Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant
Abstract
:1. Introduction
2. Results and Discussion
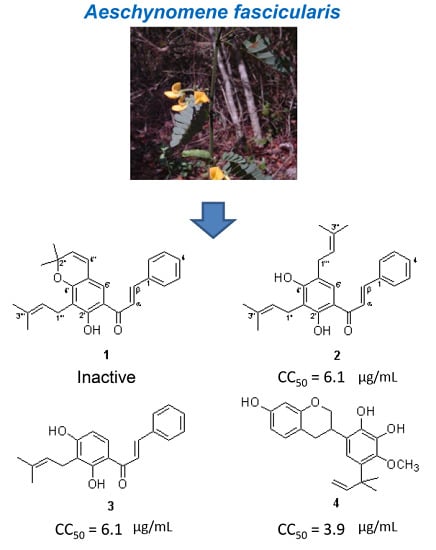
2.1. Identification of Compounds 1–4

| Position | Spinochalcone C (1) | Spinochalcone A (2) | ||
|---|---|---|---|---|
| δH (J = Hz) | δC | δH (J = Hz) | δC | |
| 1 | 135.0 | 135.2 | ||
| 2 | 7.65 m | 128.6 | 7.62 m | 128.5 |
| 3 | 7.43 m | 129.0 | 7.40 m | 129.0 |
| 4 | 7.43 m | 130.6 | 7.40 m | 130.6 |
| 5 | 7.43 m | 129.0 | 7.40 m | 129.0 |
| 6 | 7.65 m | 128.6 | 7.62 m | 128.5 |
| A | 7.57 d (15.4) | 120.6 | 7.56 d (15.4) | 120.7 |
| B | 7.86 d (15.4) | 144.0 | 7.86 d (15.4) | 144.0 |
| 1′ | 113.8 | 113.7 | ||
| 2′ | 164.4 | 162.6 | ||
| 3′ | 117.1 | 114.5 | ||
| 4′ | 158.0 | 160.4 | ||
| 5′ | 113.2 | 119.0 | ||
| 6′ | 7.40 s | 125.2 | 7.55 s | 128.6 |
| 1′′ | 3.46 d (7.0) | 22.0 | ||
| 2′′ | 77.8 | 5.30 m | 121.4 | |
| 2′′-(CH3)2 | 1.46 s | 28.6 | ||
| 3′′ | 5.59 d (9.8) | 128.7 | 135.0 | |
| 3′′-(CH3)2 | 1.76 s | 25.9 | ||
| 1.84 s | 18.0 | |||
| 4′′ | 6.32 d (9.8) | 121.7 | ||
| 1′′′ | 3.36 d (7.3) | 21.6 | 3.33 d (7.0) | 29.3 |
| 2′′′ | 5.26 m | 122.0 | 5.30 m | 122.0 |
| 3′′′ | 131.7 | 134.8 | ||
| 3′′′-(CH3)2 | 1.69 s | 25.9 | 1.79 s | 25.9 |
| 1.82 s | 18.0 | 1.80 s | 18.0 | |
| 2′-OH | 13.74 s | 13.63 s | ||
| 4′-OH | 6.31 s | |||
| C=O | 191.9 | 192.1 | ||
2.2. Biological Activities
| Fractions/Compound | Cell Lines CC50 µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|
| MDCK | Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| Crude extract | 150.3 (ND) | 55.3 (ND) | 14.0 (ND) | 16.7 (ND) | 24.0 (ND) | 23.5 (ND) | 50.2 (ND) |
| Hexane fraction | 175.4 (ND) | 33.7 (ND) | 21.5 (ND) | 18.9 (ND) | 30.1 (ND) | 29.1 (ND) | 21.6 (ND) |
| Dichloromethane fraction | - a | - | - | - | - | - | - |
| Ethyl acetate fraction | - | - | - | - | - | - | - |
| Aqueous fraction | - | - | - | - | - | - | - |
| 1 | - | - | - | - | - | - | - |
| 2 | 92.1 (245.0) | - | - | - | - | 6.1 (16.3) | 9.7 (26.0) |
| 3 | 4.2 (14.0) | 6.1 (20.0) | 11.0 (27.0) | - | - | 7.0 (23.1) | 18.4 (60.3) |
| 4 | 29.7 (83.4) | 3.9 (11.0) | 5.3 (15.0) | 8.1 (23.0) | 14.7 (41.3) | 21.5 (60.6) | 16.5 (46.4) |
| Docetaxel | 1.1 (1.4) | 0.07 (0.1) | 0.2 (0.3) | 0.19 (0.25) | 0.17 (0.22) | 0.007 (0.01) | 0.07 (0.1) |
| Compound | Cell Lines SI | |||||
|---|---|---|---|---|---|---|
| Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| Crude extract | 2.7 | 10.7 | 9.0 | 6.3 | 6.3 | 3.0 |
| Hexane fraction | 5.2 | 8.1 | 9.2 | 5.8 | 6.0 | 8.1 |
| Dichloromethane fraction | - | - | - | - | - | - |
| Ethyl acetate fraction | - | - | - | - | - | - |
| Aqueous fraction | - | - | - | - | - | - |
| 1 | - a | - | - | - | - | - |
| 2 | - | - | - | - | 15.0 | 9.4 |
| 3 | 0.7 | 0.5 | - | - | 0.6 | 0.23 |
| 4 | 7.6 | 5.5 | 3.6 | 2.0 | 1.3 | 1.8 |
| Docetaxel | 14.0 | 4.6 | 5.6 | 6.3 | 140 | 14.0 |
| Compound | Cell Lines IC50a µg/mL (µM) | ||||||
|---|---|---|---|---|---|---|---|
| MDCK | Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| 1 | - a | - | - | - | - | - | - |
| 2 | 18.7 (50.0) | - | 6.0 (16.0) | - | - | - | - |
| 3 | 18.4 (60.8) | - | - | - | - | 13.3 (43.8) | - |
| 4 | 25.4 (72.0) | - | - | - | 21.2 (60.0) | - | - |
| Docetaxel | 0.11 (0.14) | 0.05 (0.07) | 0.04 (0.06) | 0.03 (0.04) | 0.07 (0.1) | 0.01 (0.02) | 0.007 (0.01) |
| Compound | Cell Lines IC50 SI | |||||
|---|---|---|---|---|---|---|
| Hep-2 | KB | HeLa | SiHa | DU-145 | PC-3 | |
| 1 | - a | - | - | - | - | - |
| 2 | - | 3.1 | - | - | - | - |
| 3 | - | - | - | - | 1.4 | - |
| 4 | - | - | - | 1.2 | - | - |
| Docetaxel | 2.0 | 2.3 | 3.5 | 1.4 | 7 | 14 |
3. Experimental Section
3.1. General
3.2. Plant Material
3.3. Isolation of Active Compounds
3.4. Cell Culture
3.5. Cytotoxicity Assay
3.6. Antiproliferative Assay
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| λmax | wavelength of maximum absorption |
| νmax | frequency of the maximum height in the infrared spectrum |
| a.m.u. | atomic mass unit |
| COSY | correlation spectroscopy |
| DMSO | dimethyl sulfoxide |
| DEPT | distortionless enhancement by polarization transfer |
| EI-MS | electronic impact mass spectrum |
| FTIR | Fourier transform infrared spectroscopy |
| HSQC | heteronuclear single quantum coherence |
| HMBC | heteronuclear multiple bond correlation |
| NMR | nuclear magnetic resonance |
| OD | optic density |
| TLC | thin-layer chromatography |
References
- Vázquez-Domínguez, E.; Arita, H.T. The Yucatan peninsula: Biogeographical history 65 million years in the making. Ecography 2010, 33, 212–219. [Google Scholar]
- Osadao, R. El Libro del Judío o Medicina Doméstica, Descripción de las Virtudes de las Yerbas Medicinales de Yucatán, 2nd ed.; Dra. Dorothy Andrew de Zapata: Mérida, Mexico, 1834; Volume 1, pp. 20–255. [Google Scholar]
- Flores, J.; Espejel-Carvajal, I. Etnoflora Yucatanense: Tipos de Vegetación de la Península de Yucatán; Universidad Autónoma de Yucatán, Sostenibilidad Maya: Mérida, Yucatán, México, 1994; Fascículo 3. [Google Scholar]
- Flores, J. Etnoflora Yucatanense: Leguminoseae. Florística, Etnobotánica y Ecología; Universidad Autónoma de Yucatán: Mérida, Yucatán, México, 2001; Volume 18. [Google Scholar]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the 30 Years from 1981 to 2010. J. Nat. Prod. 2012, 75, 311–335. [Google Scholar] [CrossRef] [PubMed]
- Caamal-Fuentes, E.; Torres-Tapia, L.W.; Simá-Polanco, P.; Peraza-Sánchez, S.R.; Moo-Puc, R. Screening of plants used in Mayan traditional medicine to treat cancer-like symptoms. J. Ethnopharmacol. 2011, 135, 719–724. [Google Scholar]
- Fullas, F.; Kornberg, L.J.; Wani, M.C.; Wall, M.E.; Farnsworth, N.R.; Chagwedera, T.E.; Kinghorn, A.D. Two new aromatic constituents from the rootwood of Aeschynomene mimosifolia. J. Nat. Prod. 1996, 59, 190–192. [Google Scholar]
- Caamal-Fuentes, E.; Moo-Puc, R.; Torres-Tapia, L.W.; Peraza-Sanchez, S.R. Pterocarpans from the root bark of Aeschynomene fascicularis. Nat. Prod. Commun. 2013, 8, 1421–1422. [Google Scholar]
- Rao, E.V.; Prasad, Y.R. Prenylated flavonoids from Tephrosia spinosa. Phytochemistry 1992, 32, 183–185. [Google Scholar]
- Rao, E.V.; Prasad, Y.R. Two chalcones from Tephrosia spinosa. Phytochemistry 1992, 31, 2121–2122. [Google Scholar]
- De Lima, O.G.; Marini-Bettolo, G.B.; de Méllo, J.F.; delle Monache, F.; de Barros Coélho, J.S.; de Andrade Lyra, F.D.; Fernandes de Alburquerque, M.M. C- and O-prenylated chalcones from Cordoa piaca. Gazz. Chim. Ital. 1973, 103, 771–777. [Google Scholar]
- Borges-Argáez, R.; Peña-Rodríguez, L.M.; Waterman, P. Flavonoids from two Lonchocarpus species of the Yucatan Peninsula. Phytochemistry 2002, 60, 533–540. [Google Scholar]
- Tanaka, T.; Ohyama, M.; Iinuma, M.; Shirataki, Y.; Komatsu, M.; Charles, L.B. Isoflavonoids from Sophora secundiflora, S. arizonica and S. gypsophila. Phytochemistry 1998, 48, 1187–1193. [Google Scholar]
- Borges-Argáez, R.; Balnbury, L.; Flowers, A.; Giménez-Turba, A.; Ruiz, G.; Waterman, P.G.; Peña-Rodríguez, L.M. Cytotoxic and antiprotozoal activity of flavonoids from Lonchocarpus spp. Phytomedicine 2007, 14, 530–533. [Google Scholar]
- Borges-Argáez, R.; Vela-Catzín, T.; Yam-Puc, A.; Chan-Bacab, M.; Moo-Puc, R.; Cáceres-Farfán, M. Antiprotozoal and cytotoxic studies on some isocordoin derivatives. Planta Med. 2009, 75, 1336–1338. [Google Scholar]
- Wibowo, A.; Ahmat, N.; Hamzah, A.S.; Sufian, A.S.; Ismail, N.H. Malaysianol A, a new trimer resveratrol oligomer from the stem bark of Dryobalanops aromatica. Fitoterapia 2011, 82, 676–681. [Google Scholar]
- Vonthron-Sénécheau, C.; Bernard-Weniger, B.; Ouattara, M.; Tra-Bi, F.; Kamenan, A.; Lobstein, A.; Brun, R.; Anton, R. In vitro antiplasmodial activity and cytotoxicity of ethnobotanically selected Ivorian plants. J. Ethnopharmacol. 2003, 87, 221–222. [Google Scholar]
- Alvarez, L.; Rios, M.Y.; Esquivel, C.; Chávez, M.I.; Delgado, G.; Aguilar, M.I.; Villarreal, M.L.; Navarro, V. Cytotoxic isoflavans from Eysenhardtia polystachya. J. Nat. Prod. 1998, 61, 767–770. [Google Scholar]
- Choi, C.W.; Choi, Y.H.; Cha, M.R.; Kim, Y.S.; Yon, G.; Kim, Y.K.; Choi, S.U.; Kim, Y.H.; Ryu, S.Y. Antitumor components isolated from the heartwood extract of Dalbergia odorifera. J. Korean Soc. Appl. Biol. Chem. 2009, 52, 375–379. [Google Scholar]
- Sabzevari, O.; Galati, G.; Moridani, M.Y.; Siraki, A.; O’Brien, P.J. Molecular cytotoxic mechanisms of anticancer hydroxychalcones. Chem. -Biol. Interact. 2004, 148, 57–67. [Google Scholar]
- Tabata, K.; Motani, K.; Takayanagi, N.; Nishimura, R.; Asami, S.; Kimura, Y.; Ukiya, M.; Hasegawa, D.; Akihisa, T.; Suzuki, T. Xanthoangelol, a major chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma and leukemia cells. Biol. Pharm. Bull. 2005, 28, 1404–1407. [Google Scholar]
- Zi, X.; Simoneau, A.R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of Bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005, 65, 3479–3486. [Google Scholar]
- Nishimura, R.; Tabata, K.; Arakawa, M.; Ito, Y.; Kimura, Y.; Akihisa, T.; Nagai, H.; Sakuma, A.; Kohno, H.; Suzuki, T. Isobavachalcone, a chalcone constituent of Angelica keiskei, induces apoptosis in neuroblastoma. Biol. Pharm. Bull. 2007, 30, 1878–1883. [Google Scholar]
- Rahman, A.; Choudhary, M.L.; Thonsen, W.J. Bioassay Techniques for Drug Development; Harwood Academic Publishers: Amsterdam, The Netherlands, 2001; pp. 28–29. [Google Scholar]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New colorimetric cytotoxicity assay for anticancer-drug screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar]
- Mena-Rejon, G.; Caamal-Fuentes, E.; Cantillo-Ciau, Z.; Cedillo-Rivera, R.; Flores-Guido, J.; Moo-Puc, R. In vitro cytotoxic activity of nine plants used in Mayan traditional medicine. J. Ethnopharmacol. 2009, 21, 462–465. [Google Scholar]
- Sample Availability: Samples of the compounds are not available from the authors.
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caamal-Fuentes, E.E.; Peraza-Sánchez, S.R.; Torres-Tapia, L.W.; Moo-Puc, R.E. Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant. Molecules 2015, 20, 13563-13574. https://doi.org/10.3390/molecules200813563
Caamal-Fuentes EE, Peraza-Sánchez SR, Torres-Tapia LW, Moo-Puc RE. Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant. Molecules. 2015; 20(8):13563-13574. https://doi.org/10.3390/molecules200813563
Chicago/Turabian StyleCaamal-Fuentes, Edgar E., Sergio R. Peraza-Sánchez, Luis W. Torres-Tapia, and Rosa E. Moo-Puc. 2015. "Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant" Molecules 20, no. 8: 13563-13574. https://doi.org/10.3390/molecules200813563
APA StyleCaamal-Fuentes, E. E., Peraza-Sánchez, S. R., Torres-Tapia, L. W., & Moo-Puc, R. E. (2015). Isolation and Identification of Cytotoxic Compounds from Aeschynomene fascicularis, a Mayan Medicinal Plant. Molecules, 20(8), 13563-13574. https://doi.org/10.3390/molecules200813563