Google Scholar] [CrossRef]
- Deiters, A.; Martin, S.F. Synthesis of Oxygen- and Nitrogen-Containing Heterocycles by Ring-Closing Metathesis. Chem. Rev. 2004, 104, 2199–2238. [Google Scholar] [CrossRef]
- Gradillas, A.; Pérez-Castells, J. Macrocyclization by Ring-Closing Metathesis in the Total Synthesis of Natural Products: Reaction Conditions and Limitations. Angew. Chem. Int. Ed. 2006, 45, 6086–6101. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Luna, A. Grubbs’ Ruthenium-Carbenes Beyond the Metathesis Reaction: Less Conventional Non-Metathetic Utility. Chem. Rev. 2009, 109, 3817–3858. [Google Scholar] [CrossRef]
- Hassan, H.M.A. Recent applications of ring-closing metathesis in the synthesis of lactams and macrolactams. Chem. Commun. 2010, 46, 9100–9106. [Google Scholar] [CrossRef]
- Hoveyda, A.H.; Zhugralin, A.R. The remarkable metal-catalysed olefin metathesis reaction. Nature 2007, 450, 243–251. [Google Scholar] [CrossRef]
- Kotha, S.; Shirbhate, M.E. Diversity-oriented approach to macrocyclic cyclophane derivatives via ring-closing metathesis. Synlett 2012, 23, 2183–2188. [Google Scholar] [CrossRef]
- Cutignano, A.; Bruno, I.; Bifulco, G.; Casapullo, A.; Debitus, C.; Gomez-Paloma, L.; Riccio, R. Dactylolide, a New Cytotoxic Macrolide from the Vanuatu Sponge Dactylospongia sp. Eur. J. Org. Chem. 2001, 2001, 775–778. [Google Scholar]
- Tanaka, J.-I.; Higa, T. Zampanolide, a new cytotoxic marcrolide from a marine sponge. Tetrahedron Lett. 1996, 37, 5535–5538. [Google Scholar] [CrossRef]
- Field, J.J.; Singh, A.J.; Kanakkanthara, A.; Halafihi, T.I.; Northcote, P.T.; Miller, J.H. Microtubule-Stabilizing Activity of Zampanolide, a Potent Macrolide Isolated from the Tongan Marine Sponge Cacospongia mycofijiensis. J. Med. Chem. 2009, 52, 7328–7332. [Google Scholar] [CrossRef]
- Smith, A.B.; Safonov, I.G. Total Synthesis of (+)-Dactylolide. Org. Lett. 2002, 4, 635–637. [Google Scholar] [CrossRef]
- Hoye, T.R.; Hu, M. Macrolactonization via Ti(IV)-Mediated Epoxy-Acid Coupling: A Total Synthesis of (−)-Dactylolide [and Zampanolide]. J. Am. Chem. Soc. 2003, 125, 9576–9577. [Google Scholar] [CrossRef]
- Ding, F.; Jennings, M. P. An Expedient Total Synthesis of (−)-Dactylolide and Formal Synthesis of (−)-Zampanolide. Org. Lett. 2005, 7, 2321–2324. [Google Scholar] [CrossRef]
- Sanchez, C.C.; Keck, G.E. Total Synthesis of (+)-Dactylolide. Org. Lett. 2005, 7, 3053–3056. [Google Scholar] [CrossRef]
- Aubele, D.L.; Wan, S.; Floreancig, P.E. Total Synthesis of (+)-Dactylolide through an Efficient Sequential Peterson Olefination and Prins Cyclization Reaction. Angew. Chem. Int. Ed. 2005, 44, 3485–3488. [Google Scholar] [CrossRef]
- Louis, I.; Hungerford, N.L.; Humphries, E.J.; McLeod, M.D. Enantioselective Total Synthesis of (−)-Dactylolide. Org. Lett. 2006, 8, 1117–1120. [Google Scholar] [CrossRef]
- Uenishi, J.I.; Iwamoto, T.; Tanaka, J. Total Synthesis of (−)-Zampanolide and Questionable Existence of (−)-Dactylolide as the Elusive Biosynthetic Precursor of (−)-Zampanolide in an Okinawan Sponge. Org. Lett. 2009, 11, 3262–3265. [Google Scholar] [CrossRef]
- Ding, F.; Jennings, M.P. Total Synthesis of (−)-Dactylolide and Formal Synthesis of (−)-Zampanolide via Target Oriented β-C-Glycoside Formation. J. Org. Chem. 2008, 73, 5965–5976. [Google Scholar] [CrossRef]
- Wilson, M.R.; Taylor, R.E. Toward an Enantioselective Synthesis of (−)-Zampanolide: Preparation of the C9–C20 Region. Org. Lett. 2012, 14, 3408–3411. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Cheng, X. Enantioselective Total Synthesis of (−)-Zampanolide, a Potent Microtubule-Stabilizing Agent. Org. Lett. 2011, 13, 4108–4111. [Google Scholar] [CrossRef]
- Yun, S.Y.; Hansen, E.C.; Volchkov, I.; Cho, E.J.; Lo, W.Y.; Lee, D. Total Synthesis of (−)-Dactylolide. Angew. Chem. Int. Ed. 2010, 49, 4261–4263. [Google Scholar] [CrossRef]
- Evano, G.; Schaus, J.V.; Panek, J.S. A Convergent Synthesis of the Macrocyclic Core of Cytotrienins: Application of RCM for Macrocyclization. Org. Lett. 2004, 6, 525–528. [Google Scholar] [CrossRef]
- Rössle, M.; del Valle, D.J.; Krische, M.J. Synthesis of the Cytotrienin A Core via Metal Catalyzed C-C Coupling. Org. Lett. 2011, 13, 1482–1485. [Google Scholar] [CrossRef]
- Hayashi, Y.; Shoji, M.; Ishikawa, H.; Yamaguchi, J.; Tamura, T.; Imai, H.; Nishigaya, Y.; Takabe, K.; Kakeya, H.; Osada, H. The Asymmetric Total Synthesis of (+)-Cytotrienin A, an Ansamycin-Type Anticancer Drug. Angew. Chem. Int. Ed. 2008, 47, 6657–6660. [Google Scholar] [CrossRef]
- Klapars, A.; Huang, X.; Buchwald, S.L. A General and Efficient Copper Catalyst for the Amidation of Aryl Halides. J. Am. Chem. Soc. 2002, 124, 7421–7428. [Google Scholar] [CrossRef]
- Lamarre, D.; Anderson, P.C.; Bailey, M.; Beaulieu, P.; Bolger, G.; Bonneau, P.; Bose, M.; Cameron, D.R.; Cartier, M.; Cordingley, M.G.; et al. An NS3 protease inhibitor with antiviral effects in humans infected with hepatitis C virus. Nature 2003, 426, 186–189. [Google Scholar] [CrossRef]
- Faucher, A.-M.; Bailey, M.D.; Beaulieu, P.L.; Brochu, C.; Duceppe, J.-S.; Ferland, J.-M.; Ghiro, E.; Gorys, V.; Halmos, T.; Kawai, S.H.; et al. Synthesis of BILN 2061, an HCV NS3 Protease Inhibitor with Proven Antiviral Effect in Humans. Org. Lett. 2004, 6, 2901–2904. [Google Scholar] [CrossRef]
- Yee, N.K.; Farina, V.; Houpis, I.N.; Haddad, N.; Frutos, R.P.; Gallou, F.; Wang, X.-J.; Wei, X.; Simpson, R.D.; Feng, X.; et al. Efficient Large-Scale Synthesis of BILN 2061, a Potent HCV Protease Inhibitor, by a Convergent Approach Based on Ring-Closing Metathesis. J. Org. Chem. 2006, 71, 7133–7145. [Google Scholar] [CrossRef]
- Shu, C.; Zeng, X.; Hao, M.-H.; Wei, X.; Yee, N.K.; Busacca, C.A.; Han, Z.; Farina, V.; Senanayake, C.H. RCM Macrocyclization Made Practical: An Efficient Synthesis of HCV Protease Inhibitor BILN 2061. Org. Lett. 2008, 10, 1303–1306. [Google Scholar] [CrossRef]
- Wei, X.; Shu, C.; Haddad, N.; Zeng, X.; Patel, N.D.; Tan, Z.; Liu, J.; Lee, H.; Shen, S.; Campbell, S.; et al. A Highly Convergent and Efficient Synthesis of a Macrocyclic Hepatitis C Virus Protease Inhibitor BI 201302. Org. Lett. 2013, 15, 1016–1019. [Google Scholar] [CrossRef]
- Kobayashi, J.I.; Watanabe, D.; Kawasaki, N.; Tsuda, M. Nakadomarin A, a Novel Hexacyclic Manzamine-Related Alkaloid from Amphimedon Sponge. J. Org. Chem. 1997, 62, 9236–9239. [Google Scholar] [CrossRef]
- Martin, D.B.C.; Vanderwal, C.D. Concise Synthesis of (−)-Nakadomarin A. Angew. Chem. Int. Ed. 2010, 49, 2830–2832. [Google Scholar] [CrossRef]
- Nagata, T.; Nakagawa, M.; Nishida, A. The First Total Synthesis of Nakadomarin A. J. Am. Chem. Soc. 2003, 125, 7484–7485. [Google Scholar] [CrossRef]
- Ono, K.; Nakagawa, M.; Nishida, A. Asymmetric Total Synthesis of (−)-Nakadomarin A. Angew. Chem. Int. Ed. 2004, 43, 2020–2023. [Google Scholar] [CrossRef]
- Young, I.S.; Kerr, M.A. Total Synthesis of (+)-Nakadomarin A. J. Am. Chem. Soc. 2007, 129, 1465–1469. [Google Scholar] [CrossRef]
- Jakubec, P.; Cockfield, D.M.; Dixon, D.J. Total Synthesis of (−)-Nakadomarin A. J. Am. Chem. Soc. 2009, 131, 16632–16633. [Google Scholar] [CrossRef]
- Yu, M.; Wang, C.; Kyle, A.F.; Jakubec, P.; Dixon, D.J.; Schrock, R.R.; Hoveyda, A.H. Synthesis of macrocyclic natural products by catalyst-controlled stereoselective ring-closing metathesis. Nature 2011, 479, 88–93. [Google Scholar] [CrossRef]
- Wang, C.; Yu, M.; Kyle, A.F.; Jakubec, P.; Dixon, D.J.; Schrock, R.R.; Hoveyda, A.H. Efficient and Selective Formation of Macrocyclic Disubstituted Z Alkenes by Ring-Closing Metathesis (RCM) Reactions Catalyzed by Mo- or W-Based Monoaryloxide Pyrrolide (MAP) Complexes: Applications to Total Syntheses of Epilachnene, Yuzu Lactone, Ambrettolide, Epothilone C, and Nakadomarin A. Chem. Eur. J. 2013, 19, 2726–2740. [Google Scholar] [CrossRef]
- Hoye, T.R.; Jeffrey, C.S.; Tennakoon, M.A.; Wang, J.; Zhao, H. Relay Ring-Closing Metathesis (RRCM): A Strategy for Directing Metal Movement Throughout Olefin Metathesis Sequences. J. Am. Chem. Soc. 2004, 126, 10210–10211. [Google Scholar] [CrossRef]
- Roethle, P.A.; Chen, I.T.; Trauner, D. Total Synthesis of (−)-Archazolid B. J. Am. Chem. Soc. 2007, 129, 8960–8961. [Google Scholar] [CrossRef]
- Toumi, M.; Couty, F.; Evano, G. Total Synthesis of the Cyclopeptide Alkaloid Paliurine E. Insights into Macrocyclization by Ene-Enamide RCM. J. Org. Chem. 2008, 73, 1270–1281. [Google Scholar] [CrossRef]
- Nicolaou, K.C.; Bulger, P.G.; Sarlah, D. Metathesis Reactions in Total Synthesis. Angew. Chem. Int. Ed. 2005, 44, 4490–4527. [Google Scholar] [CrossRef]
- Diver, S.T.; Giessert, A.J. Enyne Metathesis (Enyne Bond Reorganization). Chem. Rev. 2004, 104, 1317–1382. [Google Scholar] [CrossRef]
- Hansen, E.C.; Lee, D. Enyne Metathesis for the Formation of Macrocyclic 1,3-Dienes. J. Am. Chem. Soc. 2003, 125, 9582–9583. [Google Scholar] [CrossRef]
- Hansen, E.C.; Lee, D. Ring Closing Enyne Metathesis: Control over Mode Selectivity and Stereoselectivity. J. Am. Chem. Soc. 2004, 126, 15074–15080. [Google Scholar] [CrossRef]
- Fu, X.; Hossain, M.B.; van der Helm, D.; Schmitz, F.J. Longithorone A: Unprecedented dimeric prenylated quinone from the tunicate aplydium longithorax. J. Am. Chem. Soc. 1994, 116, 12125–12126. [Google Scholar] [CrossRef]
- Layton, M.E.; Morales, C.A.; Shair, M.D. Biomimetic Synthesis of (−)-Longithorone A. J. Am. Chem. Soc. 2002, 124, 773–775. [Google Scholar] [CrossRef]
- Collins, S.K.; El-Azizi, Y.; Schmitzer, A.R. Development of Perfluoroarene—Arene Interactions for Macrocyclic En-yne Metathesis and the Total Synthesis of Macrocyclic Natural Products. J. Org. Chem. 2007, 72, 6397–6408. [Google Scholar] [CrossRef]
- Fürstner, A. Alkyne Metathesis on the Rise. Angew. Chem. Int. Ed. 2013, 52, 2794–2819. [Google Scholar] [CrossRef]
- Wu, X.; Tamm, M. Recent advances in the development of alkyne metathesis catalysts. Beilstein J. Org. Chem. 2011, 7, 82–93. [Google Scholar] [CrossRef]
- Brewitz, L.; Llaveria, J.; Yada, A.; Fürstner, A. Formal Total Synthesis of the Algal Toxin (−)-Polycavernoside A. Chem. Eur. J. 2013, 19, 4532–4537. [Google Scholar] [CrossRef]
- Andersson, H.; Demaegdt, H.; Johnsson, A.; Vauquelin, G.; Lindeberg, G.; Hallberg, M.; Erdélyi, M.T.; Karlén, A.; Hallberg, A. Potent Macrocyclic Inhibitors of Insulin-Regulated Aminopeptidase (IRAP) by Olefin Ring-Closing Metathesis. J. Med. Chem. 2011, 54, 3779–3792. [Google Scholar] [CrossRef]
- Andersson, H.; Demaegdt, H.; Vauquelin, G.; Lindeberg, G.; Karlén, A.; Hallberg, M.; Erdélyi, M.T.; Hallberg, A. Disulfide Cyclized Tripeptide Analogues of Angiotensin IV as Potent and Selective Inhibitors of Insulin-Regulated Aminopeptidase (IRAP). J. Med. Chem. 2010, 53, 8059–8071. [Google Scholar] [CrossRef]
- Abell, A.D.; Alexander, N.A.; Aitken, S.G.; Chen, H.; Coxon, J.M.; Jones, M.A.; McNabb, S.B.; Muscroft-Taylor, A. Synthesis of Macrocyclic β-Strand Templates by Ring Closing Metathesis. J. Org. Chem. 2009, 74, 4354–4356. [Google Scholar] [CrossRef]
- Schreiber, S.L. Target-Oriented and Diversity-Oriented Organic Synthesis in Drug Discovery. Science 2000, 287, 1964–1969. [Google Scholar] [CrossRef]
- Tan, D.S. Diversity-oriented synthesis: Exploring the intersections between chemistry and biology. Nat. Chem. Biol. 2005, 1, 74–84. [Google Scholar] [CrossRef]
- Spring, D.R. Diversity-oriented synthesis; a challenge for synthetic chemists. Org. Biomol. Chem. 2003, 1, 3867–3870. [Google Scholar] [CrossRef]
- Galloway, W.R.J.D.; Spring, D.R. Is synthesis the main hurdle for the generation of diversity in compound libraries for screening? Expert Opin. Drug Discov. 2009, 4, 467–472. [Google Scholar] [CrossRef]
- Galloway, W.R.J.D.; Isidro-Llobet, A.; Spring, D.R. Diversity-oriented synthesis as a tool for the discovery of novel biologically active small molecules. Nat. Commun. 2010, 1, 80. [Google Scholar]
- Kopp, F.; Stratton, C.F.; Akella, L.B.; Tan, D.S. A diversity-oriented synthesis approach to macrocycles via oxidative ring expansion. Nat. Chem. Biol. 2012, 8, 358–365. [Google Scholar] [CrossRef]
- Schmidt, D.R.; Kwon, O.; Schreiber, S.L. Macrolactones in diversity-oriented synthesis: Preparation of a pilot library and exploration of factors controlling macrocyclization. J. Comb. Chem. 2004, 6, 286–292. [Google Scholar] [CrossRef]
- Madsen, C.M.; Clausen, M.H. Biologically Active Macrocyclic Compounds—From Natural Products to Diversity-Oriented Synthesis. Eur. J. Org. Chem. 2011, 2011, 3107–3115. [Google Scholar] [CrossRef]
- O'Connor, C.J.; Beckmann, H.S.G.; Spring, D.R. Diversity-oriented synthesis: Producing chemical tools for dissecting biology. Chem. Soc. Rev. 2012, 41, 4444–4456. [Google Scholar] [CrossRef]
- Schreiber, S.L. Organic chemistry: Molecular diversity by design. Nature 2009, 457, 153–154. [Google Scholar] [CrossRef]
- Isidro-Llobet, A.; Murillo, T.; Bello, P.; Cilibrizzi, A.; Hodgkinson, J.T.; Galloway, W.R.J.D.; Bender, A.; Welch, M.; Spring, D.R. Diversity-oriented synthesis of macrocyclic peptidomimetics. Proc. Natl. Acad. Sci. USA 2011, 108, 6793–6798. [Google Scholar] [CrossRef]
- O'Connell, K.M.G.; Beckmann, H.S.G.; Laraia, L.; Horsley, H.T.; Bender, A.; Venkitaraman, A.R.; Spring, D.R. A two-directional strategy for the diversity-oriented synthesis of macrocyclic scaffolds. Org. Biomol. Chem. 2012, 10, 7545–7551. [Google Scholar] [CrossRef]
- Uchida, T.; Rodriquez, M.; Schreiber, S.L. Skeletally Diverse Small Molecules Using a Build/Couple/Pair Strategy. Org. Lett. 2009, 11, 1559–1562. [Google Scholar] [CrossRef]
- Hussain, A.; Yousuf, S.K.; Kumar, D.; Lambu, M.; Singh, B.; Maity, S.; Mukherjee, D. Intramolecular Base-Free Sonogashira Reaction for the Synthesis of Benzannulated Chiral Macrocycles Embedded in Carbohydrate Templates. Adv. Synth. Catal. 2012, 354, 1933–1940. [Google Scholar] [CrossRef]
- Nielsen, T.E.; Schreiber, S.L. Towards the Optimal Screening Collection: A Synthesis Strategy. Angew. Chem. Int. Ed. 2008, 47, 48–56. [Google Scholar] [CrossRef]
- Fitzgerald, M.E.; Mulrooney, C.A.; Duvall, J.R.; Wei, J.; Suh, B.-C.; Akella, L.B.; Vrcic, A.; Marcaurelle, L.A. Build/Couple/Pair Strategy for the Synthesis of Stereochemically Diverse Macrolactams via Head-to-Tail Cyclization. ACS Comb. Sci. 2012, 14, 89–96. [Google Scholar] [CrossRef]
- Dandapani, S.; Lowe, J.T.; Comer, E.; Marcaurelle, L.A. Diversity-Oriented Synthesis of 13- to 18-Membered Macrolactams via Ring-Closing Metathesis. J. Org. Chem. 2011, 76, 8042–8048. [Google Scholar] [CrossRef]
- Wang, Y.; Jimenez, M.; Hansen, A.S.; Raiber, E.-A.; Schreiber, S.L.; Young, D.W. Control of Olefin Geometry in Macrocyclic Ring-Closing Metathesis Using a Removable Silyl Group. J. Am. Chem. Soc. 2011, 133, 9196–9199. [Google Scholar] [CrossRef]
- Marx, V.M.; Herbert, M.B.; Keitz, B.K.; Grubbs, R.H. Stereoselective Access to Z and E Macrocycles by Ruthenium-Catalyzed Z-Selective Ring-Closing Metathesis and Ethenolysis. J. Am. Chem. Soc. 2012, 135, 94–97. [Google Scholar]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Umit, T. Triple click reaction strategy for macromolecular diversity. Macromol. Rapid Commun. 2013, 34, 38–46. [Google Scholar] [CrossRef]
- Sumerlin, B.S.; Vogt, A.P. Macromolecular Engineering through Click Chemistry and Other Efficient Transformations. Macromolecules 2009, 43, 1–13. [Google Scholar] [CrossRef]
- Becer, C.R.; Hoogenboom, R.; Schubert, U.S. Click Chemistry beyond Metal-Catalyzed Cycloaddition. Angew. Chem. Int. Ed. 2009, 48, 4900–4908. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A Stepwise Huisgen Cycloaddition Process: Copper(I)-Catalyzed Regioselective “Ligation” of Azides and Terminal Alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Tornøe, C.W.; Christensen, C.; Meldal, M. Peptidotriazoles on Solid Phase: [1,2,3]-Triazoles by Regiospecific Copper(I)-Catalyzed 1,3-Dipolar Cycloadditions of Terminal Alkynes to Azides. J. Org. Chem. 2002, 67, 3057–3064. [Google Scholar] [CrossRef]
- Bock, V.D.; Hiemstra, H.; van Maarseveen, J.H. CuI-Catalyzed Alkyne-Azide “Click” Cycloadditions from a Mechanistic and Synthetic Perspective. Eur. J. Org. Chem. 2006, 2006, 51–68. [Google Scholar]
- Fournier, D.; Hoogenboom, R.; Schubert, U.S. Clicking polymers: A straightforward approach to novel macromolecular architectures. Chem. Soc. Rev. 2007, 36, 1369–1380. [Google Scholar] [CrossRef]
- Hawker, C.J.; Wooley, K.L. The convengence of synthetic organic and polymer chemistries. Science 2005, 309, 1200–1205. [Google Scholar] [CrossRef]
- Tyagi, M.; Taxak, N.; Bharatam, P.V.; Kartha, K.P.R. Synthesis of self-assembling glycerotriazolophanes. RSC Adv. 2012, 2, 11366–11371. [Google Scholar] [CrossRef]
- Whiting, M.; Muldoon, J.; Lin, Y.-C.; Silverman, S.M.; Lindstrom, W.; Olson, A.J.; Kolb, H.C.; Finn, M.G.; Sharpless, K.B.; Elder, J.H.; et al. Inhibitors of HIV-1 Protease by Using In Situ Click Chemistry. Angew. Chem. Int. Ed. 2006, 45, 1435–1439. [Google Scholar] [CrossRef]
- Genin, M.J.; Allwine, D.A.; Anderson, D.J.; Barbachyn, M.R.; Emmert, D.E.; Garmon, S.A.; Graber, D.R.; Grega, K.C.; Hester, J.B.; Hutchinson, D.K.; et al. Substituent Effects on the Antibacterial Activity of Nitrogen-Carbon-Linked (Azolylphenyl)oxazolidinones with Expanded Activity Against the Fastidious Gram-Negative Organisms Haemophilus influenzae and Moraxella catarrhalis. J. Med. Chem. 2000, 43, 953–970. [Google Scholar] [CrossRef]
- Pereira, D.; Fernandes, P. Synthesis and antibacterial activity of novel 4-aryl-[1,2,3]-triazole containing macrolides. Bioorg. Med. Chem. Lett. 2011, 21, 510–513. [Google Scholar] [CrossRef]
- Zhou, C.H.; Wang, Y. Recent researches in triazole compounds as medicinal drugs. Curr. Med. Chem. 2012, 19, 239–280. [Google Scholar] [CrossRef]
- Angell, Y.L.; Burgess, K. Peptidomimetics via copper-catalyzed azide-alkyne cycloadditions. Chem. Soc. Rev. 2007, 36, 1674–1689. [Google Scholar] [CrossRef]
- Empting, M.; Avrutina, O.; Meusinger, R.; Fabritz, S.; Reinwarth, M.; Biesalski, M.; Voigt, S.; Buntkowsky, G.; Kolmar, H. "Triazole Bridge”: Disulfide-Bond Replacement by Ruthenium-Catalyzed Formation of 1,5-Disubstituted 1,2,3-Triazoles. Angew. Chem. Int. Ed. 2011, 50, 5207–5211. [Google Scholar] [CrossRef]
- Hu, T.S.; Tannert, R.; Arndt, H.D.; Waldmann, H. Solid-phase based synthesis of jasplakinolide analogs by intramolecular azide-alkyne cycloadditions. Chem. Commun. 2007, 2007, 3942–3944. [Google Scholar]
- Turner, R.A.; Oliver, A.G.; Lokey, R.S. Click chemistry as a macrocyclization tool in the solid-phase synthesis of small cyclic peptides. Org. Lett. 2007, 9, 5011–5014. [Google Scholar] [CrossRef]
- Chouhan, G.; James, K. Efficient Construction of Proline-Containing β-Turn Mimetic Cyclic Tetrapeptides via CuAAC Macrocyclization. Org. Lett. 2013, 15, 1206–1209. [Google Scholar] [CrossRef]
- Moses, J.E.; Moorhouse, A.D. The growing applications of click chemistry. Chem. Soc. Rev. 2007, 36, 1249–1262. [Google Scholar] [CrossRef]
- Bogdan, A.R.; James, K. Synthesis of 5-Iodo-1,2,3-triazole-Containing Macrocycles Using Copper Flow Reactor Technology. Org. Lett. 2011, 13, 4060–4063. [Google Scholar] [CrossRef]
- Nahrwold, M.; Bogner, T.; Eissler, S.; Verma, S.; Sewald, N. "Clicktophycin-52”: A Bioactive Cryptophycin-52 Triazole Analogue. Org. Lett. 2010, 12, 1064–1067. [Google Scholar] [CrossRef]
- Zhang, J.; Kemmink, J.; Rijkers, D.T.S.; Liskamp, R.M.J. Cu(I)- and Ru(II)-Mediated “Click” Cyclization of Tripeptides Toward Vancomycin-Inspired Mimics. Org. Lett. 2011, 13, 3438–3441. [Google Scholar] [CrossRef]
- Haridas, V.; Lal, K.; Sharma, Y.K.; Upreti, S. Design, Synthesis, and Self-Assembling Properties of Novel Triazolophanes. Org. Lett. 2008, 10, 1645–1647. [Google Scholar] [CrossRef]
- Bahulayan, D.; Arun, S. An easy two step synthesis of macrocyclic peptidotriazoles via a four-component reaction and copper catalyzed intramolecular azide-alkyne [3+2] click cycloaddition. Tetrahedron Lett. 2012, 53, 2850–2855. [Google Scholar] [CrossRef]
- Holub, J.M.; Kirshenbaum, K. Tricks with clicks: Modification of peptidomimetic oligomers via copper-catalyzed azide-alkyne [3 + 2] cycloaddition. Chem. Soc. Rev. 2010, 39, 1325–1337. [Google Scholar] [CrossRef]
- Ingale, S.; Dawson, P.E. On Resin Side-Chain Cyclization of Complex Peptides Using CuAAC. Org. Lett. 2011, 13, 2822–2825. [Google Scholar] [CrossRef]
- Ajay, A.; Sharma, S.; Gupt, M.P.; Bajpai, V.; Kumar, B.; Kaushik, M.P.; Konwar, R.; Ampapathi, R.S.; Tripathi, R.P. Diversity Oriented Synthesis of Pyran Based Polyfunctional Stereogenic Macrocyles and Their Conformational Studies. Org. Lett. 2012, 14, 4306–4309. [Google Scholar] [CrossRef]
© 2013 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Yu, X.; Sun, D. Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles. Molecules 2013, 18, 6230-6268. https://doi.org/10.3390/molecules18066230
Yu X, Sun D. Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles. Molecules. 2013; 18(6):6230-6268. https://doi.org/10.3390/molecules18066230
Chicago/Turabian StyleYu, Xufen, and Dianqing Sun. 2013. "Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles" Molecules 18, no. 6: 6230-6268. https://doi.org/10.3390/molecules18066230
APA StyleYu, X., & Sun, D. (2013). Macrocyclic Drugs and Synthetic Methodologies toward Macrocycles. Molecules, 18(6), 6230-6268. https://doi.org/10.3390/molecules18066230



