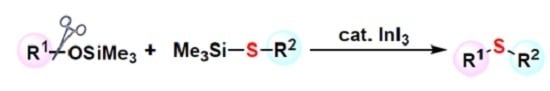
Synthesis of Thioethers by InI3-Catalyzed Substitution of Siloxy Group Using Thiosilanes
Abstract
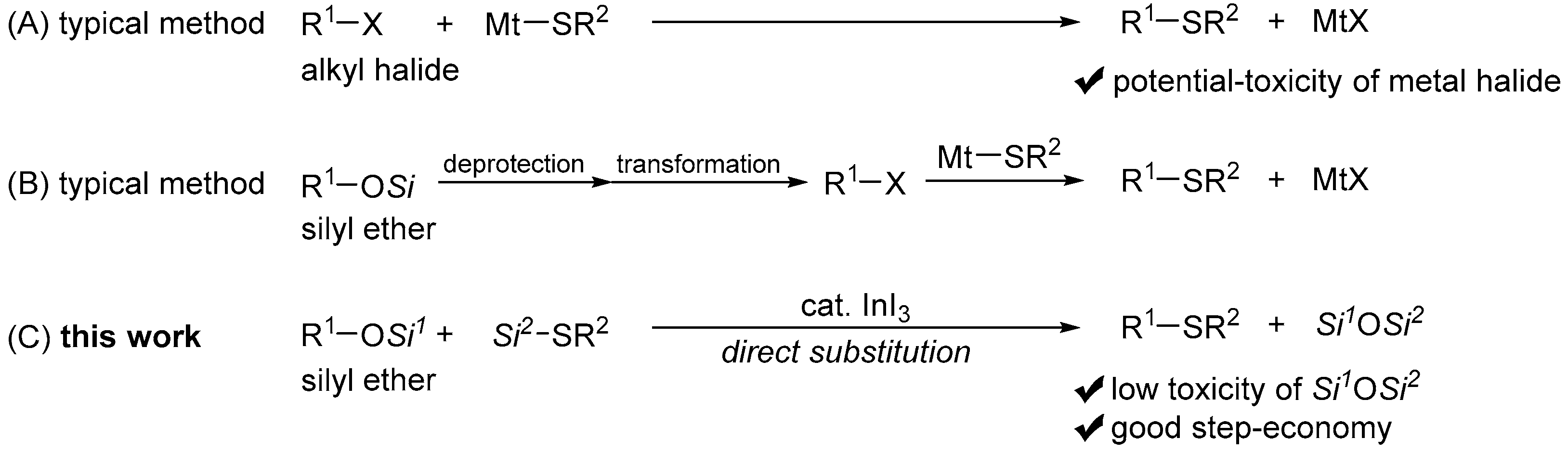
:1. Introduction
2. Results
3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References and Notes
- Peach, M.E. The Chemistry of the Thiol Group; Patai, S., Ed.; John Wiley & Sons: London, UK, 1979; pp. 721–756. [Google Scholar]
- Damani, L.A. Metabolism of Sulfur Functional Groups. In Sulfur-Containing Drugs and Related Organic Compounds: Chemistry, Biochemistry, and Toxicology; Ellis Horwood Ltd.: Chichester, UK, 1989; Volume 1, Part A. [Google Scholar]
- Oae, S. Organic Sulfur Chemistry: Structure and Mechanism; Doi, J.T., Ed.; CRC Press: Boca Raton, FL, USA, 1991. [Google Scholar]
- Cremlyn, R.J. An Introduction to Organosulfur Chemistry; John Wiley & Sons: New York, NY, USA, 1996. [Google Scholar]
- McReynolds, M.D.; Doughtery, J.M.; Hanson, P.R. Synthesis of Phosphorus and Sulfur Heterocycles via Ring-Closing Olefin Metathesis. Chem. Rev. 2004, 104, 2239–2258. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Mandal, T. Water-promoted highly selective anti-Markovnikov addition of thiols to unactivated alkenes. Synlett 2007, 925–928. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Ferreira, P.C.; Jacob, R.G.; Perin, G.; Leite, F.P.L. Solvent-free conjugated addition of thiols to citral using KF/alumina: Preparation of 3-thioorganylcitronellals, potential antimicrobial agents. Tetrahedron Lett. 2007, 48, 6763–6766. [Google Scholar] [CrossRef]
- Lenardão, E.J.; Jacob, R.G.; Mesquita, K.D.; Lara, R.G.; Webber, R.; Martinez, D.M.; Savegnago, L.; Mendes, S.R.; Alves, D.; Perin, G. Glycerol as a promoting and recyclable medium for catalyst-free synthesis of linear thioethers: New antioxidants from eugenol. Green Chem. Lett. Rev. 2013, 6, 269–276. [Google Scholar]
- Movassagh, B.; Navidi, M. Water promoted catalyst-free anti-Markovnikov addition of thiols to styrenes. Arkivoc 2008, 47–53. [Google Scholar]
- See a recent review: Ley, S.V.; Thomas, A.W. Modern synthetic methods for copper-mediated C(aryl)–O, C(aryl)–N, and C(aryl)–S Bond Formation. Angew. Chem. Int. Ed. 2003, 42, 5400–5449. [Google Scholar]
- See a review: Kondo, T.; Mitsudo, T. Metal-catalyzed carbon-sulfur bond formation. Chem. Rev. 2000, 100, 3205–3220. [Google Scholar] [CrossRef]
- See a review: Lee, C.F.; Liu, Y.C.; Badsara, S.S. Transition-metal-catalyzed C-S bond coupling reaction. Chem. Asian J. 2014, 9, 706–722. [Google Scholar]
- Parham, W.E.; Wynberg, H. Diethyl mercaptoacetal. Org. Synth. 1963, 4, 295. [Google Scholar]
- Boscato, J.F.; Catala, J.M.; Franta, E.; Brossas, J. Action of elementary sulfur onto carbanions: A new route to dialkylpolysulfides. Tetrahedron Lett. 1980, 21, 1519–1520. [Google Scholar] [CrossRef]
- Hundscheid, F.J.A.; Tandon, V.K.; Rouwette, P.H.A.M.; van Leusen, A.M. Synthesis of chiral sulfonylmethyl isocyanides, and comparison of their propensities in asymmetric induction reactions with acetophenones. Tetrahedron 1987, 43, 5073–5088. [Google Scholar] [CrossRef]
- Malmstrom, J.; Gupta, V.; Engman, L. Novel antioxidants: unexpected rearrangements in the radical cyclization approach to 2,3-dihydrobenzo[b]thiophene-5-ol derivatives. J. Org. Chem. 1998, 63, 3318–3323. [Google Scholar] [CrossRef]
- Blanchard, P.; Jousselme, B.; Frere, P.; Roncali, J. 3- and 3,4-Bis(2-cyanoethylsulfanyl)thiophenes as building blocks for functionalized thiophene-based π-conjugated systems. J. Org. Chem. 2002, 67, 3961–3964. [Google Scholar] [CrossRef] [PubMed]
- Ranu, B.C.; Jana, R. Ionic liquid as catalyst and reaction medium: A simple, convenient and green procedure for the synthesis of thioethers, thioesters and dithianes using an inexpensive ionic liquid, [pmIm]Br. Adv. Synth. Catal. 2005, 347, 1811–1818. [Google Scholar] [CrossRef]
- Greene, T.W.; Wuts, P.G.M. Protective Groups in Organic Synthesis, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1991; pp. 68–87. [Google Scholar]
- Wuts, P.G.M.; Greene, T.W. Protective Groups in Organic Synthesis, 4th ed.; John Wiley & Sons: New York, NY, USA, 2007; pp. 165–221. [Google Scholar]
- The substitution reaction of silyl ethers with Ph3P(SCN)2: Iranpoor, N.; Firouzabadi, H.; Shaterian, H. Efficient conversion of silyl ethers to thiocyanates with Ph3P(SCN)2. Synlett 2000, 65–66. [Google Scholar]
- Degl’Innocenti, A.; Ulivi, P.; Capperucci, A.; Mordini, A.; Reginato, G.; Ricci, A. Thiosilanes in organic synthesis: A novel approach to vinyl sulphides. Synlett 1992, 499–501. [Google Scholar] [CrossRef]
- Inokuchi, T.; Yamashita, H.; Date, T.; Torii, S. Generation and Michael reaction of 1,3-bis(trimethylsiloxy)-isoindoles by reduction of N-alkylphthalimides with a Zn-Me3SiCl-lutidine system. Synth. Commun. 1989, 19, 267–274. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Okita, A.; Yasuda, M.; Baba, A. Synthesis of a wide range of thioethers by indium triiodide catalyzed direct coupling between alkyl acetates and thiosilanes. Org. Lett. 2012, 14, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Nishimoto, Y.; Yasuda, M.; Baba, A. InCl3/Me3SiBr-catalyzed direct coupling between silyl ethers and enol acetates. Org. Lett. 2011, 13, 2762–2765. [Google Scholar] [CrossRef] [PubMed]
- Onishi, Y.; Nishimoto, Y.; Yasuda, M.; Baba, A. InI3/Me3SiI-catalyzed direct alkylation of enol acetates using alkyl acetates or alkyl ethers. Chem. Lett. 2011, 40, 1223–1225. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Saito, T.; Yasuda, M.; Baba, A. Indium-catalyzed coupling reaction between silyl enolates and alkyl chlorides or alkyl ethers. Tetrahedron 2009, 65, 5462–5471. [Google Scholar] [CrossRef]
- Nishimoto, Y.; Onishi, Y.; Yasuda, M.; Baba, A. α-Alkylation of carbonyl compounds by direct addition of alcohols to enol acetates. Angew. Chem. Int. Ed. 2009, 48, 9131–9134. [Google Scholar] [CrossRef] [PubMed]
- Nishimoto, Y.; Kajioka, M.; Saito, T.; Yasuda, M.; Baba, A. Direct coupling of alcohols with alkenylsilanes catalyzed by indium trichloride or bismuth tribromide. Chem. Commun. 2008, 6396–6398. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishimoto, Y.; Yasuda, M.; Baba, A. InCl3/I2-catalyzed cross-coupling of alkyl trimethylsilyl ethers and allylsilanes via an in situ derived combined Lewis acid of InCl3 and Me3SiI. J. Org. Chem. 2007, 72, 8588–8590. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Nishimoto, Y.; Yasuda, M.; Baba, A. Direct coupling reaction between alcohols and silyl compounds: Enhancement of Lewis acidity of Me3SiCl using InCl3. J. Org. Chem. 2006, 71, 8516–8522. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Yasuda, M.; Baba, A. Indium-silicon combined Lewis acid catalyst for direct allylation of alcohols with allyltrimethylsilane in non-halogenated solvent. Synlett 2005, 1737–1739. [Google Scholar] [CrossRef]
- Yasuda, M.; Saito, T.; Ueba, M.; Baba, A. Direct substitution of the hydroxyl group in alcohols with silyl nuculeophiles catalyzed by indium trichloride. Angew. Chem. Int. Ed. 2004, 43, 1414–1416. [Google Scholar] [CrossRef] [PubMed]
- Yasuda, M.; Yamasaki, S.; Onishi, Y.; Baba, A. Indium-catalyzed direct chlorination of alcohols using chlorodimethylsilane-benzil as a selective and mild system. J. Am. Chem. Soc. 2004, 126, 7186–7187. [Google Scholar] [CrossRef] [PubMed]
- Enantiopure silyl ether (R)-1d was synthesized by the silylation of (R)-1-phenylethanol (97% enantiomeric excess, purchased from Wako Pure Chemical Industries, Ltd., Osaka, Japan) using trimethylsilyl chloride and Et3N.
- NMR studies showed that AlCl3 and BF3·OEt2 transmetalated with thiosilane 2a to generate Me3SiCl and Me3SiF, respectively. In contrast, when an equivalent amount of InI3 and thiosilane 2a was mixed, no transmetalation was observed by 13C-NMR. See reference 24.
- Sample Availability: Not available.




| Entry | Catalyst (10 mol %) | Solvent | Conditions | Yield (%) b |
|---|---|---|---|---|
| 1 | InI3 | CH2Cl2 | RT c, 2 h | 27 |
| 2 | InI3 | ClCH2CH2Cl | 80 °C, 2 h | 53 |
| 3 | InCl3 | ClCH2CH2Cl | 80 °C, 2 h | 0 |
| 4 | InBr3 | ClCH2CH2Cl | 80 °C, 2 h | 27 |
| 5 | BF3·OEt2 | ClCH2CH2Cl | 80 °C, 2 h | 0 |
| 6 | AlCl3 | ClCH2CH2Cl | 80 °C, 2 h | 0 |
| 7 | TiCl4 | ClCH2CH2Cl | 80 °C, 2 h | 0 |
| 8 | InI3 | Toluene | 80 °C, 2 h | 30 |
| 9 | InI3 | Hexane | 68 °C, 2 h | 17 |
| 10 | InI3 | THF d | 66 °C, 2 h | 0 |
| 11 | InI3 | ClCH2CH2Cl | 80 °C, 8 h | 67 |
| Entry | R1OSiMe3 | Conditions | Product | Yield (%) b |
|---|---|---|---|---|
| 1 |  1b | ClCH2CH2Cl 80 °C, 8 h |  3ba | 32 |
| 2 |  1c | CH2Cl2 RT, 2 h |  3ca | 99 (95) c |
| 3 |  1d | CH2Cl2 RT, 2 h |  3da | 98 |
| 4 |  1e | ClCH2CH2Cl 80 °C, 2 h |  3ea | 85 |
| 5 |  1f | CH2Cl2 RT, 2 h |  3fa | 88 |
| 6 |  1g | ClCH2CH2Cl 80 °C, 2 h |  3ga | 83 |
| 7 |  1h | CH2Cl2 RT, 2 h |  3ha | 67 |
| 8 |  1i | ClCH2CH2Cl 80 °C, 2 h |  3ia | 36 |
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nishimoto, Y.; Okita, A.; Baba, A.; Yasuda, M. Synthesis of Thioethers by InI3-Catalyzed Substitution of Siloxy Group Using Thiosilanes. Molecules 2016, 21, 1330. https://doi.org/10.3390/molecules21101330
Nishimoto Y, Okita A, Baba A, Yasuda M. Synthesis of Thioethers by InI3-Catalyzed Substitution of Siloxy Group Using Thiosilanes. Molecules. 2016; 21(10):1330. https://doi.org/10.3390/molecules21101330
Chicago/Turabian StyleNishimoto, Yoshihiro, Aya Okita, Akio Baba, and Makoto Yasuda. 2016. "Synthesis of Thioethers by InI3-Catalyzed Substitution of Siloxy Group Using Thiosilanes" Molecules 21, no. 10: 1330. https://doi.org/10.3390/molecules21101330
APA StyleNishimoto, Y., Okita, A., Baba, A., & Yasuda, M. (2016). Synthesis of Thioethers by InI3-Catalyzed Substitution of Siloxy Group Using Thiosilanes. Molecules, 21(10), 1330. https://doi.org/10.3390/molecules21101330