3.2.3. General Procedure for the Preparation of 9
To a solution of 4a (37 mg, 0.10 mmol) in DCM (1 mL) was added Et2O·BF3 (100 μL, 1 mmol) and Et3SiH (130 μL, 1 mmol). The solution was stirred at room temperature for 4 h. Purification by column chromatography on silica gel (eluting with DCM/EA = 150:1) to give 9 as a white solid.
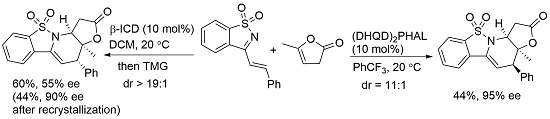
(3aS,4R,11aS)-3a-Methyl-4-phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4a) was obtained in 44% yield; the enantiomeric excess was determined to be 95% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 23.48 min, tminor = 38.66 min. = 71.0 (c = 0.4 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (d, J = 7.8 Hz, 1H), 7.72–7.67 (m, 2H), 7.63–7.59 (m, 1H), 7.35–7.29 (m, 5H), 5.81 (d, J = 4.7 Hz, 1H), 4.55 (t, J = 6.3 Hz, 1H), 3.82 (d, J = 4.7 Hz, 1H), 2.98 (dd, J = 6.1, 5.2 Hz, 2H), 1.61 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.71, 136.38, 133.51, 132.42, 131.07, 130.51, 128.94, 128.44, 128.09, 121.28, 101.14, 83.58, 54.95, 47.71, 36.26, 26.78; ESI HRMS: calcd. for C20H17NO4S + Na+ 390.0776, found 390.0775.
(3aS,4R,11aS)-3a-Methyl-4-(p-tolyl)-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridine-2(11aH)-one 10,10-dioxide (4b) was obtained in 32% yield; the enantiomeric excess was determined to be 96% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 26.39 min, tminor = 40.71 min. = 136.5 (c = 0.8 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.8 Hz, 1H), 7.74–7.67 (m, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.23 (d, J = 7.9 Hz, 2H), 7.15 (d, J = 7.8 Hz, 2H), 5.81 (d, J = 4.8 Hz, 1H), 4.55 (t, J = 6.5 Hz, 1H), 3.80 (d, J = 4.7 Hz, 1H), 2.97 (d, J = 6.3 Hz, 2H), 2.34 (s, 3H), 1.62 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.75, 137.88, 133.49, 133.19, 132.39, 130.93, 130.45, 130.36, 129.14, 128.97, 121.27, 101.29, 83.57, 54.87, 47.33, 36.32, 26.87, 21.07; ESI HRMS: calcd. for C21H19NO4S + Na+ 404.0932, found 404.0929.
(3aS,4R,11aS)-4-(2-Methoxyphenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4c) was obtained in 43% yield; the enantiomeric excess was determined to be 94% by HPLC analysis on Daicel Chiralcel IA column (10% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 33.00 min, tmajor = 41.11 min. = 19.8 (c = 0.44 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (d, J = 7.9 Hz, 1H), 7.68 (t, J = 7.4 Hz, 2H), 7.61 (d, J = 6.6 Hz, 1H), 7.34–7.27 (m, 2H), 6.95 (dd, J = 14.1, 7.7 Hz, 2H), 5.71 (d, J = 4.2 Hz, 1H), 4.60–4.55 (m, 1H), 4.47 (s, 1H), 3.89 (s, 3H), 3.12 (dd, J = 18.1, 5.2 Hz, 1H), 3.01 (dd, J = 18.1, 7.1 Hz, 1H), 1.60 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.87, 157.77, 133.38, 132.39, 130.71, 130.24, 129.29, 129.16, 125.54, 121.17, 120.67, 110.74, 84.44, 55.63, 55.27, 35.93, 26.23; ESI HRMS: calcd. for C21H19NO5S + Na+ 420.0882, found 420.0880.
(3aS,4R,11aS)-4-(3-Methoxyphenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4d) was obtained in 34% yield; the enantiomeric excess was determined to be >99% by HPLC analysis on Daicel Chiralcel IA column (10% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 42.73 min, tmajor = 50.51 min. = 89.9 (c = 0.96 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.9 Hz, 1H), 7.74–7.67 (m, 2H), 7.62 (t, J = 7.4 Hz, 1H), 7.26 (t, J = 3.8 Hz, 1H), 6.93 (d, J = 7.6 Hz, 1H), 6.91–6.84 (m, 2H), 5.80 (d, J = 4.6 Hz, 1H), 4.56 (t, J = 6.2 Hz, 1H), 3.81 (s, 1H), 3.80 (s, 3H), 3.06 (dd, J = 18.1, 5.3 Hz, 1H), 2.98 (dd, J = 18.1, 7.1 Hz, 1H), 1.62 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.69, 159.51, 137.96, 133.47, 132.46, 131.02, 130.48, 129.36, 128.93, 122.87, 121.27, 116.68, 113.11, 101.10, 83.54, 55.00, 47.66, 36.15, 26.77; ESI HRMS: calcd. for C21H19NO5S + Na+ 420.0882, found 420.0883.
(3aS,4R,11aS)-4-(3,5-Dimethoxyphenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4e) was obtained in 31% yield; the enantiomeric excess was determined to be 94% by HPLC analysis on Daicel Chiralcel IA column (10% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 47.47 min, tmajor = 51.77 min. = 116.4 (c = 0.28 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (d, J = 7.8 Hz, 1H), 7.72–7.67 (m, 2H), 7.62 (d, J = 7.3 Hz, 1H), 6.49 (d, J = 1.9 Hz, 2H), 6.42 (s, 1H), 5.79 (d, J = 4.6 Hz, 1H), 4.58–4.54 (m, 1H), 3.78 (s, 6H), 3.75 (d, J = 4.5 Hz, 1H), 3.11 (dd, J = 18.1, 5.2 Hz, 1H), 2.98 (dd, J = 18.1, 7.1 Hz, 1H), 1.62 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.73 (s), 160.60 (s), 138.75 (s), 133.46 (s), 130.96 (s), 130.48 (s), 128.92 (s), 121.27 (d, J = 11.3 Hz), 108.96 (s), 101.09 (s), 99.55 (s), 83.53 (s), 55.41 (s), 55.08 (s), 47.78 (s), 36.03 (s), 26.70 (s) ppm; ESI HRMS: calcd. for C21H21NO6S + Na+ 450.0987, found 450.0984.
(3aS,4R,11aS)-4-(2-Fluorophenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4f) was obtained in 30% yield; the enantiomeric excess was determined to be 89% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 20.02 min, tminor = 33.58 min. = 91.3 (c = 0.88 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (d, J = 7.8 Hz, 1H), 7.69 (q, J = 7.8 Hz, 2H), 7.62 (t, J = 7.2 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H), 7.32 (dd, J = 13.7, 6.9 Hz, 1H), 7.13 (dt, J = 18.2, 8.2 Hz, 2H), 5.68 (d, J = 4.1 Hz, 1H), 4.62–4.57 (m, 1H), 4.28 (d, J = 3.9 Hz, 1H), 3.16 (dd, J = 18.2, 4.5 Hz, 1H), 3.05 (dd, J = 18.2, 6.9 Hz, 1H), 1.62 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.58, 162.01, 160.37, 133.53, 132.43, 131.48, 131.38, 130.56, 129.93, 128.85 124.33, 124.19, 124.10, 121.25, 115.51, 115.36, 100.38, 83.84, 55.16, 39.58, 35.97, 26.08; ESI HRMS: calcd. for C20H16FNO4S + Na+ 408.0682, found 408.0678.
(3aS,4R,11aS)-4-(3-Bromophenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4g) was obtained in 57% yield; the enantiomeric excess was determined to be 87% by HPLC analysis on Daicel Chiralcel IA column (20% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 22.19 min, tminor = 29.23 min. = 72.8 (c = 0.88 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.8 Hz, 1H), 7.72 (t, J = 8.1 Hz, 2H), 7.66–7.61 (m, 1H), 7.51–7.46 (m, 2H), 7.30 (d, J = 7.8 Hz, 1H), 7.24 (t, J = 7.8 Hz, 1H), 5.73 (d, J = 4.2 Hz, 1H), 4.57 (dd, J = 6.6, 4.6 Hz, 1H), 3.78 (d, J = 4.2 Hz, 1H), 3.13 (dd, J = 18.2, 4.4 Hz, 1H), 3.04 (dd, J = 18.2, 6.8 Hz, 1H), 1.60 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.51, 139.05, 133.56, 133.28, 132.50, 131.38 , 131.26, 130.64, 129.96, 129.21, 128.78, 122.50, 121.30, 100.45, 83.41, 55.14, 47.34 , 36.11, 26.33; ESI HRMS: calcd. for C20H16BrNO4S + Na+ 467.9881, found 467.9882.
(3aS,4R,11aS)-4-(4-Bromophenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4h) was obtained in 42% yield; the enantiomeric excess was determined to be >99% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 21.91 min, tminor = 77.19 min. = 100.4 (c = 0.52 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (d, J = 7.8 Hz, 1H), 7.74–7.67 (m, 2H), 7.63 (dd, J = 10.5, 4.0 Hz, 1H), 7.48 (d, J = 8.4 Hz, 2H), 7.23 (d, J = 8.4 Hz, 2H), 5.74 (d, J = 4.4 Hz, 1H), 4.56 (s, 1H), 3.79 (d, J = 4.3 Hz, 1H), 3.02 (t, J = 6.4 Hz, 2H), 1.59 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.51, 135.60, 133.57, 132.47, 132.11, 131.60, 131.36, 130.65, 128.77, 122.26, 121.29, 100.54, 83.38, 55.05, 47.14, 36.19, 26.50; ESI HRMS: calcd. for C20H16BrNO4S + Na+ 467.9881, found 467.9885.
(3aS,4R,11aS)-4-(Furan-2-yl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e] pyridin-2(11aH)-one 10,10- dioxide (4i) was obtained in 29% yield; the enantiomeric excess was determined to be 92% by HPLC analysis on Daicel Chiralcel IA column (20% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 42.11 min, tminor = 58.68 min. = 5.8 (c = 0.12 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.87 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.73–7.69 (m, 1H), 7.64 (dd, J = 11.1, 4.0 Hz, 1H), 7.41 (d, J = 1.0 Hz, 1H), 6.35 (dd, J = 3.1, 1.9 Hz, 1H), 6.28 (d, J = 3.2 Hz, 1H), 5.81 (d, J = 5.7 Hz, 1H), 4.53 (t, J = 8.1 Hz, 1H), 3.97 (d, J = 5.7 Hz, 1H), 2.97 (dd, J = 18.0, 8.2 Hz, 1H), 2.83 (dd, J = 18.0, 8.1 Hz, 1H), 1.69 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.21, 149.97, 142.95, 133.52, 132.33, 130.88, 130.72, 128.72, 121.43, 121.33, 110.75, 110.47, 97.16, 82.67, 53.88, 41.71, 35.48, 27.14; ESI HRMS: calcd. for C18H15NO5S + Na+ 380.0569, found 380.0567.
(3aS,4S,11aS)-3a-Methyl-4-(thiophen-2-yl)-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4j) was obtained in 40% yield; the enantiomeric excess was determined to be >99% by HPLC analysis on Daicel Chiralcel IA column(10% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 49.55 min, tminor = 68.53 min. = 70.5 (c = 0.4 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.87 (d, J = 7.8 Hz, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.72 (t, J = 7.6 Hz, 1H), 7.65 (t, J = 7.6 Hz, 1H), 7.28 (d, J = 5.1 Hz, 1H), 7.04 (d, J = 2.8 Hz, 1H), 7.01–6.99 (m, 1H), 5.93 (d, J = 5.3 Hz, 1H), 4.54 (t, J = 7.7 Hz, 1H), 4.13 (d, J = 5.5 Hz, 1H), 2.94 (dd, J = 17.9, 7.9 Hz, 1H), 2.79 (dd, J = 17.9, 7.3 Hz, 1H), 1.68 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.58, 138.90, 133.56, 132.48, 130.88, 130.76, 128.99, 128.64, 127.22, 126.27, 121.41, 100.21, 82.85, 54.36, 42.84, 36.03, 27.08; ESI HRMS: calcd. for C18H15NO4S2 + Na+ 396.0340, found 396.0338.
(3aS,4R,11aS)-8-Chloro-3a-methyl-4-phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4k) was obtained in 31% yield; and the enantiomeric excess was determined to be 97% by HPLC analysis on Daicel Chiralcel ID column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285nm, tmajor = 16.55 min, tminor = 21.63 min. = 120.5 (c = 0.8 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.83 (s, 1H), 7.65 (s, 2H), 7.38–7.31 (m, 5H), 5.80 (d, J = 4.5 Hz, 1H), 4.58–4.53 (m, 1H), 3.82 (d, J = 4.5 Hz, 1H), 3.01 (dd, J = 11.9, 6.1 Hz, 2H), 1.61 (s, 3H) ppm; 13C-NMR (151 MHz, CDCl3) δ: 172.51, 136.71, 136.23, 133.94, 133.63, 130.46, 130.30, 128.49, 128.17, 127.32, 122.53, 121.41, 101.97, 83.45, 55.14, 47.68, 36.16, 26.58; ESI HRMS: calcd. for C20H16ClNO4S + Na+ 424.0386, found 424.0384.
(3aS,4R,11aS)-8-(tert-Butyl)-3a-methyl-4-phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4l) was obtained in 46% yield; the enantiomeric excess was determined to be 98% by HPLC analysis on Daicel Chiralcel IF column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 9.39 min, tminor = 10.78 min. = 78.5 (c = 0.6 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.85 (s, 1H), 7.73 (d, J = 8.3 Hz, 1H), 7.64 (d, J = 8.3 Hz, 1H), 7.36–7.31 (m, 5H), 5.77 (d, J = 4.8 Hz, 1H), 4.55 (s, 1H), 3.83 (d, J = 4.7 Hz, 1H), 2.96 (d, J = 6.6 Hz, 2H), 1.62 (s, 3H), 1.38 (s, 9H); 13C-NMR (151 MHz, CDCl3) δ: 172.74, 155.05, 136.44, 132.40, 131.32, 131.12, 130.53, 128.40, 128.05, 126.35, 120.98, 117.56, 100.32, 83.65, 54.94, 47.75, 36.30, 35.56, 31.07, 26.95; ESI HRMS: calcd. for C24H25NO4S + Na+ 446.1402, found 446.1400.
(3aR,4R,11aS)-3a,4-Diphenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4m) was obtained in 31% yield; and the enantiomeric excess was determined to be 94% by HPLC analysis on Daicel Chiralcel IF column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 10.98 min, tminor = 12.35 min. = 22.8 (c = 0.36 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.87 (d, J = 7.9 Hz, 1H), 7.75–7.69 (m, 2H), 7.63 (t, J = 7.5 Hz, 1H), 7.38–7.33 (m, 3H), 7.25–7.22 (m, 3H), 7.18 (t, J = 7.4 Hz, 2H), 6.93 (d, J = 7.3 Hz, 2H), 5.84 (d, J = 3.1 Hz, 1H), 5.03 (d, J = 3.8 Hz, 1H), 4.12 (d, J = 2.8 Hz, 1H), 3.39 (dd, J = 17.6, 1.4 Hz, 1H), 2.65 (dd, J = 17.6, 5.0 Hz, 1H); 13C-NMR (151 MHz, CDCl3) δ: 172.97, 139.70, 136.24, 133.54, 132.50, 131.09, 130.38, 130.23, 129.03, 128.85, 128.78, 127.89, 127.67, 125.25, 121.21, 101.61, 86.25, 56.43, 49.05, 35.65; ESI HRMS: calcd. for C25H19NO4S + Na+ 452.0932, found 452.0930.
(4R,11aS)-4-Phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (4n) was obtained in 30% yield; the enantiomeric excess was determined to be 79% by HPLC analysis on Daicel Chiralcel IC column(40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 20.60 min, tmajor = 35.62 min. = −12.8 (c = 0.4 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.84 (d, J = 7.9 Hz, 1H), 7.71 (dd, J = 17.3, 7.6 Hz, 2H), 7.61 (t, J = 7.4 Hz, 1H), 7.37 (td, J = 14.1, 7.1 Hz, 5H), 5.75 (d, J = 2.4 Hz, 1H), 4.95 (t, J = 4.0 Hz, 1H), 4.81 (s, 1H), 4.10 (d, J = 3.6 Hz, 1H), 3.46 (d, J = 17.5 Hz, 1H), 2.86 (dd, J = 17.5, 5.1 Hz, 1H); 13C-NMR (151 MHz, CDCl3) δ: 172.99, 137.45, 133.51, 132.25, 131.72, 130.48, 129.19, 129.05, 128.72, 127.95, 121.17, 99.76, 78.12, 51.24, 40.94, 36.76; ESI HRMS: calcd. for C19H15NO4S + Na+ 376.0619, found 376.0616.
(3aS,4S,11aS)-3a-Methyl-4-phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e] pyridine-2(11aH)-one 10,10-dioxide (5a) was obtained in 60% yield; the enantiomeric excess was determined to be 55% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 20.97 min, tminor = 28.28 min. = −27.6 (c = 0.84 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.87 (d, J = 7.8 Hz, 1H), 7.75 (d, J = 7.8 Hz, 1H), 7.71 (t, J = 7.5 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.39 (t, J = 7.2 Hz, 2H), 7.35 (t, J = 7.1 Hz, 1H), 7.28 (d, J = 7.2 Hz, 2H), 5.80 (d, J = 3.1 Hz, 1H), 4.55 (t, J = 8.1 Hz, 1H), 3.93 (d, J = 2.9 Hz, 1H), 3.25 (dd, J = 17.7, 7.4 Hz, 1H), 3.04 (dd, J = 17.7, 8.9 Hz, 1H), 1.19 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.4, 137.4, 133.5, 132.2, 130.5, 129.9, 129.4, 128.7, 128.0, 127.96, 121.3, 99.5, 82.9, 53.3, 46.2, 36.5, 21.8; ESI HRMS: calcd. for C20H17NO4S + Na+ 390.0776, found 390.0775.
(3aS,4S,11aS)-3a-Methyl-4-(p-tolyl)-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e] pyridin-2(11aH)-one 10,10-dioxide (5b) was obtained in 61% yield; the enantiomeric excess was determined to be 63% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 21.40 min, tminor = 30.40 min. = 73.4 (c = 0.80 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.9 Hz, 1H), 7.74 (d, J = 7.8 Hz, 1H), 7.70 (d, J = 7.3 Hz, 1H), 7.63 (d, J = 7.5 Hz, 1H), 7.19 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 8.1 Hz, 2H), 5.78 (d, J = 3.1 Hz, 1H), 4.53 (t, J = 8.1 Hz, 1H), 3.88 (d, J = 3.1 Hz, 1H), 3.22 (dd, J = 17.7, 7.4 Hz, 1H), 3.02 (ddd, J = 17.7, 8.6, 2.7 Hz, 1H), 2.36 (s, 3H), 1.18 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.46, 137.72, 134.33, 133.89, 132.17, 130.47, 129.80, 129.36, 129.28, 128.71, 121.35, 121.28, 99.87, 82.97, 53.27, 45.76, 36.46, 21.93, 21.05; ESI HRMS: calcd. for C21H19NO4S + Na+ 404.0932, found 404.0929.
(3aS,4S,11aS)-4-(4-Bromophenyl)-3a-methyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (5c) was obtained in 47% yield; the enantiomeric excess was determined to be 56% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 23.11 min, tminor = 36.52 min. = 21.3 (c = 1.60 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.88 (d, J = 7.8 Hz, 1H), 7.76–7.70 (m, 2H), 7.65 (t, J = 7.5 Hz, 1H), 7.52 (d, J = 8.3 Hz, 2H), 7.17 (d, J = 8.3 Hz, 2H), 5.72 (d, J = 3.0 Hz, 1H), 4.57–4.49 (m, 1H), 3.89 (d, J = 2.9 Hz, 1H), 3.25 (dd, J = 17.8, 7.6 Hz, 1H), 2.99 (dd, J = 17.8, 9.2 Hz, 1H), 1.18 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.11, 136.36, 133.53, 132.23, 131.82, 131.06, 130.69, 130.28, 128.45, 122.14, 121.38, 98.53, 82.40, 53.27, 45.75, 36.36, 21.81; ESI HRMS: calcd. for C20H16BrNO4S + Na+ 467.9881, found 467.9883.
(3aS,4R,11aS)-3a-Methyl-4-(thiophen-2-yl)-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (5d) was obtained in 34% yield; the enantiomeric excess was determined to be 63% by HPLC analysis on Daicel Chiralcel IA column (10% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 57.94 min, tmajor = 73.67 min. = 32.0 (c = 0.92 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.8 Hz, 1H), 7.76 (d, J = 7.8 Hz, 1H), 7.71 (t, J = 7.5 Hz, 1H), 7.64 (t, J = 7.5 Hz, 1H), 7.30 (dd, J = 4.8, 1.0 Hz, 1H), 7.07–7.03 (m, 2H), 5.84 (d, J = 3.2 Hz, 1H), 4.57 (dd, J = 8.5, 7.8 Hz, 1H), 4.22 (d, J = 3.0 Hz, 1H), 3.24 (dd, J = 17.8, 7.5 Hz, 1H), 3.00 (dd, J = 17.8, 8.9 Hz, 1H), 1.27 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.14, 139.78, 133.52, 132.29, 130.69, 129.99, 128.47, 127.18, 121.41, 98.83, 82.82, 53.22, 41.82, 36.48, 21.82; ESI HRMS: calcd. for C18H15NO4S2 + Na+ 396.0340, found 396.0338.
(3aS,4S,11aS)-3a-Methyl-4-(naphthalen-1-yl)-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo [2,3-e]pyridin-2(11aH)-one 10,10-dioxide (5e) was obtained in 44% yield; the enantiomeric excess was determined to be 66% by HPLC analysis on Daicel Chiralcel IF column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 20.51 min, tminor = 34.12 min. = 23.5 (c = 1.04 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 8.15 (d, J = 8.5 Hz, 1H), 7.93–7.88 (m, 2H), 7.85 (d, J = 8.1 Hz, 1H), 7.74–7.67 (m, 2H), 7.63 (dd, J = 15.3, 7.7 Hz, 2H), 7.54 (t, J = 7.3 Hz, 1H), 7.48 (t, J = 7.7 Hz, 1H), 7.34 (d, J = 7.0 Hz, 1H), 5.81 (d, J = 2.9 Hz, 1H), 4.93 (s, 1H), 4.60 (t, J = 6.8 Hz, 1H), 3.29 (dd, J = 22.4, 6.8 Hz, 2H), 1.22 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.56, 134.84, 133.97, 133.50, 132.21, 130.46, 129.93, 129.02, 128.89, 128.76, 127.39, 126.85, 126.11, 125.13, 123.34, 121.31, 101.64, 84.32, 53.78, 40.23, 36.23, 22.80; ESI HRMS: calcd. for C24H19NO4S + Na+ 440.0932, found 440.0928.
(3aS,4S,11aS)-8-Chloro-3a-methyl-4-phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (5f) was obtained in 37% yield; the enantiomeric excess was determined to be 55% by HPLC analysis on Daicel Chiralcel ID column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 17.39 min, tmajor = 22.99 min. = 35.9 (c = 1.16 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.84 (d, J = 1.1 Hz, 1H), 7.69–7.63 (m, 2H), 7.42–7.33 (m, 3H), 7.28–7.24 (m, 2H), 5.80 (d, J = 3.3 Hz, 1H), 4.53 (t, J = 8.0 Hz, 1H), 3.91 (d, J = 3.0 Hz, 1H), 3.23 (dd, J = 17.8, 7.4 Hz, 1H), 3.04 (dd, J = 17.7, 8.6 Hz, 1H), 1.19 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.16, 137.19, 136.75, 133.89, 133.40, 129.38, 129.18, 128.74, 128.05, 127.06, 122.61, 121.48, 100.35, 82.74, 53.40, 46.18, 36.39, 22.04; ESI HRMS: calcd. for C20H16ClNO4S + Na+ 424.0386, found 424.0384.
(4S,11aS)-4-Phenyl-3a,4-dihydro-1H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2(11aH)-one 10,10-dioxide (5g) was obtained in 58% yield; the enantiomeric excess was determined to be 54% by HPLC analysis on Daicel Chiralcel IE column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tminor = 29.40 min, tmajor = 51.53 min. = −17.5 (c = 0.72 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.86 (d, J = 7.9 Hz, 1H), 7.70 (dt, J = 15.9, 7.7 Hz, 2H), 7.62 (dd, J = 10.8, 3.9 Hz, 1H), 7.38 (t, J = 7.4 Hz, 2H), 7.33 (t, J = 7.3 Hz, 1H), 7.25 (d, J = 7.3 Hz, 2H), 5.74 (d, J = 4.3 Hz, 1H), 4.70 (t, J = 4.1 Hz, 1H), 4.64 (dd, J = 8.3, 5.1 Hz, 1H), 4.01 (t, J = 3.9 Hz, 1H), 3.42 (dd, J = 17.5, 3.1 Hz, 1H), 2.93 (dd, J = 17.6, 5.5 Hz, 1H); 13C-NMR (151 MHz, CDCl3) δ: 172.86, 139.35, 133.56, 132.26, 130.95, 130.54, 129.30, 129.02, 128.13, 121.24, 99.18, 80.44, 48.17, 41.21, 36.38; ESI HRMS: calcd. for C19H15NO4S + Na+ 376.0619, found 376.0617.
3-(2-(1,1-Dioxidobenzo[d]isothiazol-3-yl)-1-phenylethyl)-5-methylfuran-2(3H)-one (6). 1H-NMR (600 MHz, CDCl3) δ: 7.86 (t, J = 8.5 Hz, 1H), 7.71 (s, 2H), 7.64 (dd, J = 6.7, 4.5 Hz, 1H), 7.38 (t, J = 7.4 Hz, 1H), 7.29 (t, J = 7.9 Hz, 2H), 7.05 (d, J = 7.6 Hz, 2H), 6.21 (d, J = 6.5 Hz, 1H), 4.05 (t, J = 7.1 Hz, 1H), 3.77 (dd, J = 12.8, 7.7 Hz, 1H), 3.07 (dd, J = 18.5, 3.6 Hz, 1H), 2.21–2.17 (m, 1H), 2.11 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 167.35, 137.45, 134.02, 130.99, 129.44, 129.31, 128.26, 128.02, 127.92, 121.75, 106.73, 45.03, 42.37, 39.43, 30.29. ESI LRMS: calcd. for C20H17NO4S + H+ 368.1, found 368.
(3aS,12R,12aS)-12a-Methyl-12-phenyl-3,3a,12,12a-tetrahydro-2H-benzo[e]furo[2',3':5,6]pyrido [1,2-c][1,2,3]oxathiazin-2-one 5,5-dioxide (8a) was obtained in 56% yield; the enantiomeric excess was determined to be 92% by HPLC analysis on Daicel Chiralcel IA column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 5.40 min, tminor = 8.00 min. = − 2.7 (c = 1.2 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.60 (d, J = 7.9 Hz, 1H), 7.44–7.36 (m, 6H), 7.26 (dd, J = 8.9, 6.4 Hz, 1H), 7.14 (d, J = 8.3 Hz, 1H), 6.24 (d, J = 3.9 Hz, 1H), 4.68 (dd, J = 8.5, 1.7 Hz, 1H), 3.58 (d, J = 3.9 Hz, 1H), 3.14 (dd, J = 19.3, 8.5 Hz, 1H), 2.80 (dd, J = 19.3, 1.7 Hz, 1H), 1.40 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 173.19, 148.77, 136.83, 133.29, 131.14, 130.07, 128.69, 127.95, 126.46, 124.19, 119.52, 117.65, 115.56, 92.17, 62.29, 49.41, 34.60, 25.14; ESI HRMS: calcd. for C20H17NO5S + Na+ 406.0725, found 406.0723.
(3aS,12R,12aS)-8-Bromo-12a-methyl-12-phenyl-3,3a,12,12a-tetrahydro-2H-benzo[e]furo[2ʹ,3ʹ:5,6] pyrido[1,2-c][1,2,3]oxathiazin-2-one 5,5-dioxide (8b) was obtained in 45% yield; the enantiomeric excess was determined to be >99% by HPLC analysis on Daicel Chiralcel IA column (40% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 285 nm, tmajor = 6.28 min, tminor = 8.00 min. = 6.4 (c = 1.6 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.46 (d, J = 8.6 Hz, 1H), 7.42–7.35 (m, 6H), 7.32 (d, J = 1.5 Hz, 1H), 6.24 (d, J = 3.9 Hz, 1H), 4.67 (dd, J = 8.4, 1.4 Hz, 1H), 3.55 (d, J = 3.9 Hz, 1H), 3.14 (dd, J = 19.3, 8.5 Hz, 1H), 2.76 (dd, J = 19.3, 1.5 Hz, 1H), 1.39 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 172.98, 148.76, 136.60, 132.56, 130.01, 129.80, 128.74, 128.04, 125.23, 124.08, 122.81, 116.69, 116.30, 92.02, 62.36, 49.43, 34.60, 25.06; ESI HRMS: calcd. for C20H16BrNO5S + Na+ 483.9830, found 483.9827.
(3aS,4R,5aS,11aS)-3a-Methyl-4-phenyl-1,3a,4,5,5a,11a-hexahydro-2H-benzo[4,5]isothiazolo[2,3-a]furo[2,3-e]pyridin-2-one 10,10-dioxide (9) was obtained in 86% yield; the diastereomer ratio was determined to be 4:1 by 1H-NMR analysis and the enantiomeric excess was determined to be 93% by HPLC analysis on Daicel Chiralcel IA column (20% 2-propanol/n-hexane, 1 mL/min, temperature 35 °C), UV 210 nm, tmajor = 17.07 min, tminor = 25.06; = −32.5 (c = 0.36 M CH2Cl2); 1H-NMR (600 MHz, CDCl3) δ: 7.80 (d, J = 7.8 Hz, 1H), 7.63 (t, J = 7.6 Hz, 1H), 7.55 (t, J = 7.5 Hz, 1H), 7.39–7.31 (m, 6H), 4.34 (d, J = 11.3 Hz, 1H), 4.19 (d, J = 4.7 Hz, 1H), 3.79 (d, J = 18.0 Hz, 1H), 2.98 (ddd, J = 16.6, 15.5, 4.3 Hz, 2H), 2.47–2.42 (m, 1H), 2.30–2.22 (m, 1H), 1.34 (s, 3H); 13C-NMR (151 MHz, CDCl3) δ: 173.56, 138.32, 136.22, 135.09, 133.30, 129.68, 129.52, 128.53, 127.86, 122.46, 121.25, 83.36, 59.21, 58.31, 50.52, 34.65, 31.04, 23.49; ESI HRMS: calcd. for C20H19NO4S + Na+ 392.0932, found 392.0930.