Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace
Abstract
:1. Introduction
2. Results and Discussion
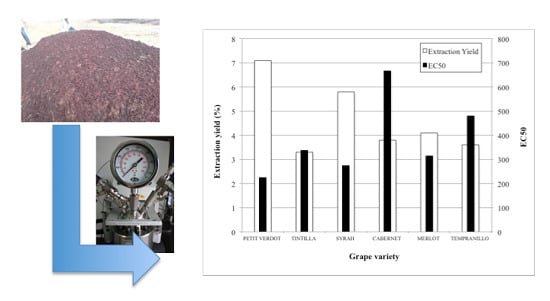
2.1. Variety Selection

| Variety | SFE CO2 + 20% EtOH a | PLE Ethanol b | ||
|---|---|---|---|---|
| AY c | PY d | AY | PY | |
| Petit Verdot | 0.3 ± 0.1 | 2.5 ± 0.1 | 16.0 ± 1.0 | 28.9 ± 1.3 |
| Tintilla | 3.8 ± 0.1 | 4.5 ± 0.1 | 49.7 ± 2.8 | 15.5 ± 0.2 |
| Syrah | 3.2 ± 0.3 | 3.6 ± 0.2 | 38.3 ± 0.6 | 24.4 ± 0.2 |
| Cabernet | 0.1 ± 0.1 | 2.3 ± 0.3 | 11.1 ± 1.2 | 23.8 ± 0.1 |
| Merlot | 0.2 ± 0.1 | 2.1 ± 0.1 | 10.1 ± 0.1 | 22.4 ± 0.1 |
| Tempranillo | 2.0 ± 0.2 | 2.2 ± 0.1 | 30.9 ± 1.0 | 19.6 ± 0.1 |

| Variety | SFE CO2 + 20% EtOH a | PLE Ethanol b | ||
|---|---|---|---|---|
| TAC c | TPC d | TAC | TPC | |
| Petit Verdot | 4.9 ± 0.3 | 34.5 ± 0.4 | 113.8 ± 7.1 | 204.9 ± 9.4 |
| Tintilla | 116.1 ± 2.0 | 135.7 ± 0.8 | 741.9 ± 41.7 | 231.6 ± 3.5 |
| Syrah | 55.1 ± 4.6 | 62.7 ± 0.7 | 292.5 ± 4.3 | 186.3 ± 1.3 |
| Cabernet | 3.5 ± 0.1 | 59.7 ± 1.8 | 107.0 ± 11.3 | 228.4 ± 0.9 |
| Merlot | 4.5 ± 0.2 | 52.2 ± 1.1 | 114.6 ± 0.2 | 254.6 ± 1.0 |
| Tempranillo | 56.8 ± 1.3 | 59.0 ± 0.9 | 278.3 ± 9.3 | 177.1 ± 1.3 |
2.2. Extraction Conditions
2.2.1. Solvent System

2.2.2. Influence of Extraction Parameters


2.2.3. Extraction Kinetics

3. Experimental Section
3.1. Samples and Chemicals
3.2. Extraction Methods
3.3. Total Phenolic Content
3.4. Anthocyanins Analysis
3.5. Antioxidant Assay with DPPH
3.6. Experimental Design
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castellucci, F. World Vitiviniculture situation in 2012. In Proceedings of the XXXVIth World Congress of Vine and Wine, Bucarest, Romanni, 2–7 June 2013; International Organisation of Vine and Wine: Paris, Francia, 2013. [Google Scholar]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural bioactive compounds from winery by-products as health promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef] [PubMed]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of Grape Waste Alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Jourdes, M.; Femenia, A.; Simal, S.; Rosselló, C.; Teissedre, P.L. Characterization of polyphenols and antioxidant potential of white grape pomace byproducts (Vitis vinifera L.). J. Agric. Food Chem. 2013, 61, 11579–11587. [Google Scholar] [CrossRef] [PubMed]
- Peralbo-Molina, A.; Priego-Capote, F.; Luque de Castro, M.D. Comparison of extraction methods for exploitation of grape skin residues from ethanol distillation. Talanta 2012, 101, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Chafer, A.; Pascual-Martí, M.C.; Salvador, A.; Berna, A. Supercritical fluid extraction and HPLC determination of relevant polyphenolic compounds in grape skin. J. Sep. Sci. 2005, 28, 2050–2056. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Lorrain, B.; Kolbas, N.; Crozier, A.; Teissedre, P.L. Wine by-products: Phenolic characterization and antioxidant activity evaluation of grapes and grape pomaces from six different French grape varieties. Molecules 2014, 19, 482–506. [Google Scholar] [CrossRef] [PubMed]
- Qingyong, L.; Wai, C.M. Supercritical fluid extraction in herbal and natural product studies—A practical review. Talanta 2001, 53, 771–782. [Google Scholar]
- Henning, J.; Core, R.; Gardea-Torresdey, J. Extracting volatile compounds from single plants using supercritical fluid extraction. Crop Sci. 1994, 34, 1120–1125. [Google Scholar] [CrossRef]
- Tena, M.T.; Ríos, A.; Valcárcel, M. Supercritical fluid extraction of t-resveratrol and other phenolics from a spiked solid. Fresenius J. Anal. Chem. 1998, 361, 143–148. [Google Scholar] [CrossRef]
- Pascual-Marti, M.C.; Salvador, A.; Chafer, A.; Berna, A. Supercritical fluid extraction of resveratrol from grape skin of Vitis vinifera and determination by HPLC. Talanta 2001, 54, 735–740. [Google Scholar] [CrossRef]
- Pinelo, M.; Ruiz-Rodríguez, A.; Sineiro, J.; Señorans, F.J.; Reglero, G.; Núñez, M.J. Supercritical fluid and solid-liquid extraction of phenolic antioxidants from grape pomace: a comparative study. Eur. Food Res. Technol. 2007, 226, 199–205. [Google Scholar] [CrossRef]
- Massias, A.; Boisard, S.; Baccaunaud, M.; Leal Calderon, F.; Subra-Paternault, P. Recovery of phenolics from apple peels using CO2 + ethanol extraction: kinetics and antioxidant activity of extracts. J. Supercrit. Fluids 2015, 98, 172–182. [Google Scholar] [CrossRef] [Green Version]
- Jaime, L.; Rodríguez-Meizoso, I.; Cifuentes, A.; Santoyo, S.; Suarez, S.; Ibáñez, E.; Señorans, F.J. Pressurized liquids as an alternative process to antioxidant carotenoids’ extraction from Haematococcus pluvialis microalgae. LWT—Food Sci. Technol. 2010, 43, 105–112. [Google Scholar] [CrossRef] [Green Version]
- Piñeiro, Z.; Palma, M.; Barroso, C.G. Determination of catechins by means of extraction with pressurized liquids. J. Chromatogr. A 2004, 1026, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Casazza, A.A.; Aliakbarian, B.; Sannita, E.; Perego, P. High-pressure high-temperature extraction of phenolic compounds from grape skins. Int. J. Food Sci. Technol. 2012, 47, 399–405. [Google Scholar] [CrossRef]
- Štavíková, L.; Polovka, M.; Hohnová, B.; Karásek, P.; Roth, M. Antioxidant activity of grape skin aqueous extracts from pressurized hot water extraction combined with electron paramagnetic resonance spectroscopy. Talanta 2011, 85, 2233–2240. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.Y.; Howard, L.R. Subcritical Water and Sulfured Water Extraction of Anthocyanins and Other Phenolics from Dried Red Grape Skin. J. Food Sci. 2005, 70, S270–S276. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; López, E.; Martínez de la Ossa, E.J. Industrial design of multifunctional supercritical extraction plant for agro-food raw materials. Chem. Eng. Trans. 2009, 17, 1585–1590. [Google Scholar]
- Santos, D.T.; Veggi, P.C.; Meireles, M.A.A. Optimization and economic evaluation of pressurized liquid extraction of phenolic compounds from jabuticaba skins. J. Food Eng. 2012, 108, 444–452. [Google Scholar] [CrossRef]
- De Campos, L.M.A.S.; Leimann, F.V.; Pedrosa, R.C.; Ferreira, S.R.C. Free radical scavenging of grape pomace extracts from Cabernet sauvingnon (Vitis vinifera). Bioresour. Technol. 2008, 99, 8413–8420. [Google Scholar] [CrossRef] [PubMed]
- Tünde, V.; Mojca, S.; Željko, K. Extraction of phenolic compounds from elder berry and different grape marc varieties using organic solvents and/or supercritical carbon dioxide. J. Food Eng. 2009, 90, 246–254. [Google Scholar]
- Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E. A screening analysis of the high-pressure extraction of anthocyanins from red grape pomace with carbon dioxide and co-solvent. Eng. Life Sci. 2003, 3, 38–42. [Google Scholar] [CrossRef]
- Casas, L.; Mantell, C.; Rodríguez, M.; Martínez de la Ossa, E.; Roldán, A.; De Ory, I.; Caro, I.; Blandino, A. Extraction of resveratrol from the pomace of Palomino fino grapes by supercritical carbon dioxide. J. Food Eng. 2010, 96, 304–308. [Google Scholar] [CrossRef]
- Fiori, L.; de Faveri, D.; Casazza, A.A.; Perego, P. Grape by-products: Extraction of polyphenolic compounds using supercritical CO2 and liquid organic solvent—a preliminary investigation. CyTA-J. Food 2009, 7, 163–171. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.; Casas, L.; Mantell, C.; Rodríguez, M. Martinez de la Ossa, E. Extraction of antioxidant compounds from different varieties of Mangifera indica leaves using green technologies. J. Supercrit. Fluids 2012, 72, 168–175. [Google Scholar] [CrossRef]
- Luque-Rodríguez, J.M.; Luque de Castro, M.D.; Pérez-Juan, P. Dynamic superheated liquid extraction of anthocyanins and other phenolics from red grape skins of winemaking residues. Bioresour. Technol. 2007, 98, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Bozan, B.; Tosun, G.; Özcan, D. Study of polyphenol content in the seeds of red grape (Vitis vinifera L.) varieties cultivated in Turkey and their antiradical activity. Food Chem. 2008, 109, 426–430. [Google Scholar] [CrossRef] [PubMed]
- Tounsi, M.S.; Ouerghemmi, I.; Wannes, W.A.; Ksouri, R.; Zemni, H.; Marzouk, B.; Kchouk, M.E. Valorization of three varieties of grape. Ind. Crops Prod. 2009, 30, 292–296. [Google Scholar] [CrossRef]
- He, L.; Zhang, X.; Xu, H.; Xu, C.; Yuan, F.; Knez, Ž.; Novak, Z.; Gaoa, Y. Subcritical water extraction of phenolic compounds from pomegranate (Punica granatum L.) seed residues and investigation into their antioxidant activities with HPLC–ABTS•+ assay. Food Bioprod. Process 2012, 90, 215–223. [Google Scholar] [CrossRef]
- Fernández-Ponce, M.; Casas, L.; Mantell, C.; Martínez de la Ossa, E. Use of high pressure techniques to produce Mangifera indica L. leaf extracts enriched in potent antioxidant phenolic compounds. Innov. Food Sci. Emerg. Technol. 2015, 29, 94–106. [Google Scholar] [CrossRef]
- EFSA. Scientific opinion on the evaluation of the substances currently on the list in the Annex to Commission Directive 96/3/EC as acceptable previous cargoes for edible fats and oils—Part I of III. EFSA J. 2011, 9, 2482. [CrossRef]
- Toxicological evaluation of some extraction solvents and certain other substances. In Proceedings of the FAO Nutrition Meetings Report Series 48A, 24 June–2 July 1970; Joint FAO/WHO Expert Committee on Food Additives: Geneva, Switzerland.
- Aliakbarian, B.; Fathi, A.; Perego, P.; Dehghani, F. Extraction of antioxidants from winery wastes using subcritical water. J. Supercrit. Fluids 2012, 65, 18–24. [Google Scholar] [CrossRef]
- Mustafa, A.; Turner, C. Pressurized liquid extraction as a green approach in food and herbal plants extraction: A review. Anal. Chim. Acta 2011, 703, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Monrad, J.K.; Howard, L.R.; King, J.W.; Srinivas, K.; Mauromoustakos, A. Subcritical solvent extraction of anthocyanins from dried red grape pomace. J. Agric. Food Chem. 2010, 58, 2862–2868. [Google Scholar] [CrossRef] [PubMed]
- Richter, B.E.; Jones, B.A.; Ezzell, J.L.; Porter, N.L.; Avdalovic, N.; Pohl, C. Accelerated solvent extraction: A technique for sample preparation. Anal. Chem. 1996, 68, 1033–1039. [Google Scholar]
- Fernández-González, V.; Concha-Graña, E.; Muniategui-Lorenzo, S.; López-Mahía, P.; Prada-Rodríguez, D. Pressurized hot water extraction coupled to solid-phase microextraction-gas chromatography-mass spectrometry for the analysis of polycyclic aromatic hydrocarbons in sediments. J. Chromatogr. A 2008, 1196–1197, 65–72. [Google Scholar] [PubMed]
- Moreno, E.; Reza, J.; Trejo, A. Extraction of polycyclic aromatic hydrocarbons from soil using water under subcritical conditions. Polycycl. Aromat. Compd. 2007, 27, 239–260. [Google Scholar] [CrossRef]
- Hawthorne, S.B.; Miller, D.J. Direct comparison of Soxhlet and low- and high-temperature supercritical CO2 extraction efficiencies of organics from environmental solids. Anal. Chem. 1994, 66, 4005–4012. [Google Scholar] [CrossRef]
- El Marsni, Z.; Casas, L.; Mantell, C.; Rodríguez, M.; Torres, A.; Macías, F.A.; Martínez de la Ossa, E.J. Allelophatic properties of the fractions obtained from sunflower leaves using supercritical carbon dioxide: The effect of co-solvent addition. J. Supercrit. Fluids 2013, 82, 221–229. [Google Scholar] [CrossRef]
- Carrera, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G. Ultrasound assisted extraction of phenolic compounds from grapes. Anal. Chim. Acta 2012, 732, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Cho, M.J.; Howard, L.R.; Prior, R.L.; Clark, J.R. Flavonoid glycosides and antioxidant capacity of various blackberry, blueberry and red grape genotypes determined by high-performance liquid chromatography/mass spectrometry. J. Sci. Food Agric. 2004, 84, 1771–1782. [Google Scholar] [CrossRef]
- Casas Cardoso, L.; Mantell Serrano, C.; Torrez Quintero, E.; Pereyra López, C.; Medrano Antezana, R.; Martínez de la Ossa, E.J. High Pressure Extraction of Antioxidants from Solanum stenotomun Peel. Molecules 2013, 18, 3137–3151. [Google Scholar] [CrossRef] [PubMed]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Scherer, R.; Godoy, H.T. Antioxidant activity index (AAI) by the 2,2-diphenyl-1-picrylhydrazyl method. Food Chem. 2009, 112, 654–658. [Google Scholar] [CrossRef]
- Calero-Rubio, C.; Stashenko, E.; René Martínez, J.; López-Giraldo, LJ. Formulation of a new generic density-based model for modeling solubility of polyphenols in supercritical carbon dioxide and ethanol. J. Supercrit. Fluids 2014, 85, 116–122. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds extracted are available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; Ossa, E.J.M.d.l. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686-9702. https://doi.org/10.3390/molecules20069686
Otero-Pareja MJ, Casas L, Fernández-Ponce MT, Mantell C, Ossa EJMdl. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules. 2015; 20(6):9686-9702. https://doi.org/10.3390/molecules20069686
Chicago/Turabian StyleOtero-Pareja, María José, Lourdes Casas, María Teresa Fernández-Ponce, Casimiro Mantell, and Enrique J. Martínez de la Ossa. 2015. "Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace" Molecules 20, no. 6: 9686-9702. https://doi.org/10.3390/molecules20069686