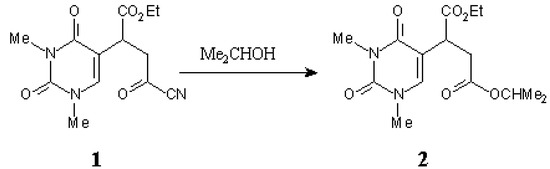
Scheme.
Scheme.
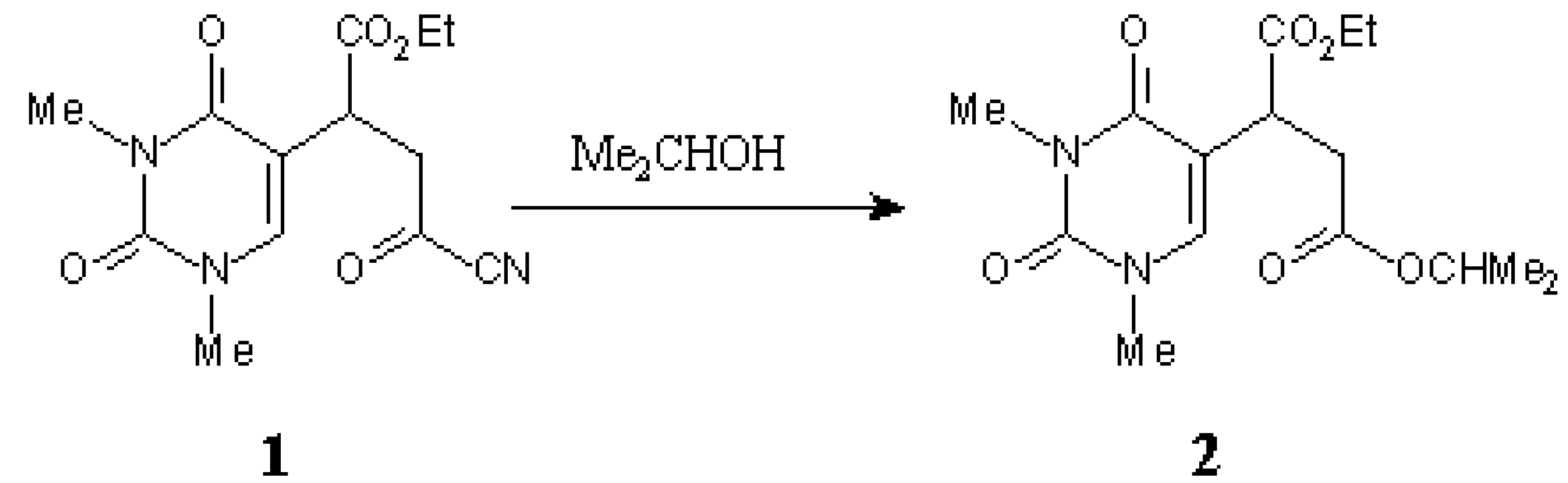
The diester 2 was prepared by the addition of iso-propanol to 1 according to the reported procedure [1].
iso-Propanol (1ml) was added to a solution of 1 (293 mg, 1mmol) in CH2Cl2 (5 ml). The mixture was left at r.t. for 1h. Evaporation of the solvent under reduced pressure afforded the title compound 2, a colourless oil: 325 mg (100 %).
IR (neat): 3070m, 1730vs, 1705vs, 1660vs, 1640vs, 1480s, 1460s, 1370s, 1300-1140br, 1105vs, 1045s, 1020s, 960s, 935s, 925s, 860s, 780s, 755s.
1H-NMR (CDCl3): 7.24 (s, 1H, H-6'); 4.96 (hep, J = 6.5, OCHMe2); 4.20 and 4.14 (2x dq, J = 11.0, 7.1, CO2CH2Me), 3.92 (dd, J = 7.2, 6.8, H-2); 3.39 (s, Me-1'); 3.33 (s, Me-3'); 3.01 (dd, J = 17.1, 6.8, 1H, H-3); 2.73 (dd, J = 17.1, 7.2, 1H, H-3); 1.24 (t, J = 7.1, CO2CH2CH3); 1.22 (d, J = 6.5, 3H, CO2CH(CH3)2); 1.19 (d, J = 6.5, 3H, CO2CH(CH3)2).
13C-NMR (CDCl3): 171.5 (CO2CH2Me), 170.7 (CO2CH(CH3)2), 162.1 (C-4'), 151.1 (C-2'), 141.4 (C-6'), 110.1 (C-5'), 67.8 (CO2CH(CH3)2), 61.0 (CO2CH2Me), 39.6 (C-2), 36.7 (Me-3'), 35.3 (C-3), 27.6 (Me-1'), 21.4 (CO2CH(CH3)2), 13.7 (CO2CH2CH3).
EI-MS: 327 (M+H+, 2), 326 (M+, 8), 281(3), 280 (13), 267 (16), 266 (9), 252 (2), 239 (24), 238 (60), 237 (22), 210 (32), 194 (12), 193 (100), 169 (7), 167 (34), 166 (45), 165 (27), 110 (26), 81 (47), 80 (25), 69 (7), 68 (11), 56 (5).
Supplementary Materials
Supplementary File 1Supplementary File 2Acknowledgment
We thank the Swiss National Foundation for financial support.
References
- Zhuo, J.-C.; Wyler, H. Helv. Chim. Acta 1993, 76, 1916. [CrossRef]
- Sample Availability: Available from MDPI, 0.3g, MDPI 10057.
1997 MDPI. All rights reserved
