Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview
Abstract
:1. Introduction
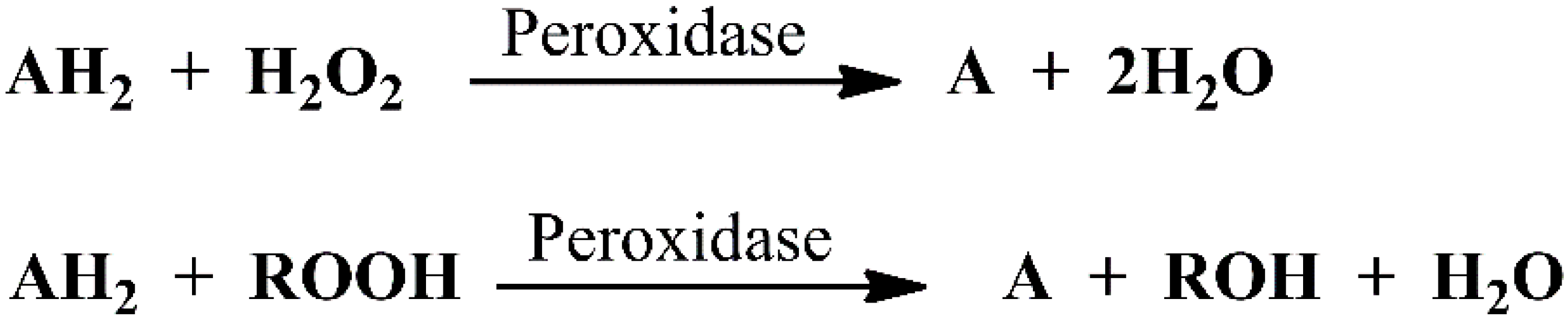
2. An Overview of Peroxidases
| Types of Peroxidases | Application(s) | References |
|---|---|---|
| LiP, MnP, and HRP | Dye decolorization | [54,55,56] |
| HRP, LiP, MnP, and microbial peroxidase | Bioremediation of waste water: removal of phenolic and amine contaminants | [57,58,59,60] |
| HRP | Deodorization of swine slurry | [61] |
| Fungal peroxidases | Degradation of lignocellulosic biomass: biofuel production | [62] |
| HRP | Detection of antigens or antibodies: ELISA | [63,64] |
| LiP | Biopulping: delignification of wood pulp | [65] |
| Fungal peroxidases | Transformation of pesticides | [66,67] |
| Chloroperoxidase | ||
| LiP, MnP | Bioremediation of polycyclic aromatic hydrocarbons | [68,69] |
| HRP | Biosensing and diagnostics: catalysis | [64,70,71] |
| Plant peroxidases | ||
| HRP | Organic and polymer synthesis | [52,72] |
| HRP, GPx, TPO, LPO, SPO, MPO, EPO, and uterine peroxidase | Cancer therapy and pathological applications | [52,73] |


3. Peroxidase Mimic@ Graphene-Based Nanomaterials (G-NMs)
3.1. Graphene Oxide, Graphene, and/or Reduced Graphene Oxide as Peroxidase Mimetic Catalysts
| Nanocarbon Oxides | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| Fullerene oxide {C60[C(COOH)2]2} | Colorimetric | TMB | 0.5 μM | Glucose detection | [76] |
| SWCNTs oxide | Colorimetric | TMB | 1 nM | SNP detection | [36] |
| CFMP | Colorimetric | TMB | 0.4 μM | H2O2 detection | [77] |
| CNDs | Colorimetric | TMB, OPD, and pyrogallol | 0.2 μM | H2O2, glucose detection | [78] |
| 0.4 μM | |||||
| CDs | Colorimetric | MO and MR | – | Degradation of dyes | [79] |

| Nanomaterial | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| GO | Colorimetric | TMB | 1.0 μM | Glucose detection | [37] |
| GO | Colorimetric | Hydroquinone | – | PSA detection | [80] |
| GO | Voltammetry | TMB | 1.0 nM | H2O2 detection | [81] |
| GO b | Fluorescence | DAB | 5.0 pg/mL | IL-5 | [82] |
| Graphene | Electrochemical | TMB | 10 nM | H2O2 detection | [83] |
| rGO (QRGO c) | Colorimetric | TMB | 1.0 μM | Glucose detection | [84] |
3.2. Graphene-Metalloprotein Conjugates as Peroxidase Mimetic Catalysts




| Nanomaterial | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| H-GNs | Colorimetric | TMB, ABTS, and OPD | – | SNPs detection | [85] |
| H-GNs | TMB | 0.2 μM | H2O2 detection | [86] | |
| Amperometric | 0.3 μM | Glucose detection | |||
| Colorimetric | 20 nM | H2O2 detection | |||
| 30 nM | Glucose detection | ||||
| GFH | Colorimetric | TMB | 1000 CC | CC detection | [87] |
| GO/Hb hydrogel | – | Pyrogallol | – | – | [88] |
| H-GCs | – | Pyrogallol | – | – | [89] |
| FeTMPyP-GCs | |||||
| H-GNs | Colorimetric | ABTS, TMB, and OPD | 9 nM | DNA detection | [90] |
| 20 nM | Cocaine detection | ||||
| FeTMPyP- streptavidin-GO BCs | Electrochemical | OPD | 22 aM | DNA detection | [91] |
| G-SO3H/cyt C Ns | Electrochemical | OPD | – | – | [92] |
| DNA-H-GNs | Colorimetric | TMB | 8 nM | Hg2+ detection | [93] |
| 0.5 nM | DNA detection | ||||
| DNA-H-GNs | Colorimetric | TMB | Protein detection: | [94] | |
| 0.5 nM | Thrombin | ||||
| 5 nM | PDGF-BB | ||||
| H-GNs | Electrochemical | HQ | 0.17 pM | microRNAs detection | [95] |
| H-GNs | Colorimetric | 4-AAP | – | Phenol detection | [96] |
3.3. Graphene-Gold Hybrid Nanostructures as Peroxidase Mimetic Catalysts



| Nanomaterial | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| G-AuNPs hybrid | Colorimetric | TMB, ABTS, and OPD | – | DNA detection | [98,99] |
| (FA)-GO-AuNCs | Colorimetric | TMB | 1000 CC | CC detection | [100] |
| GO-AuNPs hybrid | Colorimetric | TMB | 0.04 pg/mL | RSV detection | [101] |
| H-RGO-Au composite | Colorimetric | TMB | 5 nM | H2O2 detection | [102] |
| AuNPs/Cit-GNs composite | – | TMB, ABTS | – | – | [103] |
| GSHA hybrid b | – | TMB | – | – | [104] |
| G-AuNPs hybrid | Colorimetric | TMB | 0.0016 U/μL | hOGG1 detection | [105] |
| GO-AuNPs hybrid | Colorimetric | TMB | 0–50 μM | Hg2+ and Pb2+ detection | [106] |
| GSF@AuNPs hybrid | Colorimetric | TMB | 50 CC | CC detection | [107] |
| Au-rGO composite | Colorimetric | Pyrogallol | – | Dye removal | [108] |
3.4. Graphene-FexOy Magnetic Nanocomposites as Peroxidase Mimetic Catalysts


| Nanomaterial | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| GO-Fe3O4 composite | Colorimetric | TMB | 0.32 μM | H2O2 detection | [109] |
| 0.74 μM | Glucose detection | ||||
| Fe3O4 NSs-rGO composite | Colorimetric | TMB | 39 nM | Ach detection | [110] |
| GCNT-Fe3O4 composite | Colorimetric | – | H2O2 detection | [111] | |
| TMB, OPD, DAB, PAP, and HQ | – | Glucose detection | |||
| TMB | |||||
| Electrochemical | 22 μM | Glucose detection | |||
| rGO-CF composite | Colorimetric | TMB | 0.3 μM | H2O2 detection | [112] |
| GO_MNP-10-Pt-10 composite | Colorimetric | TMB | 100 CC | CC detection | [114] |
| mFe2O3-G composite | Colorimetric | TMB | 0.5 μM | Glucose detection | [115] |
| MNP-GO-H composite | Colorimetric | ABTS | 0.08 nM | GSH detection | [116] |
| AR/FeOxH-rGO composite | Fluorescence | AR | 50 nM | H2O2 detection | [117] |
| 50 nM | S2– detection | ||||
| 3DRGO_ Fe3O4-Pd composite | Colorimetric | TMB | 86 nM | H2O2 detection | [118] |
| 52 nM | GSH detection | ||||
| 0.13 μM | Glucose detection | ||||
| RGO-INs composite | Colorimetric | TMB, OPD, and THB | 0.2 μM | H2O2 detection | [119] |
| 0.8 μM | Glucose detection | ||||
| Hg2+/Au-Fe3O4-GO composite | Colorimetric | TMB | 0.15 nM | Hg2+ detection | [120] |
| >96% | Hg2+ removal |
3.5. Graphene-Based other NMs as Peroxidase Mimetic Catalysts



| Nanomaterial | Method | Substrate | LOD | Applications | Ref. |
|---|---|---|---|---|---|
| Co3O4-rGO composite | Colorimetric | TMB | 0.5 μM | H2O2 detection | [121] |
| 1.0 μM | Glucose detection | ||||
| FA/Porous Pt NPs-GO composite | Colorimetric | TMB | 125 CC | CC detection | [122] |
| Pt NPs-GO composite | Colorimetric | TMB, OPD, DAB, HQ, and 4-AAP phenol | 1.2 nM | Cys detection | [123] |
| PtPdNDs-GNs composite | Colorimetric | TMB | 0.1 μM | H2O2 detection | [124] |
| Pt-on-Pd-rGO composite | Colorimetric | TMB | 0.3 μM | H2O2 detection | [125] |
| 3DGN@WO3 NWs array | Colorimetric | TMB | – | H2O2 detection | [126] |
| – | AA detection | ||||
| Electrochemical | 238 nM | DPA detection | |||
| MWCNT@rGONR heterostructures | Colorimetric | TMB | 10 μM | Cholesterol detection | [127] |
3.6. Graphene Quantum Dots/Graphene Dots as Peroxidase Mimetic Catalysts
| GQDs | Precursor/Synthesis method | DM | SUB | LOD | Applications | Ref. |
|---|---|---|---|---|---|---|
| GQDs/GQDs-Au electrode | GO/UV-irradiation | ECHEM | TMB | 0.7 μM | H2O2 detection | [130] |
| GDs | Carbon black/Hydrothermal, 130 °C | COLM | TMB | 10 nM | H2O2 detection | [131] |
| 0.5 μM | Glucose detection | |||||
| 0.5 μM | GSH detection | |||||
| GQDs-Fe3O4 NPs composite | GQDs + FeCl3 + FeSO4/Co-precipitation | – | TMB | – | Removal of phenolic compounds | [132] |
| N-GQDs | DPA, 3D NGA/Conc. H2SO4 + HNO3 treatment | COLM | TMB | 5.3 μM | H2O2 detection | [133] |
| 16 μM | Glucose detection | |||||
| GQDs-ZnFe2O4 composite | ZnFe2O4, GO/UV-irradiation | ECHEM | TMB | 62 aM | DNA detection | [134] |
| GQDs | Graphite flakes/Conc. H2SO4 + HNO3 treatment | COLM | TMB | 6 μM | Cholesterol detection | [135] |
4. Tunable Factors in the Peroxidase-Like Activities of Graphene-Based Nanomaterials (G-NMs)
5. Conclusions and Future Perspectives
| CNMs | Key Advantages/Favorable Features | Key Disadvantages or Challenges | |
|---|---|---|---|
| G-NMs | Large surface area and abundant functional groups for further modifications, for instance, bioconjugation and as support for metal/metal oxide nanoscale structures | The frequent use of acids and other toxic chemicals in synthesis and/or functionalization is of high environmental concerns | |
| Size-(shape-, structure-, composition) dependent tunable properties | Available studies suggest certain toxicity. Data not concise at the present state | ||
| Easy in rational design, mass production, purification, recovery and recycling | Relatively low efficiency, specificity, and selectivity than natural peroxidases as reported in several cases | ||
| Tunable dispersion ability in aqueous media | Limited examples for use of peroxidase substrates other than TMB | ||
| High operational stability and Robustness to harsh environment | Much efforts are needed to be used for diversified biosensing other than glucose and H2O2 | ||
| Relatively low cost than natural peroxidases | |||
| CNTs | Large surface area (relatively low than that of graphene domain) | CNTs reveal toxic effects. Cytotoxicity and a relatively high inflammatory potential is reported in several studies. | |
| Size-(shape-, structure-, composition) dependent tunable properties in CNTs | Relatively high cost | ||
| MWCNTs are easy in rational design, mass production, purification, recovery, and recycling | Difficulties in mass production and purification of SWCNTs | ||
| Very limited examples and much efforts are needed to be used as peroxidase mimetic catalysts | |||
| Fullerenes | Excellent electron acceptor | Poor water dispersibility | |
| Unique chemical reactivity towards radicals | Relatively high cost | ||
| Difficulties in mass production | |||
| Limited functionalization | |||
| Barely used as peroxidase mimetic catalysts | |||
- (1)
- Though graphene-based NMs are increasingly used in cellular applications, the available experimental results indicate that they are not devoid of possible risks to human health or the environment [136,137]. The surface physicochemical properties, leading to possible adverse effects, warrant further studies. In line with this, more efforts are needed to translate the prominent scientific results for practical applications.
- (2)
- A further related issue is to avoid the use of toxic chemicals, for instance, hydrazine [127]. It is an undeniable fact that hydrazine is one of the most efficient reducing agent leading to the high C/O ratio in the reduction of GO to rGO. However, hydrazine is also highly toxic and can be readily absorbed orally, by inhalation, or even dermal routes of exposure. In addition, it potentially leads to serious environmental contamination. In this milieu, it would be worthwhile to mention here that U.S. Environmental Protection Agency (EPA) has identified hydrazine as a probable human carcinogen with a low threshold limit value (TLV) of 10 ppb.
- (3)
- From economic viewpoint, the use of precious metals like Pt and Pd may be replaced by smart designing of novel metal alloys containing G-NMs with similar or even higher catalytic efficiencies than those of expensive metals. Besides, visible light-driven peroxidase-like activity of G-NMs [138] is another area, which should be matured in near future.
- (4)
- Further development is needed to examine the peroxidase-mimetic activities of G-NMs in the detection of biologically important anions such as cyanide anion [139], which is known for its acute toxicity to living organisms. The other related issues are exploring efficient modulators for enhancing catalytic activities of G-NMs even at high temperature or physiological pH. Though some of the research groups have started to address this problem [100] but the research progress in this direction is still in its infancy and need more attention and efforts. Considering the Hg2+-stimulated catalytic activity of Ab-AuNPs-GO conjugates [101], an obvious question originates, how effective would be the anions in this regard? The other question of further interest is—would the catalytic efficiencies of G-NMs be the same with other substrates, aside from that of TMB, which is most frequently used?
- (5)
- Alongside, the technical loopholes/misconceptions regarding the nomenclature of G-NMs is the area of considerable concern [44] and should be tackled effectively in order to maintain scientific integrity for young and future generations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schmitt, O.H. Some Interesting and Useful Biomimetic Transform. In Proceedings of Third Internatioanl Biophysics Congress, Boston, MA, USA, 29 August–3 September 1969; 297. [Google Scholar]
- Garcia-Viloca, M.; Gao, J.; Karplus, M.; Truhlar, D.G. How enzymes work: Analysis by modern rate theory and computer simulations. Science 2004, 303, 186–195. [Google Scholar] [CrossRef] [PubMed]
- Wolfenden, R.; Snider, M.J. The depth of chemical time and the power of enzymes as catalysts. Acc. Chem. Res. 2001, 34, 938–945. [Google Scholar] [CrossRef] [PubMed]
- Hennrich, N.; Cramer, F. Inclusion compounds. XVIII.1 the catalysis of the fission of pyrophosphates by cyclodextrin. A model reaction for the mechanism of enzymes. J. Am. Chem. Soc. 1965, 87, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R.; Overman, L.E. Artificial enzyme combing a metal catalytic group and a hydrophobic binding cavity. J. Am. Chem. Soc. 1970, 92, 1075–1077. [Google Scholar] [CrossRef] [PubMed]
- Klotz, I.M.; Royer, G.P.; Scarpa, I.S. Synthetic derivatives of polyethyleneimine with enzyme-like catalytic activity (synzymes). Proc. Natl. Acad. Sci. USA 1971, 68, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, Y.; Zhao, Q.L.; Hassan, M.A.; Wei, Z.L.; Furuichi, M.; Miyamoto, Y.; Kondo, T.; Shimizu, T. SOD/catalase mimetic platinum nanoparticles inhibit heat-induced apoptosis in human lymphoma U937 and HH cells. Free Rad. Res. 2011, 45, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Bhabak, K.P.; Mugesh, G. Functional Mimics of Glutathione Peroxidase: Bioinspired Synthetic Antioxidants. Acc. Chem. Res. 2010, 43, 1408–1419. [Google Scholar] [CrossRef] [PubMed]
- Friedle, S.; Reisner, E.; Lippard, S.J. Current challenges of modeling diiron enzyme active sites for dioxygen activation by biomimetic synthetic complexes. Chem. Soc. Rev. 2010, 39, 2768–2779. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Nature of forces between large molecules of biological interest. Nature 1948, 161, 707–709. [Google Scholar] [CrossRef] [PubMed]
- Pauling, L. Molecular architecture and biological reactions. Chem. Eng. News 1946, 24, 1375–1377. [Google Scholar] [CrossRef]
- Sumner, J.B. The isolation and crystallization of the enzyme urease. J. Biol. Chem. 1926, 69, 435–441. [Google Scholar]
- Cramer, F.; Kampe, W. Inclusion compounds. XVII.1 catalysis of decarboxylation by cyclodextrins. A model reaction for the mechanism of enzymes. J. Am. Chem. Soc. 1965, 87, 1115–1120. [Google Scholar] [CrossRef] [PubMed]
- Breslow, R. Centenary lecture. Biomimetic chemistry. Chem. Soc. Rev. 1972, 1, 553–580. [Google Scholar] [CrossRef]
- Tabushi, I. Cyclodextrin catalysis as a model for enzyme action. Acc. Chem. Res. 1982, 15, 66–72. [Google Scholar] [CrossRef]
- Tabushi, I.; Shimizu, N.; Sugimoto, T.; Shiozuka, M.; Yamamura, K. Cyclodextrin flexibly capped with metal ion. J. Am. Chem. Soc. 1977, 99, 7100–7102. [Google Scholar] [CrossRef]
- Takagishi, T.; Klotz, I.M. Macromolecule-small molecule interactions; introduction of additional binding sites in polyethyleneimine by disulfide cross-linkages. Biopolymers 1972, 11, 483–491. [Google Scholar] [CrossRef] [PubMed]
- Behr, J.P.; Lehn, J.M. Enhanced rates of dihydropyridine to pyridinium hydrogen transfer in complexes of an active macrocyclic receptor molecule. J. Chem. Soc. Chem. Commun. 1978, 143–146. [Google Scholar] [CrossRef]
- Tramontano, A.; Janda, K.D.; Lerner, R.A. Catalytic antibodies. Science 1986, 234, 1566–1570. [Google Scholar] [CrossRef] [PubMed]
- Breaker, R.R.; Joyce, G.F. A DNA enzyme that cleaves RNA. Chem. Biol. 1994, 1, 223–229. [Google Scholar] [CrossRef]
- Bonar-Law, R.P.; Sanders, J.K.M. Polyol recognition by a steroid-capped porphyrin. Enhancement and modulation of misfit guest binding by added water or methanol. J. Am. Chem. Soc. 1995, 117, 259–271. [Google Scholar] [CrossRef]
- Cuevas, F.; Di Stefano, S.; Magrans, J.O.; Prados, P.; Mandolini, L.; de Mendoza, J. Toward an artificial acetylcholinesterase. Chem. Eur. J. 2000, 6, 3228–3234. [Google Scholar] [CrossRef]
- Manea, F.; Houillon, F.B.; Pasquato, L.; Scrimin, P. Nanozymes: Gold-nanoparticle-based transphosphorylation catalysts. Angew. Chem. Int. Ed. 2004, 43, 6165–6169. [Google Scholar] [CrossRef] [PubMed]
- Comotti, M.; Della Pina, C.; Matarrese, R.; Rossi, M. The catalytic activity of “naked” gold particles. Angew. Chem. Int. Ed. 2004, 43, 5812–5815. [Google Scholar] [CrossRef] [PubMed]
- Korsvik, C.; Patil, S.; Seal, S.; Self, W.T. Superoxide dismutase mimetic properties exhibited by vacancy engineered ceria nanoparticles. Chem. Commun. 2007, 1056–1058. [Google Scholar] [CrossRef] [PubMed]
- Pirmohamed, T.; Dowding, J.M.; Singh, S.; Wasserman, B.; Heckert, E.; Karakoti, A.S.; King, J.E.S.; Seal, S.; Self, W.T. Nanoceria exhibit redox state-dependent catalase mimetic activity. Chem. Commun. 2010, 46, 2736–2738. [Google Scholar] [CrossRef] [PubMed]
- Asati, A.; Santra, S.; Kaittanis, C.; Nath, S.; Perez, J.M. Oxidase-like activity of polymer-coated cerium oxide nanoparticles. Angew. Chem. Int. Ed. 2009, 48, 2308–2312. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Kim, M.I.; Cho, D.-Y.; Park, H.G. Label-free colorimetric detection of nucleic acids based on target-induced shielding against the peroxidase-mimicking activity of magnetic nanoparticles. Small 2011, 7, 1521–1525. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhang, Y.; Gu, N. Peroxidase-like catalytic activity of cubic Pt nanocrystals. Colloids Surf. A Physicochem. Eng. Asp. 2011, 373, 6–10. [Google Scholar] [CrossRef]
- Fan, J.; Yin, J.J.; Ning, B.; Wu, X.; Hu, Y.; Ferrari, M.; Anderson, G.J.; Wei, J.; Zhao, Y.; Nie, G. Direct evidence for catalase and peroxidase activities of ferritin-platinum nanoparticles. Biomaterials 2011, 32, 1611–1618. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Qi, P.; Zhang, D.; Wu, J.; Wang, Y. Manganese oxide nanowire-mediated enzyme-linked immunosorbent assay. Biosens. Bioelectron. 2012, 33, 69–74. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wu, X.; Liu, J.; Hu, X.; Zhang, K.; Hou, S.; Zhou, W.; Xie, S. Design of AgM bimetallic alloy nanostructures (M = Au, Pd, Pt) with tunable morphology and peroxidase-like activity. Chem. Mater. 2010, 22, 2988–2994. [Google Scholar] [CrossRef]
- Ali, S.S.; Hardt, J.I.; Quick, K.L.; Kim-Han, J.S.; Erlanger, B.F.; Huang, T.T.; Epstein, C.J.; Dugan, L.L. A biologically effective fullerene (C60) derivative with superoxide dismutase mimetic properties. Free Radic. Biol. Med. 2004, 37, 1191–1202. [Google Scholar] [CrossRef] [PubMed]
- Quick, K.L.; Ali, S.S.; Arch, R.; Xiong, C.; Wozniak, D.; Dugan, L.L. A carboxyfullerene SOD mimetic improves cognition and extends the lifespan of mice. Neurobiol. Aging 2008, 29, 117–128. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Wang, X.; Zhao, C.; Qu, K.; Ren, J.; Qu, X. Label-free colorimetric detection of single nucleotide polymorphism by using single-walled carbon nanotube intrinsic peroxidase-like activity. Chem. Eur. J. 2010, 16, 3617–3621. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Qu, K.; Zhao, C.; Ren, J.; Qu, X. Graphene oxide: Intrinsic peroxidase catalytic activity and its application to glucose detection. Adv. Mater. 2010, 22, 2206–2210. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; He, Y.; Liu, B.; Tang, D. NiCoBP-doped carbon nanotube hybrid: A novel oxidase mimetic system for highly efficient electrochemical immunoassay. Anal. Chim. Acta 2014, 851, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Huang, Y.; Chen, Y. Focusing on energy and optoelectronic applications: A journey for graphene and graphene oxide at large scale. Acc. Chem. Res. 2012, 45, 598–607. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Qi, X.; Boey, F.; Zhang, H. Graphene-based composites. Chem. Soc. Rev. 2012, 41, 666–686. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Chen, Q.D.; Jin, Z.; Kim, E.; Sun, H.B. Biomimetic graphene films and their properties. Nanoscale 2012, 4, 4858–4869. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Ling, Y.C. Versatilities of graphene-based catalysts in organic transformations. Green Mater. 2013, 1, 47–61. [Google Scholar] [CrossRef]
- Garg, B.; Bisht, T.; Ling, Y.C. Graphene-based nanomaterials as heterogeneous acid catalysts: A comprehensive perspective. Molecules 2014, 19, 14582–14614. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.; Bisht, T.; Ling, Y.C. Sulfonated graphene as highly efficient and reusable acid carbocatalyst for the synthesis of ester plasticizers. RSC Adv. 2014, 4, 57297–57307. [Google Scholar] [CrossRef]
- Garg, B.; Sung, C.H.; Ling, Y.C. Graphene-based nanomaterials as molecular imaging agents. WIREs Nanomed Nanobiotechnol. 2015. [Google Scholar] [CrossRef]
- Lin, Y.; Ren, J.; Qu, X. Catalytically active nanomaterials: A promising candidate for artificial enzymes. Acc. Chem. Res. 2014, 47, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wamer, W.; Xia, Q.; Yin, J.J.; Fu, P.P. Enzyme-like activity of nanomaterials. J. Environ. Sci. Health Part C Environ. Carcinog. Ecotoxicol. Rev. 2014, 32, 186–211. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, P.J. Peroxidases. Chem. Biol. Interact. 2000, 129, 113–139. [Google Scholar] [CrossRef]
- Caremel-Harel, O.; Storz, G. Roles of the glutathione- and thioredoxin-dependent reduction systems in the Escherichia coli and Saccharomyces cerevisiae responses to oxidative stress. Annu. Rev. Microbiol. 2000, 54, 439–461. [Google Scholar] [CrossRef] [PubMed]
- Brigelius-Flohe, R.; Kipp, A. Glutathione peroxidases in different stages of carcinogenesis. Biochim. Biophys. Acta 2009, 1790, 1555–1568. [Google Scholar] [CrossRef] [PubMed]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Hamid, M. Khalil-ur-Rehman Potential applications of peroxidases. Food Chem. 2009, 115, 1177–1186. [Google Scholar] [CrossRef]
- Chivukula, M.; Spadaro, J.T.; Renganathan, V. Lignin peroxidase catalyzed oxidation of sulphonated azo dyes generates novel sulphophenyl hydroperoxides. Biochemistry 1995, 34, 7765–7772. [Google Scholar] [CrossRef] [PubMed]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef]
- Bhunia, A.; Durani, S.; Wangikar, P.P. Horseradish peroxidase catalyzed degradation of industrially important dyes. Biotechnol. Bioeng. 2002, 72, 562–567. [Google Scholar] [CrossRef]
- Klibanov, A.M.; Alberti, B.N.; Morris, E.D.; Felshin, L.M. Enzymatic removal of toxic phenols and anilines from waste waters. J. Appl. Biochem. 1980, 2, 413–421. [Google Scholar]
- Aitken, M.D.; Massey, I.J.; Chen, T.; Heck, P.E. Characterization of reaction products from the enzyme catalyzed oxidation of phenolic pollutants. Water Res. 1994, 28, 1879–1889. [Google Scholar] [CrossRef]
- Al-Kassim, L.; Taylor, K.E.; Nicell, J.A.; Bewtra, J.K.; Biswas, N. Enzymatic removal of selected aromatic contaminants from waste water by a fungal peroxidase from Coprinus macrorhizus in batch reactors. J. Chem. Technol. Biotechnol. 1994, 61, 179–182. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Weber, W.J., Jr. Transformation and removal of bisphenol A from aqueous phase via peroxidase-mediated oxidative coupling reactions: Efficacy, products, and pathways. Environ. Sci. Technol. 2005, 39, 6029–6036. [Google Scholar] [CrossRef] [PubMed]
- Govere, E.M.; Tonegawa, M.; Bruns, M.A.; Wheeler, E.F.; Kephart, K.B.; Voigt, J.W.; Dec, J. Using minced horseradish roots and peroxidase for the deodorization of swine manure: A pilot scale study. Bioresour. Technol. 2007, 98, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Schoemaker, H.E.; Piontek, K. On the interaction of lignin peroxidase with lignin. Pure Appl. Chem. 1996, 68, 2089–2096. [Google Scholar] [CrossRef]
- Clarke, J.R.; Marquardt, R.R.; Oosterveld, A.; Frohlich, A.A.; Madrid, F.J.; Dawood, M. Development of a quantitative and sensitive enzyme-linked immunosorbent assay for ochratoxin—A using antibodies from the yolk of the laying hen. J. Agric. Food Chem. 1993, 41, 1784–1789. [Google Scholar] [CrossRef]
- Green, M.T.; Dawson, J.H.; Gray, H.B. Oxoiron (IV) in chloroperoxidase compound II is basic: Implications for P450 chemistry. Science 2004, 304, 1653–1656. [Google Scholar] [CrossRef] [PubMed]
- Gysin, B.; Griessmann, T. Bleaching Wood Pulp with Enzymes. European Patent No. EP 0418201 B1, 21 December 1994. [Google Scholar]
- Jauregui, J.; Valderrama, B.; Albores, A.; Vazquez-Duhalt, R. Microsomal transformation of organophosphorus pesticides by white rot fungi. Biodegradation 2003, 14, 397–406. [Google Scholar] [CrossRef] [PubMed]
- Longoria, A.; Tinoco, R.; Vázquez-Duhalt, R. Chloroperoxidase-mediated transformation of highly halogenated monoaromatic compounds. Chemosphere 2008, 72, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Weber, R.; Gaus, C.; Tysklind, M.; Johnston, P.; Forter, M.; Hollert, H.; Heinisch, E.; Holoubek, I.; Lloyd-Smith, M.; Masunaga, S.; et al. Dioxin- and POP-contaminated sites contemporary and future relevance and challenges: Overview on background, aims and scope of the series. J. Environ. Sci. Pollut. Res. 2008, 15, 363–393. [Google Scholar] [CrossRef] [PubMed]
- Harford-Cross, C.F.; Carmichael, A.B.; Allan, F.K.; England, P.A.; Rouch, D.A.; Wong, L.L. Protein engineering of cytochrome P450cam (CYP101) for the oxidation of polycyclic aromatic hydrocarbons. Protein Eng. 2000, 13, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Agostini, E.; Hernández-Ruiz, J.; Arnao, M.B.; Milrad, S.R.; Tigier, H.A.; Acosta, M. A peroxidase isoenzyme secreted by turnip (Brassica napus) hairy-root culture inactivation by hydrogen peroxide and application in diagnosis kits. J. Biotechnol. Appl. Biochem. 2002, 35, 1–7. [Google Scholar] [CrossRef]
- Ruzgas, T.; Csöregi, E.; Katakis, I.; Kenausis, G.; Gorton, L. Preliminary investigations of amperometric oligosaccharide dehydrogenase based electrode for the detection of glucose and some other low molecular weight sacharides. J. Mol. Recognit. 1996, 9, 480–484. [Google Scholar] [CrossRef]
- Oguchi, T.; Tawaki, S.I.; Uyama, H.; Kobayashi, S. Soluble polyphenol. Macromol. Rapid Commun. 1999, 20, 401–403. [Google Scholar] [CrossRef]
- Khan, A.A.; Rahmani, A.H.; Aldebasi, Y.H.; Aly, S.M. Biochemical and pathological studies on peroxidases-an updated review. Glob. J. Health Sci. 2014, 6, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Cornish-Bowden, A. Fundamentals of Enzyme Kinetics, Review ed.; Portland: London, UK, 1995; pp. 30–37, 56–57. [Google Scholar]
- Zhao, R.; Zhao, X.; Gao, X. Molecular-level insights into intrinsic peroxidase-like activity of nanocarbon oxides. Chem. Eur. J. 2015, 21, 960–964. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zhen, M.; Guan, M.; Chen, D.; Zhang, G.; Ge, J.; Gong, P.; Wang, C.; Shu, C. A novel glucose colorimetric sensor based on intrinsic peroxidase-like activity of C60-carboxyfullerenes. Biosens. Bioelectron. 2013, 47, 502–507. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Wang, L.; Zhai, J.; Luo, Y.; Sun, X. Carboxyl functionalized mesoporous polymer: A novel peroxidase-like catalyst for H2O2 detection. Anal. Methods 2011, 3, 1475–1477. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Q.; Long, Y.; Cheng, Z.; Chen, S.; Zheng, H.; Huang, Y. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem. Commun. 2011, 47, 6695–6697. [Google Scholar] [CrossRef] [PubMed]
- Safavi, A.; Sedaghati, F.; Shahbaazi, H.; Farjami, E. Facile approach to the synthesis of carbon nanodots and their peroxidase mimetic function in azo dyes degradation. RSC Adv. 2012, 2, 7367–7370. [Google Scholar] [CrossRef]
- Qu, F.; Li, T.; Yang, M. Colorimetric platform for visual detection of cancer biomarker based on intrinsic peroxidase activity of graphene oxide. Biosens. Bioelectron. 2011, 26, 3927–3931. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Ju, X.; Zhang, Y.; Sun, X.; Li, G.; Sun, Z. Application of carboxyl functionalized graphene oxide as mimetic peroxidase for sensitive voltammetric detection of H2O2 with 3,3′,5,5′-tetramethylbenzidine. Electrochem. Commun. 2013, 26, 113–116. [Google Scholar] [CrossRef]
- Lim, S.Y.; Ahn, J.; Lee, J.S.; Kim, M.-G.; Park, C.B. Graphene-oxide-based immunosensing through fluorescence quenching by peroxidase-catalyzed polymerization. Small 2012, 8, 1994–1999. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Lv, X.; Weng, J. High peroxidase catalytic activity of exfoliated few-layer grapheme. Carbon 2013, 62, 51–60. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, X.; Zhou, J.; Wang, A.; Wang, J.; Jin, R.; Lv, H. Quinone-mediated microbial synthesis of reduced graphene oxide with peroxidase-like activity. Bioresour. Technol. 2013, 149, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Deng, L.; Li, J.; Guo, S.; Wang, E.; Dong, S. Hemin-graphene hybrid nanosheets with intrinsic peroxidase-like activity for label-free colorimetric detection of single-nucleotide polymorphism. ACS Nano 2011, 5, 1282–1290. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Li, J.; Dong, S. Hemin functionalized graphene nanosheets-based dual biosensor platforms for hydrogen peroxide and glucose. Sens. Actuators B Chem. 2011, 160, 295–300. [Google Scholar] [CrossRef]
- Song, Y.; Chen, Y.; Feng, L.; Ren, J.; Qu, X. Selective and quantitative cancer cell detection using target-directed functionalized graphene and its synergistic peroxidase-like activity. Chem. Commun. 2011, 47, 4436–4438. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Bai, H.; Li, C.; Shi, G. A graphene oxide/hemoglobin composite hydrogel for enzymatic catalysis in organic solvents. Chem. Commun. 2011, 47, 4962–4964. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.; Jiang, S.; Qu, Y.; Su, Q.; Cheng, R.; Dubin, S.; Chiu, C.Y.; Kaner, R.; Huang, Y.; Duan, X. Graphene-supported hemin as a highly active biomimetic oxidation catalyst. Angew Chem. Int. Ed. 2012, 51, 3822–3825. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Xi, Q.; Ge, J.; Luo, F.Y.; Tang, L.J.; Jiang, J.H.; Yu, R.Q. Graphene-hemin hybrid nanosheets as a label-free colorimetric platform for DNA and small molecule assays. RSC Adv. 2014, 4, 64252–64257. [Google Scholar] [CrossRef]
- Wang, Q.; Lei, J.; Deng, S.; Zhang, L.; Ju, H. Graphene-supported ferric porphyrin as a peroxidase mimic for electrochemical DNA biosensing. Chem. Commun. 2013, 49, 916–918. [Google Scholar] [CrossRef] [PubMed]
- Hua, B.Y.; Wang, J.; Wang, K.; Li, X.; Zhu, X.J.; Xia, X.H. Greatly improved catalytic activity and direct electron transfer rate of cytochrome C due to the confinement effect in a layered self-assembly structure. Chem. Commun. 2012, 48, 2316–2318. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lin, Y.; Ren, J.; Qu, X. Self-assembled, functionalized graphene and DNA as a universal platform for colorimetric assays. Biomaterials 2013, 34, 4810–4817. [Google Scholar] [CrossRef] [PubMed]
- Lin, B.; Sun, Q.; Liu, K.; Lu, D.; Fu, Y.; Xu, Z.; Zhang, W. Label-free colorimetric protein assay and logic gates design based on the self-assembly of hemin-graphene hybrid nanosheet. Langmuir 2014, 30, 2144–2151. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, M.; Xu, Z.; Ni, C.; Yin, H.; Ai, S. Investigation of the effect of phytohormone on the expression of microRNA-159a in Arabidopis thaliana seedlings based on mimic enzyme catalysis systematic electrochemical biosensor. Biosens. Bioelectron. 2014, 54, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, Y.; Ni, Y.; Kokot, S. Spectrophotometric analysis of phenols, which involves a hemin-graphene hybrid nanoparticles with peroxidase-like activity. J. Hazard. Mater. 2014, 266, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Ren, J.; Qu, X. Nano-gold as artificial enzymes: Hidden talents. Adv. Mater. 2014, 26, 4200–4217. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Interface engineering catalytic graphene for smart colorimetric biosensing. ACS Nano 2012, 6, 3142–3151. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Stimuli-responsive peroxidase mimicking at a smart graphene interface. Chem. Commun. 2012, 48, 7055–7057. [Google Scholar] [CrossRef] [PubMed]
- Tao, Y.; Lin, Y.; Huang, Z.; Ren, J.; Qu, X. Incorporating graphene oxide and gold nanoclusters: A synergistic catalyst with surprisingly high peroxidase-like activity over a broad pH range and its application for cancer cell detection. Adv. Mater. 2013, 25, 2594–2599. [Google Scholar] [CrossRef] [PubMed]
- Zhan, L.; Li, C.M.; Wu, W.B.; Huang, C.Z. A colorimetric immunoassay for respiratory syncytial virus detection based on gold nanoparticles-graphene oxide hybrids with mercury-enhanced peroxidase-like activity. Chem. Commun. 2014, 50, 11526–11528. [Google Scholar] [CrossRef] [PubMed]
- Lv, X.; Weng, J. Ternary composite of hemin, gold, nanoparticles and graphene for highly efficient decomposition of hydrogen peroxide. Sci. Rep. 2013, 3, 3285–3294. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Tian, X.; Su, B.; Huang, Z.; Chen, X.; Oyama, M. Au nanoparticles on citrate-functionalized graphene nanosheets with a high peroxidase-like performance. Dalton Trans. 2014, 43, 7449–7454. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Wu, L.; Huang, Y.; Ren, J.; Qu, X. Positional assembly of hemin and gold nanoparticles in graphene mesoporous silica nanohybrids for tandem catalysis. Chem. Sci. 2015, 6, 1272–1276. [Google Scholar] [CrossRef]
- Yuan, F.; Zhao, H.; Liu, M.; Quan, X. Visible assay for glycosylase based on intrinsic catalytic ability of graphene/gold nanoparticles hybrids. Biosens. Bioelectron. 2015, 68, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, N.; Synder, J.H.; Chen, Q.; Liu, P.; Jin, L.; Zheng, Q.; Lin, F.; Hu, J.; Zhou, H. Colorimetric detection of Hg2+ and Pb2+ based on peroxidase-like activity of graphene oxide-gold nanohybrids. Anal. Methods 2015, 7, 1951–1957. [Google Scholar] [CrossRef]
- Maji, S.K.; Mandal, A.K.; Nguyen, K.T.; Borah, P.; Zhao, Y. Cancer cell detection and therapeutics using peroxidase-active nanohybrid of gold nanoparticle-loaded mesoporous silica-coated graphene. ACS Appl. Mater. Interfaces 2015, 7, 9807–9816. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sarkar, S.; Ray, C.; Pal, T. Benzoin derived reduced graphene oxide (rGO) and its nanocomposite: Application in dye removal and peroxidase-like activity. RSC Adv. 2013, 3, 21475–21483. [Google Scholar] [CrossRef]
- Dong, Y.; Zhang, H.; Rahman, Z.U.; Su, L.; Chen, X.; Hu, J.; Chen, X. Graphene oxide-Fe3O4 magnetic nanocomposites with peroxidase-like activity for colorimetric detection of glucose. Nanoscale 2012, 4, 3969–3976. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, X.; Jiang, L.; Zhu, C.; Mao, H.; Wang, K. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine. Sens. Actuators B Chem. 2014, 201, 160–166. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Si, Y.; Sun, Z.; Li, S.; Lin, Y. Recyclable enzyme mimic of cubic Fe3O4 nanoparticles loaded on graphene oxide-dispersed carbon nanotubes with enhanced peroxidase-like catalysis and electrocatalysis. J. Mater. Chem. B 2014, 2, 4442–4448. [Google Scholar] [CrossRef]
- Hao, J.; Zhang, Z.; Yang, W.; Lu, B.; Ke, X.; Zhang, B.; Tang, J. In situ controllable growth of CoFe2O4 ferrite nanocubes on graphene for colorimetric detection of hydrogen peroxide. J. Mater. Chem. A 2013, 1, 4352–4357. [Google Scholar] [CrossRef]
- Wang, C.; Daimon, H.; Sun, S. Dumbbell-like Pt-Fe3O4 nanoparticles and their enhanced catalysis for oxygen reduction reaction. Nano Lett. 2009, 9, 1493–1496. [Google Scholar] [CrossRef] [PubMed]
- Kim, M., II; Kim, M.S.; Woo, M.A.; Ye, Y.; Kang, K.S.; Lee, J.; Park, H.G. Highly efficient colorimetric detection of target cancer cells utilizing superior catalytic activity of graphene-oxide-magnetic-platinum nanohybrids. Nanoscale 2014, 6, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Tian, J.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Two-dimensional hybrid mesoporous Fe2O3-graphene nanostructures: A highly active and reusable peroxidase mimetic toward rapid, highly sensitive optical detection of glucose. Biosens. Bioelectron. 2014, 52, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Zhao, T.; Jia, X.; He, P. Magnetic graphene oxide-supported hemin as peroxidase probe for sensitive detection of thiols in extracts of cancer cells. Biosens. Bioelectron. 2014, 57, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Hsu, K.; Lien, C.W.; Lin, C.H.; Chang, H.T.; Huang, C.C. Immobilization of iron hydroxide/oxide on reduced graphene oxide: Peroxidase-like activity and selective detection of sulfide ions. RSC Adv. 2014, 4, 37705–37713. [Google Scholar] [CrossRef]
- Zheng, X.; Zhu, Q.; Song, H.; Zhao, X.; Yi, T.; Chen, H.; Chen, X. In situ synthesis of self-assembled three-dimensional graphene-magnetic palladium nanohybrids with dual-enzyme activity through one-pot strategy and its application in glucose probe. ACS Appl. Mater. Interfaces 2015, 7, 3480–3491. [Google Scholar] [PubMed]
- Li, L.; Zeng, C.; Ai, L.; Jiang, J. Synthesis of reduced graphene oxide-iron nanoparticles with superior enzyme-mimetic activity for biosensing application. J. Alloys Comps. 2015, 639, 470–477. [Google Scholar] [CrossRef]
- Zhang, S.; Li, H.; Wang, Z.; Liu, J.; Zhang, H.; Wang, B.; Yang, Z. A strongly coupled Au/Fe3O4/GO hybrid material with enhanced nanozyme activity for highly sensitive colorimetric detection, and rapid and efficient removal of Hg2+ in aqueous solutions. Nanoscale 2015, 7, 8495–8502. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Cao, H.; Jiang, H.; Chen, Y.; Shi, W.; Zheng, H.; Huang, Y. Co3O4-reduced graphene oxide nanocomposite as an effective peroxidase mimetic and its application in visual biosensing of glucose. Anal. Chim. Acta 2013, 796, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.N.; Deng, H.H.; Lin, F.L.; Xu, X.W.; Weng, S.H.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. In situ growth of porous platinum nanoparticles on graphene oxide for colorimetric detection of cancer cells. Anal. Chem. 2014, 86, 2711–2718. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.Q.; Deng, H.H.; Wu, G.W.; Peng, H.P.; Liu, A.L.; Lin, X.H.; Xia, X.H.; Chen, W. Platinum nanoparticles/graphene-oxide hybrid with excellent peroxidase-like activity and its application for cysteine detection. Analyst 2015. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Su, B.; Cai, Z.; Chen, X.; Oyama, M. PtPd nanodendrites supported on graphene nanosheets: A peroxidase-like catalyst for colorimetric detection of H2O2. Sens. Actuators B Chem. 2014, 201, 286–292. [Google Scholar] [CrossRef]
- Zhang, X.; Wu, G.; Cai, Z.; Chen, X. Dual-functional Pt-on-Pd supported on reduced graphene oxide hybrids: Peroxidase-mimic activity and an enhanced electrocatalytic oxidation characteristic. Talanta 2015, 134, 132–135. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhao, M.; Cai, B.; Wang, W.; Ye, Z.; Huang, J. 3D graphene network@WO3 nanowire composites: A multifunctional colorimetric and electrochemical biosensing platform. Chem. Commun. 2014, 50, 11135–11138. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Yang, X.; Yang, Z.; Zhu, G.; Mao, H.; Wang, K. Multiwalled carbon nanotube@reduced graphene oxide nanoribbonheterostructure: Synthesis, intrinsic peroxidase-like catalytic activity, and its application in colorimetric biosensing. J. Mater. Chem. B 2015, 3, 1624–1632. [Google Scholar] [CrossRef]
- Sun, H.; Wu, L.; Wei, W.; Qu, X. Recent advances in graphene quantum dots in sensing. Mater. Today 2013, 16, 433–442. [Google Scholar] [CrossRef]
- Bacon, M.; Bradley, S.J.; Nann, T. Graphene Quantum Dots. Part. Part. Syst. Charact. 2014, 31, 415–428. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, C.; Zhou, X.; Wu, X.; Yang, Y.; Wu, H.; Guo, S.; Zhang, J. Graphene quantum dots/gold electrode and its application in living cell H2O2 detection. Nanoscale 2013, 5, 1816–1819. [Google Scholar] [CrossRef] [PubMed]
- Zheng, A.X.; Cong, Z.X.; Wang, J.R.; Li, J.; Yang, H.H.; Chen, G.N. Highly-efficient peroxidase-like activity of graphene dots for biosensing. Biosens. Bioelectron. 2013, 49, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Zhang, Y.; Han, T.; Wu, H.; Guo, S.; Zhang, J. Composite of graphene quantum dots and Fe3O4 nanoparticles: Peroxidase activity and application in phenolic compound removal. RSC Adv. 2014, 4, 3299–3305. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yang, H.; Ma, C.; Ding, Y.; Ge, S.; Yu, J.; Yan, M. Graphene-palladium nanowires based electrochemical sensor using ZnFe2O4-graphene quantum dots as an effective peroxidase mimic. Anal. Chim. Acta 2014, 852, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Nirala, N.R.; Abraham, S.; Kumar, V.; Bansal, A.; Srivastava, A.; Saxena, P.S. Colorimetric detection of cholesterol based on highly efficient peroxidase mimetic activity of graphene quantum dots. Sens. Actuators B Chem. 2015, 218, 42–50. [Google Scholar] [CrossRef]
- Bianco, A. Graphene: Safe or toxic? The two faces of the medal. Angew. Chem. Int. Ed. 2013, 52, 4986–4997. [Google Scholar] [CrossRef] [PubMed]
- Seabra, A.B.; Paula, A.J.; de Lima, R.; Alves, O.L.; Durán, N. Nanotoxicity of graphene and graphene oxide. Chem. Res. Toxicol. 2014, 27, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Xu, X.; Wu, X.; Cao, G.; Dong, Y.; Li, Z. Visible-light-stimulated enzymelike activity of graphene oxide and its application for facile glucose sensing. J. Phys. Chem. C 2014, 118, 28109–28117. [Google Scholar] [CrossRef]
- Garg, B.; Ling, Y.C. A highly selective phenothiazine-based fluorescence ‘turn-on’ indicator based on cyanide-promoted novel protection/deprotection mechanism. Chem. Commun. 2015, 51, 8809–8812. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garg, B.; Bisht, T.; Ling, Y.-C. Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview. Molecules 2015, 20, 14155-14190. https://doi.org/10.3390/molecules200814155
Garg B, Bisht T, Ling Y-C. Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview. Molecules. 2015; 20(8):14155-14190. https://doi.org/10.3390/molecules200814155
Chicago/Turabian StyleGarg, Bhaskar, Tanuja Bisht, and Yong-Chien Ling. 2015. "Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview" Molecules 20, no. 8: 14155-14190. https://doi.org/10.3390/molecules200814155
APA StyleGarg, B., Bisht, T., & Ling, Y. -C. (2015). Graphene-Based Nanomaterials as Efficient Peroxidase Mimetic Catalysts for Biosensing Applications: An Overview. Molecules, 20(8), 14155-14190. https://doi.org/10.3390/molecules200814155






