Kinugasa Reactions in Water: From Green Chemistry to Bioorthogonal Labelling
Abstract
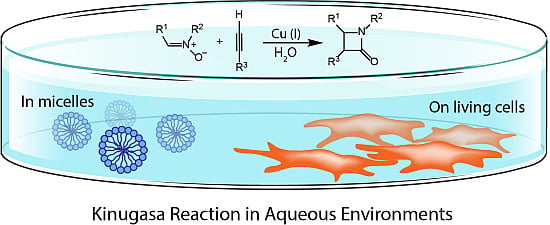
:1. Introduction
2. Factors Affecting Catalysis in the Kinugasa Reaction


2.1. Water as the Solvent

2.2. Micelle-Promoted Kinugasa Reaction

3. Kinugasa Reaction for Bioorthogonal Chemistry
4. Bioorthogonal Labelling Applications

5. Conclusions/Summary
Acknowledgments
Author Contributions
Conflicts of Interest
References
- McKay, C.S.; Moran, J.; Pezacki, J.P. Nitrones as dipoles for rapid strain-promoted 1,3-dipolar cycloadditions with cyclooctynes. Chem. Commun. 2010, 46, 931–933. [Google Scholar] [CrossRef]
- Dommerholt, J.; Schmidt, S.; Temming, R.; Hendriks, L.J.A.; Rutjes, F.P.J.T.; van Hest, J.C.M.; Lefeber, D.J.; Friedl, P.; van Delft, F.L. Readily accessible bicyclononynes for bioorthogonal labeling and three-dimensional imaging of living cells. Angew. Chem. Int. Ed. 2010, 49, 9422–9425. [Google Scholar] [CrossRef]
- Colombo, M.; Sommaruga, S.; Mazzucchelli, S.; Polito, L.; Verderio, P.; Galeffi, P.; Corsi, F.; Tortora, P.; Prosperi, D. Site-specific conjugation of scFvs antibodies to nanoparticles by bioorthogonal strain-promoted alkyne-nitrone cycloaddition. Angew. Chem. Int. Ed. 2012, 51, 496–499. [Google Scholar] [CrossRef]
- McKay, C.S.; Chigrinova, M.; Blake, J.A.; Pezacki, J.P. Kinetics studies of rapid strain-promoted [3+2]-cycloadditions of nitrones with biaryl-aza-cyclooctynone. Org. Biomol. Chem. 2012, 10, 3066–3070. [Google Scholar] [CrossRef] [PubMed]
- Temming, R.P.; Eggermont, L.; van Eldijk, M.B.; van Hest, J.C.M.; van Delft, F.L. N-terminal dual protein functionalization by strain-promoted alkyne-nitrone cycloaddition. Org. Biomol. Chem. 2013, 11, 2772–2779. [Google Scholar] [CrossRef] [PubMed]
- MacKenzie, D.A.; Pezacki, J.P. Kinetics studies of rapid strain-promoted [3+2] cycloadditions of nitrones with bicyclo[6.1.0]nonyne. Can. J. Chem. 2014, 92, 337–340. [Google Scholar] [CrossRef]
- Ding, L.K.; Irwin, W.J. Cis- and trans-azetidin-2-ones from nitrones and copper acetylide. J. Chem. Soc. Perkin Trans. 1 1976, 2382–2386. [Google Scholar] [CrossRef]
- Kinugasa, M.; Hashimoto, S. The reactions of copper(I) phenylacetylide with nitrones. J. Chem. Soc. Chem. Commun. 1972, 466–467. [Google Scholar] [CrossRef]
- Stecko, S.; Furman, B.; Chmielewski, M. Kinugasa reaction: An ‘ugly duckling’ of β-lactam chemistry. Tetrahedron 2014, 70, 7817–7844. [Google Scholar] [CrossRef]
- Alcaide, B.; Almendros, P.; Luna, A. Novel achievements with an old metal: Copper-promoted synthesis of four-membered azacycles. RSC Adv. 2014, 4, 1689–1707. [Google Scholar] [CrossRef]
- Khangarot, R.K.; Kaliappan, K.P. Kinugasa reaction: A direct one-pot route to highly functionalized β-lactams. Eur. J. Org. Chem. 2013, 2013, 7664–7677. [Google Scholar] [CrossRef]
- McKay, C.S.; Kennedy, D.C.; Pezacki, J.P. Studies of multicomponent Kinugasa reactions in aqueous media. Tetrahedron Lett. 2009, 50, 1893–1896. [Google Scholar] [CrossRef]
- Sherratt, A.R.; Chigrinova, M.; McKay, C.S.; Beaulieu, L.-P.B.; Rouleau, Y.; Pezacki, J.P. Copper-catalysed cycloaddition reactions of nitrones and alkynes for bioorthogonal labelling of living cells. RSC Adv. 2014, 4, 46966–46969. [Google Scholar] [CrossRef]
- Hang, H.C.; Yu, C.; Kato, D.L.; Bertozzi, C.R. A metabolic labeling approach toward proteomic analysis of mucin-type o-linked glycosylation. Proc. Natl. Acad. Sci. USA 2003, 100, 14846–14851. [Google Scholar] [CrossRef] [PubMed]
- Sletten, E.M.; Bertozzi, C.R. Bioorthogonal chemistry: Fishing for selectivity in a sea of functionality. Angew. Chem. Int. Ed. 2009, 48, 6974–6998. [Google Scholar] [CrossRef]
- Agard, N.J.; Baskin, J.M.; Prescher, J.A.; Lo, A.; Bertozzi, C.R. A comparative study of bioorthogonal reactions with azides. ACS Chem. Biol. 2006, 1, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular consequences of copper complexes used to catalyze bioorthogonal click reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; Lyn, R.K.; Pezacki, J.P. Cellular lipid metabolism is influenced by the coordination environment of copper. J. Am. Chem. Soc. 2009, 131, 2444–2445. [Google Scholar] [CrossRef] [PubMed]
- McKay, C.S.; Finn, M.G. Click chemistry in complex mixtures: Bioorthogonal bioconjugation. Chem. Biol. 2014, 21, 1075–1101. [Google Scholar] [CrossRef] [PubMed]
- Baskin, J.M.; Prescher, J.A.; Laughlin, S.T.; Agard, N.J.; Chang, P.V.; Miller, I.A.; Lo, A.; Codelli, J.A.; Bertozzi, C.R. Copper-free click chemistry for dynamic in vivo imaging. Proc. Natl. Acad. Sci. USA 2007, 104, 16793–16797. [Google Scholar] [CrossRef] [PubMed]
- Ning, X.; Temming, R.P.; Dommerholt, J.; Guo, J.; Ania, D.B.; Debets, M.F.; Wolfert, M.A.; Boons, G.-J.; van Delft, F.L. Protein modification by strain-promoted alkyne-nitrone cycloaddition. Angew. Chem. Int. Ed. 2010, 49, 3065–3068. [Google Scholar] [CrossRef]
- Blackman, M.L.; Royzen, M.; Fox, J.M. Tetrazine ligation: Fast bioconjugation based on inverse-electron-demand Diels-Alder reactivity. J. Am. Chem. Soc. 2008, 130, 13518–13519. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, N.K.; Weissleder, R.; Hilderbrand, S.A. Tetrazine-based cycloadditions: Application to pretargeted live cell imaging. Bioconjugate Chem. 2008, 19, 2297–2299. [Google Scholar] [CrossRef]
- Saxon, E.; Armstrong, J.I.; Bertozzi, C.R. A “traceless” Staudinger ligation for the chemoselective synthesis of amide bonds. Org. Lett. 2000, 2, 2141–2143. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click chemistry: Diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Rostovtsev, V.V.; Green, L.G.; Fokin, V.V.; Sharpless, K.B. A stepwise Huisgen cycloaddition process: Copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002, 41, 2596–2599. [Google Scholar] [CrossRef]
- Chen, Z.; Lin, L.; Wang, M.; Liu, X.; Feng, X. Asymmetric synthesis of trans-β-lactams by a Kinugasa reaction on water. Chem. Eur. J. 2013, 19, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Yi, H.; Zhang, G.; Deng, Y.; Bai, R.; Zhang, H.; Miller, J.T.; Kropf, A.J.; Bunel, E.E.; Lei, A. Direct observation of reduction of Cu(II) to Cu(I) by terminal alkynes. J. Am. Chem. Soc. 2014, 136, 924–926. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Enna, M.; Okuro, K.; Nomura, M. Copper-catalyzed reaction of terminal alkynes with nitrones. Selective synthesis of 1-aza-1-buten-3-yne and 2-azetidinone derivatives. J. Org. Chem. 1995, 60, 4999–5004. [Google Scholar] [CrossRef]
- Ye, M.-C.; Zhou, J.; Huang, Z.-Z.; Tang, Y. Chiral tris(oxazoline)/Cu(II) catalyzed coupling of terminal alkynes and nitrones. Chem. Commun. 2003, 2554–2555. [Google Scholar] [CrossRef]
- Basak, A.; Chandra, K.; Pal, R.; Ghosh, S.C. Kinugasa reaction under click chemistry conditions. Synlett 2007, 2007, 1585–1588. [Google Scholar] [CrossRef]
- Tra, V.N.; Dube, D.H. Glycans in pathogenic bacteria - potential for targeted covalent therapeutics and imaging agents. Chem. Commun. 2014, 50, 4659–4673. [Google Scholar] [CrossRef]
- Grammel, M.; Hang, H.C. Chemical reporters for biological discovery. Nat. Chem. Biol. 2013, 9. [Google Scholar] [CrossRef] [PubMed]
- Maes, J.; Verlooy, L.; Buenafe, O.E.; de Witte, P.A.M.; Esguerra, C.V.; Crawford, A.D. Evaluation of 14 organic solvents and carriers for screening applications in zebrafish embryos and larvae. PLoS ONE 2012, 7, e43850. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.H.; Kavanagh, C.; Chang, C.C.; Trosko, J.E. Inhibition of metabolic cooperation in Chinese hamster V79 cells by various organic solvents and simple compounds. Cell Biol. Toxicol. 1984, 1, 155–171. [Google Scholar] [CrossRef] [PubMed]
- Torres, S.; Pandey, A.; Castro, G.R. Organic solvent adaptation of gram positive bacteria: Applications and biotechnological potentials. Biotechnol. Adv. 2011, 29, 442–452. [Google Scholar] [CrossRef] [PubMed]
- Zlatopolskiy, B.D.; Krapf, P.; Richarz, R.; Frauendorf, H.; Mottaghy, F.M.; Neumaier, B. Synthesis of 18F-labelled β-lactams by using the Kinugasa reaction. Chem. Eur. J. 2014, 20, 4697–4703. [Google Scholar] [CrossRef] [PubMed]
- Ledin, P.A.; Kolishetti, N.; Boons, G.-J. Multifunctionalization of polymers by strain-promoted cycloadditions. Macromolecules 2013, 46, 7759–7768. [Google Scholar] [CrossRef] [PubMed]
- Dumont, A.; Malleron, A.; Awwad, M.; Dukan, S.; Vauzeilles, B. Click-mediated labeling of bacterial membranes through metabolic modification of the lipopolysaccharide inner core. Angew. Chem. Int. Ed. 2012, 51, 3143–3146. [Google Scholar] [CrossRef]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chigrinova, M.; MacKenzie, D.A.; Sherratt, A.R.; Cheung, L.L.W.; Pezacki, J.P. Kinugasa Reactions in Water: From Green Chemistry to Bioorthogonal Labelling. Molecules 2015, 20, 6959-6969. https://doi.org/10.3390/molecules20046959
Chigrinova M, MacKenzie DA, Sherratt AR, Cheung LLW, Pezacki JP. Kinugasa Reactions in Water: From Green Chemistry to Bioorthogonal Labelling. Molecules. 2015; 20(4):6959-6969. https://doi.org/10.3390/molecules20046959
Chicago/Turabian StyleChigrinova, Mariya, Douglas A. MacKenzie, Allison R. Sherratt, Lawrence L. W. Cheung, and John Paul Pezacki. 2015. "Kinugasa Reactions in Water: From Green Chemistry to Bioorthogonal Labelling" Molecules 20, no. 4: 6959-6969. https://doi.org/10.3390/molecules20046959
APA StyleChigrinova, M., MacKenzie, D. A., Sherratt, A. R., Cheung, L. L. W., & Pezacki, J. P. (2015). Kinugasa Reactions in Water: From Green Chemistry to Bioorthogonal Labelling. Molecules, 20(4), 6959-6969. https://doi.org/10.3390/molecules20046959