Native Chemical Ligation: A Boon to Peptide Chemistry
Abstract
:1. Introduction
2. History of Ligation Strategies
2.1. Hydrazone Ligation
2.2. Oxime Ligation
2.3. Thiazolidine Ligation
2.4. Thioester Ligation
| Technique | Advantages | Disadvantages | Ref. |
|---|---|---|---|
Hydrazone Ligation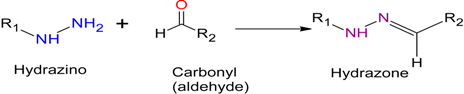 |
|
| [7,8,9,16] |
Oxime Ligation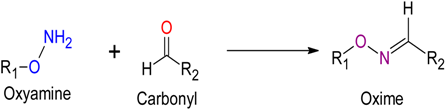 |
|
| [9,10,11,16] |
Thiazolidine Ligation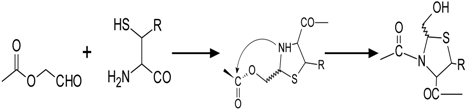 |
|
| [11,12,13] |
Thioester Ligation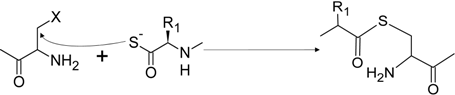 |
|
| [13,14,15] |
3. Native Chemical Ligation
3.1. Native Chemical Ligation at Cysteine

3.2. Native Chemical Ligation at Non-Cysteine Sites: Auxiliary Mediated Ligation

3.3. Ligation at Non Cysteine Amino Acids
4. Sequential and Convergent Ligation

5. Application of Native Chemical Ligation
5.1. Cyclotides and Conotoxins

5.2. NCL in Overcoming Length Limitation Associated with SPPS
| Toxin/Peptide | Application | Sequence | Ref. |
|---|---|---|---|
| Human interleukin 8 (IL-8) | Chemokine, chemoattractants for leukocytes | SAKELRCQCIKTYSKPFHPKFIKELRVIESGPHCANTEIIVKLSDGRELCLDPKEWVQRVVEKFLKRAENS | [5,90] |
| Human group II secretory phospholipase A2 (sPLA2) | Hydrolysis of the fatty acid side chain ester bond | NLVNFHRMIKLTTGKEAALSYGFYGCHCGVGGRGSPKDATDRCCVTHDCCYKRLEKRGCGTKFLSYKFSNSGSRITCAKQDSCRSQLCECDKAAATCFARNKTTYNKKYQYYSNKHCRGSTPRC | [22] |
| Microprotein S | Anticoagulant cofactor activity | NSLLγγTKQGNLγRγCIγγLCNKγγARγVFγNDPγTDYFYPKYLGCLRSFQTGLFTAARQSTNAYPDLRSCVNAIPDQCSPLPCNEDGYMSCKDGKASFTCTCKPGWQG EKCEFD | [89] |
| Barnase (Lys49-Cys49) | Microbial ribonuclease | AQVINTFDGVADYLQTYHKLPNDYITKSEAQALGWVASKGNLADVAPGCSIGGDIFSNREGKLPGKSGRTWREADINYTSGFRNSDRILYSSDWLIYKTTDHYQTFTKIR | [24] |
5.3. Synthesis of Challenging Sequences
| Toxin/Peptide | Application | Sequence | Ref. |
|---|---|---|---|
| Ec-MscL | Mechanosensitive ion channel | MSIIKEFREFAMRGNVVDLAVGVIIGAAFGKIVSSLVADIIMPPLGLLIGGIDFKCFAVTLRDAQGDIPAVVMHYGVFIQNVFDFLIVAFAIFMAIKLINKLCRKKEEPAAAPAPTKEEVLLTEIRDLLKEQNNRS | [92] |
| IbTx V16A/D19-Cys-4-MeOBzl | Binding to BK Ca2+-activated K+ channel (KCa1.1) | ZFTDVDCSVSKECWSACKX2LFGVDRGKCMGKKCRCYQ (X2 = Cys-4-MeOBzl) | [98] |
| Snow Flea Antifreeze Protein (sfAFP) | Inhibition of Ice Crystal Formation | CLGADGAHGVNGCPGTAGAAGSVGGPGCDGGHGGNGGNGNPGCAGGVGGAGGASGGTGVGGRGGKGGSGTPKGADGAPGAP | [96,97] |
| KcsA (Pro2-Ala2, Gln58-Ala58, Thr61-Ser61, Arg64-Asp64) | Potassium Channel | MAPMLSGLLARLVKLLLGRHGSALHWRAAGAATVLLVIVLLAGSYLAVLAERGAPGAALISYPDALWWSVETACTVGYGDLYPVTLWGRLVAVVVMVAGITSFGLVTAALATWFVGREQERRGK | [99] |
5.4. Use in Doing Alanine Scans to Study Structure Activity Relationships
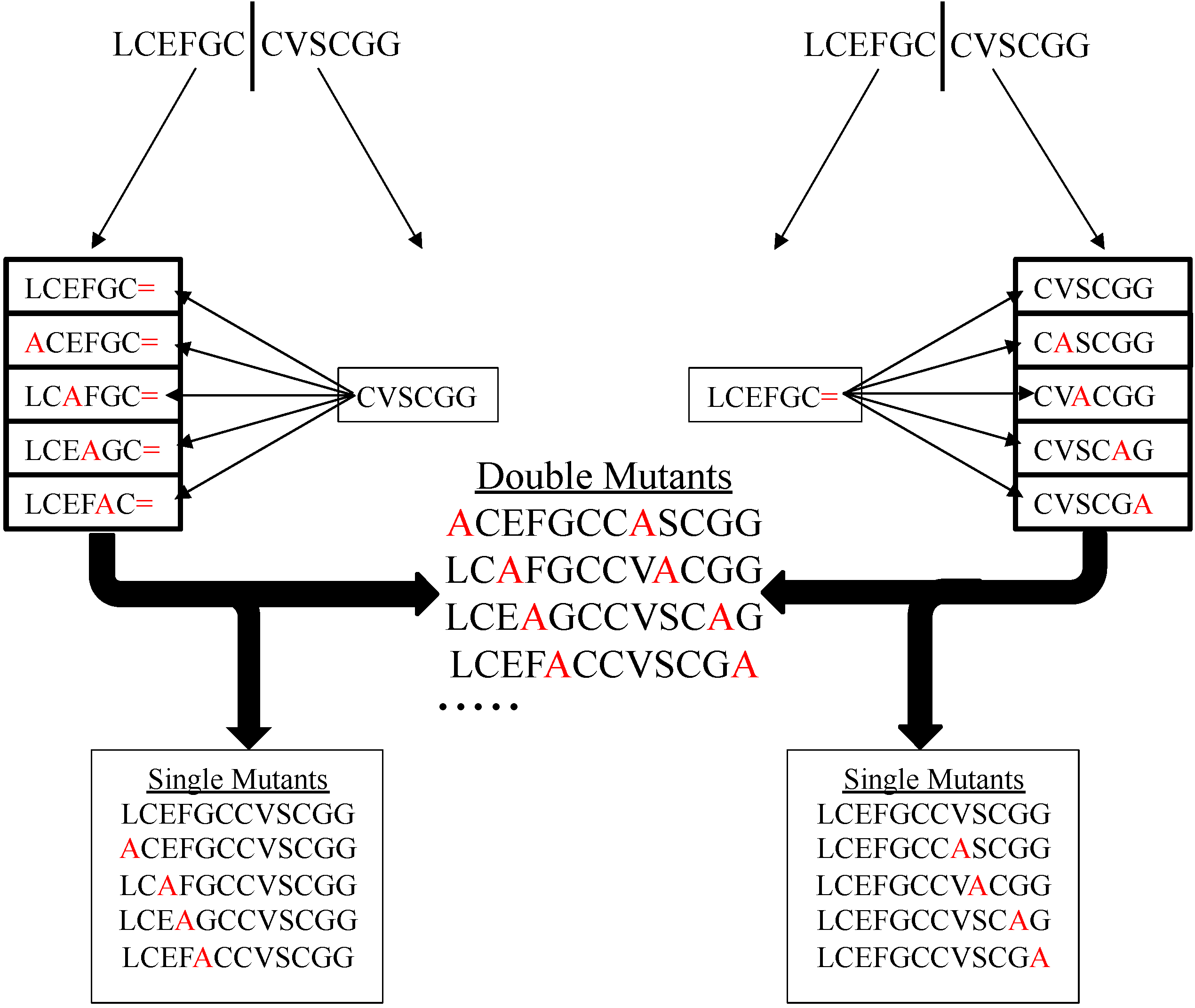
5.5. Conjugation with Recombinant Technology to Make Glycopeptides and Peptidomemetics
| Toxin/Peptide | Application | Sequence | Ref. |
|---|---|---|---|
| Diptericin ε (Cys25,Glu29,Glu45) | Antibacterial glycopeptide | DEKPKLILPTPAPPNLPQLVGGGGCNRKEGFGVSVDAHQKVWTSENGRHSIGVTPGYSQHLGGPYGNSRPDYRIGAGYSYNF | [109] |
| Lymphotactin (Lptn) | Chemoattractant (for T- cell and natural killer cell) | VGSEVSDKRTCVSLTTQRLPVSRIKTYTITEGSLRAVIFITKRGLKVCADPQATWVRDVVRSMDRKSNTRNNMIQTKPTGTQQSTNTAVTLTG | [112] |
| RNase B Fragment | Cleavage of N-linked carbohydrates | MKSRNLTKDRCKPVNTFVHESLADVQAVCSQKNVACKNG | [115] |
| CCL7 (MCP-3) | Monocyte specific chemotactic protein-3 | QPVGINTSTTCCYRFINKKIPKQRLESYRRTTSSHCPREAVIFKTKLDKEICADPTQKWVQDFMKHLDKKTQTPKL | [116] |
6. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Merrifield, R.B. Solid phase peptide synthesis. I. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963, 85, 2149–2154. [Google Scholar]
- Dawson, P.E.; Kent, S.B. Synthesis of native proteins by chemical ligation. Ann. Rev. Biochem. 2000, 69, 923–960. [Google Scholar] [CrossRef] [PubMed]
- Grant, G.A. Synthetic Peptides: A User’s Guide, 2nd ed.; Oxford University Press: Oxford, UK, 2002; p. 390. [Google Scholar]
- Chan, W.C.; White, P.D. Fmoc Solid Phase Peptide Synthesis: A Practical Approach; Oxford University Press: Oxford, UK, 2000; p. 346. [Google Scholar]
- Dawson, P.E.; Muir, T.W.; Clark-Lewis, I.; Kent, S.B. Synthesis of proteins by native chemical ligation. Science 1994, 266, 776–779. [Google Scholar] [CrossRef] [PubMed]
- Chandrudu, S.; Simerska, P.; Toth, I. Chemical methods for peptide and protein production. Molecules 2013, 18, 4373–4388. [Google Scholar] [CrossRef] [PubMed]
- Day, A.C.; Whiting, M.C. Acetone hydrazone. Org. Synth. 1988, 50, 10–12. [Google Scholar]
- Cabezas, E.; Wang, M.; Parren, P.W.; Stanfield, R.L.; Satterthwait, A.C. A structure-based approach to a synthetic vaccine for HIV-1. Biochemistry 2000, 39, 14377–14391. [Google Scholar] [CrossRef] [PubMed]
- Rose, K. Facile synthesis of homogeneous artificial proteins. J. Am. Chem. Soc. 1994, 116, 30–33. [Google Scholar] [CrossRef]
- Dirksen, A.; Hackeng, T.M.; Dawson, P.E. Nucleophilic catalysis of oxime ligation. Angew. Chem. Int. Ed. 2006, 45, 7581–7584. [Google Scholar] [CrossRef]
- Shao, J.; Tam, J.P. Unprotected peptides as building-blocks for the synthesis of peptide dendrimers with oxime, hydrazone, and thiazolidine linkages. J. Am. Chem. Soc. 1995, 117, 3893–3899. [Google Scholar] [CrossRef]
- Liu, C.F.; Tam, J.P. Chemical ligation approach to form a peptide-bond between unprotected peptide segments—Concept and model study. J. Am. Chem. Soc. 1994, 116, 4149–4153. [Google Scholar] [CrossRef]
- Liu, C.F.; Rao, C.; Tam, J.P. Orthogonal ligation of unprotected peptide segments through pseudoproline formation for the synthesis of HIV-1 protease. J. Am. Chem. Soc. 1996, 118, 307–312. [Google Scholar] [CrossRef]
- Schnolzer, M.; Kent, S.B. Constructing proteins by dovetailing unprotected synthetic peptides: Backbone-Engineered HIV protease. Science 1992, 256, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Kent, S.B. Total chemical synthesis of proteins. Chem. Soc. Rev. 2009, 38, 338–351. [Google Scholar] [CrossRef] [PubMed]
- Algar, W.R.; Prasuhn, D.E.; Stewart, M.H.; Jennings, T.L.; Blanco-Canosa, J.B.; Dawson, P.E.; Medintz, I.L. The controlled display of biomolecules on nanoparticles: a challenge suited to bioorthogonal chemistry. Bioconjug. Chem. 2011, 22, 825–858. [Google Scholar] [CrossRef] [PubMed]
- Muir, T.W.; Dawson, P.E.; Kent, S.B. Protein synthesis by chemical ligation of unprotected peptides in aqueous solution. Methods Enzymol. 1997, 289, 266–298. [Google Scholar] [PubMed]
- Lu, W.; Randal, M.; Kossiakoff, A.; Kent, S.B. Probing intermolecular backbone H-bonding in serine proteinase-protein inhibitor complexes. Chem. Biol. 1999, 6, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Ayers, B.; Cowburn, D.; Muir, T.W. Chemical ligation of folded recombinant proteins: Segmental isotopic labeling of domains for NMR studies. Proc. Natl. Acad. Sci. USA 1999, 96, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Qasim, M.A.; Kent, S.B.H. Comparative total syntheses of turkey ovomucoid third domain by both stepwise solid phase peptide synthesis and native chemical ligation. J. Am. Chem. Soc. 1996, 118, 8518–8523. [Google Scholar] [CrossRef]
- Kent, S.; Sohma, Y.; Liu, S.; Bang, D.; Pentelute, B.; Mandal, K. Through the looking glass–A new world of proteins enabled by chemical synthesis. J. Pept. Sci. off. Publ. Eur. Pept. Soc. 2012, 18, 428–436. [Google Scholar]
- Hackeng, T.M.; Mounier, C.M.; Bon, C.; Dawson, P.E.; Griffin, J.H.; Kent, S.B. Total chemical synthesis of enzymatically active human type II secretory phospholipase A2. Proc. Natl. Acad. Sci. USA 1997, 94, 7845–7850. [Google Scholar] [CrossRef] [PubMed]
- Hackeng, T.M.; Griffin, J.H.; Dawson, P.E. Protein synthesis by native chemical ligation: Expanded scope by using straightforward methodology. Proc. Natl. Acad. Sci. USA 1999, 96, 10068–10073. [Google Scholar] [CrossRef] [PubMed]
- Dawson, P.E.; Churchill, M.J.; Ghadiri, M.R.; Kent, S.B.H. Modulation of Reactivity in Native Chemical Ligation through the Use of Thiol Additives. J. Am. Chem. Soc. 1997, 119, 4325–4329. [Google Scholar] [CrossRef]
- Johnson, E.C.; Kent, S.B. Studies on the insolubility of a transmembrane peptide from signal peptide peptidase. J. Am. Chem. Soc. 2006, 128, 7140–7141. [Google Scholar] [CrossRef] [PubMed]
- Torbeev, V.Y.; Kent, S.B.H. Convergent Chemical Synthesis and Crystal Structure of a 203 Amino Acid “Covalent Dimer” HIV-1 Protease Enzyme Molecule. Angew. Chem. Int. Ed. Engl. 2007, 46, 1667–1670. [Google Scholar] [CrossRef] [PubMed]
- Boerema, D.J.; Tereshko, V.A.; Kent, S.B.H. Total synthesis by modern chemical ligation methods and high resolution (1.1 Å) X-ray structure of ribonuclease A. Pept. Sci. 2008, 90, 278–286. [Google Scholar]
- Rohde, H.; Seitz, O. Ligation-desulfurization: A powerful combination in the synthesis of peptides and glycopeptides. Biopolymers 2010, 94, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Akaji, K.; Aimoto, S. Peptide bond formation mediated by 4,5-dimethoxy-2-mercaptobenzylamine after periodate oxidation of the N-terminal serine residue. Org. lett. 2001, 3, 1403–1405. [Google Scholar] [CrossRef] [PubMed]
- Low, D.W.; Hill, M.G.; Carrasco, M.R.; Kent, S.B.; Botti, P. Total synthesis of cytochrome b562 by native chemical ligation using a removable auxiliary. Proc. Natl. Acad. Sci. USA 2001, 98, 6554–6559. [Google Scholar] [CrossRef] [PubMed]
- Offer, J.; Boddy, C.N.; Dawson, P.E. Extending synthetic access to proteins with a removable acyl transfer auxiliary. J. Am. Chem. Soc. 2002, 124, 4642–4646. [Google Scholar] [CrossRef] [PubMed]
- Cardona, V.M.; Hartley, O.; Botti, P. Synthesis of cyclic peptides from unprotected precursors using removable N alpha-(1-(4-methoxyphenyl)-2-mercaptoethyl) auxiliary. J. Pept. Res. 2003, 61, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Wu, B.; Chen, J.; Warren, J.D.; Chen, G.; Hua, Z.; Danishefsky, S.J. Building complex glycopeptides: Development of a cysteine-free native chemical ligation protocol. Angew. Chem. Int. Ed. 2006, 45, 4116–4125. [Google Scholar] [CrossRef]
- Payne, R.J.; Ficht, S.; Tang, S.; Brik, A.; Yang, Y.Y.; Case, D.A.; Wong, C.H. Extended sugar-assisted glycopeptide ligations: Development, scope, and applications. J. Am. Chem. Soc. 2007, 129, 13527–13536. [Google Scholar] [CrossRef] [PubMed]
- Tchertchian, S.; Hartley, O.; Botti, P. Synthesis of N alpha-(1-phenyl-2-mercaptoethyl) amino acids, new building blocks for ligation and cyclization at non-cysteine sites: Scope and limitations in peptide synthesis. J. Org. Chem 2004, 69, 9208–9214. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Xu, J.; Eom, K.D. Methods and strategies of peptide ligation. Biopolymers 2001, 60, 194–205. [Google Scholar] [CrossRef]
- Yan, L.Z.; Dawson, P.E. Synthesis of peptides and proteins without cysteine residues by native chemical ligation combined with desulfurization. J. Am. Chem. Soc. 2001, 123, 526–533. [Google Scholar] [CrossRef] [PubMed]
- Pentelute, B.L.; Kent, S.B. Selective desulfurization of cysteine in the presence of Cys(Acm) in polypeptides obtained by native chemical ligation. Org. Lett. 2007, 9, 687–690. [Google Scholar] [CrossRef] [PubMed]
- Wan, Q.; Danishefsky, S.J. Free-radical-based, specific desulfurization of cysteine: A powerful advance in the synthesis of polypeptides and glycopolypeptides. Angew. Chem. Int. Ed. 2007, 46, 9248–9252. [Google Scholar] [CrossRef]
- Crich, D.; Banerjee, A. Native chemical ligation at phenylalanine. J. Am. Chem. Soc. 2007, 129, 10064–10065. [Google Scholar] [CrossRef] [PubMed]
- Botti, P.; Tchertchian, S. Side-Chain Extended Ligation. WO2006133962 A1, 21 December 2006. [Google Scholar]
- Haase, C.; Rohde, H.; Seitz, O. Native chemical ligation at valine. Angew. Chem. Int. Ed. 2008, 47, 6807–6810. [Google Scholar] [CrossRef]
- Kent, S. Origin of the chemical ligation concept for the total synthesis of enzymes (proteins). Biopolymers 2010, 94, iv–ix. [Google Scholar] [CrossRef] [PubMed]
- Bang, D.; Kent, S.B. A one-pot total synthesis of crambin. Angew. Chem. Int. Ed. 2004, 43, 2534–2538. [Google Scholar] [CrossRef]
- Lee, J.Y.; Bang, D. Challenges in the chemical synthesis of average sized proteins: Sequential vs. convergent ligation of multiple peptide fragments. Biopolymers 2010, 94, 441–447. [Google Scholar] [CrossRef]
- Bang, D.; Chopra, N.; Kent, S.B. Total chemical synthesis of crambin. J. Am. Chem. Soc. 2004, 126, 1377–1383. [Google Scholar] [CrossRef]
- Ueda, S.; Fujita, M.; Tamamura, H.; Fujii, N.; Otaka, A. Photolabile Protection for One-Pot Sequential Native Chemical Ligation. Chem. Biochem. 2005, 6, 1983–1986. [Google Scholar]
- Bang, D.; Kent, S.B. His6 tag-assisted chemical protein synthesis. Proc. Natl. Acad. Sci. USA 2005, 102, 5014–5019. [Google Scholar] [CrossRef]
- Brik, A.; Keinan, E.; Dawson, P.E. Protein synthesis by solid-phase chemical ligation using a safety catch linker. J. Org. Chem. 2000, 65, 3829–3835. [Google Scholar] [CrossRef] [PubMed]
- Johnson, E.C.; Durek, T.; Kent, S.B. Total chemical synthesis, folding, and assay of a small protein on a water-compatible solid support. Angew. Chem. Int. Ed. 2006, 45, 3283–3287. [Google Scholar] [CrossRef]
- Canne, L.E.; Botti, P.; Simon, R.J.; Chen, Y.; Dennis, E.A.; Kent, S.B. Chemical Protein Synthesis by Solid Phase Ligation of Unprotected Peptide Segments. J. Am. Chem. Soc. 1999, 121, 8720–8727. [Google Scholar] [CrossRef]
- Bang, D.; Pentelute, B.L.; Kent, S.B. Kinetically controlled ligation for the convergent chemical synthesis of proteins. Angew. Chem. Int. Ed. 2006, 45, 3985–3988. [Google Scholar] [CrossRef]
- Albericio, F. Developments in peptide and amide synthesis. Curr. Opin. Chem. Biol. 2004, 8, 211–221. [Google Scholar] [PubMed]
- Canne, L.E.; Ferre-D’Amare, A.R.; Burley, S.K.; Kent, S.B. Total Chemical Synthesis of a Unique Transcription Factor-Related Protein: cMyc-Max. J. Am. Chem. Soc. 1995, 117, 2998–3007. [Google Scholar] [CrossRef]
- Baca, M.; Muir, T.W.; Schnolzer, M.; Kent, S.B. Chemical Ligation of Cysteine-Containing Peptides: Synthesis of a 22 kDa Tethered Dimer of HIV-1 Protease. J. Am. Chem. Soc. 1995, 117, 1881–1887. [Google Scholar] [CrossRef]
- Zhang, L.; Tam, J.P. Synthesis and Application of Unprotected Cyclic Peptides as Building Blocks for Peptide Dendrimers. J. Am. Chem. Soc. 1997, 119, 2363–2370. [Google Scholar] [CrossRef]
- Durek, T.; Torbeev, V.Y.; Kent, S.B. Convergent chemical synthesis and high-resolution x-ray structure of human lysozyme. Proc. Natl. Acad. Sci. USA 2007, 104, 4846–4851. [Google Scholar] [PubMed]
- Torbeev, V.Y.; Kent, S.B. Convergent chemical synthesis and crystal structure of a 203 amino acid “covalent dimer” HIV-1 protease enzyme molecule. Angew. Chem. Int. Ed. 2007, 46, 1667–1670. [Google Scholar]
- Craik, D.J. Seamless proteins tie up their loose ends. Science 2006, 311, 1563–1564. [Google Scholar] [CrossRef] [PubMed]
- Trabi, M.; Craik, D.J. Circular proteins-no end in sight. Trends Biochem. Sci. 2002, 27, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Daly, N.L.; Bond, T.; Waine, C. Plant cyclotides: A unique family of cyclic and knotted proteins that defines the cyclic cystine knot structural motif. J. Mol. Biol. 1999, 294, 1327–1336. [Google Scholar] [CrossRef] [PubMed]
- Svangard, E.; Burman, R.; Gunasekera, S.; Lovborg, H.; Gullbo, J.; Goransson, U. Mechanism of action of cytotoxic cyclotides: Cycloviolacin O2 disrupts lipid membranes. J. Nat. Prod. 2007, 70, 643–647. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Daly, N.L.; Waine, C. The cystine knot motif in toxins and implications for drug design. Toxicon 2001, 39, 43–60. [Google Scholar] [CrossRef] [PubMed]
- Mylne, J.S.; Chan, L.Y.; Chanson, A.H.; Daly, N.L.; Schaefer, H.; Bailey, T.L.; Nguyencong, P.; Cascales, L.; Craik, D.J. Cyclic peptides arising by evolutionary parallelism via asparaginyl-endopeptidase-mediated biosynthesis. Plant Cell 2012, 24, 2765–2778. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; Craik, D.J. Native chemical ligation applied to the synthesis and bioengineering of circular peptides and proteins. Biopolymers 2010, 94, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Daly, N.L.; Rosengren, K.J.; Craik, D.J. Discovery, structure and biological activities of cyclotides. Adv. Drug Deliv. Rev. 2009, 61, 918–930. [Google Scholar] [CrossRef] [PubMed]
- Craik, D.J.; Swedberg, J.E.; Mylne, J.S.; Cemazar, M. Cyclotides as a basis for drug design. Expert. Opin. Drug Discov. 2012, 7, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Tam, J.P.; Lu, Y.A.; Yang, J.L.; Chiu, K.W. An unusual structural motif of antimicrobial peptides containing end-to-end macrocycle and cystine-knot disulfides. Proc. Natl. Acad. Sci. USA 1999, 96, 8913–8918. [Google Scholar] [CrossRef] [PubMed]
- Gustafson, K.R.; McKee, T.C.; Bokesch, H.R. Anti-HIV cyclotides. Curr. Protein Pept. Sci. 2004, 5, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Goransson, U.; Svangard, E.; Claeson, P.; Bohlin, L. Novel strategies for isolation and characterization of cyclotides: The discovery of bioactive macrocyclic plant polypeptides in the Violaceae. Curr. Protein Pept. Sci. 2004, 5, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Barbeta, B.L.; Marshall, A.T.; Gillon, A.D.; Craik, D.J.; Anderson, M.A. Plant cyclotides disrupt epithelial cells in the midgut of lepidopteran larvae. Proc. Natl. Acad. Sci. USA 2008, 105, 1221–1225. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; Daly, N.L.; Craik, D.J. Structural plasticity of the cyclic-cystine-knot framework: implications for biological activity and drug design. Biochem. J. 2006, 394, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, S.; Foley, F.M.; Clark, R.J.; Sando, L.; Fabri, L.J.; Craik, D.J.; Daly, N.L. Engineering stabilized vascular endothelial growth factor-A antagonists: Synthesis, structural characterization, and bioactivity of grafted analogues of cyclotides. J. Med. Chem. 2008, 51, 7697–7704. [Google Scholar] [CrossRef] [PubMed]
- Thongyoo, P.; Roque-Rosell, N.; Leatherbarrow, R.J.; Tate, E.W. Chemical and biomimetic total syntheses of natural and engineered MCoTI cyclotides. Org. Biomol. Chem. 2008, 6, 1462–1470. [Google Scholar] [CrossRef] [PubMed]
- Terlau, H.; Olivera, B.M. Conus venoms: A rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004, 84, 41–68. [Google Scholar] [CrossRef] [PubMed]
- Miljanich, G.P. Ziconotide: Neuronal calcium channel blocker for treating severe chronic pain. Curr. Med. Chem. 2004, 11, 3029–3040. [Google Scholar] [CrossRef] [PubMed]
- Olivera, B.M.; Cruz, L.J.; de Santos, V.; LeCheminant, G.W.; Griffin, D.; Zeikus, R.; McIntosh, J.M.; Galyean, R.; Varga, J.; Gray, W.R.; et al. Neuronal calcium channel antagonists. Discrimination between calcium channel subtypes using omega-conotoxin from Conus magus venom. Biochemistry 1987, 26, 2086–2090. [Google Scholar]
- Lee, S.; Kim, Y.; Back, S.K.; Choi, H.W.; Lee, J.Y.; Jung, H.H.; Ryu, J.H.; Suh, H.W.; Na, H.S.; Kim, H.J.; et al. Analgesic effect of highly reversible omega-conotoxin FVIA on N type Ca2+ channels. Mol. Pain 2010, 6, 97. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H. Pharmacokinetics of biotech drugs: Peptides, proteins and monoclonal antibodies. Curr. Drug Metab. 2009, 10, 661–691. [Google Scholar] [CrossRef] [PubMed]
- Haines, D.J.; Swan, C.H.; Green, J.R.; Woodley, J.F. Mucosal peptide hydrolase and brush-border marker enzyme activities in three regions of the small intestine of rats with experimental uraemia. Clin. Sci. 1990, 79, 663–668. [Google Scholar] [PubMed]
- Woodley, J.F. Enzymatic barriers for GI peptide and protein delivery. Crit. Rev. Ther. Drug Carr. Syst. 1994, 11, 61–95. [Google Scholar]
- Reichert, J.M. Monoclonal Antibodies as Innovative Therapeutics. Curr. Pharm. Biotechnol. 2008, 9, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; Fischer, H.; Dempster, L.; Daly, N.L.; Rosengren, K.J.; Nevin, S.T.; Meunier, F.A.; Adams, D.J.; Craik, D.J. Engineering stable peptide toxins by means of backbone cyclization: Stabilization of the alpha-conotoxin MII. Proc. Natl. Acad. Sci. USA 2005, 102, 13767–13772. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.J.; Jensen, J.; Nevin, S.T.; Callaghan, B.P.; Adams, D.J.; Craik, D.J. The engineering of an orally active conotoxin for the treatment of neuropathic pain. Angew. Chem. Int. Ed. 2010, 49, 6545–6548. [Google Scholar] [CrossRef]
- Lovelace, E.S.; Armishaw, C.J.; Colgrave, M.L.; Wahlstrom, M.E.; Alewood, P.F.; Daly, N.L.; Craik, D.J. Cyclic MrIA: A stable and potent cyclic conotoxin with a novel topological fold that targets the norepinephrine transporter. J. Med. Chem. 2006, 49, 6561–6568. [Google Scholar] [CrossRef] [PubMed]
- Deechongkit, S.; Kelly, J.W. The effect of backbone cyclization on the thermodynamics of beta-sheet unfolding: Stability optimization of the PIN WW domain. J. Am. Chem. Soc. 2002, 124, 4980–4986. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.J. The structural aspects of limited proteolysis of native proteins. Biochim. Biophys. Acta 1998, 1382, 191–206. [Google Scholar] [CrossRef] [PubMed]
- Quik, M.; Polonskaya, Y.; Kulak, J.M.; McIntosh, J.M. Vulnerability of 125I-alpha-conotoxin MII binding sites to nigrostriatal damage in monkey. J. Neurosci. 2001, 21, 5494–5500. [Google Scholar] [PubMed]
- Hackeng, T.M.; Fernandez, J.A.; Dawson, P.E.; Kent, S.B.; Griffin, J.H. Chemical synthesis and spontaneous folding of a multidomain protein: anticoagulant microprotein S. Proc. Natl. Acad. Sci. USA 2000, 97, 14074–14078. [Google Scholar] [CrossRef] [PubMed]
- Clark-Lewis, I.; Dewald, B.; Loetscher, M.; Moser, B.; Baggiolini, M. Structural requirements for interleukin-8 function identified by design of analogs and CXC chemokine hybrids. J. Biol. Chem. 1994, 269, 16075–16081. [Google Scholar]
- Kimmerlin, T.; Seebach, D. “100 years of peptide synthesis”: Ligation methods for peptide and protein synthesis with applications to beta-peptide assemblies. J. Pept. Res. 2005, 65, 229–260. [Google Scholar] [CrossRef] [PubMed]
- Clayton, D.; Shapovalov, G.; Maurer, J.A.; Dougherty, D.A.; Lester, H.A.; Kochendoerfer, G.G. Total chemical synthesis and electrophysiological characterization of mechanosensitive channels from Escherichia coli and Mycobacterium tuberculosis. Proc. Natl. Acad. Sci. USA 2004, 101, 4764–4769. [Google Scholar] [CrossRef] [PubMed]
- Sukharev, S.I.; Blount, P.; Martinac, B.; Blattner, F.R.; Kung, C. A large-conductance mechanosensitive channel in E. coli encoded by mscL alone. Nature 1994, 368, 265–268. [Google Scholar]
- Cole, S.T.; Brosch, R.; Parkhill, J.; Garnier, T.; Churcher, C.; Harris, D.; Gordon, S.V.; Eiglmeier, K.; Gas, S.; Barry, C.E.; et al. Deciphering the biology of Mycobacterium tuberculosis from the complete genome sequence. Nature 1998, 393, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.H.; Graham, L.A.; Campbell, R.L.; Davies, P.L. Structural modeling of snow flea antifreeze protein. Biophys. J. 2007, 92, 1717–1723. [Google Scholar] [CrossRef] [PubMed]
- Pentelute, B.L.; Gates, Z.P.; Tereshko, V.; Dashnau, J.L.; Vanderkooi, J.M.; Kossiakoff, A.A.; Kent, S.B. X-ray structure of snow flea antifreeze protein determined by racemic crystallization of synthetic protein enantiomers. J. Am. Chem. Soc. 2008, 130, 9695–9701. [Google Scholar] [CrossRef] [PubMed]
- Pentelute, B.L.; Gates, Z.P.; Dashnau, J.L.; Vanderkooi, J.M.; Kent, S.B. Mirror image forms of snow flea antifreeze protein prepared by total chemical synthesis have identical antifreeze activities. J. Am. Chem. Soc. 2008, 130, 9702–9707. [Google Scholar] [CrossRef] [PubMed]
- Bingham, J.P.; Chun, J.B.; Ruzicka, M.R.; Li, Q.X.; Tan, Z.Y.; Kaulin, Y.A.; Englebretsen, D.R.; Moczydlowski, E.G. Synthesis of an iberiotoxin derivative by chemical ligation: a method for improved yields of cysteine-rich scorpion toxin peptides. Peptides 2009, 30, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Valiyaveetil, F.I.; MacKinnon, R.; Muir, T.W. Semisynthesis and Folding of the Potassium Channel KcsA. J. Am. Chem. Soc. 2002, 124, 9113–9120. [Google Scholar] [CrossRef] [PubMed]
- Morrison, K.L.; Weiss, G.A. Combinatorial alanine-scanning. Curr. Opin. Chem. Biol. 2001, 5, 302–307. [Google Scholar] [CrossRef] [PubMed]
- Weiss, G.A.; Watanabe, C.K.; Zhong, A.; Goddard, A.; Sidhu, S.S. Rapid mapping of protein functional epitopes by combinatorial alanine scanning. Proc. Nat. Acad. Sci. USA 2000, 97, 8950–8954. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, B.C.; Wells, J.A. High-resolution epitope mapping of hGH-receptor interactions by alanine-scanning mutagenesis. Science 1989, 244, 1081–1085. [Google Scholar] [CrossRef]
- Matthews, B.W. Structural and genetic analysis of the folding and function of T4 lysozyme. FASEB J. 1996, 10, 35–41. [Google Scholar]
- Gregoret, L.M.; Sauer, R.T. Additivity of mutant effects assessed by binomial mutagenesis. Proc. Nat. Acad. Sci. USA 1993, 90, 4246–4250. [Google Scholar] [CrossRef] [PubMed]
- Payne, R.J.; Wong, C.H. Advances in chemical ligation strategies for the synthesis of glycopeptides and glycoproteins. Chem. Commun. 2010, 46, 21–43. [Google Scholar] [CrossRef]
- Masania, J.; Li, J.; Smerdon, S.J.; Macmillan, D. Access to phosphoproteins and glycoproteins through semi-synthesis, Native Chemical Ligation and N-->S acyl transfer. Org. Biomol. Chem. 2010, 8, 5113–5119. [Google Scholar] [CrossRef] [PubMed]
- Davis, B.G. Synthesis of glycoproteins. Chem. Rev. 2002, 102, 579–602. [Google Scholar] [CrossRef] [PubMed]
- Pratt, M.R.; Bertozzi, C.R. Synthetic glycopeptides and glycoproteins as tools for biology. Chem. Soc. Rev. 2005, 34, 58–68. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.; Winans, K.A.; Backes, B.J.; Kent, S.B.H.; Ellman, J.A.; Bertozzi, C.R. Fmoc-Based Synthesis of Peptide-αThioesters: Application to the Total Chemical Synthesis of a Glycoprotein by Native Chemical Ligation. J. Am. Chem. Soc. 1999, 121, 11684–11689. [Google Scholar] [CrossRef]
- Backes, B.J.; Ellman, J.A. An Alkanesulfonamide “Safety-Catch” Linker for Solid-Phase Synthesis. J. Org. Chem. 1999, 64, 2322–2330. [Google Scholar] [CrossRef]
- Backes, B.J.; Virgilio, A.A.; Ellman, J.A. Activation Method to Prepare a Highly Reactive Acylsulfonamide “Safety-Catch” Linker for Solid-Phase Synthesis1. J. Am. Chem. Soc. 1996, 118, 3055–3056. [Google Scholar] [CrossRef]
- Marcaurelle, L.A.; Mizoue, L.S.; Wilken, J.; Oldham, L.; Kent, S.B.; Handel, T.M.; Bertozzi, C.R. Chemical synthesis of lymphotactin: a glycosylated chemokine with a C-terminal mucin-like domain. Chemistry 2001, 7, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Hedrick, J.A.; Saylor, V.; Figueroa, D.; Mizoue, L.; Xu, Y.; Menon, S.; Abrams, J.; Handel, T.; Zlotnik, A. Lymphotactin is produced by NK cells and attracts both NK cells and T cells in vivo. J. Immunol. 1997, 158, 1533–1540. [Google Scholar] [PubMed]
- Davis, B.G. Biochemistry. Mimicking posttranslational modifications of proteins. Science 2004, 303, 480–482. [Google Scholar]
- Mezzato, S.; Schaffrath, M.; Unverzagt, C. An orthogonal double-linker resin facilitates the efficient solid-phase synthesis of complex-type N-glycopeptide thioesters suitable for native chemical ligation. Angew. Chem. Int. Ed. 2005, 44, 1650–1654. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tanabe, Y.; Okamoto, R.; Dawson, P.E.; Kajihara, Y. Chemical Synthesis of a Glycoprotein Having an Intact Human Complex-Type Sialyloligosaccharide under the Boc and Fmoc Synthetic Strategies. J. Am. Chem. Soc. 2007, 130, 501–510. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Thapa, P.; Zhang, R.-Y.; Menon, V.; Bingham, J.-P. Native Chemical Ligation: A Boon to Peptide Chemistry. Molecules 2014, 19, 14461-14483. https://doi.org/10.3390/molecules190914461
Thapa P, Zhang R-Y, Menon V, Bingham J-P. Native Chemical Ligation: A Boon to Peptide Chemistry. Molecules. 2014; 19(9):14461-14483. https://doi.org/10.3390/molecules190914461
Chicago/Turabian StyleThapa, Parashar, Rui-Yang Zhang, Vinay Menon, and Jon-Paul Bingham. 2014. "Native Chemical Ligation: A Boon to Peptide Chemistry" Molecules 19, no. 9: 14461-14483. https://doi.org/10.3390/molecules190914461
APA StyleThapa, P., Zhang, R.-Y., Menon, V., & Bingham, J.-P. (2014). Native Chemical Ligation: A Boon to Peptide Chemistry. Molecules, 19(9), 14461-14483. https://doi.org/10.3390/molecules190914461




