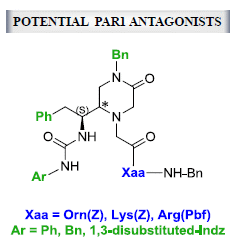
Exploring the Phe-Gly Dipeptide-Derived Piperazinone Scaffold in the Search for Antagonists of the Thrombin Receptor PAR1
Abstract
Share and Cite
Valdivielso, Á.M.; García-López, M.T.; Gutiérrez-Rodríguez, M.; Herranz, R. Exploring the Phe-Gly Dipeptide-Derived Piperazinone Scaffold in the Search for Antagonists of the Thrombin Receptor PAR1. Molecules 2014, 19, 4814-4846. https://doi.org/10.3390/molecules19044814
Valdivielso ÁM, García-López MT, Gutiérrez-Rodríguez M, Herranz R. Exploring the Phe-Gly Dipeptide-Derived Piperazinone Scaffold in the Search for Antagonists of the Thrombin Receptor PAR1. Molecules. 2014; 19(4):4814-4846. https://doi.org/10.3390/molecules19044814
Chicago/Turabian StyleValdivielso, Ángel M., M. Teresa García-López, Marta Gutiérrez-Rodríguez, and Rosario Herranz. 2014. "Exploring the Phe-Gly Dipeptide-Derived Piperazinone Scaffold in the Search for Antagonists of the Thrombin Receptor PAR1" Molecules 19, no. 4: 4814-4846. https://doi.org/10.3390/molecules19044814
APA StyleValdivielso, Á. M., García-López, M. T., Gutiérrez-Rodríguez, M., & Herranz, R. (2014). Exploring the Phe-Gly Dipeptide-Derived Piperazinone Scaffold in the Search for Antagonists of the Thrombin Receptor PAR1. Molecules, 19(4), 4814-4846. https://doi.org/10.3390/molecules19044814