Abstract
Recent studies have shown that sulforaphane (SFN) selectively inhibits the growth of ALDH+ breast cancer stem-like cells.Herein, a series of SFN analogues were synthesized and evaluated against breast cancer cell lines MCF-7 and SUM-159, and the leukemia stem cell-like cell line KG-1a. These SFN analogues were characterized by the replacement of the methyl group with heterocyclic moieties, and the replacement of the sulfoxide group with sulfide or sulfone. A growth inhibitory assay indicated that the tetrazole analogs 3d, 8d and 9d were significantly more potent than SFN against the three cancer cell lines. Compound 14c, the water soluble derivative of tetrazole sulfide 3d, demonstrated higher potency against KG-1a cell line than 3d. SFN, 3d and 14c significantly induced the activation of caspase-3, and reduced the ALDH+ subpopulation in the SUM159 cell line, while the marketed drug doxrubicin(DOX) increased the ALDH+ subpopulation.
Keywords:
sulforaphane (SFN); analogues; water soluble derivative; KG-1a; SUM-159; MCF-7; caspase-3; ALDH+ 1. Introduction
The natural compound, sulforaphane (SFN), was first isolated from broccoli in 1992. Since then, SFN has been found to be an effective chemo-preventive agent, and it exhibits anti-inflammatory, antioxidant, anti-proliferative and anti-cancer activities [1,2,3,4,5,6,7]. Recently, Sun et al. reported that SFN also inhibits the growth of the ALDH+ subpopulation of the breast cancer stem cell line, SUM-159, via down regulation of the Wnt/β-catenin self-renewal pathway [8]. Analogs of SFN were subsequently synthesized and their anti-cancer activities against various cancer cell lines were examined in the literature [1,4,9,10,11,12,13,14,15,16,17,18,19], and it was found that the replacement of the methyl group yielded compounds with significant activity [1,19]. Herein, a series of SFN analogues containing a heterocyclicring were synthesized, and then were evaluated for their activities against the breast cancer cell lines MCF-7 and SUM-159, and the leukemia stem cell-like KG-1a.
2. Results and Discussion
2.1. Synthesis of Sulforaphane Analogues and Water Soluble Derivatives
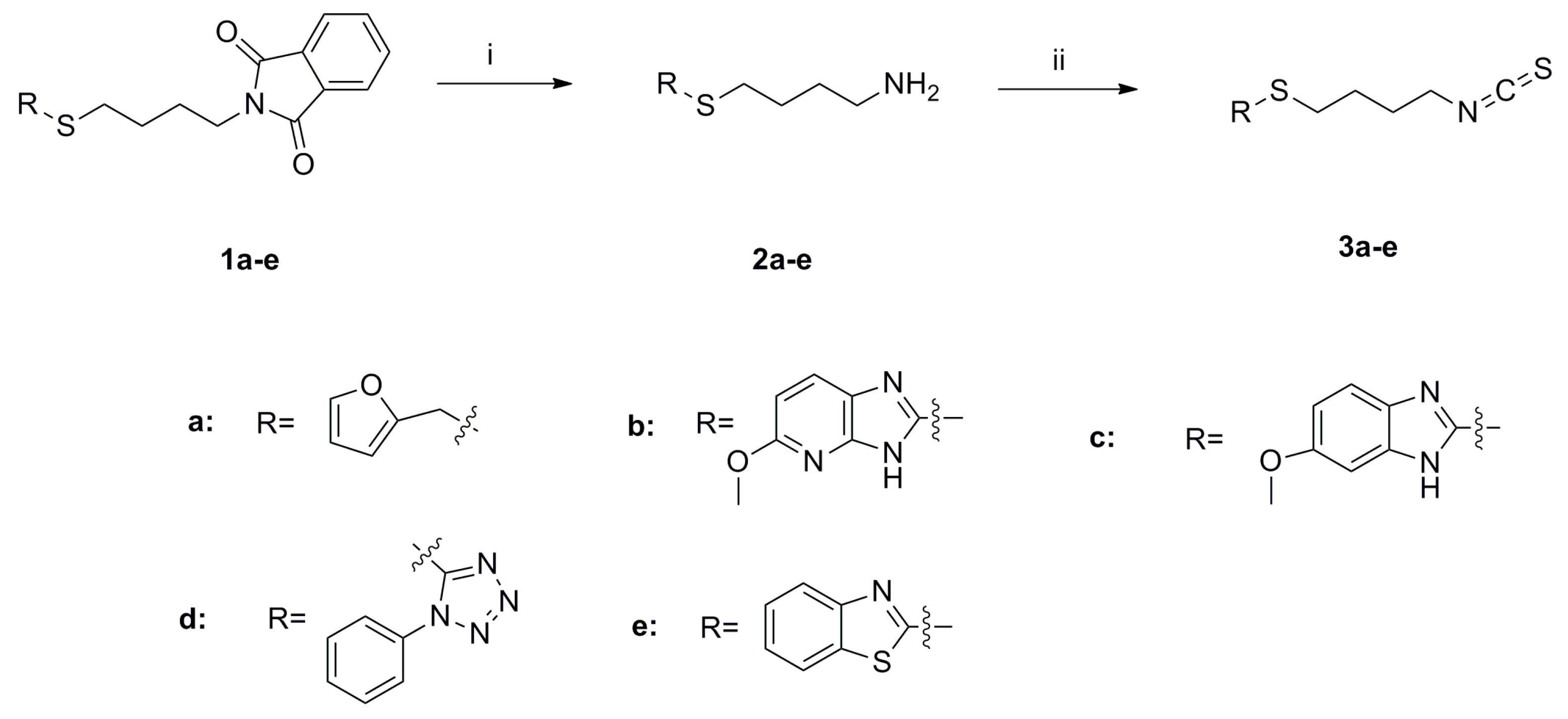
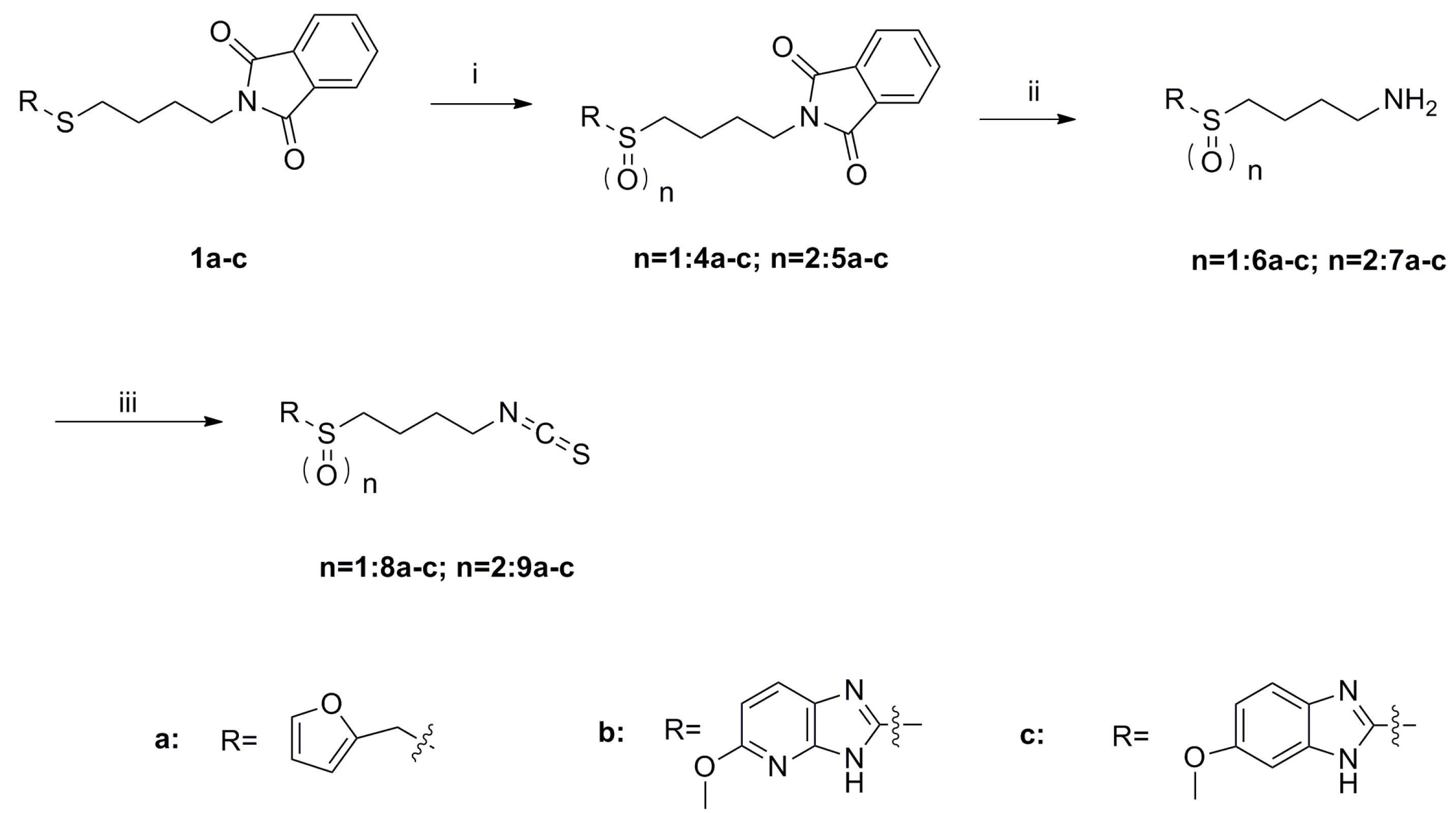
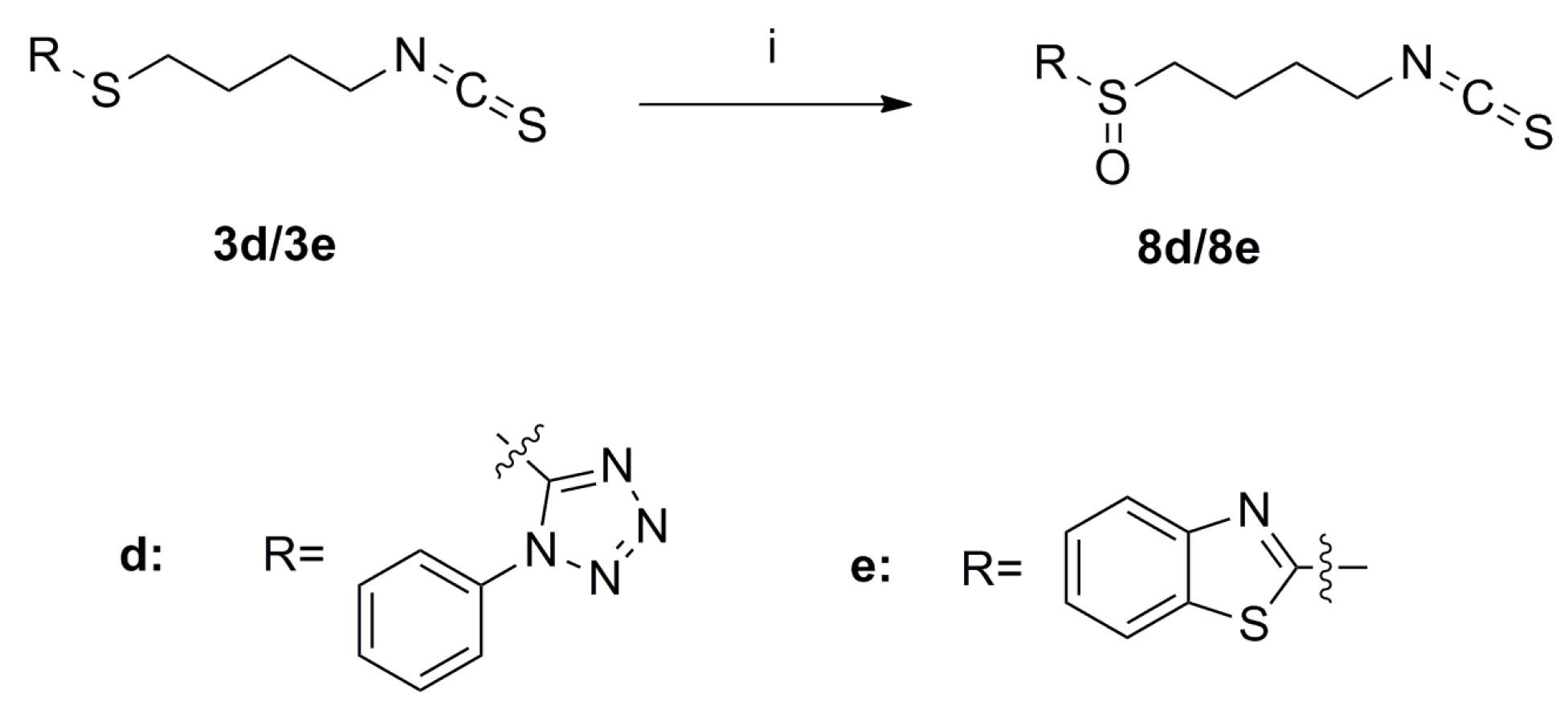
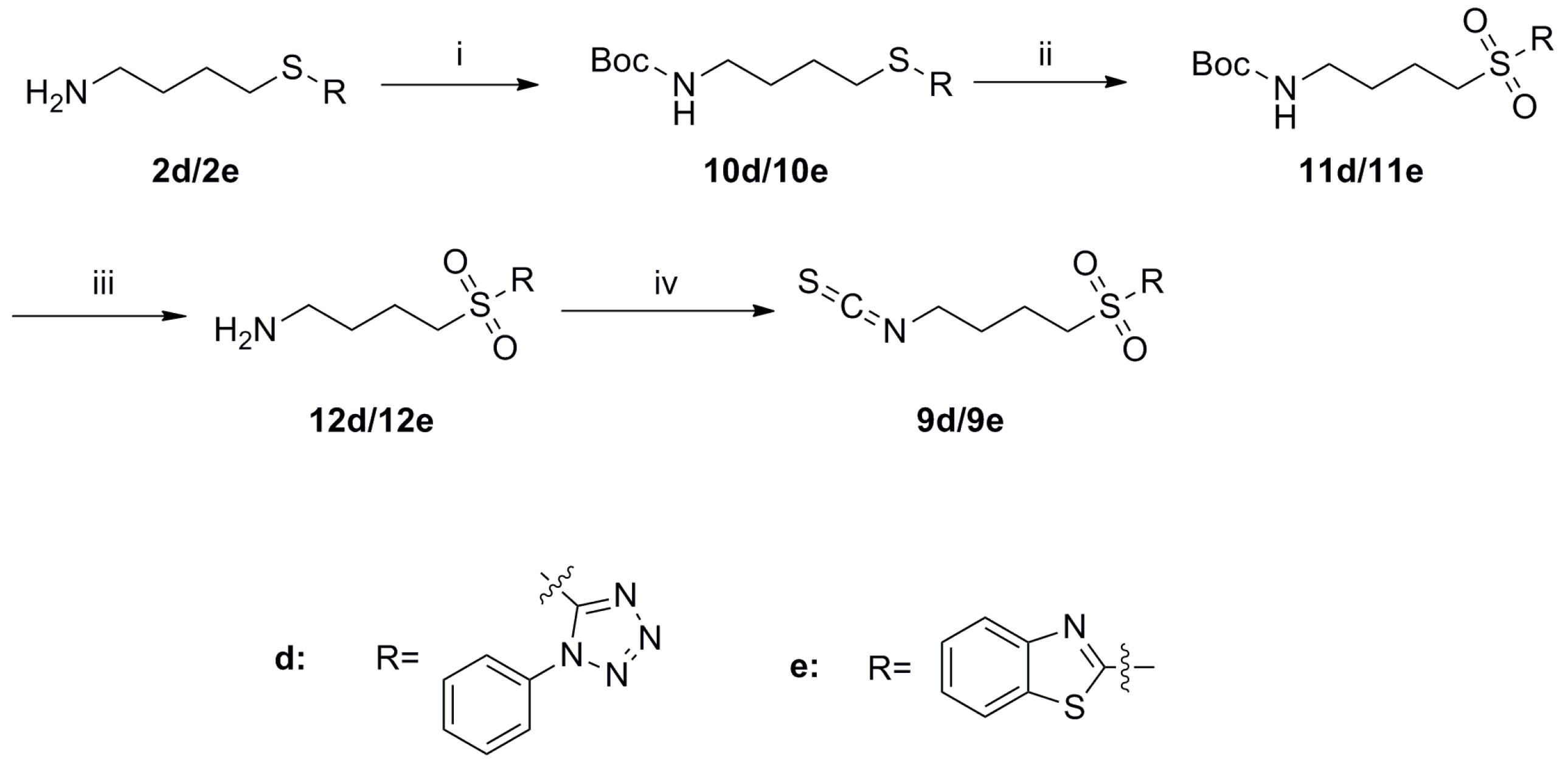
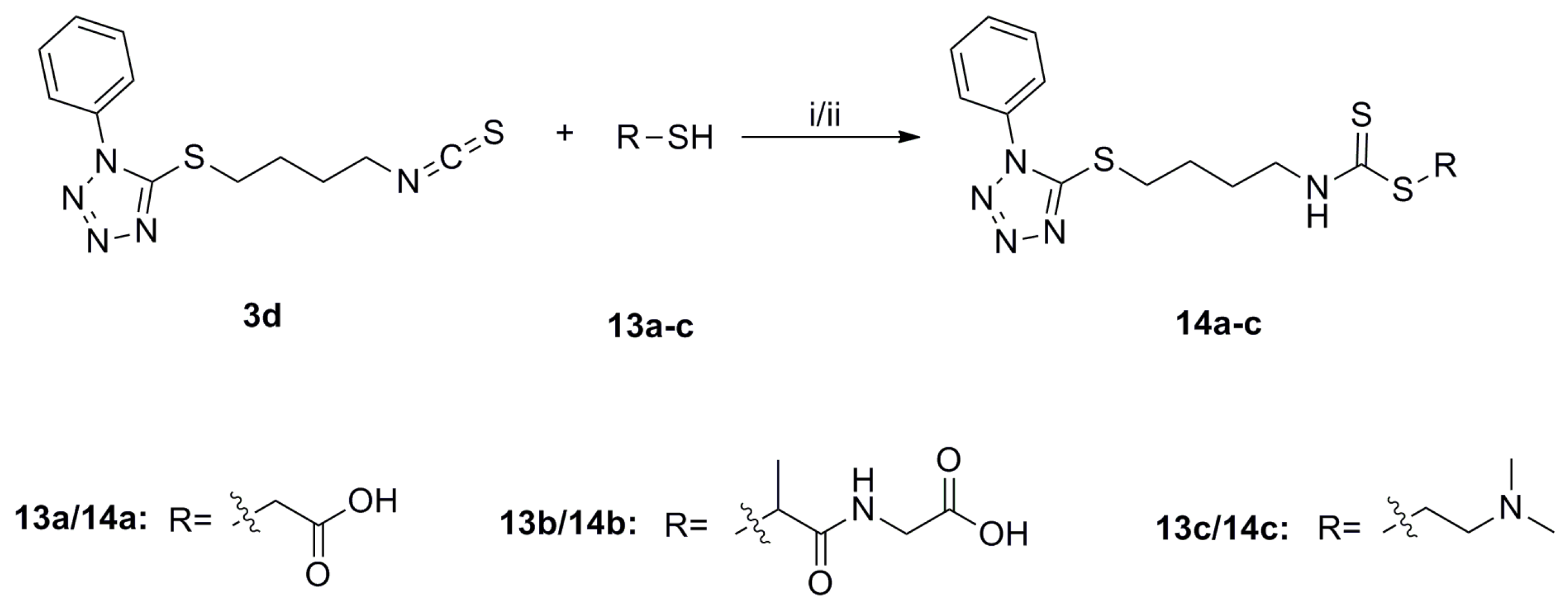
SFN analogues with heterocyclic ring were prepared as shown in Scheme 1, Scheme 2, Scheme 3 and Scheme 4, and water soluble derivatives were prepared as shown in Scheme 5.
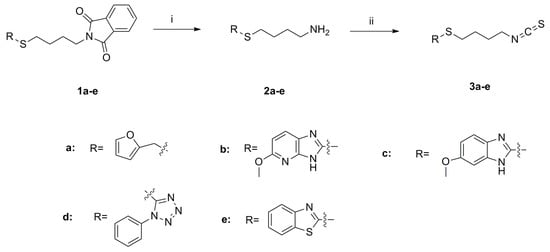
Scheme 1.
Synthesis of isothiocyanates 3a–e. Reagents and conditions: (i) Me-NH2, MeOH, r.t., 70%–85% (ii) CSCl2, NaOH (aq, 1 mol/L), CH2Cl2, 0 °C to r.t., 37%–81%.
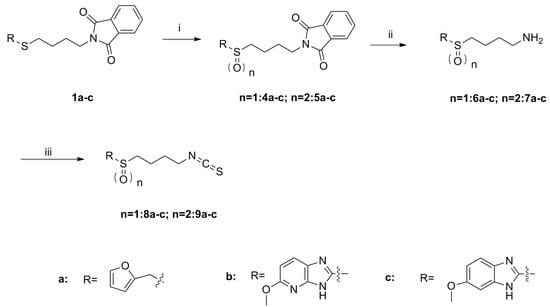
Scheme 2.
Synthesis of isothiocyanates 8a–c and 9a–c. Reagents and conditions: (i) TBHP, Ti(O-i-Pr)4, CH2Cl2, −20 to −10 °C, 58%–95%; (ii) Me-NH2, MeOH, r.t., 48%–64%; (iii) CSCl2, NaOH (aq, 1 mol/L), CH2Cl2, 0 °C to r.t., 53%–80%.
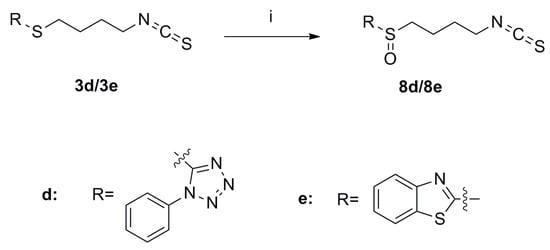
Scheme 3.
Synthesis of isothiocyanates 8d–e. Reagents and conditions: (i) m-CPBA, CH2Cl2, r.t., Yield: 8d: 20% and 8e: 40%.
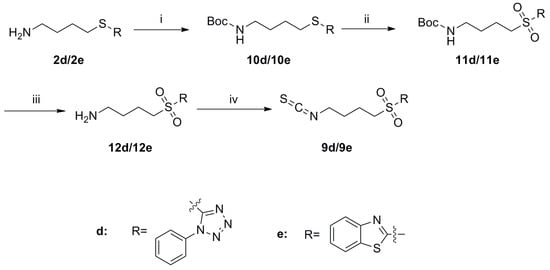
Scheme 4.
Synthesis of isothiocyanates 9d–e. Reagents and conditions: (i) (Boc)2O, Et3N, CH2Cl2, r.t., Yield: 10d: 63% and 10e: 77%; (ii) TBHP, Ti(O-i-Pr)4, CH2Cl2, r.t., Yield: 11d: 73% and 11e: 94%; (iii) CF3COOH, CH2Cl2, NaOH (aq,1 mol/L), r.t., crude; (iv) CSCl2, NaOH (aq,1 mol/L), CH2Cl2, 0 °C to r.t., Yield: 9d: 15% and 9e: 22%.
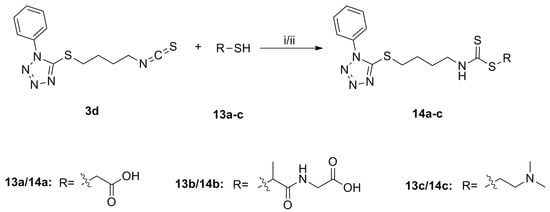
Scheme 5.
Synthesis of water soluble derivatives 14a–c. Reagents and conditions: (i) for 14a–b: DMAP, CH2Cl2, r.t., Yield: 14a: 16% and 14b: 23%; (ii) for 14c: EtOH, NaOH (aq.), 40 °C, r.t., 20%.
As shown in Scheme 1, the phthalimide groups in compounds 1a–e were hydrolyzed to yield amines 2a–e, which were then converted to isothiocyanates 3a–e with CSCl2 under basic conditions. Sulfides 1a–1c were oxidized by tert-butyl hydroperoxide (TBHP) to provide compounds 4a–c and 5a–c (Scheme 2) [20]. These compounds were subsequently hydrolyzed to give amines 6a–c and 7a–c, respectively. Amines 6a–c and 7a–c were further converted to isothiocyanates 8a–c and 9a–c.
Sulfides 3d and 3e were oxidized with m-chloroperoxybenzoic acid (m-CPBA) to provide compounds 8d and 8e (Scheme 3) [16].
As shown in Scheme 4, protection of the free amines 2d and 2e with Boc groups generated compounds 10d and 10e, respectively. Sulfides 10d and 10e were then oxidized with TBHP to provide sulfones 11d and 11e.
Following removal of the Bocgroups in compounds 11d and 11e, the free amines 12d and 12e were obtained, respectively. Treatment of 12d and 12e with CSCl2 then converted these compounds to the final isothiocyanate compounds 9d and 9e.
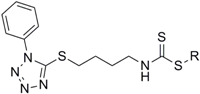
However, all of the tetrazole SFN analogues showed low solubility in water, and thus, were further modified to generate water soluble derivatives (Scheme 5). Sulfide 3d was converted to the water soluble derivatives, 14a–c, via treatment with thiol 13a–c under basic conditions. Unfortunately, water soluble derivatives of sulfoxide 8d and sulfone 9d could not be generated, because 8d and 9d were unstable under basic conditions, and cleavage of the heterocyclic ring occurred in each case.
2.2. Activity of Sulforaphane Analogues and Water Soluble Derivatives
These series of SFN analogues 3a–e, 8a–e, 9a–e and water soluble derivatives 14a–c were evaluated for their activities against MCF-7, SUM-159 and KG-1a cell lines, using SFN and parent molecule 3d as a positive controls. The results are summarized in Table 1 and Table 2, respectively.

Table 1.
Inhibitory effects of SFN analogues 3a–e, 8a–e, 9a–e on MCF-7, SUM-159 and KG-1a cell lines.
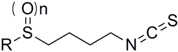


Table 2.
Inhibitory effects (IC50) of water soluble compounds 14a–c on MCF-7, SUM-159 and KG-1a cell lines.

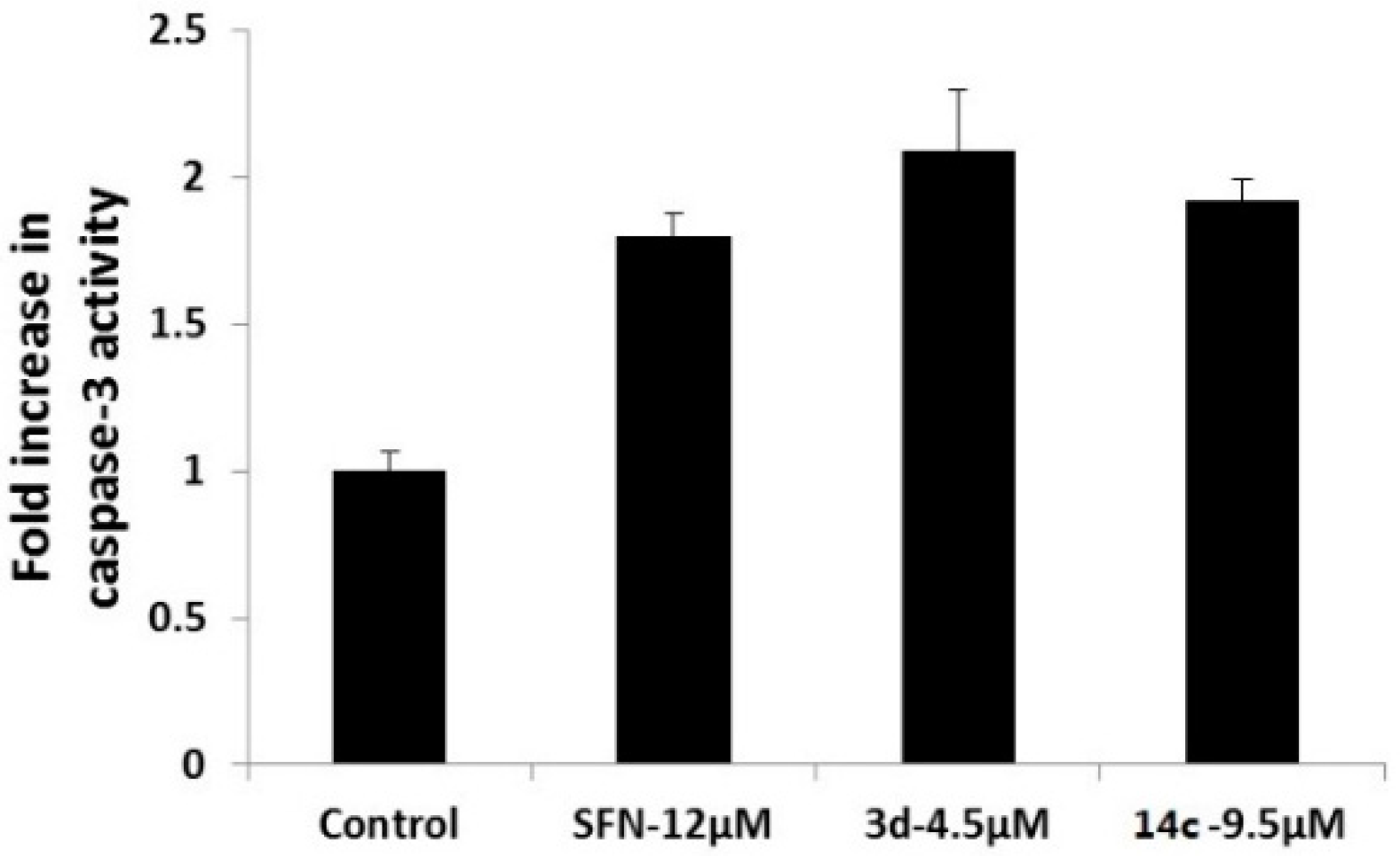
Moreover, the cytotoxicities of the water soluble compounds 14a–c were assayed, and their inhibitory effects on 293T cell line are shown in Table 3. SFN, 3d and 14c enhanced caspase-3 activity in SUM-159 cells is shown in Figure 1, and inhibitory effects on ALDH-positive cell subpopulation in SUM-159 cells is shown in Figure 2.

Table 3.
Effect of compounds 14a–c on 293T cell.
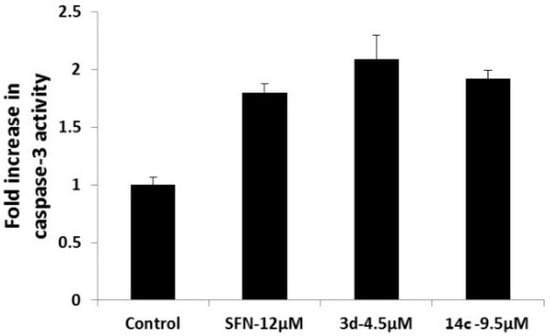
Figure 1.
SFN, 3d and 14c enhanced caspase-3 activity in SUM-159 cells.
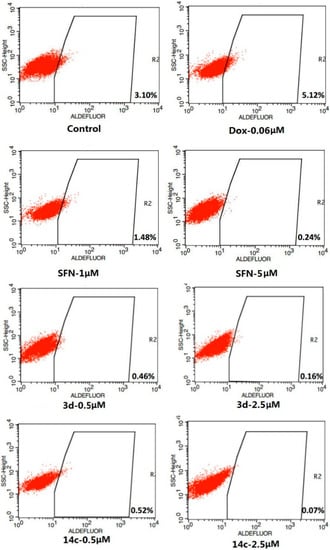
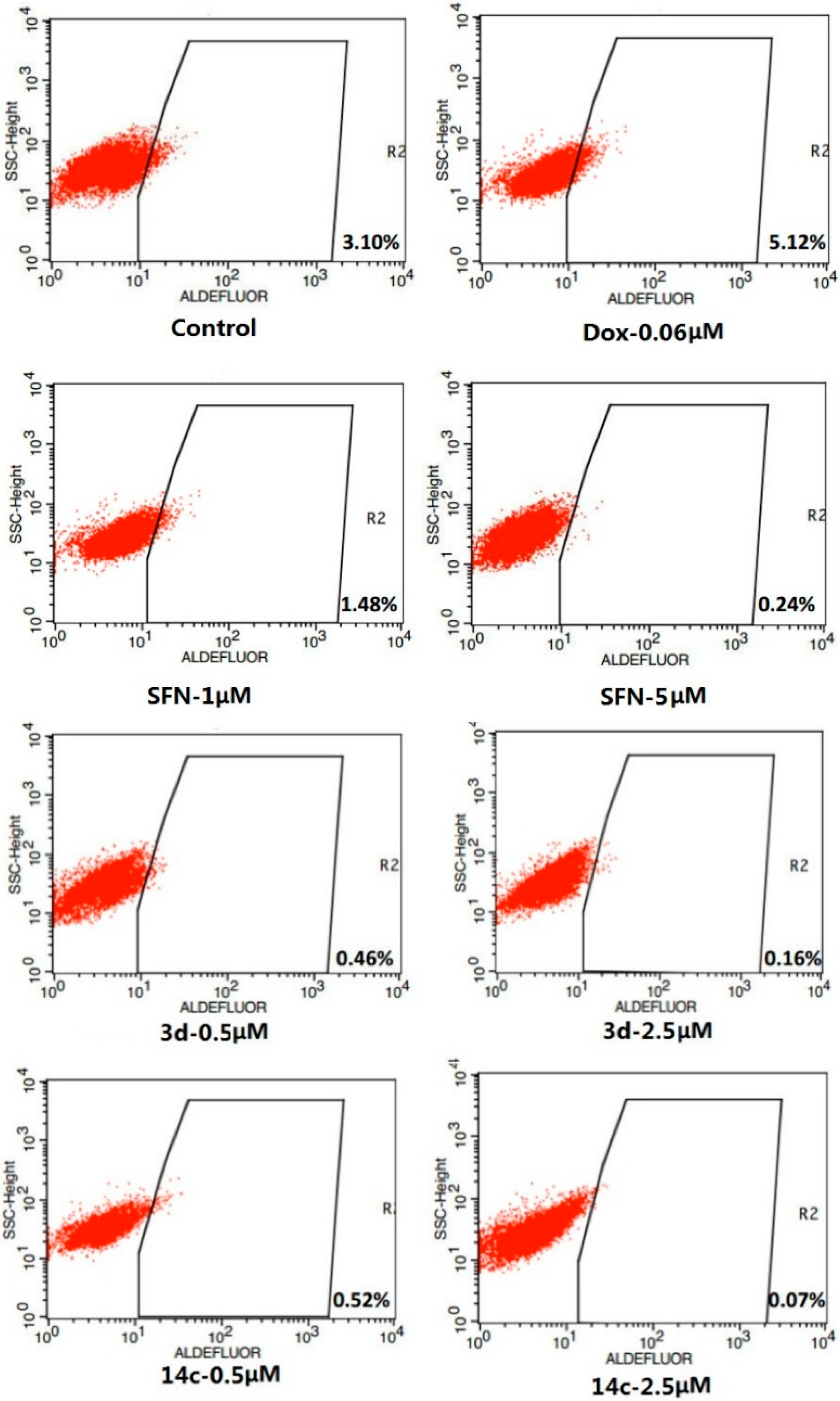
Figure 2.
Inhibitory effects of SFN, 3d and 14c on ALDH-positive cell subpopulation.
SFN demonstrated a higher potency against the stem cell-like KG-1a cell line (IC50 = 8.24 µM) compared with the breast cancer cell lines, MCF-7 (IC50 = 24.11 µM) and SUM-159 (IC50 = 7.69 µM). The furan compounds, 3a, 8a and 9a, exhibited lower levels of activity compared with SFN against the MCF-7 and SUM-159 cell lines, while sulfoxide 8a (IC50 = 10.87 µM) and sulfone 9a (IC50 = 11.98 µM) exhibited comparable inhibitory activities to SFN against the KG-1a cell line. The methoxypyridine compounds 3b, 8b and 9b exhibited lower activities compared with SFN, yet sulfide 3b was as potent as SFN against all three of the cell lines evaluated. Among the methoxy-substituted heteroaromatic compounds, 3c, 8c and 9c, sulfide 3c and sulfoxide 8c were slightly more potent than SFN against the MCF-7, SUM-159 and KG-1a cell lines, while sulfone 9c exhibited comparable inhibitory activity compared with SFN. Thetetrazole compounds, 3d, 8d, and 9d exhibited IC50 values of 2.66, 4.11, and 1.66 µM, respectively, against the MCF-7 cell line, and were approximately 4.9–13.5 times more potent than SFN (IC50 = 24.11 µM). Sulfide 8d (IC50 = 1.54 µM) was approximately 4 times more potent than SFN (IC50 = 7.69 µM) against the SUM-159 cell line. Against the KG-1a cell line, 8d and 9d exhibited IC50 values of 0.51 µM and 0.88 µM, respectively, which were 15.2 and 8.4 times more potent than SFN (IC50 = 8.24 µM).All of the thiazole compounds exhibited comparable activity levels to SFN, except sulfide 3e and sulfone 9e (IC50 > 33.33 µM) were much less active against the SUM-159 cell line than SFN.
Among the five types of heterocyclic SFN analogues that were tested, the tetrazole SFN analogues were the most active against the MCF-7, SUM-159 and KG-1a cell lines. Against the stem cell-like KG-1a cell line, IC50 values within 1 µM were observed for 8d and 9d.
The activities of water soluble compounds of 3d, i.e., 14a–c, were assayed against the MCF-7, SUM-159 and KG-1a cell lines in an independent experiment, and both SFN and 3d were applied as the positive controls.The water soluble derivative 14a, was found to be comparably active against MCF-7 cell lines, and less active against SUM-159 and KG-1a cell lines, and it was less active than the parent molecule 3d against all three of the cell lines. Compounds 14b exhibited higher activity than SFN against MCF-7, SUM-159 and KG-1a cell lines, with IC50 values of 7.84, 5.96, and 4.73 µM, respectively. Compared with 3d, compound 14b exhibited comparable activity against all three cell lines. Compound 14c exhibited higher activities than SFN against all three cell lines, with IC50 values of 7.30, 6.71 and 1.88 µM, respectively. As for inhibitory effect against the stem cell-like KG-1a cell line, water soluble derivative 14c was about 6.4 times more potent than SFN, and it was also more potent than its parent molecule 3d.
Compound 14a (CC50 = 10.78 μΜ) exhibited comparable activity to SFN (CC50 = 7.84 μΜ) against 293T cell. Compounds 14b and 14c exhibited higher activities than SFN against 293T cell, with CC50 of 5.39 μΜ and 4.56 μΜ, respectively. Finally, Compounds 14a–c exhibited less effect than the parent molecule 3d (CC50 = 2.12 μΜ) against 293T cell.
In the caspase-3 activity assays, SFN, 3d and 14c were found to significantly induce the activation of caspase-3 in the SUM-159 cell line (Figure 1). Based on these results, it appears that SFN, 3d and 14c potentially induce apoptosis in cancer cells by increasing caspase-3 activity.
In addition, SFN and doxorubicin (DOX) as well as 3d and 14c were assayed for their affects on the ALDH+ subpopulation in the SUM-159 cell line (Figure 2). SUM-159 cells were treated with SFN (1 and 5 µM), 3d (0.5 and 2.5 µM), 14c (0.5 and 2.5 µM) and DMSO for 4 days and subject to Aldefluor assay and flow cytometry analysis.
Compared to the negative control (containing 3.10% ALDH+ cells), treatment with 1 µM SFN significantly decreased the ALDH+ population to 1.48%, while 5 µM SFN further reduced the ALDH+ population to 0.24% (p < 0.05). At 0.5 µM of 3d or 14c, the ALDH+ population decreased to 0.46% and 0.52%, respectively (p < 0.001).At 2.5 µM of 3d or 14c, the ALDH+ population further decreased to 0.22% and 0.07%, respectively (p < 0.001). In contrast, a concentration of 0.06 µM DOX increased the ratio of ALDH+ cells to 5.12%.
In general, conventional drugs such as DOX are usually less active against ALDH+ cells, thereby leading to an increase in this cell population. In contrast, SFN, 3d, and 14c were found to selectively inhibit the growth of ALDH+ cells at concentrations that were 10-fold lower than their IC50 values against the SUM-159 cell.
3.Experimental Section
3.1.General Information
1H- and 13C-NMR spectra were obtained using a AV 400 spectrometer (Bruker, Madison, WI, USA) using CDCl3 as the solvent.Chemical shifts are reported in parts per million (ppm) relative to either a tetramethylsilane internal standard or solvent signals. Data are reported as follows: chemical shift, multiplicity (s = singlet, d = doublet, t = triplet, q = quartet, br. = broad, m = multiplet), coupling constants and integration.
3.2. General Procedure for the Synthesis of Compounds 1a–e
Compounds 1a–e were prepared according to a literature methid according to the literature method [19].
2-(4-((Furan-2-ylmethyl)thio)butyl)isoindoline-1,3-dione (1a): yield 90%, white solid; 1H-NMR (CDCl3) δ 7.86 (q, J1 = 3.2 Hz, J2 = 5.6 Hz, 2H), 7.73 (q, J1 = 2.8 Hz, J2 = 5.2 Hz, 2H), 7.34 (d, J = 0.8 Hz, 1H), 6.29 (t, J = 3.2 Hz, 1H), 6.18 (d, J = 2.8 Hz, 1H), 3.69–3.72 (m, 4H), 2.56 (t, J = 7.2 Hz, 2H), 1.75–1.83 (m, 2H), 1.60–1.66 (m, 3H); MS: [M + Na]+ 338.14.
2-(4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)thio)butyl)isoindoline-1,3-dione (1b): yield 89%, yellow solid; 1H-NMR (CDCl3) δ 7.86 (q, J1 = 3.2 Hz, J2 = 5.6 Hz, 2H), 7.72–7.75 (m, 3H), 6.64 (d, J = 8.8 Hz, 1H), 3.98 (s, 3H), 3.75 (t, J = 6.8 Hz, 2H), 3.39 (t, J = 6.8 Hz, 2H), 1.81–1.93 (m, 4H); MS: [M + H]+ 383.71.
2-(4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)thio)butyl)isoindoline-1,3-dione (1c): yield 74.1%, yellow solid; 1H-NMR (CDCl3) δ 7.84 (q, J1 = 3.6 Hz, J2 = 5.6 Hz, 2H), 7.72 (q, J1 = 3.2 Hz, J2 = 5.2 Hz, 2H), 7.40 (d, J = 8.8 Hz, 1H), 7.02 (s, 1H), 6.84 (dd, J1 =2.0 Hz, J2 = 8.8 Hz, 1H), 3.84 (s, 3H), 3.72 (t, J = 6.4 Hz, 2H), 3.33 (t, J = 6.8 Hz, 2H), 1.79–1.90 (m, 4H); 13C-NMR (CDCl3) δ 168.59, 156.28, 149.16, 134.09, 132.07, 123.34, 111.55, 55.94, 37.37, 32.36, 27.61, 27.01; MS: [M + H]+ 382.19; Mp: 87.6–88.9 °C.
2-(4-((5-Phenyl-1H-tetrazol-1-yl)thio)butyl)isoindoline-1,3-dione (1d): yield 87%, yellow solid; 1H-NMR (CDCl3) δ 7.86 (q, J1 = 3.2 Hz, J2 = 5.6 Hz, 2H), 7.74 (q, J1 = 3.2 Hz, J2 = 5.6 Hz, 2H), 7.54–7.60 (m, 5H), 3.76 (t, J = 6.8 Hz, 2H), 3.47 (t, J = 6.8 Hz, 2H), 1.84–1.98 (m, 4H).
2-(4-(Benzo[d]thiazol-2-ylthio)butyl)isoindoline-1,3-dione (1e): yield 92%, yellow solid; 1H-NMR (CDCl3) δ 7.85–7.91 (m, 3H), 7.72–7.77 (m, 3H), 7.44 (t, J = 8.0 Hz, 1H), 7.32 (t, J = 8.0 Hz, 1H), 3.78 (t, J = 6.4 Hz, 2H), 3.43–3.47 (m, 2H), 1.91–1.94 (m, 4H); 13C-NMR (CDCl3) δ 168.50, 166.81, 153.40, 135.34, 134.07, 132.21, 126.11, 124.28, 123.37, 121.65, 121.05, 37.48, 33.01, 27.79, 26.75; MS: [M + H]+ 369.12; Mp: 87.5–88.6 °C.
3.3. General Procedure for the Synthesis of Amines 2a–e, 6a–c and 7a–c
To a solution of compound 1 (1 mmol) in anhydrous MeOH (10 mL) was added methylamine (10 mL, 40% water solution) at room temperature. The mixture was stirred at room temperature overnight, and the solvent was removed under reduced pressure to give crude residue, which was purified by columnchromatography to obtain 2.The synthesis of 6 and 7 were similar to that of 2.
4-((Furan-2-ylmethyl)thio)butan-1-amine (2a): yield 71%, yellow oil; 1H-NMR (CDCl3) δ 7.35 (dd, J1 = 0.8 Hz, J2 = 2.0 Hz, 1H), 6.30 (dd, J1 = 2.0 Hz, J2 = 3.2 Hz, 1H), 6.16 (d, J = 3.2 Hz, 1H), 3.71 (s, 2H), 2.68 (t, J = 6.8 Hz, 2H), 2.51 (t, J = 7.2 Hz, 2H), 1.64–1.47 (m, 4H).
4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)thio)butan-1-amine (2b): yield 71%, white oil; 1H-NMR (CDCl3) δ 7.69 (d, J = 8.6 Hz, 1H), 6.59 (d, J = 8.6 Hz, 1H), 3.95 (s, 3H), 3.25 (t, J = 7.2 Hz, 2H), 2.85 (t, J = 6.7 Hz, 2H), 1.89 (td, J1 = 6.5 Hz, J2 = 8.0 Hz, J3 = 14.8 Hz, 2H), 1.69–1.62 (m, 2H); MS: [M + H]+ 253.24, [M − H]− 251.25.
4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)thio)butan-1-amine (2c): yield 71%, white solid; 1H-NMR (MeOH) δ 7.30 (d, J = 8.7 Hz, 1H), 6.93 (d, J = 2.4 Hz, 1H), 6.77 (dd, J1 = 2.4 Hz, J2 = 8.8 Hz, 1H), 3.76 (s, 3H), 3.17 (t, J = 7.2 Hz, 2H), 2.60 (t, J = 7.1 Hz, 2H), 1.78–1.70 (d, J = 7.2 Hz, 2H), 1.63–1.49 (m, 2H); 13C-NMR (CDCl3) δ 156.05, 149.93, 140.08, 134.69, 114.74, 111.06, 97.54, 55.95, 41.14, 32.09, 31.39, 27.11; MS: [M + H]+ 252.20; Mp: 105.4–106.9°C.
4-((5-Phenyl-1H-tetrazol-1-yl)thio)butan-1-amine (2d): yield 70%, yellow oil; 1H-NMR (CDCl3) δ 7.59–7.52 (m, 5H), 3.41 (t, J = 7.4 Hz, 2H), 2.75 (t, J = 7.0 Hz, 2H), 1.91–1.86 (m, 2H), 1.63–1.58 (m, 2H); MS: [M + H]+ 250.15.
4-(Benzo[d]thiazol-2-ylthio)butan-1-amine(2e): yield 85%, yellow oil; 1H-NMR (CDCl3) δ 7.59 (d, J = 8.1 Hz, 1H), 7.48 (d, J = 7.9 Hz, 1H), 7.14 (t, J = 7.7 Hz, 1H), 7.05–6.98 (m, 1H), 3.10 (t, J = 7.3 Hz, 2H), 1.65–1.58 (m, 2H), 1.41–1.33 (m, 2H), 1.19 (bs, 2H); 13C-NMR (CDCl3) δ 167.16, 153.41, 135.28, 126.11, 124.26, 121.56, 121.03, 41.67, 33.40, 32.77, 26.80; MS: [M + H]+ 239.26.
4-((Furan-2-ylmethyl)sulfinyl)butan-1-amine (6a): yield 48%, yellow oil; 1H-NMR (CDCl3): δ 7.41 (s, 1H), 6.39 (s, 2H), 4.04 (d, J = 3.0 Hz, 2H), 2.72 (t, J = 6.9 Hz, 2H), 2.68 (t, J = 7.6 Hz, 2H), 1.75–1.87 (m, 2H), 1.51–1.62 (m, 4H); 13C-NMR (CDCl3) δ 143.96, 143.42, 111.20, 51.25, 50.54, 41.42, 32.39, 19.79; MS: [M + H]+ 202.08.
4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)sulfinyl)butan-1-amine (6b): yield 47%, yellow oil; 1H-NMR (CD3OD) δ 7.78 (d, J = 8.6 Hz, 1H), 6.52 (d, J = 8.6 Hz, 1H), 5.44 (s, 1H), 3.88 (s, 3H), 3.28–3.23 (m, 2H), 2.88–2.75 (m, 2H), 1.78–1.61 (m, 4H); 13C-NMR (CD3OD) δ 162.12, 159.16, 155.91, 133.19, 129.19, 106.07, 53.82, 53.29, 40.52, 28.81, 20.70; MS: [M + H]+ 269.25.
4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)sulfinyl)butan-1-amine (6c): yield 77%, white oil; 1H-NMR (CD3OD) δ 7.36 (d, J = 8.9 Hz, 1H), 6.96 (d, J = 2.2 Hz, 1H), 6.73 (dd, J = 8.9, 2.3 Hz, 1H), 3.67 (s, 3H), 3.21 (s, 1H), 3.19–3.12 (m, 2H), 2.66–2.60 (m, 2H), 1.69–1.47 (m, 4H); 13C-NMR (CD3OD) δ 158.23, 155.34, 142.58, 138.03, 118.61, 114.48, 98.57, 56.12, 54.11, 40.90, 29.89, 20.50; MS: [M + H]+ 268.23.
4-((Furan-2-ylmethyl)sulfonyl)butan-1-amine (7a): yield 64%, white solid; 1H-NMR (CDCl3): δ 7.45 (d, J = 1.6 Hz, 1H), 6.51 (d, J = 3.2 Hz, 1H), 6.43 (dd, J1 = 2.0 Hz, J2 = 3.2 Hz, 1H), 4.29 (s, 2H), 2.93 (t, J = 8.0 Hz, 2H), 2.71 (t, J = 7.2 Hz, 2H), 1.82–1.90 (m, 2H), 1.52–1.59 (m, 2H); 13C-NMR (CDCl3) δ 143.91, 142.64, 112.33, 111.63, 52.70, 51.85, 41.49, 32.31, 19.45; MS: [M + H]+ 218.15; Mp: 105.9–107.1 °C.
4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)sulfonyl)butan-1-amine (7b): yield 46%, yellow oil; 1H-NMR (CD3OD) δ 7.85 (d, J = 8.7 Hz, 1H), 6.61 (d, J = 8.7 Hz, 1H), 3.93 (s, 3H), 3.39 (t, J = 6.9 Hz, 2H), 3.35 (s, 1H), 2.92 (t, J = 6.8 Hz, 2H), 2.68 (d, J = 2.0 Hz, 1H), 2.55 (s, 1H), 1.86–1.71 (m, 4H); 13C-NMR (CD3OD) δ 162.79, 155.12, 155.07, 132.83, 130.14, 107.59, 55.02, 53.75, 40.10, 27.18, 21.07; MS: [M + H]+ 285.23.
4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)sulfonyl)butan-1-amine (7c): yield 60%, white solid; 1H-NMR (DMSO-d6) δ 7.40 (d, J = 8.7 Hz, 1H), 6.99 (d, J = 2.3 Hz, 1H), 6.69 (dd, J = 8.8, 2.4 Hz, 1H), 3.73 (s, 3H), 3.41–3.33 (m, 2H), 2.79 (t, J = 7.1 Hz, 2H), 1.74–1.69 (m, 2H), 1.64–1.59 (m, 2H); 13C-NMR (DMSO-d6) δ 154.95, 154.81, 144.21, 139.14, 118.27, 111.25, 98.92, 55.23, 52.74, 38.70, 26.66, 19.55; MS: [M + H]+ 284.31; Mp: 138.6–139.9 °C.
3.4. General Procedure for the Synthesis of Sulfides 3a–e, Sulfoxides 8a–c and Sulfones 9a–c
To a solution of 2 (1 mmol) and NaOH (1 mol/L, 1.66 mmol) in anhydrous CH2Cl2 (10 mL) was added CSCl2 (2.55 mmol) at 0 °C. The mixture was stirred for 20 min, warmed to room temperature, and continued to stir for 3 h. The reaction mixture was diluted with brine (10 mL) and CH2Cl2 (10 mL), and extracted with CH2Cl2(10 mL × 2). The organic layer was dried over anhydrous Na2SO4, and solvent was removed under reduced pressure. The residue was purified by columnchromatography to obtain isothiocyanate 3. The synthesis of compounds 8 and 9 were similar to 3.
2-(((4-Isothiocyanatobutyl)thio)methyl)furan (3a): yield 78%, yellow oil; 1H-NMR (CDCl3): δ 7.36 (d, J1 = 1.8 Hz, J2 = 0.9 Hz,1H), 6.31 (dd, J1 = 3.2 Hz, J2 = 1.9 Hz, 1H), 6.18 (d, J = 2.8 HZ, 1H), 3.72 (s, 2H), 3.51 (t, J = 6.4 Hz, 2H), 2.53 (t, J = 7.0 Hz, 2H), 1.81–1.74 (m, 2H), 1.71–1.64 (m, 2H); 13C-NMR (CDCl3): δ 151.5, 142.2, 130.2, 110.5, 107.6, 44.7, 30.8, 28.9, 28.3, 26.0; MS:[M + H]+ 228.2.
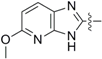
2-((4-Isothiocyanatobutyl)thio)-5-methoxy-3H-imidazo[4,5-b]pyridine (3b): yield 66%, yellow soild; 1H-NMR (CDCl3) δ 9.75 (bs,1H), 7.76 (d, J = 8.8 Hz, 1H), 6.63 (d, J = 8.4 Hz, 1H), 3.96 (s, 3H), 3.51 (t, J = 6.4 Hz, 2H), 3.34 (t, J = 6.4 Hz, 2H), 1.93–1.87 (m, 2H), 1.85–1.77 (m, 2H); 13C-NMR (CDCl3): δ 161.3, 149.2, 130.5, 127.6, 126.0, 105.6, 54.2, 44.7, 31.7, 28.9, 26.8; MS: [M + H]+ 295.14. Mp: 79.3–81.6 °C.
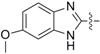
2-((4-Isothiocyanatobutyl)thio)-6-methoxy-1H-benzo[d]imidazole (3c): yield 37%, yellow soild; 1H-NMR (CDCl3) δ 7.40 (d, J = 8.8 Hz, 1H), 7.01 (s, 1H), 6.85 (dd, J1 = 8.8 Hz, J2 = 2.4 Hz, 1H), 3.83 (s, 3H), 3.53 (t, J = 6.4 Hz, 2H), 3.32 (t, J = 6.8 Hz, 2H), 1.94–1.79 (m, 4H); 13C-NMR (CDCl3): δ 156.5, 148.8, 111.7, 56.0, 44.7, 32.2, 28.9, 26.9. HRMS-ESI (+) for C13H16N3OS2, calculated 294.0735, found 294.0745 [M + H]+; Mp: 58.9–60.4°C.
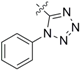
1-((4-Isothiocyanatobutyl)thio)-5-phenyl-1H-tetrazole (3d): yield 81%, yellow solid; 1H-NMR (CDCl3): δ 7.52–7.58 (m, 5H), 3.59 (t, J = 6.4 Hz, 2H), 3.43 (t, J = 7.0 Hz, 2H), 1.96–2.03 (m, 2H), 1.83–1.90 (m, 2H); 13C-NMR (CDCl3): δ 154.0, 133.7, 130.2, 129.9, 123.9, 44.5, 32.3, 28.9, 26.4; HRMS-ESI (+) for C12H14N5S2, calculated 292.0691, found 292.0964 [M + H]+; Mp: 52.8–55.0 °C.
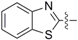
2-((4-Isothiocyanatobutyl)thio)benzo[d]thiazole (3e): yield 64%, yellow soild; 1H-NMR (CDCl3) δ 7.87 (d, J = 8.4 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.41 (t, J = 7.6 Hz, 1H), 7.29 (t, J = 7.6 Hz, 1H), 3.57 (t, J = 6.4 Hz, 2H), 3.38 (t, J = 7.2 Hz, 2H), 1.84–2.00 (m, 4H); 13C-NMR (CDCl3) δ 166.3, 153.2, 135.3, 126.1, 124.4, 121.6, 121.1, 44.6, 32.5, 28.9, 26.5; HRMS-ESI (+) for C12H13N2S3, ca lculated 281.0241, found 281.0250 [M + H]+; Mp: 60.2–61.9 °C.
2-(((4-Isothiocyanatobutyl)sulfinyl)methyl)furan (8a): yield 53%, yellow oil; 1H-NMR (CDCl3): δ 7.44 (s, 1H), 6.41 (s, 2H), 4.08 (d, J = 7.6 Hz, 2H), 3.57 (t, J = 6.0 Hz, 2H), 2.71–2.60 (m, 2H), 1.95–1.78 (m, 4H); 13C-NMR (CDCl3): δ 143.6, 111.4, 111.3, 50.8, 50.5, 44.6, 29.1, 19.9; HRMS-ESI (+) for C10H13NO2S2Na, calculated 266.0285, found 266.0294 [M + Na]+.
2-((4-Isothiocyanatobutyl)sulfinyl)-5-methoxy-3H-imidazo[4,5-b]pyridine (8b): yield 58%, yellow solid; 1H-NMR (CDCl3): δ 7.89 (d, J = 8.8 Hz, 1H),6.78 (d, J = 8.8 Hz, 1H), 4.00 (s, 3H), 3.56 (t, J = 5.6 Hz, 2H), 3.37–3.43 (m, 2H), 2.18–2.04 (m, 1H), 1.94–1.78 (m, 3H); 13C-NMR (CDCl3): δ 162.6, 150.8, 131.5, 127.8, 108.9, 54.1, 53.5, 44.7, 29.0, 19.1; HRMS-ESI (+) for C12H14N4O2S2Na, calculated 333.0456, found 333.0467 [M + Na]+; Mp: 144.5–146.1 °C.
2-((4-Isothiocyanatobutyl)sulfinyl)-6-methoxy-1H-benzo[d]imidazole (8c): yield 51%, yellow soild; 1H-NMR (CDCl3): δ 7.58 (d, J = 8.9 Hz, 1H), 7.09 (d, J = 2.4 Hz, 1H), 7.00 (dd, J1 = 8.8 Hz, J2 = 2.0 Hz, 1H), 3.87 (s, 3H), 3.56–3.52 (m, 2H), 3.44–3.28 (m, 2H), 2.10–2.02 (m, 1H), 1.92–1.76 (m, 3H); 13C-NMR (CDCl3): δ 157.6, 151.0, 131.5, 114.5, 56.0, 53.8, 44.7, 29.0, 19.3; HRMS-ESI (+) for C13H15N3O2S2Na, calculated 332.0504, found 332.0488 [M + Na]+; Mp: 93.4–95.2 °C.
2-(((4-Isothiocyanatobutyl)sulfonyl)methyl)furan (9a): yield 63%, white solid; 1H-NMR (CDCl3): δ 7.49 (s, 1H) 6.54 (d, J = 3.2 Hz, 1H), 6.48–6.44 (m, 1H), 4.33 (s, 2H), 3.57 (t, J = 6.4 Hz, 2H), 2.96 (t, J = 7.6 Hz, 2H), 2.00–1.93 (m, 2H), 1.88–1.82 (m, 2H); 13C-NMR (CDCl3): δ 144.1, 142.4, 112.6, 111.8, 53.1, 51.1, 44.6, 28.8, 19.5; HRMS-ESI (+) for C10H13NO3S2Na, calculated 282.0235, found 282.0242 [M + Na]+; Mp: 67.7–68.9 °C.
2-((4-Isothiocyanatobutyl)sulfonyl)-5-methoxy-3H-imidazo[4,5-b]pyridine (9b): yield 80%, white soild; 1H-NMR (CDCl3): δ 8.00 (d, J = 8.8 Hz, 1H), 6.85 (d, J = 8.8 Hz, 1H), 3.98 (s, 3H) , 3.54–3.58 (m, 4H), 2.03–1.95 (m, 2H), 1.89–1.82 (m, 2H); 13C-NMR (CDCl3): δ 171.6, 163.9, 145.1, 131.3, 110.8, 54.3, 54.1, 44.5, 28.5, 19.9; HRMS-ESI (+) for C12H14N4O3S2Na, calculated 349.0405, found 349.0414 [M + Na]+; Mp: 65.5–67.3 °C.
2-((4-Isothiocyanatobutyl)sulfonyl)-6-methoxy-1H-benzo[d]imidazole (9c): yield 56%, yellow soild; 1H-NMR (CDCl3) δ 7.75–7.56 (m, 1H), 7.06–7.08 (m, 2H), 3.86 (s, 3H), 3.52–3.57 (m, 4H), 1.95–2.03 (m, 2H), 1.82–1.89 (m, 2H); 13C-NMR (CDCl3): δ 158.9, 14598, 131.4, 116.7, 56.0, 54.2, 44.6, 28.5, 20.0; HRMS-ESI (+) for C13H15N3O3S2Na, calculated 348.0453, found 348.0467 [M + Na]+; Mp: 112.2–113.5 °C.
3.5. General Procedure for the Synthesis of Amides 4a–c and 5a–c
To a solution of compound 1 (1 mmol) in anhydrous CH2Cl2 (20 mL) under an argon atmosphere was added Ti(O-i-Pr)4 (1 mmol), and the reaction mixture was stirred at room temperature for 15 min. Then the reaction mixture was cooled to−20 °C for 20 min, and a solution of TBHP (2 mmol) in anhydrous CH2Cl2 (3.2 mL) was added slowly. The mixture was stirred at −20 °C for 8–12 h, and water (20 mL) was added, the reaction mixture was stirred for 1 h, and the resulting gel was dissolved with ethyl acetate (30 mL× 2). After remove the solvent under reduced pressure, and obtain crude 4 or 5, which was purified withcolumnchromatography.
2-(4-((Furan-2-ylmethyl)sulfinyl)butyl)isoindoline-1,3-dione (4a): yield 90%, white soild; 1H-NMR (CDCl3): δ 7.84 (dd, J1 = 5.6 Hz, J2 = 3.2 Hz, 2H), 7.72 (dd, J1 = 5.5 Hz, J2 = 3.0 Hz, 2H), 7.41 (dd, J1 = 0.8 Hz, J2 = 2.0 Hz, 1H), 6.40–6.37 (m, 2H), 4.05 (d, J = 2.8 Hz, 2H), 3.71–3.74 (m, 2H), 2.62–2.74 (m, 2H), 1.76–1.89 (m, 4H); 13C-NMR (CDCl3) δ 168.31, 143.93, 143.48, 134.05, 132.04, 123.30, 111.26, 50.96, 50.69, 37.20, 27.82, 19.81; MS: [M + H]+ 332.23; Mp: 75.6–77.1°C.
2-(4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)sulfinyl)butyl)isoindoline-1,3-dione (4b): yield 58%, white soild; 1H-NMR (CDCl3) δ 7.86 (d, J = 8.8 Hz, 1H), 7.79 (dd, J1 = 2.0 Hz, J2=3.6 Hz, 2H), 7.70 (q, J1 = 2.0 Hz, J2 = 3.6 Hz, 2H), 6.77 (d, J = 6.0 Hz, 1H), 4.00 (s, 3H), 3.70 (t, J = 4.4 Hz, 2H), 3.39–3.44 (m, 1H), 3.28–3.33 (m, 1H), 2.00–2.08 (m, 2H), 1.85–1.93 (m, 2H); 13C-NMR (CDCl3) δ 168.34, 162.54, 134.08, 131.96, 130.97, 123.32, 109.13, 108.32, 54.04, 37.17, 29.81, 27.60, 19.34; MS: [M + H]+ 399.18; Mp: 80.6–81.7 °C.
2-(4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)sulfinyl)butyl)isoindoline-1,3-dione (4c): yield 82%, white soild; 1H-NMR (CDCl3) δ 11.84 (s, 1H), 7.65–7.62 (m, 2H), 7.57–7.55 (m, 2H), 7.43 (bs, 1H), 6.83 (d, J = 8.0 Hz, 2H), 3.75 (s, 3H), 3.57 (t, J = 6.9 Hz, 2H), 3.37–3.14 (m, 2H), 1.92–1.63 (s, 4H); 13C-NMR (CDCl3) δ 168.35, 157.42, 151.15, 134.07, 131.96, 123.33, 114.39, 55.90, 54.19, 37.16, 27.58, 19.37; MS: [M + H]+ 398.40; Mp: 141.3–142.8°C.
2-(4-((Furan-2-ylmethyl)sulfonyl)butyl)isoindoline-1,3-dione (5a): yield 60%, white soild; 1H-NMR (CDCl3) δ 7.84 (dd, J = 5.4, 3.0 Hz, 2H), 7.72 (dd, J = 5.4, 3.1 Hz, 2H), 7.42 (s, 1H), 6.51 (d, J = 3.2 Hz, 1H), 6.41–6.40 (m, 1H), 4.30 (s, 2H), 3.71 (t, J = 6.2 Hz, 2H), 2.98 (t, J = 7.2 Hz, 2H), 1.89–1.80 (m, 4H); 13C-NMR (CDCl3) δ 168.42, 143.98, 142.56, 134.20, 132.12, 123.46, 112.44, 111.67, 52.88, 51.52, 37.06, 27.49, 19.39; MS: [M + H]+ 348.22; Mp: 81.9–83.2°C.
2-(4-((5-Methoxy-3H-imidazo[4,5-b]pyridin-2-yl)sulfonyl)butyl)isoindoline-1,3-dione (5b): yield 95%, white soild; 1H-NMR (CDCl3): δ 7.94 (d, J = 8.8 Hz, 1H), 7.76 (q, J1 = 5.2 Hz, J2 = 3.2 Hz, 2H), 7.68 (dd, J1 = 3.2 Hz, J2 = 5.6 Hz, 2H), 6.82 (d, J = 9.2 Hz, 1H), 3.99 (s, 3H), 3.68 (t, J = 6.4 Hz, 2H), 3.56 (t, J = 6.8 Hz, 2H), 1.80–1.97 (m, 4H); 13C-NMR (DMSO-d6) δ 167.90, 162.32, 146.48, 134.35, 131.49, 122.97, 109.50, 53.45, 48.65, 36.64, 26.45, 19.66; MS: [M + H]+ 415.17; Mp: 91.3–92.8 °C.
2-(4-((6-Methoxy-1H-benzo[d]imidazol-2-yl)sulfonyl)butyl)isoindoline-1,3-dione (5c): yield 60%, white soild; 1H-NMR (CDCl3): δ 7.69–7.72 (m, 2H), 7.64–7.67 (m, 2H), 7.59 (d, J = 8.8 Hz, 1H), 7.05 (s, 1H), 7.00 (dd, J1 = 9.0 Hz, J2 = 2.4 Hz, 1H), 3.82 (s, 3H), 3.66 (t, J = 6.6 Hz, 2H), 3.56 (t, J = 6.8 Hz, 2H), 1.72–1.92 (m, 4H); 13C-NMR (CDCl3) δ 168.42, 164.11, 144.77, 144.14, 134.18, 132.52, 132.01, 130.38, 123.44, 110.29, 54.42, 54.32, 36.88, 27.10, 19.80; MS: [M + H]+ 414.18; Mp: 101.5–102.4°C.
3.6. General Procedure for the Synthesis of Sulfoxide Compounds 8d and 8e
To a solution of compound 3 (1 mmol) in anhydrous CH2Cl2 (20 mL) under an argon atmosphere was added MCPBA (2 mmol) at 0 °C, and the resulting reaction mixture was stirred at room temperature for overnight. Saturated sodium bicarbonate solution was added, and the aqueous layer was extracted with CH2Cl2 (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure, the residue was purified with columnchromatography to obtain 8.
1-((4-Isothiocyanatobutyl)sulfinyl)-5-phenyl-1H-tetrazole (8d): yield 20%, yellow soild; 1H-NMR (CDCl3): δ 7.73–7.70 (m, 2H), 7.65–7.60 (m, 3H), 3.72 (dt, J1 = 13.4 Hz, J2 = 7.4 Hz, 1H), 3.61 (t, J = 6.3 Hz, 2H), 3.51–3.58 (m, 1H), 1.99–2.07 (m, 2H), 1.89–1.96 (m, 2H); 13C-NMR (CDCl3) δ 160.0, 133.1, 131.5, 130.2, 125.1, 51.8, 44.6, 29.0, 19.9; HRMS-ESI (+) for C12H14N5OS2, calculated 308.0640, found 308.0644 [M + H]+; Mp: 69.5–71.2 °C.
2-((4-Isothiocyanatobutyl)sulfinyl)benzo[d]thiazole (8e): yield 40%, yellow soild; 1H-NMR (CDCl3): δ 8.08 (d, J = 8.0 Hz, 1H), 8.01 (d, J = 8.0 Hz, 1H), 7.58 (m, 1H), 7.50 (m, 1H), 3.57 (t, J = 6.4 Hz, 2H), 3.36–3.20 (m, 2H), 2.15–2.06 (m, 1H), 1.81–1.96 (m, 3H); 13C-NMR (CDCl3) δ 176.9, 153.9, 136.0, 131.3, 127.1, 126.3, 124.0, 122.4, 55.2, 44.5, 28.9, 19.1; HRMS-ESI (+) for C12H12N2OS3Na, calculated 319.000, found 319.0014 [M + Na]+; Mp: 79.5–81.1 °C.
3.7. General Procedure for the Synthesis of Tert-Butyl Carbonate Sulfide Compounds 10d and 10e
To a solution of compound 2 (1 mmol) in anhydrous CH2Cl2 (20 mL) was added Et3N (2 mmol), and the reaction mixture was stirred at room temperature for 30 min. (Boc)2O was added, and then the mixture was stirred at room temperature for another 8 h. Water (10 mL) was added to quench the reaction, and the aqueous layer was extracted with CH2Cl2 (10 mL × 2). The combined organic layer was dried over anhydrous Na2SO4, and the solvent was removed under reduced pressure, and the crude residue was purified with columnchromatography to obtain 10.
tert-Butyl (4-((5-phenyl-1H-tetrazol-1-yl)thio)butyl)carbamate (10d): yield 63%, yellow oil; 1H-NMR (CDCl3) δ 7.59–7.53 (m, 5H), 3.40 (t, J = 7.6 Hz, 2H), 3.17 (d, J = 6.2 Hz, 2H), 1.92–1.84 (m, 2H), 1.61–1.68 (m, 2H), 1.43 (s, 9H); 13C-NMR (CDCl3) δ 156.04, 154.35, 133.74, 130.18, 129.86, 123.89, 79.25, 39.86, 32.86, 29.13, 28.47, 26.53; MS: [M + H]+ 350.20.
tert-Butyl (4-(benzo[d]thiazol-2-ylthio)butyl)carbamate (10e): yield 77%, yellow oil; 1H-NMR (CDCl3) δ 7.89 (d, J = 8.0 Hz, 1H), 7.75 (d, J = 8.0 Hz, 1H), 7.41 (m, 1H), 7.35–7.27 (m, 1H), 3.36 (t, J = 7.2 Hz, 2H), 3.21 (m, 2H), 1.85–1.92 (m, 2H), 1.64–1.71 (m, 2H), 1.44 (s, 9H); 13C-NMR (CD3OD) δ 169.29, 158.51, 154.37, 136.17, 127.34, 125.54, 122.26, 122.11, 79.88, 40.66, 34.03, 30.00, 28.77, 27.79; MS: [M + H]+ 339.16.
3.8. General Procedure for the Synthesis of Tert-Butyl Carbonate Sulfone Compounds 11d and 11e
To a solution of compound 10 (1 mmol) in anhydrous CH2Cl2 (20 mL) under an argon atmosphere was added titanium tetraisopropoxide (1 mmol), and the reaction mixture was stirred at room temperature for 15 min, and then was cooled to −20 °C for 20 min. A solution of TBHP (2 mmol) in anhydrous CH2Cl2 (3.2 mL) was added slowly, the mixture was stirred for 8 to 12 h at room temperature, and then water (20 mL) was added. The mixture was stirred for another 1 h, the resulting gel was dissolved with ethyl acetate (30 mL × 2). After the solvent was removed under reduced pressure, the crude oil was purified by columnchromatography.
tert-Butyl (4-((5-phenyl-1H-tetrazol-1-yl)sulfonyl)butyl)carbamate (11d): yield 73%, yellow soild; 1H-NMR (CDCl3): δ 7.68–7.70 (m, 2H), 7.63–7.58(m, 3H), 3.78 (t, J = 8.0 Hz, 2H), 3.18 (dd, J1 = 6.4 Hz, J2 = 12.4 Hz, 2H), 2.05–1.97 (m, 2H), 1.74–1.67 (m, 2H); 13C-NMR (CDCl3) δ 156.08, 153.53, 133.14, 131.60, 129.84, 125.21, 79.60, 55.68, 39.58, 28.80, 28.50, 19.64; MS: [M + H]+ 382.22; Mp: 94.1–95.5°C.
tert-Butyl (4-(benzo[d]thiazol-2-ylsulfonyl)butyl)carbamate (11e): yield 94%, yellow soild; 1H-NMR (CDCl3) δ 8.27–8.19 (m, 1H), 8.02 (d, J = 7.6 Hz, 1H), 7.67–7.55 (m, 2H), 3.55 (t, J = 8.0 Hz, 2H), 3.14 (d, J = 5.5 Hz, 2H), 1.97–1.89 (m, 2H), 1.66 (p, J = 7.1 Hz, 2H), 1.41 (s, 9H); 13C-NMR (CDCl3) δ 165.81, 156.01, 152.77, 136.83, 128.16, 127.78, 125.57, 122.45, 79.44, 54.34, 39.71, 28.84, 28.45, 19.84; MS: [M + H]+ 369.01; Mp: 103.6–104.5 °C.
3.9. General Procedure for the Synthesis of Sulfone Compounds 9d and 9e
To a solution of compound 11 (1 mmol) in dry CH2Cl2 (10 mL) was added CF3COOH (2 mmol) and stirred at room temperature for 2 h. Then NaOH (1 mmol/L) was added until pH = 9, extracted with CH2Cl2 (10 mL). Then organic layer 12 was applied directly to the next step reaction followed as synthesis of compound 9.
1-((4-Isothiocyanatobutyl)sulfonyl)-5-phenyl-1H-tetrazole (9d): yield 15%, yellow soild; 1H-NMR (CDCl3): δ 7.79–7.58 (m, 5H), 3.83 (t, J = 7.7 Hz, 2H), 3.64 (t, J = 6.4 Hz, 2H), 2.19–2.11 (m, 2H), 1.98–1.91 (m, 2H); 13C-NMR (CDCl3) δ 153.4, 133.0, 131.7, 129.9, 125.2, 55.2, 44.5, 28.5, 19.9; HRMS-ESI (+) for C12H14N5O2S2, calculated 324.0589, found 324.0613 [M + H]+; Mp: 91.4–92.7 °C.
2-((4-Isothiocyanatobutyl)sulfonyl)benzo[d]thiazole (9e): yield 22%, yellow soild; 1H-NMR (CDCl3) δ 8.24 (d, J = 8.0 Hz, 1H), 8.04 (d, J = 7.6 Hz, 1H), 7.60–7.68 (m, 2H), 3.56–3.60 (m, 4H), 2.09–2.02 (m, 2H), 1.94–1.87 (m, 2H); 13C-NMR (CDCl3) δ 165.5, 152.8, 136.9, 128.3, 127.9, 125.7, 122.5, 53.8, 44.5, 28.6, 20.1; HRMS-ESI (+) for C12H12N2O2S3Na, calculated 334.9958, found 334.9978 [M + Na]+; Mp: 101.1–102.6 °C.
3.10. General Procedure for the Synthesis of Tetrazole Acids Compounds 14a–b
To a solution of compound 3d (1 mmol) in dried CH2Cl2 (2 mL) was added DMAP (0.2 mmol) and stirred at room temperature for 5 min. Then N-acetylcysteine (0.8 mmol) or thiopronin (0.8 mmol) was added, and the reaction mixture was stirred for 4 h. Added 10% aqueous citric acid solution (10 mL) and CH2Cl2 (10 mL). Then organic layer was dried with anhydrous sodium sulfate, then purified by silica gel column and got compound 14.
2-(((4-((5-Phenyl-1H-tetrazol-1-yl)thio)butyl)carbamothioyl)thio)acetic acid (14a): yield 16%, yellow oil; 1H-NMR (CDCl3): δ 7.57–7.55 (m, 5H), 4.04 (q, J1 = 8.5 Hz, J2 = 6.9 Hz, 2H), 4.00 (s, 2H), 3.41 (q, J1 = 8.3 Hz, J2 = 7.5 Hz, 2H), 2.04–7.73 (m, 4H); 13C-NMR (CDCl3) δ 201.4, 174.0, 154.2, 133.6, 130.2, 129.8, 123.9, 43.8, 35.5, 32.6, 27.0, 26.3. HRMS-ESI (+) for C14H18N5O2S3, calculated 384.0623, found 384.0625 [M + H]+.
2-(2-(((4-((5-Phenyl-1H-tetrazol-1-yl)thio)butyl)carbamothioyl)thio)propanamido)acetic acid (14b): yield 23%, yellow oil; 1H-NMR (CDCl3): δ 8.75 (t, J = 5.2 Hz, 1H), 7.57–7.56 (m, 5H), 7.44 (t, J = 5.6 Hz, 1H), 4.55 (q, J = 7.2 Hz, 1H), 4.15 (dd, J1 = 6.0 Hz, J2 = 18.0 Hz, 1H), 3.97 (dd, J1 = 5.2 Hz, J2 = 18.4 Hz, 1H), 3.91–3.83 (m, 1H), 3.80–3.72 (m, 1H), 3.44–3.34 (m, 2H), 1.97–1.91 (m, 2H), 1.88–1.83 (m, 2H), 1.55 (d, J = 7.6 Hz, 3H); 13C-NMR (CDCl3) δ 195.5, 177.2, 173.5, 154.6, 133.5, 130.3, 129.9, 124.0, 46.7, 32.7, 29.8, 27.0, 26.7, 20.9, 16.5; HRMS-ESI (+) for C17H23N6O3S3, calculated 455.0994, found 455.0995 [M + H]+.
3.11. General Procedure for the Synthesis Tetrazole N-dimethyl Compound 14c
To a solution of 2-(dimethylamino)ethanethiol (0.71 mmol) in 95% EtOH (1 mL) was added 3d (0.71 mmol), and the reaction mixture was stirred for 10 h at 40 °C. Then organic layer was dried with anhydrous sodium sulfate, then purified by silica gel column to give compound 14c.
2-(Dimethylamino)ethyl(4-((5-phenyl-1H-tetrazol-1-yl)thio)butyl)carbamodithioate (14c): yield 20%, yellow oil; 1H-NMR (CDCl3): δ 11.07 (s, 1H), δ 7.55–7.51 (m, 5H), 3.71–3.66 (m, 2H), 3.39 (t, J = 7.2 Hz, 2H), 3.01 (t, J = 5.2 Hz, 2H), 2.72 (t, J = 5.6 Hz, 2H). 2.30 (s, 6H), 1.96–1.84 (m, 2H), 1.82–1.75 (m, 2H); 13C-NMR (CDCl3) δ 196.4, 154.2, 133.5, 130.1, 129.8, 123.8, 60.9, 46.2, 45.1, 34.0, 32.6, 27.2, 26.7; HRMS-ESI (+) for C16H25N6S3, calculated 397.1303, found 397.1298 [M + H]+.
3.12. Bioogical Activity
3.12.1. Inhibition of Cell Growth in Vitro
MCF-7, SUM-159, KG-1a and 293T cellswere from GuangzhouJennio Biotech Co.Ltd. (Guangzhou, China). They were cultured in RPMI 1640 supplemented with 10% FBS at 37 °C in a 5% CO2 incubator. Cells were seeded in 96-well plates at a density of 3000 cells per well. All cells were treated for 48 h, with increasing concentrations of different compounds.Cell viability was measured using an MTT assay which was performed following the manufacturer’s protocol. The number of living cells was directly proportional to the absorbance at 490 nm of a formazan product reduced from MTT by living cells. The IC50 value was obtained using SPSS 11.5 software. The results were derived from three independent experiments performed in triplicate.
3.12.2. Caspase-3 Activity Assay
SUM-159 cells were treated with SFN (12 µM), 3d (4.5 µM), 14c (9.5 µM), respectively, and collected after 48 h. The caspase-3 activity assay was determined using a caspase-3 activity kit (Beyotime, Nanjing, China). Cellular protein was extracted with the supplied lysis buffer, followed by the determination of protein concentration using BCA Protein Assay Reagents (Beyotime). The assay is based on the ability of caspase-3 to change acetyl-Asp-Glu-Val-Asp p-nitroanilide into the yellow formazan product, p-nitroaniline. The absorbance at 405 nm was determined, and the activity of caspase-3 was assessed by calculating the ratio at OD405nm of the drug-treated cells to the untreated cells. The results were derived from three independent experiments performed in triplicate.
3.12.3. Aldefluor Assay
A cell population with a high aldehyde dehydrogenase (ALDH) enzyme activity was previously reported to enrich mammary stem/progenitor cells. SUM-159 cells were treated with SFN (1 and 5 µM), 3d (0.5 and 2.5 µM), 14c (0.5 and 2.5 µM) and DMSO for 4 days and subject to Aldefluor assay and flow cytometry analysis. (Stem Cell Technologies, BD, New York, NY, USA). Single cells obtained from the drug-treated cells were incubated in an Aldefluor assay buffer containing an ALDH substrate, bodipy-aminoacetaldehyde (1 µM per 1,000,000 cells), for 40 to 50 min at 37 °C. As a negative control, a fraction of cells from each sample was incubated under identical condition in the presence of the ALDH inhibitor diethylaminobenzaldehyde. Flow cytometry was used to measure ALDH-positive cell population. The results were derived from three independent experiments performed in triplicate.
4. Conclusions
The ALDH+ population in breast cancer cell line SUM-159 was stem cell-like cells, and it was recently reported that SFN can selectively inhibit the growth of this cell population [8]. To further investigate the structure-activity relationship of SFN, a series of SFN analogues with heterocyclic moieties were synthesized in this study, and then were assayed against the breast cancer cell lines, MCF-7 and SUM-159, and the acute leukemia stem-cell like cell line KG-1a. Among the furan, methoxypyridine, methoxybenzene, tetrazole, and thiazole classes of SFN analogues, the tetrazole SFN analogues 3d, 8d, and 9d were generally the most potent. In particular, 8d exhibited up to a 15-fold greater potency than SFN against KG-1a cell line. The water soluble derivatives 14c exhibited comparable activity with the parent molecule, 3d, against MCF-7 and SUM-159, and higher potency against the KG-1a cell line. In addition, compounds 14c exhibited less effect than 3d on the human embryonic kidney cell line 293T.Caspase-3 activity data also indicated that SFN, 3d and 14c induced apoptosis in the SUM-159 cell line by increasing caspase-3 activity. Like SFN, analogues 3d and 14c also significantly reduced the ALDH+ subpopulation in the SUM-159 cell line from 3.10% to 0.16% and 0.07%, respectively. In contrast, treatment with DOX increased the ALDH+ population to 5.12%. Based on the results obtained for the leukemia stem-cell like cell line KG-1a, and the observed effects on the stem cell-like ALDH+ subpopulation in breast cancer cells, the biological activities of 3d and 14c should be further investigated.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (NSFC) (21372129), Program for New Century Excellent Talents in University to Y.C., Hundred Young Academic Leaders Program of Nankai University to Y.C., National Biomedical Special Project of International Innovation Park (13ZCZDSY03000), National Science & Technology Pillar Program (2015BAI12B15), National Nature Science Foundation of China(NSFC) (81302080), Anticancer Key Technologies R & D Program of Tianjin (12ZCDZSY16200), Research Fund for the Doctoral Program of Higher Education of China (20131202120003), Natural Science Foundation of Tianjin (14JCQNJC11100) and Foundation of Tianjin Medical University (2013KYQ06).
Author Contributions
Yue Chen, Cui-Gai Bai and Zhong-Sheng Tong conceived and designed the experiments; Ye-Hui Shi, Dong-Fang Dai, Jing Li, Yan-Wei Dong, Yin Jiang, Huan-Gong Li, Yuan Gao, Chuan-Ke Chong, Hui-Ying Li, Xiao-Qian Chu performed the experiments; Yue Chen, Cui-Gai Bai, Zhong-Sheng Tong, Cheng Yang, Quan Zhang analyzed the data, Yue Chen, Zhong-Sheng Tong and Cheng Yang contributed reagents, materials and analysis tools; Yue Chen, Cui-Gai Bai wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Milelli, A.; Fimognari, C.; Ticchi, N.; Neviani, P.; Minarini, A.; Tumiatti, V. Isothiocyanate Synthetic Analogs: Biological Activities, Structure-Activity Relationships and Synthetic Strategies. Mini-Rev. Med. Chem. 2014, 14, 963–977. [Google Scholar] [CrossRef] [PubMed]
- Elhalem, E.; Recio, R.; Werner, S.; Lieder, F.; Calderon-Montano, J.M.; Lopez-Lazaro, M.; Fernandez, I.; Khiar, N. Sulforaphane homologues: Enantiodivergent synthesis of both enantiomers, activation of the Nrf2 transcription factor and selective cytotoxic activity. Eur.J. Med. Chem. 2014, 87, 552–563. [Google Scholar] [CrossRef] [PubMed]
- Kallifatidis, G.; Labsch, S.; Rausch, V.; Mattern, J.; Gladkich, J.; Moldenhauer, G.; Buechler, M.W.; Salnikov, A.V.; Herr, I. Sulforaphane Increases Drug-mediated Cytotoxicity Toward Cancer Stem-like Cells of Pancreas and Prostate. Mol. Ther. 2011, 19, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Khiar, N.; Werner, S.; Mallouk, S.; Lieder, F.; Alcudia, A.; Fernandez, I. Enantiopure Sulforaphane Analogues with Various Substituents at the Sulfinyl Sulfur: Asymmetric Synthesis and Biological Activities. J. Org. Chem. 2009, 74, 6002–6009. [Google Scholar] [CrossRef] [PubMed]
- Giacoppo, S.; Galuppo, M.; Iori, R.; Nicola, G.R.D.; Bramanti, P.; Mazzon, E. (R,S)-glucoraphanin purified from Tuscan black kale and bioactivated with myrosinase enzyme protects against cerebral ischemia/reperfusion injury in rats. Fitoterapia 2014, 99, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.; Huang, M.-T.; Liu, Y.; Khor, T.O.; Conney, A.H.; Kong, A.-N. Impact of Nrf2 on UVB-Induced Skin Inflammation/Photoprotection and Photoprotective Effect of Sulforaphane. Mol. Carcinog. 2011, 50, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Chhavi, S.; Lida, S.; Anita, P.; Musthaq, A.; Ahmad, H.H.; Arif, H. Anti-carcinogenic effects of sulforaphane in association with its apoptosis-inducing and anti-inflammatory properties in human cervical cancer cells. Cancer Epidemiol. 2011, 35, 272–278. [Google Scholar]
- Li, Y.Y.; Zhang, T.; Korkaya, H.; Liu, S.; Lee, H.-F.; Newman, B.; Yu, Y.; Clouthier, S.G.; Schwartz, S.J.; Wicha, M.S.; et al. Sulforaphane, a Dietary Component of Broccoli/Broccoli Sprouts, Inhibits Breast Cancer Stem Cells. Clin. Cancer Res. 2010, 16, 2580–2590. [Google Scholar] [CrossRef] [PubMed]
- Whitesell, J.K.; Wong, M.S. Asymmetric-synthesis of Chiral Sulfinate Esters and Sulfoxides-synthesis of Sulforaphane. J. Org. Chem. 1994, 59, 597–601. [Google Scholar] [CrossRef]
- Holland, H.L.; Brown, F.M.; Larsen, B.G. Preparation of (R)-Sulforaphane ByBiotransformation Using Helminthosporium Species Nrrl 4671. Tetrahedron-Asymmetry 1994, 5, 1129–1130. [Google Scholar] [CrossRef]
- Holland, H.L.; Brown, F.M.; Larsen, B.G.; Zabic, M. Biotransformation of Organic Sulfides Part 7 Formation of Chiral Isothiocyanato Sulfoxides and Related-Compounds by Microbial Biotransformation. Tetrahedron Asymmetry 1995, 6, 1569–1574. [Google Scholar] [CrossRef]
- Schenk, W.A.; Durr, M. Synthesis of (R)-sulforaphane using [CpRu((R,R)-CHIRAPHOS)]+ as Chiral Auxiliary. Chem. Eur. J. 1997, 3, 713–716. [Google Scholar] [CrossRef]
- Kuhnert, N.; Holst, B.; Williamson, G. Synthesis of 14C-labelled Sulforaphane. J. Label. Comp. Radiopharm. 2001, 44, 347–354. [Google Scholar] [CrossRef]
- Vermeulen, M.; Zwanenburg, B.; Chittenden, G.J.F.; Verhagen, H. Synthesis of Isothiocyanate-derived Mercapturic Acids. Eur. J. Med. Chem. 2003, 38, 729–737. [Google Scholar] [CrossRef]
- Kuhnert, N.; Lu, Y. Synthesis of 1,1′,2,2′3,3′4,4′-Octadeutero-Sulforaphane. J. Label. Comp. Radiopharm 2004, 47, 501–507. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.Y.; Sun, X.Q.; Ma, H.Z.; Chen, X.X.; Ren, J.; Hu, K. New Method for the Synthesis of Sulforaphane and Related Isothiocyanates. Synthesis 2011, 24, 3991–3996. [Google Scholar] [CrossRef]
- Hu, K.; Qi, Y.J.; Zhao, J.; Jiang, H.F.; Chen, X.; Ren, J. Synthesis and Biological Evaluation of Sulforaphane Derivatives as Potential Antitumor Aagents. Eur. J. Med. Chem. 2013, 64, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Kielbasinski, P.; Luczak, J.; Cierpial, T.; Blaszczyk, J.; Sieron, L.; Wiktorska, K.; Lubelska, K.; Milczarek, M.; Chilmonczyk, Z. New enantiomeric fluorine-containing derivatives of sulforaphane: Synthesis, absolute configurations and biological activity. Eur. J. Med. Chem. 2014, 76, 332–342. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.Z.; Xu, P.; Liu, L.; Zheng, D.; Lei, P.S. Synthesis and antibacterial activity of novel ketolides with 11,12-sulfur contained aryl alkyl side chains. Eur. J. Med. Chem. 2011, 46, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Massa, A.; Siniscalchi, F.R.; Bugatti, V.; Lattanzi, A.; Scettri, A. New procedures for the enantioselective oxidation of sulfides under stoichiometric and catalytic conditions. Tetrahedron Asymmetry 2002, 13, 1277–1283. [Google Scholar] [CrossRef]
- Sample Availability: Samples of the compounds 3a–e, 8a–e, 9a–e are available from the authors.
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).