Abstract
Muntingia calabura (Tiliaceae) is commercially used in healthcare for the improvement of hypertension, myocardial infarction, spasm, and inflammatory conditions. Its fruits can be processed into jam and the leaves can be used for making tea. In the work reported herein a new biflavan, (M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-decamethoxy-5,5'' biflavan (1), a new flavone, 4'-hydroxy-7,8,3',5'-tetramethoxyflavone (2), and a new dihydrochalcone, (R)-2',β-dihydroxy-3',4'-dimethoxydihydrochalcone (3), have been isolated from the stem wood of M. calabura, together with 12 known compounds (4–15). The structures of these new compounds were elucidated by the interpretations of extensive spectroscopic data. Among the isolated compounds, 5-hydroxy-7-methoxyflavone (5), quercetin (6), and (2S)-7-hydroxyflavanone (10) exhibited potent inhibition of fMLP-induced superoxide anion generation by human neutrophils, with IC50 values of 1.77 ± 0.70, 3.82 ± 0.46, and 4.92 ± 1.71 μM, respectively.
1. Introduction
Muntingia calabura L. (Tiliaceae) is an evergreen tree originally distributed in tropical America [1]. In Mexico, the fruits of M. calabura are eaten and sold in markets. The fruits can be processed into jams and the leaves can be used for making tea. Past studies have revealed flavones, flavanones, flavans, and biflavans to be the major constituents of this species, some of which have displayed anti-platelet aggregation [2] and cytotoxic [3,4,5,6] activities.
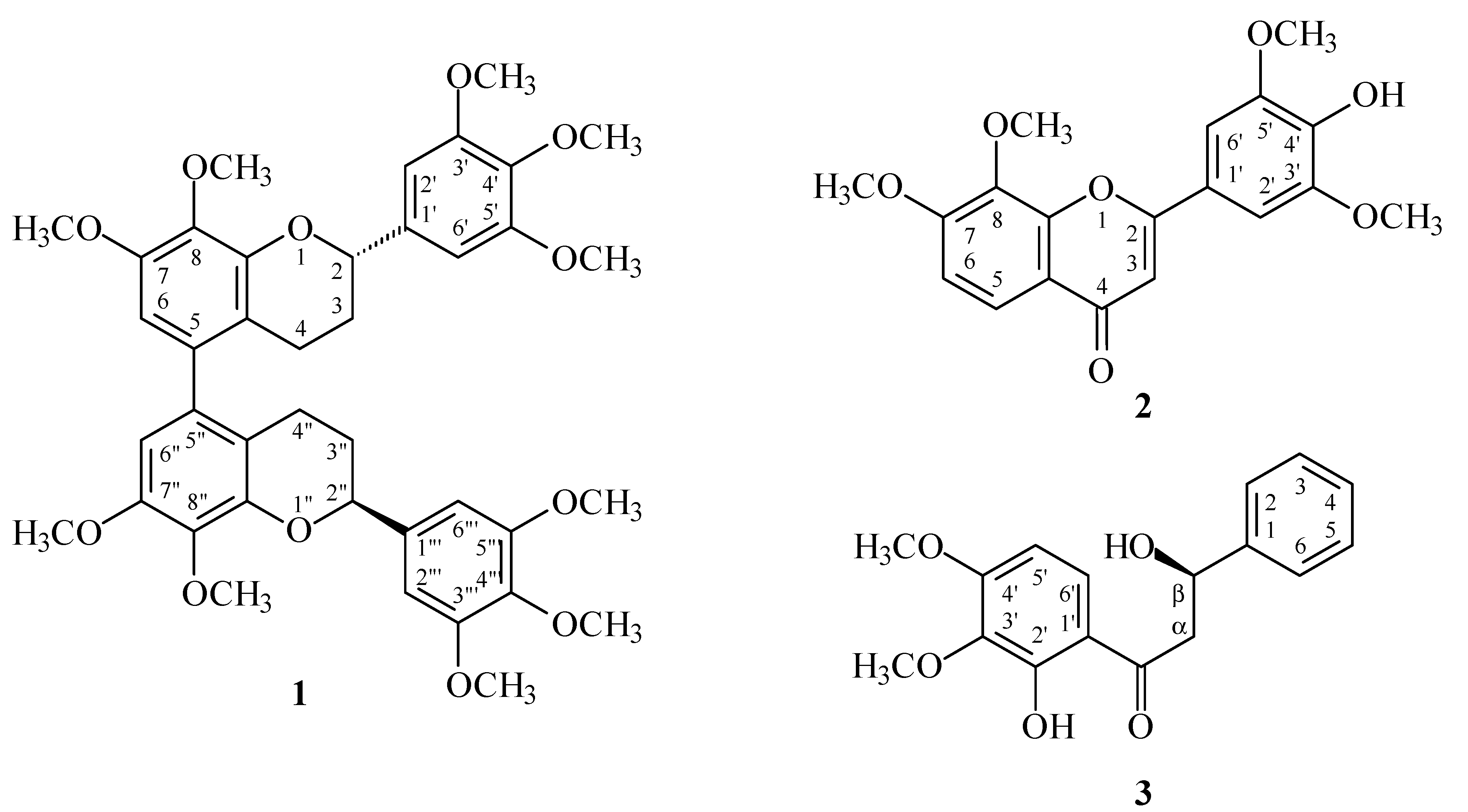
Reactive oxygen species (ROS) (e.g., superoxide anion (O2•−) and hydrogen peroxide) produced by human neutrophils are involved in the pathogenesis of a variety of inflammatory diseases. In a screening program searching for anti-inflammatory compounds from Formosan plants [7,8,9,10,11], M. calabura was found to be an active species. The MeOH extract of the stem wood of M. calabura showed potent inhibitory effects on superoxide anion generation by human neutrophils in response to formyl-l-methionyl-l-leucyl-l-phenylalanine (fMLP). Figure 1 shows the structures of three new compounds, (M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-decamethoxy-5,5''-biflavan (1), 4'-hydroxy-7,8,3',5'-tetramethoxyflavone (2), and (R)-2',β-dihydroxy-3',4'-dimethoxydihydrochalcone (3) that have been isolated and identified from the stem wood of M. calabura together with the 12 known compounds 4–15 (Figure 2). This paper describes the structural elucidation of the compounds 1–3, and the inhibitory activities of all isolates on superoxide generation by neutrophils.
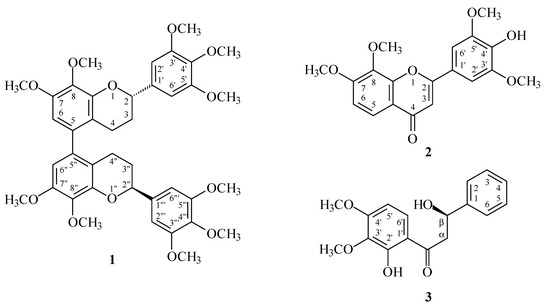
Figure 1.
The chemical structures of new compounds 1–3 isolated from M. calabura.
Figure 1.
The chemical structures of new compounds 1–3 isolated from M. calabura.
2. Results and Discussion
2.1. Results
Chromatographic purification of the CH2Cl2-soluble fraction of a MeOH extract of stem wood of M. calaburaon a silica gel column and preparative thin-layer chromatography (TLC) afforded three new compounds 1–3 and twelve known compounds 4–15.
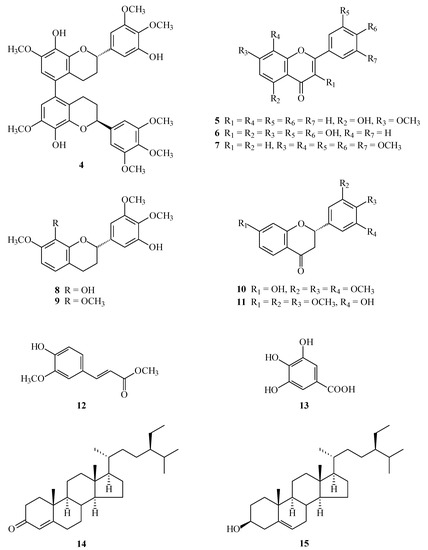
Figure 2.
The chemical structures of known compounds 4–15 isolated from M. calabura.
Figure 2.
The chemical structures of known compounds 4–15 isolated from M. calabura.
(M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-Decamethoxy-5,5''-biflavan (1) was obtained as optically active ( = +12.8) colorless prisms. The molecular formula C40H46O12 was deduced from a sodium adduct ion at m/z 741.2889 [M + Na]+ (calcd. 741.2887) in the HR-ESI mass spectrum. The presence of an aromatic ring C=C stretch was revealed by the bands at 1602, 1515, and 1452 cm−1 in the IR spectrum. The 1H-NMR spectrum (Table 1) of 1 indicated the presence of five methoxy groups [δ 3.85 (3H, s, OMe-4'), 3.86 (3H, s, OMe-7), 3.87 (6H, s, OMe-3' and OMe-5'), 3.90 (3H, s, OMe-8)], three aromatic protons [δ 6.42 (1H, s, H-6), 6.64 (2H, s, H-2' and H-6' )], an oxymethine proton [δ 5.02 (1H, dd, J = 9.6, 3.0 Hz, H-2)], and four methylene protons [δ 1.90–2.08 (1H, m, Hax-3), 2.09-2.18 (1H, m, Heq-3), 2.23 (1H, ddd, J = 16.4, 4.4, 4.4 Hz, Heq-4), 2.66 (1H, ddd, J = 16.4, 11.0, 6.0 Hz, Hax-4)]. Comparison of the 1H-, 13C-NMR, and MS data of 1 with those of (M),(2S),(2''S)-, (P),(2S),(2''S)-8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4) [5] (Table 1) suggested that their structures were closely related, except that the 8-methoxy [δH 3.90 (3H, s); δC 60.8 (OMe-8)], 5'-methoxy [δH 3.87 (3H, s); δC 56.1 (OMe-5')], and 8′′-methoxy [δH 3.90 (3H, s); δC 60.8 (OMe-8'')] groups of 1 replaced the 8,5',8''-trihydroxy groups of 4 [5]. This was supported by both HMBC correlations (Figure 3) between OMe-8 (δ 3.90)/C-8 (δ 141.0), OMe-5' (δ 3.87)/C-5' (δ 153.4), and OMe-8'' (δ 3.90)/C-8'' (δ 141.0), and NOESY correlations (Figure 3) between OMe-5' (δ 3.87) and H-6' (δ 6.64). The 1H- and 13C-NMR spectra (Table 1 and Table 2) of 1 also showed two diastereomers (M and P in terms of helicity) as in the case of (M),(2S),(2''S)-,(P),(2S),(2''S)-8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4) [5]. The absolute configurations at C-2 and C-2'' were determined as 2S,2''S by CD comparison with the analogous biflavan, (M),(2S),(2''S)-, (P),(2S),(2''S)-8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4) [5]. The full assignment of 1H- and 13C-NMR resonances (Table 1 and Table 2) was supported by 1H-1H COSY, DEPT, HSQC, NOESY (Figure 3), and HMBC (Figure 3) spectral analyses. According to the above data, the structure of 1 was elucidated as (M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-decamethoxy-5,5''-biflavan.

Table 1.
1H-NMR data of 1 and 4 [5].
| Position | δH | |||
|---|---|---|---|---|
| 1a | 4 b | |||
| M | P | M | P | |
| 2 | 5.02 dd (9.6, 3.0) | 5.03 dd (9.6, 3.0) | 4.90 d (9.4) | 4.90 d (9.4) |
| 3ax | 1.90–2.08 m | 1.90–2.08 m | 1.70–2.13 m | 1.70–2.13 m |
| 3eq | 2.09–2.18 m | 2.09–2.18 m | 1.70–2.13 m | 1.70–2.13 m |
| 4ax | 2.66 ddd (16.4, 11.0, 6.0) | 2.54 ddd (16.4, 11.0, 6.0) | 2.25–2.72 m | 2.25–2.72 m |
| 4eq | 2.23 ddd (16.4, 4.4, 4.4) | 2.50 ddd (16.4, 4.4, 4.4) | 2.25–2.72 m | 2.25–2.72 m |
| 6 | 6.42 s | 6.37 s | 6.38 s | 6.30 s |
| 2' | 6.64 s | 6.66 s | 6.59 d (2.1) | 6.59 d (2.1) |
| 6' | 6.64 s | 6.66 s | 6.61 s | 6.61 s |
| 2'' | 5.02 dd (9.6, 3.0) | 5.03 dd (9.6, 3.0) | 4.97 d (9.9) | 4.97 d (9.9) |
| 3''ax | 1.90–2.08 m | 1.90–2.08 m | 1.70–2.13 m | 1.70–2.13 m |
| 3''eq | 2.09–2.18 m | 2.09–2.18 m | 1.70–2.13 m | 1.70–2.13 m |
| 4''ax | 2.66 ddd (16.4, 11.0, 6.0) | 2.54 ddd (16.4, 11.0, 6.0) | 2.25–2.72 m | 2.25–2.72 m |
| 4''eq | 2.23 ddd (16.4, 4.4, 4.4) | 2.50 ddd (16.4, 4.4, 4.4) | 2.25–2.72 m | 2.25–2.72 m |
| 6'' | 6.42 s | 6.37 s | 6.38 s | 6.32 s |
| 2''' | 6.64 s | 6.66 s | 6.79 s | 6.82 s |
| 6''' | 6.64 s | 6.66 s | 6.79 s | 6.82 s |
| OMe-7 | 3.86 s | 3.87 s | 3.75 s | 3.74 s |
| OH-8 | 8.13 s | 8.13 s | ||
| OMe-8 | 3.90 s | 3.89 s | ||
| OMe-3' | 3.87 s | 3.88 s | 3.77 s | 3.79 s |
| OMe-4' | 3.85 s | 3.86 s | 3.66 s | 3.68 s |
| OH-5' | 9.17 s | 9.20 s | ||
| OMe-5' | 3.87 s | 3.88 s | ||
| OMe-7'' | 3.86 s | 3.87 s | 3.75 s | 3.74 s |
| OH-8'' | 8.13 s | 8.13 s | ||
| OMe-8'' | 3.90 s | 3.89 s | ||
| OMe-3''' | 3.87 s | 3.88 s | 3.77 s | 3.79 s |
| OMe-4''' | 3.85 s | 3.86 s | 3.66 s | 3.68 s |
| OMe-5''' | 3.87 s | 3.88 s | 3.81 s | 3.79 s |
a Recorded in CDCl3 at 400 MHz. b Recorded in DMSO-d6 at 300 MHz. Values in ppm (δ). J (in Hz) in parentheses.

Table 2.
13C-NMR data of 1 and 4 [5].
| Position | δC | |||
|---|---|---|---|---|
| 1 a | 4 b | |||
| M | P | M | P | |
| 2 | 78.5 | 78.6 | 76.8 | 76.9 |
| 3 | 30.4 | 30.1 | 29.7 | 29.5 |
| 4 | 23.6 | 23.0 | 23.3 | 22.7 |
| 5 | 130.8 | 130.4 | 130.0 | 130.5 |
| 6 | 109.6 | 109.2 | 106.2 | 105.7 |
| 7 | 146.5 | 146.5 | 145.9 | 145.9 |
| 8 | 141.0 | 141.0 | 133.7 | 133.7 |
| 9 | 147.6 | 147.4 | 143.7 | 143.6 |
| 10 | 113.8 | 113.2 | 113.7 | 114.2 |
| 1' | 136.9 | 136.9 | 137.2 | 137.2 |
| 2' | 103.2 | 103.3 | 101.6 | 101.7 |
| 3' | 153.4 | 153.4 | 153.0 | 153.0 |
| 4' | 136.8 | 136.8 | 135.7 | 135.8 |
| 5' | 153.4 | 153.4 | 150.3 | 150.4 |
| 6' | 103.2 | 103.3 | 107.2 | 107.2 |
| 2'' | 78.5 | 78.6 | 77.0 | 77.1 |
| 3'' | 30.4 | 30.1 | 29.7 | 29.5 |
| 4'' | 23.6 | 23.0 | 23.5 | 22.9 |
| 5'' | 130.8 | 130.4 | 130.1 | 130.6 |
| 6'' | 109.6 | 109.2 | 106.4 | 105.9 |
| 7'' | 146.5 | 146.5 | 145.9 | 145.9 |
| 8'' | 141.0 | 141.0 | 133.7 | 133.7 |
| 9'' | 147.6 | 147.4 | 143.6 | 143.6 |
| 10'' | 113.8 | 113.2 | 113.7 | 114.2 |
| 1''' | 136.9 | 136.9 | 137.4 | 137.4 |
| 2''' | 103.2 | 103.3 | 103.7 | 103.8 |
| 3''' | 153.4 | 153.4 | 152.8 | 152.8 |
| 4''' | 136.8 | 136.8 | 136.9 | 137.0 |
| 5''' | 153.4 | 153.4 | 152.8 | 152.8 |
| 6''' | 103.2 | 103.3 | 103.7 | 103.8 |
| OMe-7 | 56.3 | 56.3 | 56.1 | 56.1 |
| OMe-8 | 60.8 | 60.8 | ||
| OMe-3' | 56.1 | 56.1 | 55.7 | 55.7 |
| OMe-4' | 60.9 | 60.9 | 59.9 | 59.9 |
| OMe-5' | 56.1 | 56.1 | ||
| OMe-7'' | 56.3 | 56.3 | 56.1 | 56.1 |
| OMe-8'' | 60.8 | 60.8 | ||
| OMe-3''' | 56.1 | 56.1 | 55.7 | 55.9 |
| OMe-4''' | 60.9 | 60.9 | 59.9 | 60.0 |
| OMe-5''' | 56.1 | 56.1 | 55.9 | |
a Recorded in CDCl3 at 100 MHz. b Recorded in DMSO-d6 at 75.6 MHz. Values in ppm (δ).
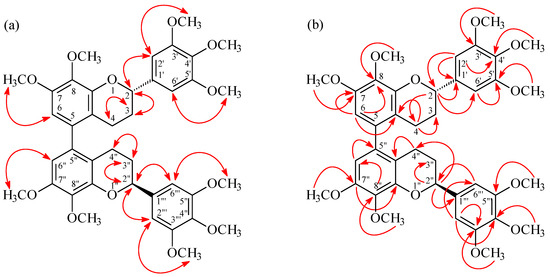
Figure 3.
Key NOESY (a) and HMBC (b) correlations of 1.
Figure 3.
Key NOESY (a) and HMBC (b) correlations of 1.
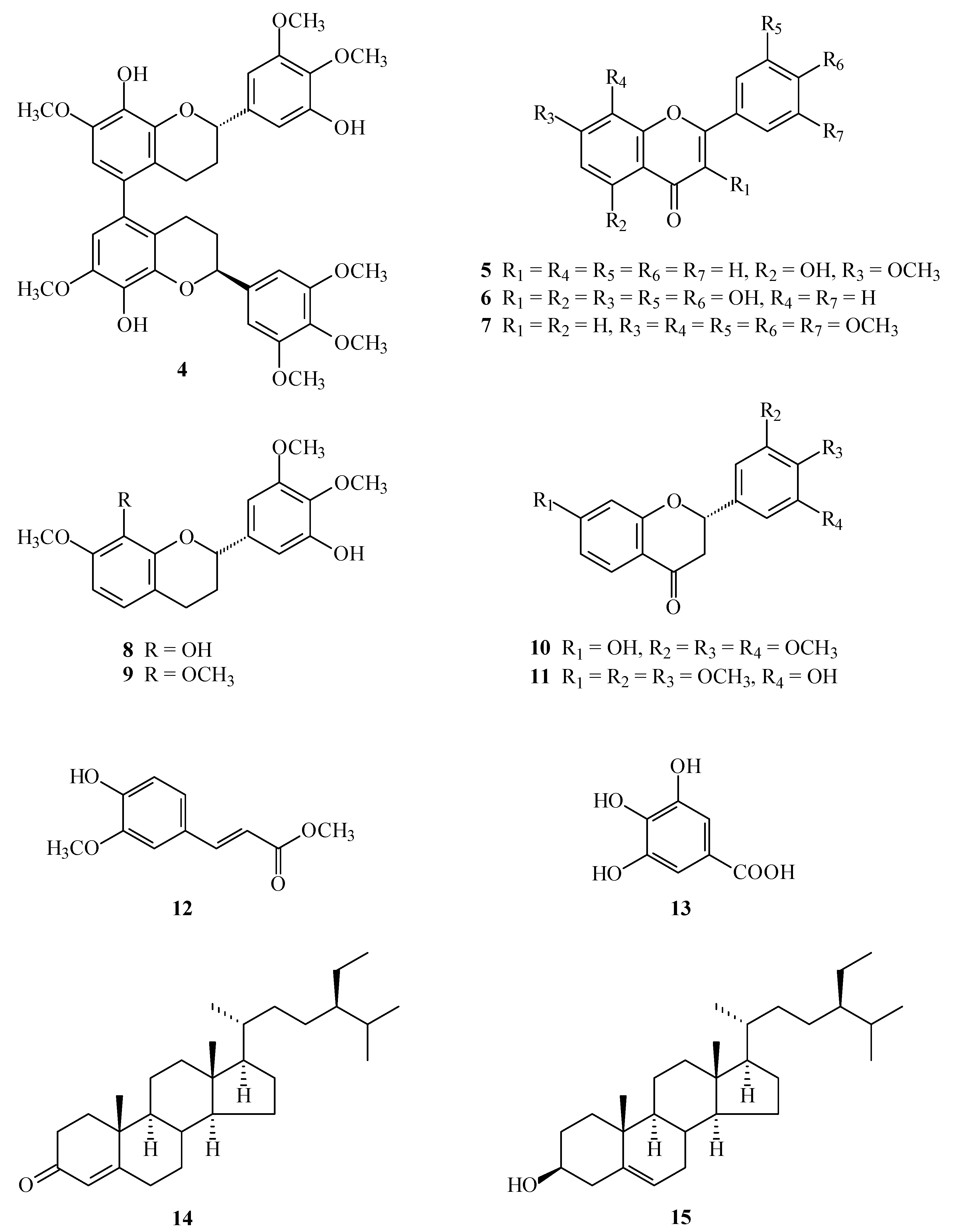
4'-Hydroxy-7,8,3',5'-tetramethoxyflavone (2) was isolated as yellowish needles with molecular formula C19H18O7 as determined by positive-ion HR-ESI-MS, showing an [M + Na]+ ion at m/z 381.0952 (calcd for C19H18O7Na, 381.0950). The presence of a carbonyl group was revealed by a band at 1630 cm−1 in the IR spectrum, and was confirmed by the resonance at δ 178.0 in the 13C-NMR spectrum. The 1H-NMR spectrum of 2 showed the presence of four methoxy groups [δ 3.99 (6H, s, OMe-3' and OMe-5'), 4.02 (3H, s, OMe-8), and 4.05 (3H, s, OMe-7)], five aromatic protons [δ 6.72 (1H, s, H-3), 7.22 (2H, s, H-2' and H-6'), and 7.07, 7.96 (each 1H, each d, J = 8.8 Hz, H-6 and H-5)], and a hydroxy group [δ 5.88 (1H, br s, D2O exchangeable, OH-4')]. The 1H- and 13C-NMR data of 2 were similar to those of 7,8,3',4',5'-pentamethoxyflavone (7) [5], except that the 4'-hydroxy group [δH 5.88 (1H, br s); δC 136.5 (C-4')] of 2 replaced the 4'-methoxy group [δH 3.94 (3H, s); δC 61.1 (OMe-4'), 141.1 (C-4')] of 7. This was supported by HMBC correlation observed between H-2'/H-6' (δ 7.22) and C-4' (δ 136.5). On the basis of the evidence above, the structure of 2 was elucidated as 4'-hydroxy-7,8,3',5'-tetramethoxyflavone, which was further substantiated through 2D-experiments, including HSQC, 1H-1H COSY, HMBC (Figure 4), and NOESY (Figure 4) spectra. This is the first report of the occurrence of 2 in a natural source, although it has been synthesized by Bellini and Venturella [12].
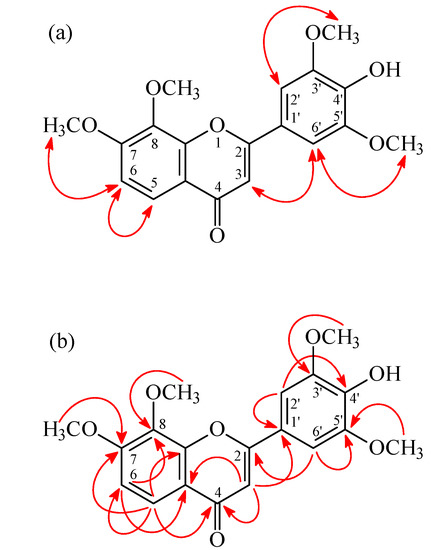
Figure 4.
Key NOESY (a) and HMBC (b) correlations of 2.
Figure 4.
Key NOESY (a) and HMBC (b) correlations of 2.
(R)-2',β-Dihydroxy-3',4'-dimethoxydihydrochalcone (3) was isolated as amorphous powder. The ESI-MS of 3 afforded an [M + Na]+ ion at m/z 325, implying a molecular formula of C17H18O5, which was confirmed by HR-ESI-MS (m/z 325.1053 [M + Na]+, calcd for C17H18O5Na, 325.1052). The IR spectrum showed the presence of OH (3412 cm−1) and carbonyl (1625 cm−1) groups. Comparison of the 1H-NMR data of 3 with those of 3'-methoxy-2',4',β-trihydroxydihydrochalcone (3a) [4] suggested that their structures were closely related, except that 4'-methoxy group [δ 3.91 (3H, s)] of 3 replaced the 5'-hydroxy group [δ 6.41 (1H, br s)] of 3'-methoxy-2',4',β-trihydroxydihydrochalcone. This was supported by the NOESY correlations between OMe-4' (δ 3.91) and H-5' (δ 6.46) and by the HMBC correlations between OMe-4' (δ 3.91) and C-4' (δ 158.5). Furthermore, the absolute configuration of 3 was proposed to be R by comparing specific rotation data ( +60.5) of 3 with that reported for (R)-β-hydroxydihydrochalcone ( +60.8) [13]. By the aid of DEPT, HSQC, HMBC (Table 3), 1H-1H COSY, and NOESY (Table 3) techniques, and the full 1H- and 13C-NMR signals of 3 were unambiguously assigned. Therefore, the structure of 3 was determined as (R)-2',β-dihydroxy-3',4'-dimethoxydihydrochalcone.
The known isolates were readily identified by a comparison of physical and spectroscopic data (UV, IR, 1H-NMR, [α]D, and MS) with corresponding authentic samples or literature values, and this included a biflavan, (M),(2S),(2''S)-,(P),(2S),(2''S)-8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptame-thoxy-5,5''-biflavan (4) [5], three flavones, 5-hydroxy-7-methoxyflavone (5) [14], quercetin (6) [15], and 7,8,3',4',5'-pentamethoxyflavone (7) [5], two flavans, (2S)-8,5'-dihydroxy-7,3',4'-trimethoxyflavan (8) [5] and (2S)-5'-hydroxy-7,8,3',4'-tetramethoxyflavan (9) [5], two flavanones, (2S)-7-hydroxy-flavanone (10) [16] and (2S)-7-hydroxy-8-methoxyflavanone (11) [4], two benzenoids, (E)-ferulic acid (12) [17] and gallic acid (13) [18], and two steroids, β-sitostenone (14) [19] and β-sitosterol (15) [20].

Table 3.
1H-NMR data of 3 and 3a [4].
| Position | 3 a | 3a a,b | ||
|---|---|---|---|---|
| δH J (Hz) | NOESY | HMBC | δH J (Hz) | |
| 2 | 7.44 br d (7.6) | 3, α, β | 4, 6, β | 7.44 br d (7.6) |
| 3 | 7.39 br t (7.6) | 2, 4 | 1, 5 | 7.39 br t (7.6) |
| 4 | 7.31 br t (7.6) | 3, 5 | 2, 3, 5, 6 | 7.31 br t (7.6) |
| 5 | 7.39 br t (7.6) | 4, 6 | 1, 3 | 7.39 br t (7.6) |
| 6 | 7.44 br d (7.6) | 5, α, β | 2, 4, β | 7.44 br d (7.6) |
| α | 3.29 dd (17.2, 3.2) | 6, β | 1, 1', C=O | 3.29 dd (17.4, 3.2) |
| 3.36 dd (17.2, 8.8) | β, 6' | 1, 1', β | 3.36 dd (17.4, 8.8) | |
| β | 5.33 dd (8.8, 3.2) | 2, 6, α | 1, 2, 6, α, C=O | 5.34 dd (8.8, 3.2) |
| 5' | 6.46 d (9.0) | 6', OMe-4' | 1', 3', 4' | 6.50 d (9.0) |
| 6' | 7.47 d (9.0) | α, 5' | 2', 4', C=O | 7.40 d (9.0) |
| OH-β | 3.64 br s | 3.57 br s | ||
| OH-2' | 12.62 s | 1', 2', 3' | 12.67 s | |
| OH-4' | 6.41 br s | |||
| OMe-3' | 3.87 s | 3' | 4.00 s | |
| OMe-4' | 3.91 s | 5' | 4' | |
a Recorded in CDCl3 at 400 MHz. Values in ppm (δ). J (in Hz) in parentheses. b 3a = 3′-methoxy-2',4',β-trihydroxydihydrochalcone [4].
Reactive oxygen species (ROS) (e.g., superoxide anion (O2•−), hydrogen peroxide) and granule proteases (e.g., elastase, cathepsin G) produced by human neutrophils contribute to the pathogenesis of inflammatory diseases. Inhibition of neutrophil O2•− generation by drugs has been proposed as a way to ameliorate inflammatory diseases. The anti-inflammatory effects of the isolated compounds from the stem wood of M. calabura were evaluated by suppressing fMet-Leu-Phe (fMLP)-induced O2•− generation by human neutrophils. The anti-inflammatory activity data are shown in Table 4. Ibuprofen, a clinically used anti-inflammatory agent, was used as the positive control. From Table 4, six conclusions can be drawn: (a) 4'-Hydroxy-7,8,3',5'-tetramethoxyflavone (2), (M),(2S),(2''S)-, (P),(2S),(2''S)-8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4), 5-hydroxy-7-methoxyflavone (5), quercetin (6), (2S)-8,5'-dihydroxy-7,3',4'-trimethoxyflavan (8), (2S)-7-hydroxy-flavanone (10), and (E)-ferulic acid (12) exhibited inhibition (IC50 ≤ 58.4 μM) of superoxide anion generation by human neutrophils in response to formyl-L-methionyl-L-leucyl-L-phenylalanine (fMLP). (b) Biflavan 4 (with 8,5',8''-trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy groups) exhibited more effective inhibition than its analogue 1 (with 7,8,3',4',5',7'',8'',3''',4''',5'''-decamethoxy groups) against fMLP-induced O2•− generation. (c) Flavones 5 (with 5-hydroxy-7-methoxy groups) and 6 (with 3,5,7,3',4'-pentahydroxy groups) showed strong inhibition against fMLP-induced superoxide production, but its analogue 7 (with 7,8,3',4',5'-pentamethoxy groups) was inactive. (d) Flavan 8 (with 8,5'-dihydroxy-7,3',4'-trimethoxy groups) exhibited more effective inhibition than its analogue 9 (with 5'-hydroxy-7,8,3',4'-tetramethoxy groups) against fMLP-induced O2•− generation. (e) Flavanone 10 (with 7-hydroxy group) exhibited more effective inhibition than its analogue 11 (with 5'-hydroxy-7,3',4'-trimethoxy groups) against fMLP-induced O2•− generation. (f) 5-Hydroxy-7-methoxyflavone (5), quercetin (6), and (2S)-7-hydroxyflavanone (10) are the most effective among the isolated compounds, with IC50values of 1.77 ± 0.70, 3.82 ± 0.46, and 4.92 ± 1.71 μM, respectively, against fMLP-induced superoxide anion generation.

Table 4.
Inhibitory effects of compounds 1–15 from the stem wood of M. calabura on superoxide radical anion generation by human neutrophils in response to fMet-Leu-Phe a.
| Compounds | IC50 (μM) a |
|---|---|
| (M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-Decamethoxy-5,5''-biflavan (1) | >100 |
| 4'-Hydroxy-7,8,3',5'-tetramethoxyflavone (2) | 58.4 ± 6.2 |
| (R)-2',β-Dihydroxy-3',4'-dimethoxydihydrochalcone (3) | >100 |
| (M),(2S),(2''S)-,(P),(2S),(2''S)-8,5',8''-Trihydroxy-7,3',4',7'',3''',4''',5'''-heptamethoxy-5,5''-biflavan (4) | 54.2 ± 5.3 |
| 5-Hydroxy-7-methoxyflavone (5) | 1.77 ± 0.70 |
| Quercetin (6) | 3.82 ± 0.46 |
| 7,8,3',4',5'-Pentamethoxyflavone (7) | >100 |
| (2 S)-8,5'-Dihydroxy-7,3',4'-trimethoxyflavan (8) | 56.6 ± 6.2 |
| (2 S)-5'-Hydroxy-7,8,3',4'-tetramethoxyflavan (9) | >100 |
| (2 S)-7-Hydroxyflavanone (10) | 4.92 ± 1.71 |
| (2 S)-5'-Hydroxy-7,3',4'-trimethoxyflavanone (11) | >100 |
| (E)-Ferulic acid (12) | 24.18 ± 1.54 |
| Gallic acid (13) | >100 |
| β-Sitostenone (14) | >100 |
| β-Sitosterol (15) | >100 |
| Ibuprofen b | 27.5 ± 3.2 |
a The IC50 values were calculated from the slope of the dose-response curves (SigmaPlot). Values are expressed as average ± SEM (n = 4); b Ibuprofen was used as a positive control.
2.2. Discussion
A new biflavan 1, a new flavone 2, a new dihydrochalcone 3, and twelve known compounds 4–15 were isolated from the stem wood of M. calabura. The structures of new compounds 1–3 were determined by NMR and MS analyses. Among the known isolates, compound 12 has been found for the first time in this plant species. More discovery of new compounds from the genus Muntingia may not only provide more structure-activity data of these isolates, but also contribute to enhancing our understanding of the taxonomy and evolution of the genus Muntingia.
Human neutrophils are known to play a significant role in the host defense against microorganisms and in the pathogenesis of various diseases such as ischemia-reperfusion injury, asthma, rheumatoid arthritis, and chronic obstructive pulmonary disease [21,22]. In response to different stimuli, activated neutrophils secrete a series of cytotoxins, such as superoxide anion (O2•−), a precursor of other reactive oxygen species (ROS), and bioactive lipids [21,23,24]. Suppression of the extensive or inappropriate activation of neutrophils by drugs has been proposed as a way to ameliorate inflammatory diseases. Based on the results of our biological tests (Table 4), 5-hydroxy-7-methoxyflavone (5), quercetin (6), and (2S)-7-hydroxyflavanone (10) exhibited potent inhibition with IC50 values of 1.77 ± 0.70, 3.82 ± 0.46, and 4.92 ± 1.71 μM, respectively, against fMLP-induced superoxide anion generation. These findings indicated that the promising inhibitory activity against fMLP-induced O2•− generation of M. calabura and its isolates (especially 5, 6, and 10) could stimulate future development of new anti-inflammatory agents.
3. Experimental Section
3.1. General Procedures
Melting points were determined on a Yanaco micro-melting point apparatus and were uncorrected. Optical rotations were measured using a Jasco DIP-370 polarimeter in CHCl3. Ultraviolet (UV) spectra were obtained on a Jasco UV-240 spectrophotometer. Circular dichroism (CD) spectra were recorded on a Jasco J-810 spectropolarimeter. Infrared (IR) spectra (neat or KBr) were recorded on a Perkin Elmer 2000 FT-IR spectrometer. Nuclear magnetic resonance (NMR) spectra, including correlation spectroscopy (COSY), nuclear Overhauser effect spectrometry (NOESY), heteronuclear multiple-bond correlation (HMBC), and heteronuclear single-quantum coherence (HSQC) experiments, were acquired using a Varian Unity 400 or a Varian Inova 500 spectrometer operating at 400 and 500 MHz (1H) and 100 and 125 MHz (13C), respectively, with chemical shifts given in ppm (δ) using tetramethylsilane (TMS) as an internal standard. Electrospray ionisation (ESI) and high-resolution electrospray ionization (HRESI)-mass spectra were recorded on a Bruker APEX II or a VG Platform Electrospray ESI/MS mass spectrometer. Silica gel (70–230, 230–400 mesh, Merck) was used for column chromatography (CC). Silica gel 60 F-254 (Merck, Darmstadt, Germany) was used for thin-layer chromatography (TLC) and preparative thin-layer chromatography (PTLC).
3.2. Plant Material
The stem wood of M. calabura was collected from Changjhih Township, Pingtung County, Taiwan, in August 2011 and identified by Dr. J. J. Chen. A voucher specimen (MC-201108) was deposited in the Department of Pharmacy, Tajen University, Pingtung, Taiwan.
3.3. Extraction and Isolation
The dried stem wood (3.7 kg) of M. calabura was pulverized and extracted three times with MeOH (30 L each) for 3 days. The MeOH extracts were concentrated under reduced pressure at 35 °C, and the residue (360 g) was partitioned between CH2Cl2 and H2O (1:1). The CH2Cl2 layer was concentrated to give a residue (fraction A, 105 g). The water layer was further extracted with n-BuOH, and the n-BuOH-soluble part (fraction B, 121 g) and the water-solubles (fraction C, 134 g) were separated. Fraction A (105 g) was chromatographed on silica gel (70–230 mesh, 5.1 kg), eluting with CH2Cl2, gradually increasing the polarity with MeOH to give 11 fractions: A1 (3 L, CH2Cl2), A2 (3.5 L, CH2Cl2/MeOH, 98:1), A3 (3 L, CH2Cl2/MeOH, 95:1), A4 (3 L, CH2Cl2/MeOH, 90:1), A5 (4 L, CH2Cl2/MeOH, 80:1), A6 (3 L, CH2Cl2/MeOH, 70:1), A7 (4 L, CH2Cl2/MeOH, 50:1), A8 (4 L, CH2Cl2/MeOH, 10:1), A9 (3 L, CH2Cl2/MeOH, 5:1), A10 (4 L, CH2Cl2/MeOH, 1:1), A11 (3.5 L, MeOH). Fraction A2 (9.4 g) was washed with MeOH and filtered to yield 14 (71 mg) after recrystallization (MeOH). The filtrate was chromatographed on silica gel (230–400 mesh) eluting with n-hexane/EtOAc (15:1–0:1) to give 10 fractions (each 800 mL, A2-1–A2-10). Fraction A2-3 (102 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 10:1) to obtain 15 (7.2 mg). Fraction A3 (8.8 g) was chromatographed further on silica gel (230–400 mesh, 398 g) eluting with CH2Cl2/MeOH (15:1–0:1) to give 9 fractions (each 850 mL, A3-1–A3-9). Fraction A3-3 (95 mg) was purified by preparative TLC (silica gel, CHCl3/acetone, 15:1) to obtain 5 (4.7 mg). Fraction A3-5 (104 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 2:1) to afford 9 (3.8 mg). Fraction A3-8 (102 mg) was purified by preparative TLC (silica gel, n-hexane/acetone, 1:1) to afford 11 (4.6 mg). Fraction A4 (10.1 g) was chromatographed further on silica gel (230–400 mesh, 462 g) eluting with CHCl3/MeOH (10:1–0:1) to give 12 fractions (each 750 mL, A4-1–A4-12). Fr. A4-2 (113 mg) was further purified by preparative TLC (silica gel, n-hexane/acetone, 5:1) to obtain 1 (3.5 mg). Fraction A4-3 (364 mg) was purified by MPLC (16.7 g of SiO2, 230–400 mesh, n-hexane/EtOAc 15:1–0:1, 180-mL fractions) to give 8 subfractions: Frs. A4-3-1–A4-3-8. Fr. A4-3-3 (48 mg) was further purified by preparative TLC (silica gel, n-hexane/acetone, 3:1) to afford 4 (4.2 mg). Fraction A4-4 (136 mg) was purified further by preparative TLC (silica gel, CH2Cl2/acetone, 4:1) to obtain 7 (5.3 mg). Fraction A4-5 (145 mg) was purified further by preparative TLC (silica gel, CHCl3/acetone, 4:1) to give 2 (3.6 mg). Fraction A4-6 (133 mg) was purified further by preparative TLC (silica gel, CHCl3/acetone, 5:1) to give 12 (5.7 mg). Fraction A5 (9.2 g) was chromatographed further on silica gel (230–400 mesh, 437 g) eluting with CHCl3/MeOH (10:1–0:1) to give 10 fractions (each 750 mL, A5-1–A5-10). Fraction A5-2 (132 mg) was further purified by preparative TLC (n-hexane/acetone, 4:3) to obtain 3 (3.7 mg). Fraction A5-3 (124 mg) was further purified by preparative TLC (n-hexane/acetone, 3:2) to obtain 10 (4.8 mg). Fraction A5-4 (116 mg) was purified further by preparative TLC (silica gel, n-hexane/EtOAc, 1:1) to afford 8 (4.1 mg). Fraction A8 (8.4 g) was chromatographed further on silica gel (230–400 mesh, 383 g) eluting with CH2Cl2/MeOH (6:1–0:1) to give 8 fractions (each 800 mL, A8-1–A8-8). Fraction A8-1 (108 mg) was purified by preparative TLC (silica gel, EtOAc/MeOH, 1:1) to afford 13 (4.4 mg). Fraction A8-4 (122 mg) was purified by preparative TLC (silica gel, CH2Cl2/MeOH, 2:1) to afford 6 (4.5 mg).
(M),(2S),(2''S)-,(P),(2S),(2''S)-7,8,3',4',5',7'',8'',3''',4''',5'''-Decamethoxy-5,5''-biflavan (1). Colorless prisms (CH2Cl2/MeOH), m.p. > 225 °C (dec). : +12.8 (c 0.16, CHCl3). UV (MeOH): λmax (log ε) = 246 (4.21), 268 (3.86) nm. CD (MeOH, Δε): 275 (−0.4), 289 (−2.0), 304 (0) nm. IR (KBr): υmax = 1602, 1515, 1452 (aromatic ring C=C stretch) cm−1. 1H-NMR spectroscopic data, see Table 1. 13C-NMR spectroscopic data, see Table 2. ESI-MS: m/z = 741 [M + Na]+. HR-ESI-MS: m/z = 741.2889 [M + Na]+ (calcd for C40H46O12Na: 741.2887).
4'-Hydroxy-7,8,3',5'-tetramethoxyflavone (2). Yellowish needles (MeOH); m.p. 221–223 °C. UV (MeOH): λmax (log ε) = 242 (4.23), 269 (4.29), 312 (4.28) nm. IR (KBr): υmax 3290 (OH), 1630 (C=O) cm−1. 1H-NMR (CDCl3, 500 MHz): δ = 3.99 (6H, s, OMe-3' and OMe-5'), 4.02 (OMe-8), 4.05 (OMe-7), 6.72 (H-3), 7.07 (1H, d, J = 8.8 Hz, H-6), 7.22 (2H, s, H-2' and H-6'), 7.96 (1H, d, J = 8.8 Hz, H-5). 13C-NMR (CDCl3, 125 MHz): δ = 56.3 (OMe-3'), 56.3 (OMe-5'), 56.4 (OMe-7), 61.4 (OMe-8), 104.1 (C-2'), 104.1 (C-6'), 106.4 (C-3), 110.0 (C-6), 118.5 (C-10), 121.0 (C-5), 126.9 (C-1'), 136.5 (C-4'), 136.8 (C-8), 147.8 (C-3'), 147.8 (C-5'), 150.4 (C-9), 156.6 (C-7), 162.7 (C-2), 178.0 (C-4). ESI-MS: m/z = 381 [M + Na]+. HR-ESI-MS: m/z = 381.0952 [M + Na]+ (calcd for C19H18O7Na: 381.0950).
(R)-2',β-Dihydroxy-3',4'-dimethoxydihydrochalcone (3). Amorphous powder. : +60.5 (c 0.16, CHCl3). UV (MeOH): λmax (log ε) = 211 (4.05), 230 (sh, 3.75), 287 (3.85) nm. IR (KBr): υmax = 3412 (OH), 1625 (C=O) cm−1. 1H-NMR spectroscopic data, see Table 3. 13C-NMR (CDCl3, 100 MHz): δ = 52.6 (C-α), 70.2 (C-β), 56.1 (OMe-4'), 60.6 (OMe-3'), 102.9 (C-5'), 114.1 (C-1'), 125.9 (C-4), 126.0 (C-6'), 127.8 (C-2 and C-6), 128.7 (C-3 and C-5), 136.3 (C-3'), 143.2 (C-1), 156.9 (C-2'), 158.5 (C-4'), 200.6 (C=O). ESI-MS: m/z = 325 [M + Na]+. HR-ESI-MS: m/z = 325.1053 [M + Na]+ (calcd for C17H18O5Na: 325.1052).
3.4. Biological Assay
The effect of the isolated compounds on neutrophil pro-inflammatory response was evaluated by monitoring the inhibition of superoxide anion generation in fMLP-activated human neutrophils in a concentration-dependent manner. The purity of the tested compounds was >98% as identified by NMR and MS.
3.4.1. Preparation of Human Neutrophils
Human neutrophils from venous blood of healthy, adult volunteers (20–30 years old) obtained by venipuncture were isolated using a standard method of dextran sedimentation prior to centrifugation in a Ficoll Hypaque gradient and hypotonic lysis of erythrocytes [25]. Purified neutrophils containing >98% viable cells, as determined by the trypan blue exclusion method [26], were re-suspended in a calcium (Ca2+)-free HBSS buffer at pH 7.4 and were maintained at 4 °C prior to use. The protocol was approved by the Institutional Review Board at Chang Gung Memorial Hospital. All donors gave written consent. The Medical Ethics Committee of Chang Gung Memorial Hospital approved this consent procedure.
3.4.2. Measurement of Superoxide Anion Generation
The assay for measurement of O2•− generation was based on the SOD-inhibitable reduction of ferricytochrome c [27]. In brief, neutrophils (1 × 106 cells/mL) pretreated with the various test agents at 37 °C for 5 min were stimulated with fMLP (1 μmol/L) in the presence of ferricytochrome c (0.5 mg/mL). Extracellular O2•− production was assessed with a UV spectrophotometer at 550 nm (Hitachi U-3010, Tokyo, Japan). The percentage of superoxide inhibition of the test compound was calculated as the percentage of inhibition = {(control − resting) − (compound − resting)}/(control − resting) × 100. The software, SigmaPlot was used for determining the IC50 values.
3.4.3. Statistical Analysis
Results were expressed as the mean ± SEM, and comparisons were made using Student’s t-test. A probability of 0.05 or less was considered significant. The software SigmaPlot was used for the statistical analysis.
4. Conclusions
Fifteen compounds, including three new compounds 1–3, were isolated from the stem wood of M. calabura. The structures of these compounds were established on the basis of spectroscopic data. Reactive oxygen species (ROS) (e.g., superoxide anion (O2•−), hydrogen peroxide) produced by human neutrophils contribute to the pathogenesis of inflammatory diseases. The effects on neutrophil pro-inflammatory responses of isolates were evaluated by suppressing fMLP/CB-induced O2•− generation by human neutrophils. The results of anti-inflammatory experiments indicate that compounds 2, 4–6, 8, 10, and 12 can significantly inhibit fMLP-induced O2•− generation. 5-Hydroxy-7-methoxyflavone (5), quercetin (6), and (2S)-7-hydroxyflavanone (10) are the most effective among the isolated compounds, with IC50values of 1.77 ± 0.70, 3.82 ± 0.46, and 4.92 ± 1.71 μM, respectively, against fMLP-induced superoxide anion generation. Our study suggests M. calabura and its isolates (especially 5, 6, and 10) could be further developed as potential candidates for the treatment or prevention of various inflammatory diseases.
Supplementary Materials
Supplementary materials can be accessed at: http://www.mdpi.com/1420-3049/19/12/20521/s1.
Acknowledgments
This research was supported by grants from the National Science Council of the Republic of China (No. NSC 98-2320-B-127-001-MY3 and NSC 101-2320-B-127-001-MY3), awarded to J.-J. Chen. This work was also supported by the grant from Chung-Jen Junior College of Nursing, Health Sciences and Management (No. Chung-Jen 103001).
Author Contributions
W.-L.K. and J.-J.C. designed the research; W.-L.K., H.-R.L. and J.-J.C. performed the experiments and analyzed the data; and W.-L.K. and J.-J.C. wrote the paper. All authors have read and approved the final manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Liu, T.S.; Lo, H.C. Tiliaceae. In Flora of Taiwan, 2nd ed.; Editorial Committee of the Flora of Taiwan: Taipei, Taiwan, 1993; Volume 3, pp. 722–733. [Google Scholar]
- Chen, J.J.; Lee, H.H.; Shih, C.D.; Liao, C.H.; Chen, I.S.; Chou, T.H. New dihydrochalcones and anti-platelet aggregation constituents from the leaves of Muntingia calabura. Planta Med. 2007, 73, 572–577. [Google Scholar]
- Chen, J.J.; Lin, R.W.; Duh, C.Y.; Huang, H.Y.; Chen, I.S. Flavones and cytotoxic constituents from the stem bark of Muntingia calabura. J. Chin. Chem. Soc. 2004, 51, 665–670. [Google Scholar]
- Chen, J.J.; Lee, H.H.; Duh, C.Y.; Chen, I.S. Cytotoxic chalcones and flavonoids from the leaves of Muntingia calabura. Planta Med. 2005, 71, 970–973. [Google Scholar]
- Kaneda, N.; Pezzuto, J.M.; Soejarto, D.D.; Kinghorn, A.D.; Farnsworth, N.R.; Santisuk, T.; Tuchinda, P.; Udchachon, J.; Reutrakul, V. New cytotoxic flavonoids from Muntingia calabura roots. J. Nat. Prod. 1991, 54, 196–206. [Google Scholar]
- Nshimo, C.M.; Pezzuto, J.M.; Kinghorn, A.D.; Farnsworth, N.R. Cytotoxic constituents of Muntingia calabura leaves and stems collected in Thailand. Int. J. Pharm. 1993, 31, 77–81. [Google Scholar]
- Chen, J.J.; Cho, J.Y.; Hwang, T.L.; Chen, I.S. Benzoic acid derivatives, acetophenones, and anti-inflammatory contituents from Melicope semecarpifolia. J. Nat. Prod. 2008, 71, 71–75. [Google Scholar]
- Chen, J.J.; Luo, Y.T.; Hwang, T.L.; Sung, P.J.; Wang, T.C.; Chen, I.S. A new indole alkaloid and anti-inflammatory constituents from Strychnos cathayensis. Chem. Biodivers. 2008, 5, 1345–1352. [Google Scholar]
- Chen, J.J.; Ting, C.W.; Hwang, T.L.; Chen, I.S. Benzophenone derivatives from the fruits of Garcinia multiflora and their anti-inflammatory activity. J. Nat. Prod. 2009, 72, 253–258. [Google Scholar]
- Chen, J.J.; Lin, Y.H.; Day, S.H.; Hwang, T.L.; Chen, I.S. New benzenoids and anti-inflammatory constituents from Zanthoxylum nitidum. Food Chem. 2011, 125, 282–287. [Google Scholar]
- Chen, J.J.; Tsai, Y.C.; Hwang, T.L.; Wang, T.C. Thymol, benzofuranoid, and phenylpropanoid derivatives: Anti-inflammatory constituents from Eupatorium annabinum. J. Nat. Prod. 2011, 74, 1021–1027. [Google Scholar]
- Bellini, A.; Venturella, P. The synthesis of hydroxychalcone and hydroxyflavone derivatives. VI. Flavones derived from syringic aldehyde. Ann. Chim. 1958, 48, 716–722. [Google Scholar]
- Cheon, C.H.; Yamamoto, H. N-Triflylthiophosphoramide catalyzed enantioselective mukaiyama aldol reaction of aldehydes with silyl enol ethers of ketones. Org. Lett. 2010, 12, 2476–2479. [Google Scholar]
- Thusoo, A.; Raina, N.; Minhaj, N.; Ahmed, S.R.; Zaman, A. Crystalline constituents from Daphne oleoides. Indian J. Chem. B 1981, 20, 937–938. [Google Scholar]
- Kim, S.M.; Kang, K.; Jho, E.H.; Jung, Y.J.; Nho, C.W.; Um, B.H.; Pam, C.H. Hepatoprotective effect of flavonoid glycosides from Lespedeza cuneata against oxidative stress induced by tert-butyl hyperoxide. Phytother. Res. 2011, 25, 1011–1017. [Google Scholar]
- Tanrisever, N.; Fronczek, F.R.; Fischer, N.H.; Williamson, G.B. Ceratiolin and other flavonoids from Ceratiola ericoides. Phytochemistry 1987, 26, 175–179. [Google Scholar]
- Machida, K.; Kikuchi, M. Norisoprenoids from Viburnum dilatatum. Phytochemistry 1992, 41, 1333–1336. [Google Scholar]
- Wang, Y.S.; Huang, R.; Yang, J.H. Chemical constituents of Litsea szemaois. Chem. Nat. Compd. 2011, 47, 122–123. [Google Scholar]
- Chen, J.J.; Huang, S.S.; Liao, C.H.; Wei, D.C.; Sung, P.J.; Wang, T.C.; Cheng, M.J. A new phragmalin-type limonoid and anti-inflammatory constituents from the fruits of Swietenia macrophylla. Food Chem. 2010, 120, 379–384. [Google Scholar]
- Chen, J.J.; Wang, T.C.; Yang, C.K.; Liao, H.R.; Sung, P.J.; Chen, I.S.; Cheng, M.J.; Peng, C.F.; Chen, J.F. New pterosin sesquiterpenes and antitubercular constituents from Pteris ensiformis. Chem. Biodivers. 2013, 10, 1903–1908. [Google Scholar]
- Witko-Sarsat, V.; Rieu, P.; Descamps-Latscha, B.; Lesavre, P.; Halbwachs-Mecarelli, L. Neutrophils: Molecules, functions and pathophysiological aspects. Lab. Investig. 2000, 80, 617–653. [Google Scholar]
- Ennis, M. Neutrophils in asthma pathophysiology. Curr. Allergy Asthma Rep. 2003, 3, 159–165. [Google Scholar]
- Borregaard, N. The human neutrophil. Function and dysfunction. Eur. J. Haematol. 1998, 41, 401–413. [Google Scholar]
- Roos, D.; van Bruggen, R.; Meischl, C. Oxidative killing of microbes by neutrophils. Microbes Infect. 2003, 5, 1307–1315. [Google Scholar]
- Boyum, A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand. J. Clin. Lab. Investig. 1968, 97, 77–89. [Google Scholar]
- Jauregui, H.O.; Hayner, N.T.; Driscoll, J.L.; Williams-Holland, R.; Lipsky, M.H.; Galletti, P.M. Trypan blue dye uptake and lactate dehydrogenase in adult rat hepatocytes-freshly isolated cells, cell suspensions, and primary monolayer cultures. In Vitro 1981, 17, 1100–1110. [Google Scholar]
- Babior, B.M.; Kipnes, R.S.; Curnutte, J.T. Biological defense mechanisms. The production by leukocytes of superoxide, a potential bactericidal agent. J. Clin. Investig. 1973, 52, 741–744. [Google Scholar]
- Sample Availability: Samples of the all compounds are available from the authors.
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
