Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities
Abstract
:1. Introduction
2. Occurrence
| Bis-naphtho-γ-pyrone | Fungal Species | Reference |
|---|---|---|
| Chaetochromin A (1) | Endophytic fungus Chaetomium chiversii | [11] |
| Chaetomium gracile | [10] | |
| Chaetomium microcephalum | [12] | |
| Chaetomium virenscens (C. thielavioideum) | [13] | |
| Isochaetochromin A1 (2) | Penicillium sp. FK I-4942 | [14,15] |
| Isochaetochromin A2 (3) | Chaetomium microcephalum | [12] |
| Chaetochromin B (4) | Chaetomium gracile | [6,10] |
| Chaetomium microcephalum | [12] | |
| Isochaetochromin B1 (5) | Endophytic fungus Fusarium sp. | [3] |
| Penicillium sp. FKI-4942 | [14] | |
| Isochaetochromin B2 (6) | Endophytic fungus Fusarium sp. | [3] |
| Sponge-derived fungus Metarhizium anisopliae | [16] | |
| Penicillium sp. FKI-4942 | [14] | |
| Oxychaetochromin B (7) | Endophytic fungus Fusarium sp. | [3] |
| Chaetochromin C (8) | Chaetomium gracile | [10] |
| Chaetochromin D (9) | Chaetomium gracile | [10] |
| Isochaetochromin D1 (10) | Endophytic fungus Fusarium sp. | [3] |
| Cephalochromin = Sch 45752 (11) | Cephalosporium sp. | [17] |
| Cosmospora vilior YMJ89051501 | [18] | |
| Nectria flavoviridis | [19] | |
| Nectria viridescens | [19] | |
| Endophytic fungus Pseudoanguillospora sp. | [20] | |
| Verticillium sp. K-113 | [21] | |
| Unidentified fungal isolate SCF-125 | [22] | |
| Hypochromin A (12) | Marine-derived fungus Hypocrea vinosa | [23] |
| Hypochromin B (13) | Marine-derived fungus Hypocrea vinosa | [23] |
| SC2051 (14) | Marine-derived fungus Hypocrea vinosa | [23] |
| Ustilaginoidin A (15) | Villosiclava virens (Ustilaginoidea virens) | [24] |
| Isoustilaginoidin A (16) | Verticillium sp. K-113 | [21] |
| Dihydroisoustilaginoidin A (17) | Verticillium sp. K-113 | [21] |
| Unidentified fungal isolate SCF-125 | [22] | |
| Ustilaginoidin B (18) | Villosiclava virens (Ustilaginoidea virens) | [25] |
| Ustilaginoidin C (19) | Villosiclava virens (Ustilaginoidea virens) | [25] |
| Ustilaginoidin D (20) | Sponge-derived fungus Metarhizium anisopliae | [16] |
| Villosiclava virens (Ustilaginoidea virens) | [26] | |
| Ustilaginoidin E (21) | Villosiclava virens (Ustilaginoidea virens) | [26] |
| Ustilaginoidin F (22) | Villosiclava virens (Ustilaginoidea virens) | [26] |
| Ustilaginoidin G (23) | Villosiclava virens (Ustilaginoidea virens) | [26] |
| Ustilaginoidin H (24) | Villosiclava virens (Ustilaginoidea virens) | [26] |
| Ustilaginoidin I (25) | Villosiclava virens (Ustilaginoidea virens) | [26] |
| Ustilaginoidin J (26) | Villosiclava virens (Ustilaginoidea virens) | [26] |


| Bis-naphtho-γ-pyrone | Fungal Species | Reference |
|---|---|---|
| Asperpyrone A (27) | Endophytic fungus Aspergillus sp. | [34] |
| Endophytic fungus Aspergillus sp. DCS31 | [35] | |
| Aspergillus niger | [30] | |
| Endophytic fungus Aspergillus niger | [8] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Asperpyrone B (28) | Aspergillus niger | [30] |
| Aspergillus niger IFB-E003 | [31] | |
| Aspergillus niger ATCC 11414 | [33] | |
| Asperpyrone C (29) | Aspergillus niger | [30] |
| Asperpyrone D (30) | Endophytic fungus Aspergillus sp. DCS31 | [35] |
| Endophytic fungus Aspergillus niger | [8] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Asperpyrone E (31) | Endophytic fungus Aspergillus niger | [8] |
| Aurasperone A (32) | Alternaria alternata | [27] |
| Aspergillus sp. FKI-3451 | [4] | |
| Aspergillus awamori | [28,37] | |
| Endophytic fungus Aspergillus auleatus | [32] | |
| Aspergillus fonsecaeus | [29] | |
| Aspergillus niger | [28,37] | |
| Aspergillus niger | [38] | |
| Aspergillus niger | [30] | |
| Aspergillus niger IFB-E003 | [31] | |
| Aspergillus niger ATCC 11414 | [33] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Isoaurasperone A (33) | Endophytic fungus Aspergillus sp. | [34] |
| Aspergillus niger | [38] | |
| Endophytic fungus Aspergillus niger | [8] | |
| Aurasperone B (34) | Alternaria alternata | [27] |
| Aspergillus sp. FKI-3451 | [4] | |
| Aspergillus awamori | [28,39] | |
| Aspergillus fonsecaeus | [29] | |
| Aspergillus niger | [28,39] | |
| Aspergillus niger | [38] | |
| Aspergillus niger ATCC 11414 | [33] | |
| Aspergillus niger C-433 | [40] | |
| Aurasperone B (34) | Aspergillus niger ATCC 11414 | [33] |
| Aspergillus vadensis | [41] | |
| Aurasperone C (35) | Alternaria alternata | [27] |
| Aspergillus awamori | [28,39] | |
| Aspergillus niger | [28,39] | |
| Aspergillus niger | [38] | |
| Aspergillus niger C-433 | [40] | |
| Aspergillus niger ATCC 11414 | [33] | |
| Dianhydro-aurasperone C (36) | Endophytic fungus Aspergillus sp. | [34] |
| Aspergillus niger | [38] | |
| Endophytic fungus Aspergillus niger | [8] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Aurasperone D (37) | Aspergillus niger | [42] |
| Aspergillus niger | [38] | |
| Endophytic fungus Aspergillus niger | [8] | |
| Aspergillus niger C-433 | [40] | |
| Aurasperone E (38) | Aspergillus niger | [38] |
| Aspergillus niger C-433 | [40] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Aurasperone F (39) | Alternaria alternata | [27] |
| Aspergillus niger C-433 | [40,43] | |
| Isoaurasperone F (40) | Endophytic fungus Aspergillus niger | [8] |
| Aurasperone G (41) | Aspergillus niger C-433 | [43] |
| Fonsecinone A (42) | Endophytic fungus Aspergillus sp. | [34] |
| Endophytic fungus Aspergillus auleatus | [32] | |
| Aspergillus fonsecaeus | [29] | |
| Aspergillus niger | [30] | |
| Aspergillus niger IFB-E003 | [31] | |
| Aspergillus niger ATCC 11414 | [33] | |
| Endophytic fungus Aspergillus tubingensis | [36] | |
| Endophytic fungus Cladosporium herbarum IFB-E002 | [44] | |
| Fonsecinone B (43) | Aspergillus fonsecaeus | [29] |
| Aspergillus niger ATCC 11414 | [33] | |
| Fonsecinone C (44) | Aspergillus fonsecaeus | [29] |
| Aspergillus niger ATCC 11414 | [33] | |
| Fonsecinone D (45) | Aspergillus fonsecaeus | [29] |
| Nigerasperone B (46) | Aspergillus niger EN-13 | [45] |
| Nigerasperone C (47) | Aspergillus niger EN-13 | [45] |
| Rubasperone A (48) | Endophytic fungus Aspergillus tubingensis | [46] |
| Rubasperone B (49) | Endophytic fungus Aspergillus tubingensis | [46] |
| Rubasperone C (50) | Endophytic fungus Aspergillus tubingensis | [46] |
| Rubasperone D (51) | Endophytic fungus Aspergillus tubingensis (GX1-5E) | [47] |
| Rubasperone E (52) | Endophytic fungus Aspergillus tubingensis (GX1-5E) | [47] |
| Rubasperone F (53) | Endophytic fungus Aspergillus tubingensis (GX1-5E) | [47] |


| Bis-naphtho-γ-pyrone | Fungal Species | Reference |
|---|---|---|
| 10,10'-Bifonsecin B (54) | Marine-derived fungus Aspergillus carbonarius | [7] |
| Nigerone (55) | Marine-derived fungus Aspergillus carbonarius | [7] |
| Aspergillus niger | [5] | |
| 6'-O-Demethylnigerone (56) | Marine-derived fungus Aspergillus carbonarius | [7] |
| Aspergillus niger | [5] | |
| 8'-O-Demethylnigerone (57) | Marine-derived fungus Aspergillus carbonarius | [7] |
| 8'-O-Demethylisonigerone (58) | Marine-derived fungus Aspergillus carbonarius | [7] |
| Isonigerone (59) | Marine-derived fungus Aspergillus carbonarius | [7] |
| Aspergillus niger | [5] |

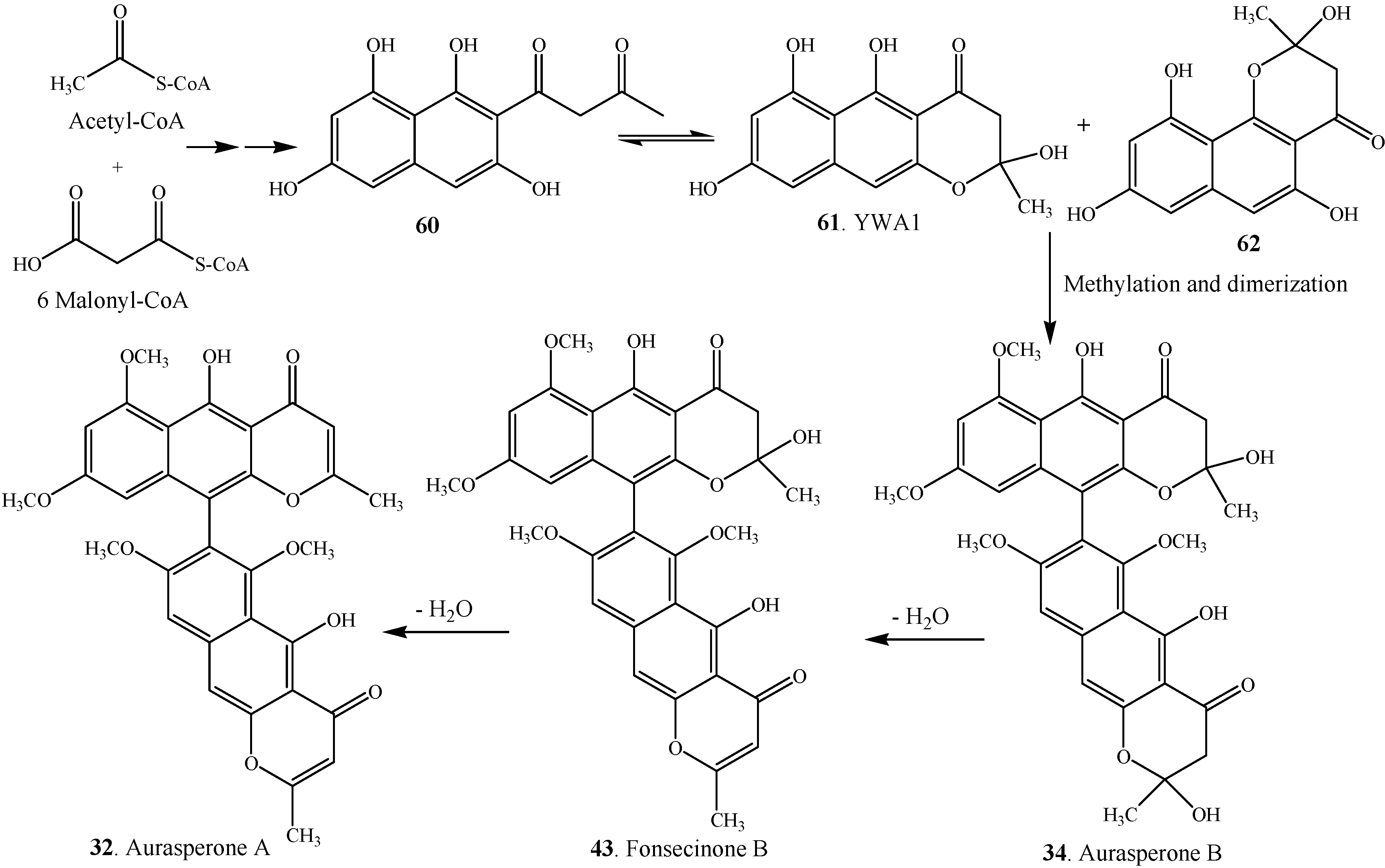
3. Biosynthesis
| Monomeric Naphtho-γ-pyrone | Fungal Species | Reference | |
|---|---|---|---|
| Nigerasperone A (63) | Aspergillus niger EN-13 | [45] | |
| Rubrofusarin (64) | Aspergillus niger | [38] | |
| Endophytic fungus Aspergillus tubingensis GX1-5E | [46] | ||
| Rubrofusarin B = Heminigerone (65) | Alternaria alternata | [27] | |
| Endophytic fungus Aspergillus sp. | [34] | ||
| Endophytic fungus Aspergillus niger IFB-E003 | [31] | ||
| Marine-derived fungus Aspergillus carbonarius | [7] | ||
| Aspergillus niger var. niger TC 1629 | [52] | ||
| Endophytic fungus Aspergillus tubingensis GX1-5E | [46] | ||
| Endophytic fungus Aspergillus tubingensis NRRC 4700 | [36] | ||
| Endophytic fungus Cladosporium herbarum IFB-E002 | [44] | ||
| Rubrofusarin-6-O-α-D-ribofuranoside (66) | Endophytic fungus Aspergillus niger | [8] | |
| Fonsecin (67) | Alternaria alternata | [27] | |
| Marine-derived fungus Aspergillus carbonarius | [7] | ||
| Aspergillus niger | [38] | ||
| Aspergillus niger C-433 | [40] | ||
| Aspergillus niger var.niger TC 1629 | [52] | ||
| Endophytic fungus Aspergillus tubingensis GX1-5E | [47] | ||
| Endophytic fungus Aspergillus tubingensis NRRC 4700 | [36] | ||
| Fonsecin B = Fonsecin monomethyl ether (68) | Alternaria alternata | [27] | |
| Aspergillus niger | [38] | ||
| Aspergillus niger var. niger TC 1629 | [52] | ||
| Endophytic fungus Aspergillus tubingensis NRRC 4700 | [36] | ||
| TMC-256A1 (69) | Marine-derived fungus Aspergillus carbonarius | [7] | |
| Aspergillus niger var. niger TC 1629 | [52] | ||
| Endophytic fungus Aspergillus tubingensis GX1-5E | [46] | ||
| Endophytic fungus Aspergillus tubingensis NRRC 4700 | [36] | ||
| (R)-10-(3-succinimidyl)-TMC-256A1 (70) | Endophytic fungus Aspergillus niger | [8] | |
| TMC-256C1 (71) | Aspergillus niger var. niger TC 1629 | [52] | |
| Flavasperone = Asperxanthon = TMC-256C2 (72) | Endophytic fungus Aspergillus sp. DCS31 | [35] | |
| Aspergillus sp. FKI-3451 | [4] | ||
| Marine-derived fungus Aspergillus carbonarius | [7] | ||
| Aspergillus niger | [38] | ||
| Aspergillus niger var. niger TC 1629 | [52] | ||
| Endophytic fungus Aspergillus tubingensis | [47] | ||
| Indigotide B (73) | Entomopathogenic fungus Cordyceps indigotica | [53] | |
| Sponge-derived fungus Metarhizium anisopliae mxh-99 | [16] | ||
| 8-O-Methylindigotide B (74) | Entomopathogenic fungus Cordyceps indigotica | [53] | |
| Indigotide G (75) | Sponge-derived fungus Metarhizium anisopliae mxh-99 | [16] | |
| Indigotide H (76) | Sponge-derived fungus Metarhizium anisopliae mxh-99 | [16] |

4. Biological Activities
4.1. Cytotoxic and Antitumor Activity
4.2. Antimicrobial Activity
4.3. Other Biological Activities
| Bis-naphtho-γ-pyrone | Biological Activity | Reference |
|---|---|---|
| Chaetochromin A (1) | Cytotoxic and antitumor activity | [2,54,55] |
| Teratogenicity to mice embryo | [59] | |
| Inhibitory effects on nitric oxide (NO) production by activated macrophages | [60] | |
| Antibacterial activity | [12] | |
| Immunological activity | [12] | |
| Inhibitory activity on botulinum neurotoxin serotype A | [58] | |
| Isochaetochromin A1 (2) | Inhibitory activity on triacylglycerol synthesis | [14] |
| Isochaetochromin A2 (3) | Antibacterial activity | [12] |
| Immunological activity | [12] | |
| Chaetochromin B (4) | Cytotoxic and antitumor activity | [2,54] |
| Antibacterial activity | [12] | |
| Immunological activity | [12] | |
| Isochaetochromin B1 (5) | HIV-1 integrase inhibitory activity | [3] |
| Inhibitory activity on triacylglycerol synthesis | [14] | |
| Isochaetochromin B2 (6) | Anti-tubercular activity | [16] |
| HIV-1 integrase inhibitory activity | [3] | |
| Inhibitory activity on triacylglycerol synthesis | [14] | |
| Oxychaetochromin B (7) | HIV-1 integrase inhibitory activity | [3] |
| Chaetochromin C (8) | Cytotoxic and antitumor activity | [2,54] |
| Chaetochromin D (9) | Cytotoxic and antitumor activity | [2,54] |
| Impairing effects on mitochondrial respiration and structure | [61] | |
| Chaetochromin D1 (10) | HIV-1 integrase inhibitory activity | [3] |
| Cephalochromin (11) | Cytotoxic and antitumor activity | [18,55] |
| Inhibitory effects on nitric oxide (NO) production by activated macrophages | [57] | |
| Antimicrobial activity | [21,57] | |
| Inhibitory activity on calmodulin-sensitive cyclic nucleotide phosphodiestease | [22] | |
| Inhibitory activity on botulinum neurotoxin serotype A | [58] | |
| Hypochromin A (12) | Tyrosine kinase inhibitory activity | [23] |
| Hypochromin B (13) | Tyrosine kinase inhibitory activity | [23] |
| SC2051 (14) | Tyrosine kinase inhibitory activity | [23] |
| Ustilaginoidin A (15) | Cytotoxic and antitumor activity | [54,55] |
| Isoustilaginoidin A (16) | Antimicrobial activity | [21] |
| Dihydroisoustilaginoidin A (17) | Antimicrobial activity | [21] |
| Inhibitory effects on nitric oxide (NO) production by activated macrophages | [62] | |
| Ustilaginoidin D (20) | Cytotoxic and antitumor activity | [2] |
| Anti-tubercular activity | [16] | |
| Ustilaginoidin E (21) | Cytotoxic and antitumor activity | [2] |
| Asperpyrone A (27) | Antimicrobial activity | [34] |
| Inhibitory activity on Taq DNA polymerase | [30] | |
| Asperpyrone B (28) | Antimicrobial activity | [31] |
| Aurasperone A (32) | Antimicrobial activity | [31] |
| Inhibitory activity on xanthine oxidase | [31] | |
| Inhibitory activity on acyl-CoA:cholesterol acyltransferase | [4] | |
| Inhibitory activity on Taq DNA polymerase | [30] | |
| Isoaurasperone A (33) | Antimicrobial activity | [34] |
| Dianhydro-aurasperone C (36) | Antibacterial activity | [34] |
| Drug resistance-reversing activity | [59] | |
| Aurasperone D (37) | Central nervous system depressant effects | [42] |
| Inhibitory activity on acyl-CoA:cholesterol acyltransferase | [4] | |
| Fonscinone A (42) | Antimicrobial activity | [31,34] |
| Inhibitory activity on Taq DNA polymerase | [30] | |
| 8'-O-Demethylnigerone (57) | Anti-tubercular activity | [7] |
| 8'-O-Demethylisonigerone (58) | Anti-tubercular activity | [7] |
5. Conclusions and Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Nielsen, K.F.; Mogensen, J.M.; Johansen, M.; Larsen, T.O.; Frisvad, J.C. Review of secondary metabolites and mycotoxins from the Aspergillus niger group. Anal. Bioanal. Chem. 2009, 395, 1225–1242. [Google Scholar] [CrossRef]
- Koyama, K.; Ominato, K.; Natori, S.; Tashiro, T.; Tsuruo, T. Cytotoxicity and antitumor activities of fungal bis(naphtho-γ-pyrone) derivatives. J. Pharmacobiodyn. 1988, 11, 630–635. [Google Scholar] [CrossRef]
- Singh, S.B.; Zink, D.L.; Bills, G.F.; Teran, A.; Silverman, K.C.; Lingham, R.B.; Felock, P.; Hazuda, D. Four novel bis-(naphtho-γ-pyrones) isolated from Fusarium species as inhibitors of HIV-1 integrase. Bioorg. Med. Chem. Lett. 2003, 13, 713–717. [Google Scholar] [CrossRef]
- Sakai, K.; Ohte, S.; Ohshiro, T.; Matsuda, D.; Masuma, R.; Rudel, L.L.; Tomoda, H. Selective inhibition of Acyl-CoA:Cholesterol acyltransferase 2 isozyme by flavasperone and sterigmatocystin from Aspergillus species. J. Antibiot. 2008, 61, 568–572. [Google Scholar] [CrossRef]
- Gorst-Allman, C.P.; Steyn, P.S.; Rabie, C.J. Structural elucidation of the nigerones, four new naphthopyrones from cultures of Aspergillus niger. J. Chem. Soc. Perkin Trans. 1 1980, 1980, 2474–2479. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S.; Iitaka, Y. Absolute configurations of chaetochromin A and related bis(naphtho-γ-pyrone) mold metabolites. Chem. Pharm. Bull. 1987, 35, 4049–4055. [Google Scholar] [CrossRef]
- Zhang, Y.; Ling, S.; Fang, Y.; Zhu, T.; Gu, Q.; Zhu, W.-M. Isolation, structure elucidation, and antimycobacterial properties of dimeric naphtho-γ-pyrones from the marine-derived fungus Aspergillus carbonarius. Chem. Biodivers. 2008, 5, 93–100. [Google Scholar] [CrossRef]
- Li, X.-B.; Xie, F.; Liu, S.-S.; Li, Y.; Zhou, J.-C.; Liu, Y.-Q.; Yuan, H.-Q.; Lou, H.-X. Naphtho-γ-pyrones from endophyte Aspergillus niger occurring in the liverwort Heteroscyphus tener (Steph.) Schiffn. Chem. Biodivers. 2013, 10, 1193–1201. [Google Scholar] [CrossRef]
- Koyama, K.; Aida, S.; Natori, S. Supplemental observations on atropisomerism of fungal bis(naphtho-γ-pyrone)s. Chem. Pharm. Bull. 1990, 38, 2259–2261. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S. Chaetochromins B, C and D, bis(naphtho-γ-pyrone) derivatives from Chaetomium gracile. Chem. Pharm. Bull. 1987, 35, 578–584. [Google Scholar] [CrossRef]
- Paranagama, P.A.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Uncovering biosynthetic potential of plant-associated fungi: Effect of culture conditions on metabolite production by Paraphaeosphaeria quadriseptata and Chaetomium chiversii. J. Nat. Prod. 2007, 70, 1939–1945. [Google Scholar] [CrossRef]
- Xu, G.-B.; Yang, T.; Bao, J.-K.; Fang, D.-M.; Li, G.-Y. Isochaetochromin A2, a new bis(naphthodihydropyran-4-one) with antimicrobial and immunological activities from fungus Chaetomium microcephalum. Arch. Pharm. Res. 2014, 37, 575–579. [Google Scholar] [CrossRef]
- Sekita, S.; Yoshihira, K.; Natori, S. Chaetochromin, a bis(naphthodihydropyran-4-one) mycotoxin from Chaetomium thielavioideum: Application of 13C-1H long-range coupling to the structure elucidation. Chem. Pharm. Bull. 1980, 28, 2428–2435. [Google Scholar] [CrossRef]
- Ugaki, N.; Matsuda, D.; Yamazaki, H.; Nonaka, K.; Masuma, R.; Omura, S.; Tomoda, H. New isochaetochromin, an inhibitor of triacylalycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: I. Taxonomy, fermentation, isolation and biological properties. J. Antibiot. 2012, 65, 15–19. [Google Scholar] [CrossRef]
- Ugaki, N.; Yamazaki, H.; Uchida, R.; Tomoda, H. New isochaetochromin, an inhibitor of triacylglycerol synthesis in mammalian cells, produced by Penicillium sp. FKI-4942: II. Structure elucidation. J. Antibiot. 2012, 65, 21–24. [Google Scholar] [CrossRef]
- Kong, X.; Ma, X.; Xie, Y.; Cai, S.; Zhu, T.; Gu, Q.; Li, D. Aromatic polyketides from a sponge-derived fungus Metarhizium anisopliae mxh-99 and their antitubercular activities. Arch. Pharm. Res. 2013, 36, 739–744. [Google Scholar] [CrossRef]
- Haskins, R.H.; Knapp, C. Cephalosporium species (PRL 2070) and the production of cephalochromin. Can. J. Microbiol. 1969, 15, 435–437. [Google Scholar] [CrossRef]
- Hsiao, C.-J.; Hsiao, G.; Chen, W.-L.; Wang, S.-W.; Chiang, C.-P.; Liu, L.-Y.; Guh, J.-H.; Lee, T.-H.; Chung, C.-L. Cephalochromin induces G0/G1 cell cycle arrest and apoptosis in A549 human non-small-cell lung cancer cells by inflicting mitochondrial disruption. J. Nat. Prod. 2014, 77, 758–765. [Google Scholar] [CrossRef]
- Carey, S.T.; Nair, M.S.R. Metabolites of Pyrenomycetes. V. identification of an antibiotic from two species of Nectria, as cephalochromin. Lloydia 1975, 38, 448–449. [Google Scholar]
- Kock, I.; Draeger, S.; Schulz, B.; Elsasser, B.; Kurtan, T.; Kenez, A.; Antus, S.; Pescitelli, G.; Salvadori, P.; Speakman, J.-B.; et al. Pseudoanguillosporin A and B: Two new isochromans isolated from the endophytic fungus Pseudoanguillospora sp. Eur. J. Org. Chem. 2009, 2009, 1427–1434. [Google Scholar] [CrossRef]
- Matsumoto, M.; Minato, H.; Kondo, E.; Mitsugi, T.; Katagiri, K. Cephalochromin, dihydroisoustilaginoidin A, and isoustilaginoidin A from Verticillium sp. K-113. J. Antibiot. 1975, 28, 602–604. [Google Scholar] [CrossRef]
- Hegde, V.R.; Miller, J.R.; Patel, M.G.; King, A.H.; Puar, M.S.; Horan, A.; Hart, R.; Yarborough, R.; Gullo, V. Sch 45752—An inhibitor of calmodulin-sensitive cyclic nucleotide phosphodiesterase activities. J. Antibiot. 1993, 46, 207–213. [Google Scholar] [CrossRef]
- Ohkawa, Y.; Miki, K.; Suzuki, T.; Nishio, K.; Sugita, T.; Kinoshita, K.; Takhashi, K.; Koyama, K. Antiangiogenic metabolites from a marine-derived fungus, Hypocrea vinosa. J. Nat. Prod. 2010, 73, 579–582. [Google Scholar] [CrossRef]
- Shibata, S.; Ogihara, Y.; Ohta, A. Metabolic products of fungi. XXII. On ustilaginoidins. 2. The structure of ustilaginoidin A. Chem. Pharm. Bull. 1963, 11, 1179–1182. [Google Scholar] [CrossRef]
- Shibata, S.; Ogihara, Y. Metabolic products of fungi. XXIII. Ustilaginoidins. 3. The structure of ustilaginoidins B and C. Chem. Pharm. Bull. 1963, 11, 1576–1578. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S. Further characterization of seven bis(naphtho-γ-pyrone) congeners of ustilaginoidins, coloring matters of Claviceps virens (Ustilaginoidea virens). Chem. Pharm. Bull. 1988, 36, 146–152. [Google Scholar] [CrossRef]
- Shaaban, M.; Shaaban, K.A.; Abdel-Aziz, M.S. Seven naphtho-γ-pyrones from the marine derived fungus Alternaria alternata: Structure elucidation and biological properties. Org. Med. Chem. Lett. 2012, 2, 6. [Google Scholar] [CrossRef]
- Tanaka, H.; Wang, P.-L.; Yamada, O.; Tamura, L.T. Yellow pigments of Aspergillus niger and Aspergillus awamori. Part I. Isolation of aurasperone A and related pigments. Agric. Biol. Chem. 1966, 30, 107–113. [Google Scholar] [CrossRef]
- Priestap, H.A. New naphthopyrones from Aspergillus fonsecaeus. Tetrahedron 1984, 40, 3617–3624. [Google Scholar] [CrossRef]
- Akiyama, K.; Teraguchi, S.; Hamasaki, Y.; Mori, M.; Tatsumi, K.; Ohnishi, K.; Hayashi, H. New dimeric naphthopyrones from Aspergillus niger. J. Nat. Prod. 2003, 66, 136–139. [Google Scholar] [CrossRef]
- Song, Y.C.; Li, H.; Ye, Y.H.; Shan, C.Y.; Yang, Y.M.; Tan, R.X. Endophytic naphthopyrone metabolites are co-inhibitors of xanthine oxidase, SW1116 cell and some microbial growths. FEMS Microbiol. Lett. 2004, 241, 67–72. [Google Scholar] [CrossRef]
- Campos, F.R.; Barison, A.; Daolio, C.; Ferreira, A.G.; Rodrigues-Fo, E. Complete 1H- and 13C-NMR assignments of aurasperone A and fonsecinone A, two bis-naphthopyrones produced by Aspergillus aculeatus. Magn. Reson. Chem. 2005, 43, 962–965. [Google Scholar]
- Chiang, Y.-M.; Meyer, K.M.; Praseuth, M.; Baker, S.E.; Bruno, K.S.; Wang, C.C.C. Characterization of a polyketide synthase in Aspergillus niger whose product is a precursor for both dihydroxynaphthalene (DHN) melanin and naphtho-γ-pyrone. Fungal Genet. Biol. 2011, 48, 430–437. [Google Scholar] [CrossRef]
- Xiao, J.; Zhang, Q.; Gao, Y.-Q.; Shi, X.-W.; Gao, J.-M. Antifungal and antibacterial metabolites from an endophytic Aspergillus sp. associated with Melia azedarach. Nat. Prod. Res. 2014, 28. [Google Scholar] [CrossRef]
- Meng, X.; Ge, F.; Zeng, Y.; Zhao, P. Chemical constituents from the endophytic fungus Aspergillus sp. DCS31 of Daphniphyllum longeracemosum. Nat. Prod. Res. Dev. 2013, 25, 190–192. [Google Scholar]
- Zhan, J.; Gunaherath, G.M.K.B.; Wijeratne, E.M.K.; Gunatilaka, A.A.L. Asperpyrone D and other metabolites of the plant-associated fungal strain Aspergillus tubingensis. Phytochemistry 2007, 68, 368–372. [Google Scholar] [CrossRef]
- Wang, P.-L.; Tanaka, H. Yellow pigments of Aspergillus niger and Aperigillus awamori. Part II. Chemical structure of aurasperone A. Agric. Biol. Chem. 1966, 30, 683–687. [Google Scholar] [CrossRef]
- Ehrlich, K.C.; DeLucca, A.J; Ciegler, A. Naphtho-γ-pyrone production by Aspergillus niger isolated from stored cottonseed. Appl. Environ. Microbiol. 1984, 48, 1–4. [Google Scholar]
- Tanaka, H.; Wang, P.-L.; Namiki, M. Structure of aurasperone C. Agric. Biol. Chem. 1972, 36, 2511–2517. [Google Scholar] [CrossRef]
- Bouras, N.; Mathieu, F.; Coppel, Y.; Lebrihi, A. Aurasperone F—A new member of the naphtho-gamma-pyrone class isolated from a cultured microfungus, Aspergillus niger C-433. Nat. Prod. Res. 2005, 19, 653–659. [Google Scholar] [CrossRef]
- De Vries, R.P.; Frisvad, J.C.; van de Vondervoort, P.J.I.; Burgers, K.; Kuijpers, A.F.A.; Samson, R.A.; Visser, J. Aspergillus vadensis, a new species of the group of black Aspergilli. Antonie Van Leeuwenkoek 2005, 87, 195–203. [Google Scholar] [CrossRef]
- Ghosal, S.; Biswas, K.; Chakrabarti, D.K. Toxic naphtho-γ-pyrones from Aspergillus niger. J. Agric. Food. Chem. 1979, 27, 1347–1351. [Google Scholar] [CrossRef]
- Bouras, N.; Mathieu, F.; Coppel, Y.; Strelkov, S.E.; Lebrihi, A. Occurrence of naphtho-gamma-pyrones- and ochratoxin A—Producing fungi in French grapes and characterization of new naphtho-gamma-pyrone polyketide (aurasperone G) isolated from Aspergillus niger C-433. J. Agric. Food Chem. 2007, 55, 8920–8927. [Google Scholar] [CrossRef]
- Ye, Y.H.; Zhu, H.L.; Song, Y.C.; Liu, S.J.; Tan, R.X. Structural revision of aspernigrin A, reisolated from Cladosporium herbarum IFB-E002. J. Nat. Prod. 2005, 68, 1106–1108. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.-M.; Wang, B.-G. Nigerasperones A-C, new monomeric and dimeric naphtho-γ-pyrones from marine alga-derived endophytic fungus Aspergillus niger EN-13. J. Antibiot. 2007, 60, 204–210. [Google Scholar] [CrossRef]
- Huang, H.-B.; Feng, X.-J.; Liu, L.; Chen, B.; Lu, Y.-J.; Ma, L.; She, Z.-G.; Lin, Y.-C. Three dimeric naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis isolated from Pongamia pinnata. Planta Med. 2010, 76, 1888–1891. [Google Scholar] [CrossRef]
- Huang, H.-B.; Xiao, Z.-E.; Feng, X.-J.; Huang, C.-H.; Zhu, X.; Ju, J.-H.; Li, M.-F.; Lin, Y.-C.; Liu, L.; She, Z.-G. Cytotoxic naphtho-γ-pyrones from the mangrove endophytic fungus Aspergillus tubingensis (GX1–5E). Helv. Chim. Acta 2011, 94, 1732–1740. [Google Scholar] [CrossRef]
- Geiser, D.M.; Klich, M.A.; Frisvad, J.C.; Peterson, S.W.; Varga, J.; Samson, R.A. The current status of species recognition and identification in Aspergillus. Stud. Mycol. 2007, 59, 1–10. [Google Scholar] [CrossRef]
- Perrone, G.; Susca, A.; Cozzi, G.; Ehrlich, K.; Varga, J.; Frisvad, J.C.; Meijer, M.; Noonim, P.; Mahakarnchanakul, W.; Samson, R.A. Biodiversity of Aspergillus species in some important agricultural products. Stud. Mycol. 2007, 59, 53–66. [Google Scholar] [CrossRef]
- Samson, R.A.; Varga, J. What is a species in Aspergillus? Med. Mycol. 2009, 47, S13–S20. [Google Scholar] [CrossRef]
- Koyama, K.; Natori, S. Biosynthesis of chaetochromin A, a bis(naphtho-γ-pyrone), in Chaetomium spp. Chem. Pharm. Bull. 1989, 37, 2022–2025. [Google Scholar]
- Sakurai, M.; Kohno, J.; Yamamoto, K.; Okuda, T.; Nishio, M.; Kawano, K.; Ohnuki, T. TMC-256A1 and C1, new inhibitors of IL-4 signal transduction produced by Aspergillus niger var. niger TC 1629. J. Antibiot. 2002, 55, 685–692. [Google Scholar] [CrossRef]
- Asai, T.; Yamamoto, T.; Oshima, Y. Aromatic polyketide production in Cordyceps indigotica, an entomopathogenic fungus, induced by exposure to a histone deacetylase inhibitor. Org. Lett. 2012, 14, 2006–2009. [Google Scholar] [CrossRef]
- Tsuchiya, T.; Sekita, S.; Koyama, K.; Natori, S.; Takahashi, A. Effect of chaetochromin A, chaetochromin D and ustilaginoidin A, bisnaphtho-γ-pyrone derivatives, on the mouse embryo limb bud and midbrain cells in culture. Cong. Anom. 1987, 27, 245–250. [Google Scholar] [CrossRef]
- Kawai, K.; Hisada, K.; Mori, S.; Nozawa, Y.; Koyama, K.; Natori, S. The impairing effect of chaetochromin A and related mycotoxins on mitochondrial respiration. Proc. Jpn. Assoc. Mycotoxicol. 1991, 33, 31–35. [Google Scholar]
- Campbell, J.W.; Cronan, J.E. Bacterial fatty acid biosynthesis: Targets for antibacterial drug discovery. Annu. Rev. Microbiol. 2001, 55, 305–332. [Google Scholar] [CrossRef]
- Zheng, C.J.; Sohn, M.-J.; Lee, S.; Hong, Y.-S.; Kwak, J.-H.; Kim, W.-G. Cephalochromin, a FabI-directed antibacterial of microbial origin. Biochem. Biophys. Res. Commun. 2007, 362, 1107–1112. [Google Scholar] [CrossRef]
- Cardellina, J.H.; Roxas-Duncan, V.I.; Montgomery, V.; Eccard, V.; Campbell, Y.; Hu, X.; Khavrutskii, I.; Tawa, G.J.; Wallqvist, A.; Gloer, J.B.; et al. Fungal bis-naphthopyrones as inhibitors of botulinum neurotoxin serotype A. ACS Med. Chem. Lett. 2012, 3, 387–391. [Google Scholar] [CrossRef]
- Ikeda, S.-I.; Sugita, M.; Yoshimura, A.; Sumizawa, T.; Douzono, H.; Nagata, Y.; Akiyama, S.-I. Aspergillus species strain M39 produces two naphtho-γ-pyrones that reverse drug resistance in human KB cells. Int. J. Cancer 1990, 45, 508–513. [Google Scholar] [CrossRef]
- Ito, Y.; Ohtsubo, K. Teratogenicity of oral chaetochromin, a polyphenolic mycotoxin produced by Chaetomium spp., to mice embryo. Bull. Environ. Contam. Toxicol. 1987, 39, 299–303. [Google Scholar] [CrossRef]
- Mori, S.; Kawai, K.; Nozawa, Y.; Koyama, K.; Natori, S. The impairing effects of chaetochromin D on mitochondrial respiration and structure. Mycotoxin Res. 1993, 9, 85–93. [Google Scholar] [CrossRef]
- Ishii, R.; Horie, M.; Koyama, K.; Ishikawa, Y.; Kitanaka, S. Inhibitory effects of fungal bis(naphtho-γ-pyrone) derivatives on nitric oxide production by a murine macrophage-like cell line, RAW 264.7, activated by lipopolysaccharide and interferon-γ. Biol. Pharm. Bull. 2005, 28, 786–790. [Google Scholar] [CrossRef]
- Zhao, J.; Shan, T.; Mou, Y.; Zhou, L. Plant-derived bioactive compounds produced by endophytic fungi. Mini Rev. Med. Chem. 2011, 11, 159–168. [Google Scholar] [CrossRef]
- Gutierrez, R.M.P.; Gonzalez, A.M.N.; Ramirez, A.M. Compounds derived from endophytes: A review of phytochemistry and pharmacology. Curr. Med. Chem. 2012, 19, 2992–3030. [Google Scholar] [CrossRef]
- Debbab, A.; Aly, A.H.; Proksch, P. Mangrove derived fungal endophytes—A chemical and biological perception. Fungal Divers. 2013, 61, 1–27. [Google Scholar] [CrossRef]
- Lee, Y.M.; Kim, M.J.; Li, H.; Zhang, P.; Bao, B; Lee, K.J.; Jung, J.H. Marine-derived Aspergillus species as a source of bioactive secondary metabolites. Mar. Biotechnol. 2013, 15, 499–519. [Google Scholar] [CrossRef]
- Morishita, E.; Shibatam, S. Metabolic products of fungi. XXVII. Synthesis of racemic unstilaginoidin A and its related compounds. Chem. Pharm. Bull. 1967, 15, 1772–1775. [Google Scholar] [CrossRef]
- DiVirgilio, E.S.; Dugan, E.C.; Mulrooney, C.A.; Kozlowski, M.C. Asymmetric total synthesis of nigerone. Org. Lett. 2007, 9, 385–388. [Google Scholar] [CrossRef]
- Kozlowski, M.C.; Dugan, E.C.; DiVirgilio, E.S.; Maksimenka, K.; Bringmann, G. Asymmetric total synthesis of nigerone and ent-nigerone: Enantioselective oxidative biaryl coupling highly hindered naphthols. Adv. Synth. Catal. 2007, 349, 583–594. [Google Scholar] [CrossRef]
- Marin, P.; de Ory, A.; Cruz, A.; Magan, N.; Gonzalez-Jaen, M.T. Potential effects of environmental conditions on the efficiency of the antifungal tebuconazole controlling Fusarium verticillioides and Fusarium proliferatum growth rate and fumonisin biosynthesis. Int. J. Food Microbiol. 2013, 165, 251–258. [Google Scholar] [CrossRef]
- Jorgensen, T.R.; Park, J.; Arentshorst, M.; van Welzen, A.M.; Lamers, G.; van Kuyk, P.A.; Damveld, R.A.; van den Hondel, C.A.M.; Nielsen, K.F.; Frisvad, J.C.; et al. The molecular and genetic basis of conidial pigmentation in Aspergillus niger. Fungal Genet. Biol. 2011, 48, 544–553. [Google Scholar] [CrossRef]
- Zhou, L.; Lu, S.; Shan, T.; Wang, P.; Sun, W.; Chen, Z.; Wang, S. Chemistry and biology of mycotoxins from rice false smut pathogen. In Mycotoxins: Properties, Applications and Hazards; Melborn, B.J., Greene, J.C., Eds.; Nova Science Publishers: New York, NY, USA, 2012; pp. 109–130. [Google Scholar]
© 2014 by the authors. licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Lu, S.; Tian, J.; Sun, W.; Meng, J.; Wang, X.; Fu, X.; Wang, A.; Lai, D.; Liu, Y.; Zhou, L. Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities. Molecules 2014, 19, 7169-7188. https://doi.org/10.3390/molecules19067169
Lu S, Tian J, Sun W, Meng J, Wang X, Fu X, Wang A, Lai D, Liu Y, Zhou L. Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities. Molecules. 2014; 19(6):7169-7188. https://doi.org/10.3390/molecules19067169
Chicago/Turabian StyleLu, Shiqiong, Jin Tian, Weibo Sun, Jiajia Meng, Xiaohan Wang, Xiaoxiang Fu, Ali Wang, Daowan Lai, Yang Liu, and Ligang Zhou. 2014. "Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities" Molecules 19, no. 6: 7169-7188. https://doi.org/10.3390/molecules19067169
APA StyleLu, S., Tian, J., Sun, W., Meng, J., Wang, X., Fu, X., Wang, A., Lai, D., Liu, Y., & Zhou, L. (2014). Bis-naphtho-γ-pyrones from Fungi and Their Bioactivities. Molecules, 19(6), 7169-7188. https://doi.org/10.3390/molecules19067169







