Abstract
The totality of chemical space is so immense that only a small fraction can ever be explored. Computational searching has indicated that bioactivity is associated with a comparatively small number of ring-containing structures. Pyrrole, indole, pyridine, quinoline, quinazoline and related 6-membered ring-containing aza-arenes figure prominently. This review focuses on the search for fast, efficient and environmentally friendly preparative methods for these rings with specific emphasis on iminyl radical-mediated procedures. Oxime derivatives, particularly oxime esters and oxime ethers, are attractive precursors for these radicals. Their use is described in conventional thermolytic, microwave-assisted and UV-vis based preparative procedures. Photoredox-catalyzed protocols involving designer oxime ethers are also covered. Choice can be made amongst these synthetic strategies for a wide variety of 5- and 6-membered ring heterocycles including phenanthridine and related aza-arenes. Applications to selected natural products and bioactive molecules, including trispheridine, vasconine, luotonin A and rutaecarpine, are included.
1. Introduction
Several recent articles have drawn attention to the virtually boundless extent of chemical space; the domain that contains the totality of all possible compounds [1,2]. The total number of molecules that could be made from only 30 atoms is in the range 1020 to 1024 [3], “drug-like” chemical space comprises over 1060 molecules [4,5] and, of course, even these huge numbers are insignificant in comparison with the protein or nucleic acid spaces. The number of polypeptide chains of modest (250 unit) length, drawn from the 20 natural amino acids, exceeds the ‘trans-astronomical’ number of 10325 [6]. The CAS registry currently contains about 108 chemical substances. Its present rate of growth is about 5 × 106 substances per year, so that at this rate more than 1054 years would be needed just to explore “drug-like” chemical space! It is abundantly evident that only a minute fraction of chemical space can ever be preparatively accessed. To address this problem, computational algorithms are being devised capable of virtual screening and/or for locating, within the total space, “islands” or “trees” of substances with potentially desirable properties such as bioactivity [1,2,7]. Ertl and co-workers developed self-organising neural networks which showed that a comparatively small number of ring structural units is associated with bioactivity. They listed 30 heterocyclic moieties as of crucial importance and 22 of these contained one or more N-atoms [7]. Pyrrole, indole and related structures figured prominently, as did pyridine, quinoline, quinazoline and analogous 6-membered ring-containing aza-arenes. The immense size of chemical space presents exciting opportunities of discovering hitherto unknown and extraordinary substances with properties beneficial to human society. Its size also represents a huge challenge for preparative chemists such that it is imperative to open up every possible avenue that might facilitate the task. The exploration and exploitation of identified “islands” depends critically, of course, on the availability of practical preparative methods. Thus, the development of synthetic strategies for the ring systems associated with bioactivity that are fast, efficient and of low environmental impact, deserves special attention.
The advantages of free-radical based preparative methods include the usually neutral conditions, the tolerance for many unprotected functional groups and the availability of much kinetic, thermodynamic and mechanistic data to guide the design of experimental methodology. During the last two decades a great deal of research has been directed towards making radical-mediated synthetic methods safer, more efficient and more convenient [8,9,10,11,12,13]. New tactics have been devised for avoiding hazardous initiator peroxides or azo-compounds and for dispensing with toxic tin, mercury, copper and other metal reagents. For example, ‘pro-aromatic’ reagents, based on the cyclohexadiene structure, release many radical types without the need for metals [14]. Murphy and co-workers’ development of organic super electron donors unlocked completely new ways of generating radicals and radical-ions and harnessing them synthetically [15,16,17]. The unique properties of organoboron compounds have led to the design of several different reagent types for radical release including B-alkylcatecholboranes [18,19] and N-heterocyclic carbene boranes [20,21,22,23]. The discovery of homogeneous photoredox catalysts (PCs) has had huge impact on radical-mediated preparations. The most popular are complexes of Ru or Ir [24,25,26,27] that re-introduce metals, albeit in small quantities. However, organic dyes and other donor molecules are also coming into use as PCs [28,29]. Heterogeneous photoredox catalysts, particularly titanium dioxide (titania, TiO2), possess the added convenience of easy removal after use by filtration or centrifugation. Their exploitation for radical mediated preparations is also developing rapidly [30,31,32].
The N–O bonds in oximes and in oxime derivatives are comparatively weak and break homolytically with production of a pair of N- and O-centered radicals. Aldehydes and ketones are available as starting materials in huge variety from natural and commercial sources. Oximes can be prepared essentially quantitatively from them simply by treatment with hydroxylamine hydrochloride. The consequence is that oxime esters of many types containing the >C=N–OC(O)– structural unit (carbonyl oximes) and oxime ethers containing the >C=N–O– unit are very readily accessible. Most members of both classes are stable to moderate heat and hydrolysis, are non-toxic and non-hazardous, are easily handled and have long shelf lives. Suitably functionalised, they have proved amazingly adaptable for radical generation by an unprecedentedly wide range of methods [33]. These include conventional thermolyses, microwave irradiations, UV photolyses, sensitised UV photolyses and with several types of photoredox catalysis. Oxime derivatives are therefore particularly flexible, convenient and benign and stand as very attractive alternatives to other more hazardous radical precursors. This article reviews the use of both carbonyl oximes and oxime ethers in radical mediated organic syntheses.
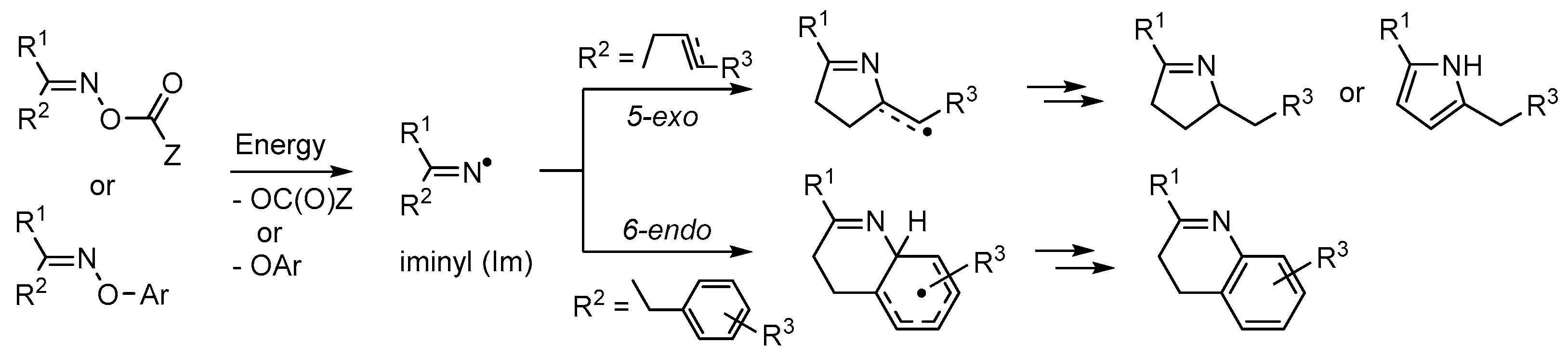
When fittingly stimulated, both compound types initially yield N-centred iminyl radicals >C=N• (Im, Scheme 1). Those suitably accoutred with acceptor groups can, when appropriately manipulated, yield azaheterocycles. An O-centred radical [•OC(O)Z] is released from a carbonyl oxime together with the iminyl radical and can be chosen to end up as volatile or otherwise easily separable by-products. For the oxime ether precursors, best results are usually achieved with O-aryl substituents. In this case the by-product is usually a phenol (ArOH) which can readily be removed because of its mild acidity. Iminyl radicals with butene or butyne type side chains selectively undergo 5-exo cyclisation to produce 5-member ring containing dihydropyrrole type products. By way of contrast, iminyl radicals with aromatic or heteroaromatic acceptor substituents preferentially yield 6-membered ring pyridine, quinoline etc. products. In some instances this results from an initial 5-exo spiro cyclization followed by ring expansion via an aziridinyl type intermediate (see for example Section 4.1). Preparations of many different azaheterocycle types may therefore be achieved by careful choice of the acceptor substituent(s), and by tuning the reaction conditions and methodology.
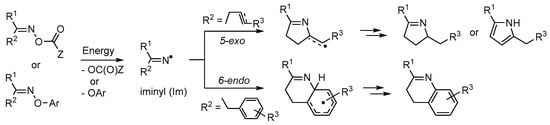
Scheme 1.
Generation of iminyl radicals from oxime derivatives and subsequent ring closures.
This review also focuses on the iminyl radical based synthetic methodology developed for the sets of aza-arenes identified above as of importance in the bioactivity islands of chemical space.
2. Syntheses of Dihydropyrroles and Pyrroles
Organotin promoted radical methodology is justly famed because it works seamlessly in so many situations and has proved so dependable. Many ingenious syntheses of azaheterocycles have employed organotin hydrides or ditins for the generation of iminyl or aminyl radicals. Zard, for example, described tin hydride-mediated syntheses of dihydropyrroles, indolizidines and other aza-heterocycles from sulfenimines (PhS–N=C<), thionocarbazones and other derivatives [34,35]. Nanni and co-workers generated iminyl radicals by ring closures of C-centred radicals onto organic nitriles and hence prepared many heterocyclic systems. They also employed tin-free thermolytic and other processes [36,37,38]. Much of this earlier research has been reviewed by Bowman and Aldabbagh [39,40,41] and/or by Fallis and Brinza [42]. Recently, Zhang and Studer have published an outstanding review of aza-arene syntheses flowing from radical additions to organic isonitriles [43]. This methodology exploits the formation and ring closures of amidoyl radicals (Ar–N=C•–R).
2.1. Pyrrole and Dihydropyrrole Preparations from Carbonyl Oximes
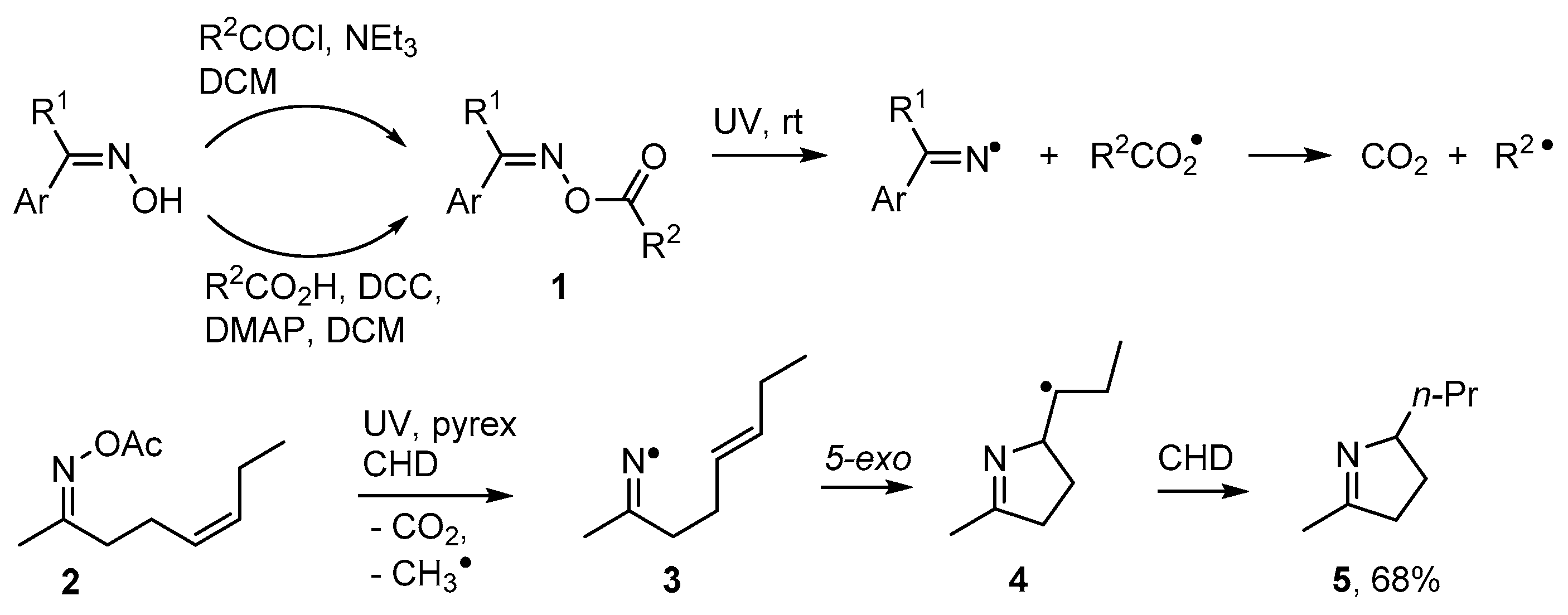
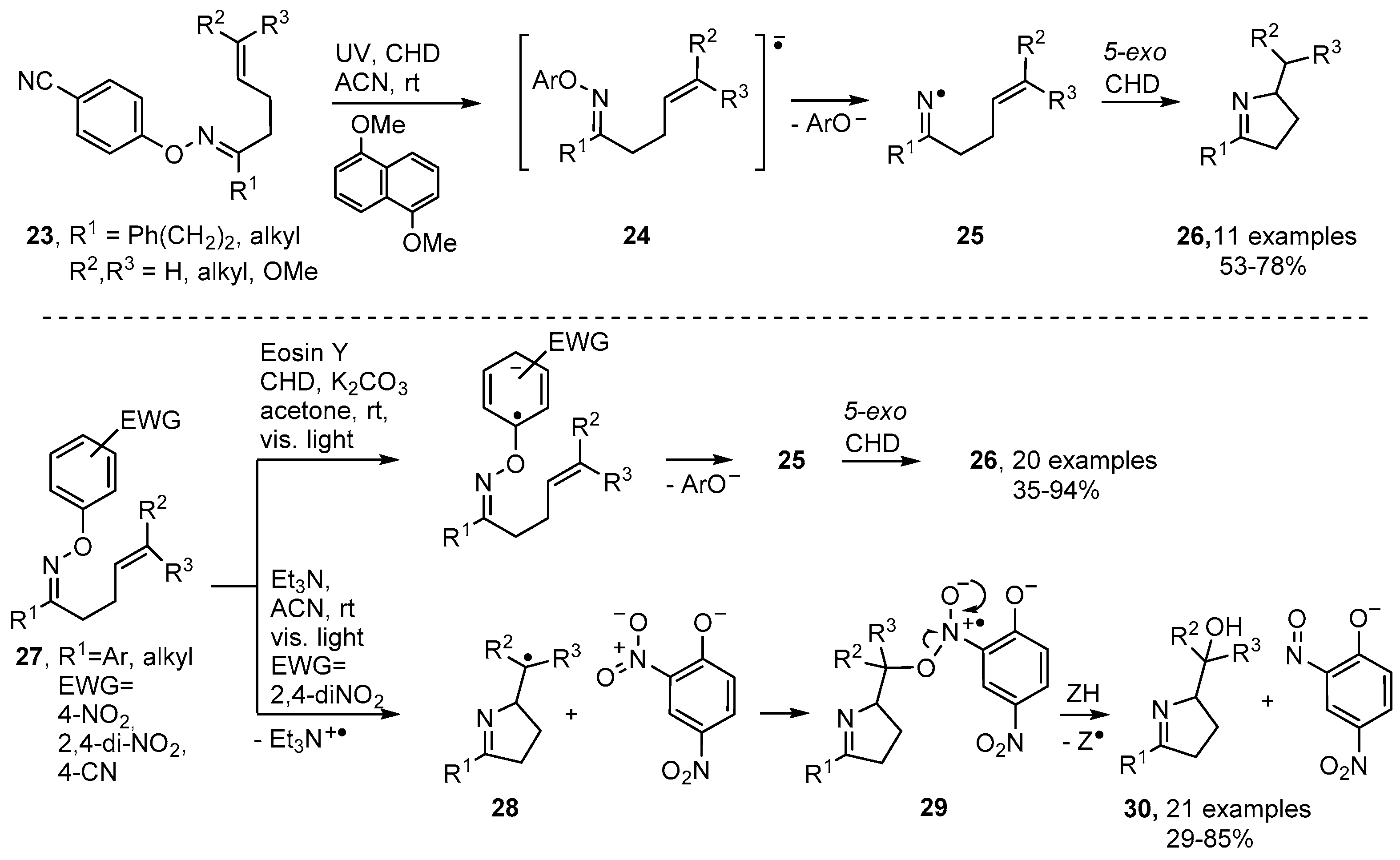
Numerous alkaloids contain pyrrole, dihydropyrrole or related rings and many biological roles are associated with these structures [44,45,46]. Oxime esters 1 can easily be prepared from oximes reacting with either carboxylic acids or acyl halides [47,48]. On photolysis they release an iminyl radical together with an acyloxyl radical and the latter rapidly extrudes CO2 with production of a C-centred radical [48,49] (Scheme 2). Rodrigues, Sampedro and co-workers used acyl oximes such as 2 as efficient sources of iminyl radicals [50,51,52]. With this precursor type, the radical co-produced with the iminyl during UV photolyses was acyloxyl [CH3CO2•] that simply furnished volatile CH4 and CO2 as by-products. They reported that the iminyls could be conscripted into syntheses of many types of heterocycles including dihydropyrroles. UV photolysis of 2 through Pyrex released iminyl radical 3 with an alkenyl side chain. These selectively cyclised in the 5-exo mode with production of pyrolidinylmethyl radical 4 that subsequently abstracted an H-atom from co-reactant cyclohexa-1,4-diene (CHD) to yield 3,4-dihydropyrrole derivative 5 (Scheme 2).
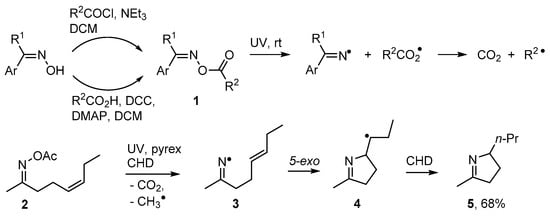
Scheme 2.
Photo-dissociation of oxime esters and preparation of dihydropyrroles [48,51].
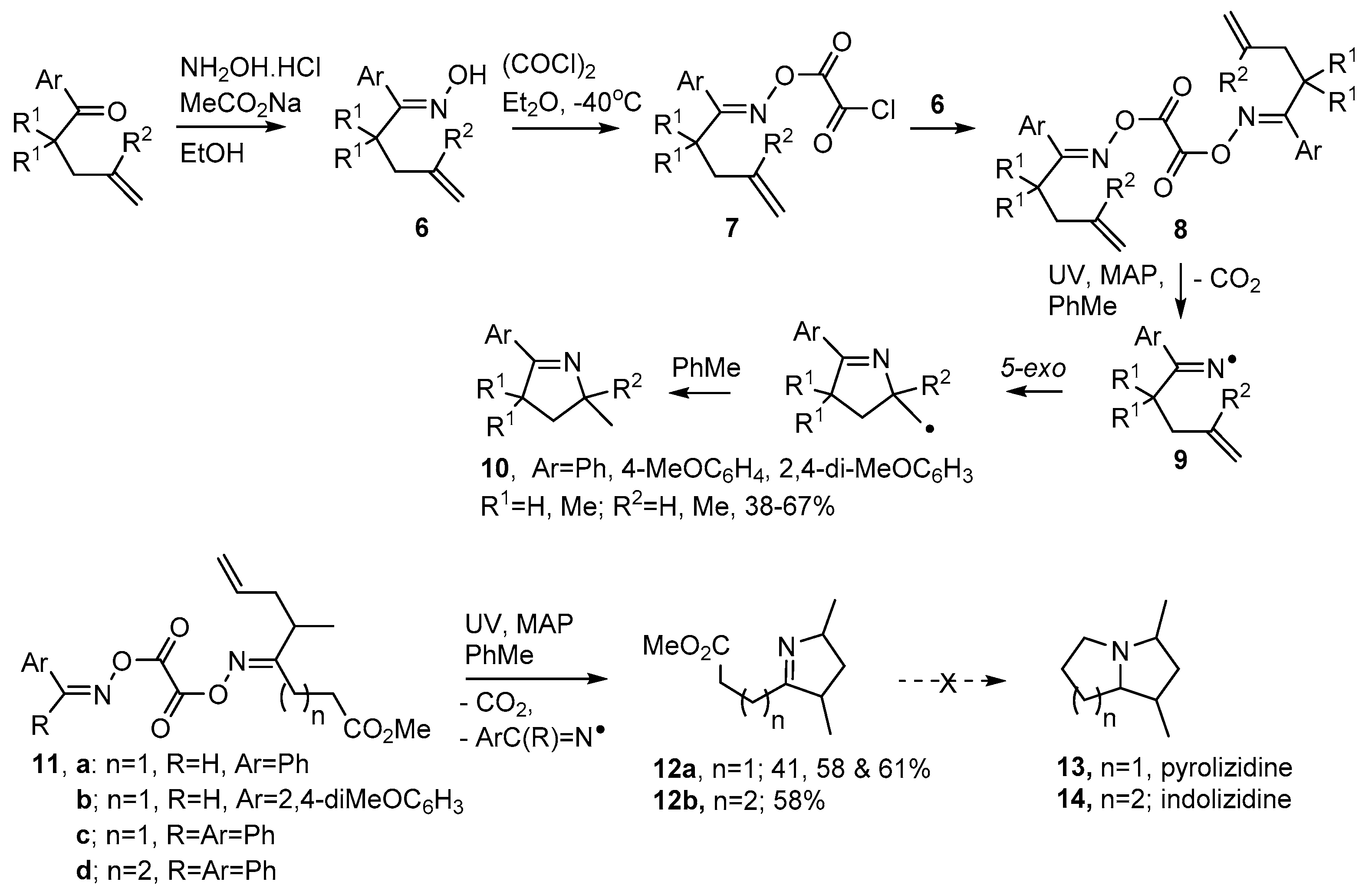
Dioxime oxalates 8 are another type of oxime ester that proved convenient for clean generation of iminyl radicals. Symmetrical types were made by treatment of an oxime 6 with oxalyl chloride to yield oxime oxalyl chlorides 7 as intermediates (Scheme 3). Although these could be isolated, they hydrolysed and degraded quickly, so immediate treatment with another equivalent of either the same oxime 6, or a different oxime, yielded the symmetrical dioxime oxalates 8 or unsymmetrical types such as 11a–d respectively [53,54]. Dioxime oxalates 8 were “clean” and atom-efficient because, apart from CO2, they delivered only iminyl radicals 9 on UV photolysis. Photo-dissociations were aided by inclusion of 4-methoxyacetophenone (MAP) as photosensitizer and by the presence of an aryl substituent adjacent to the C=N bond.
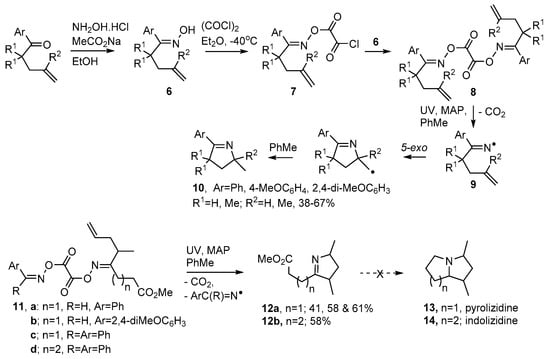
Scheme 3.
Preparation of dioxime oxalates and their use in dihydropyrrole syntheses [54].
3,4-Dihydropyrrole derivatives 10 were obtained in moderate yields with toluene acting as both solvent and H-atom donor (Scheme 3). Unsymmetrical dioxime oxalates such as 11 incorporated an aryl-oxime unit, to promote photo-dissociation, as well as an alkenic acceptor unit. These precursors enabled non-aromatic dihydropyrroles such as 12a,b to be accessed. Attempts to convert the latter to pyrolizidines 13 and indolizidines 14 were unsuccessful.
2.2. Pyrrole and Dihydropyrrole Preparations from Oxime Ethers
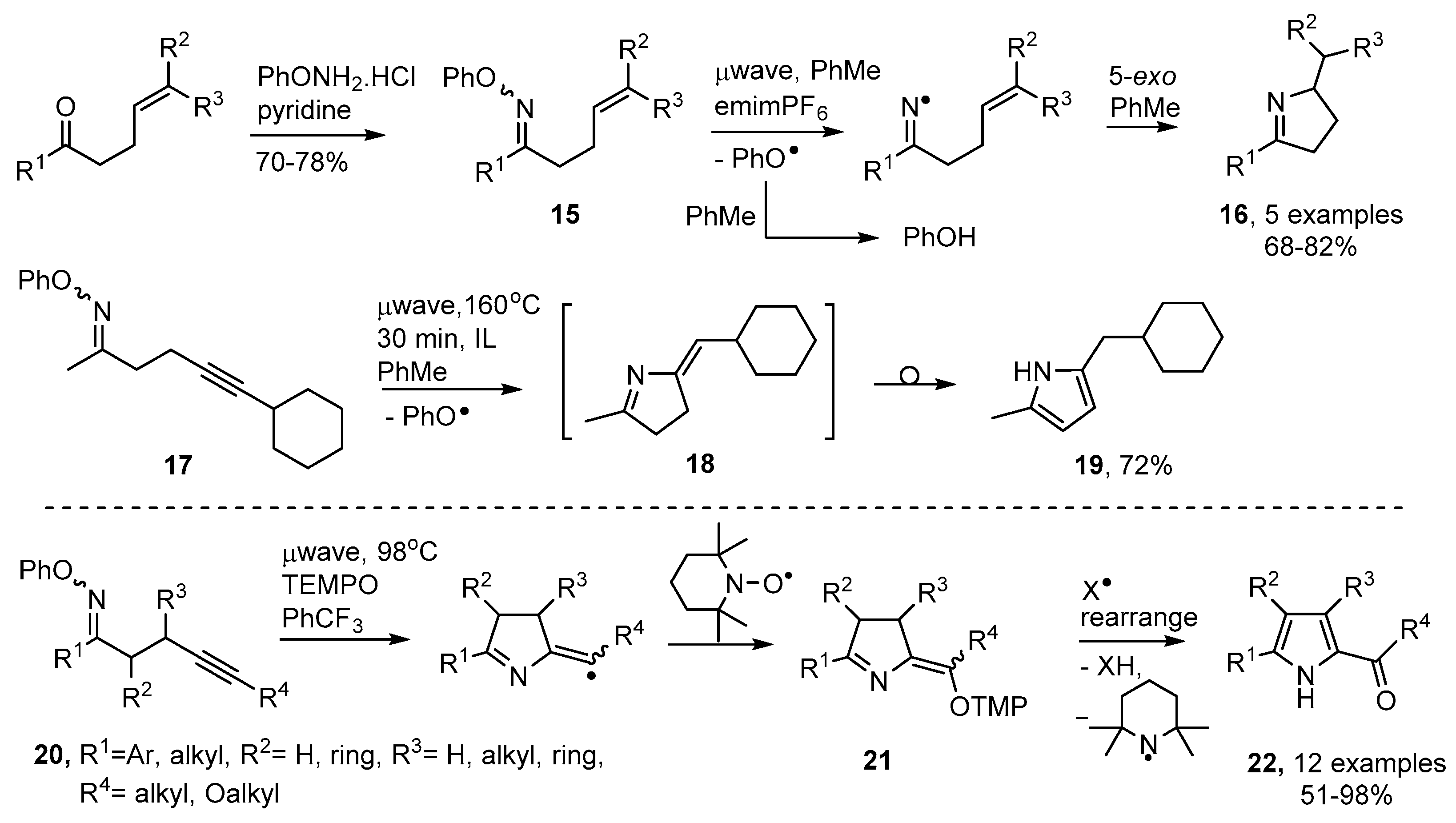
Thermal preparative methods are often superior because of their simplicity and non-hazardous nature. O-Phenyl oxime ethers 15 can easily be made by treatment of carbonyl compounds with the commercially available O-phenylhydroxylamine hydrochloride. Conventional thermolyses of appropriate derivatives were shown to provide dialkyl- or diaryl-iminyl radicals [55]. Subsequently it was established that microwave heating (μwave) was a particularly efficient means of releasing iminyl radicals and mediating dihydropyrrole preparations [56,57].
The optimum procedure utilized toluene as both solvent and H-donor together with an equivalent of the ionic liquid (IL) 1-ethyl-3-methyl-1H-imidazol-3-ium hexafluorophosphate (emimPF6) to promote microwave absorbance. This method enabled ketones with but-3-enyl type side chains to be converted to dihydropyrroles 16 in good yields in two steps (Scheme 4). The phenoxyl radicals released from 15 also abstracted H-atoms from the solvent to afford phenol as an easily separable by-product. When oxime ether 17 with an alkyne side chain was microwave irradiated under similar conditions, pyrrole 19 was isolated in good yield. Evidently the first-formed methylene-dihydropyrrole 18 rearranged under the reaction conditions.
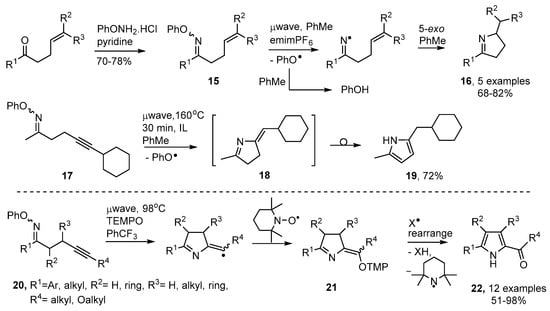
Scheme 4.
Preparation of oxime ethers and microwave-promoted syntheses of dihydropyrroles and pyrroles [56,57,58].
Castle and co-workers prepared a set of alkyne-substituted oxime ethers 20 and carried out microwave irradiations of mixtures with tetramethylpiperidine-N-oxide (TEMPO) in benzotrifluoride solvent [58]. The ring closed radicals were trapped by the TEMPO with production of intermediates 21 (Scheme 4). These also rearranged, with loss of a piperidinyl radical, so providing 2-acylpyrroles 22 in good to excellent yields.
2.3. Photoredox Catalyzed Preparations of Pyrroles and Dihydropyrroles from Oxime Derivatives
Photoredox catalytic methodology [25,27,59] has also been developed for production of dihydropyrroles from O-aryl oxime ethers substituted with electron withdrawing groups (EWG) in their O-aryl units. Narasaka and co-workers prepared oxime ethers 23 containing 4-CN (or 2,4-di-NO2 or 4-CF3) aryl substituents. On inclusion of a catalytic amount of 1,5-dimethoxynaphthalene (DMN) and irradiation with UV light in 1,4-cyclohexadiene, dihydropyrroles 25 were isolated in good yields (Scheme 5) [60]. The incident light raised the photocatalyst to an excited triplet state (PC*) that then transferred an electron to the oxime ethers with production of the radical anions 24 (Scheme 5). Loss of the stable phenolate type anions then occurred with release of the corresponding iminyl radicals that subsequently underwent 5-exo cyclisation and H-atom transfer with CHD to afford dihydropyrroles 25.
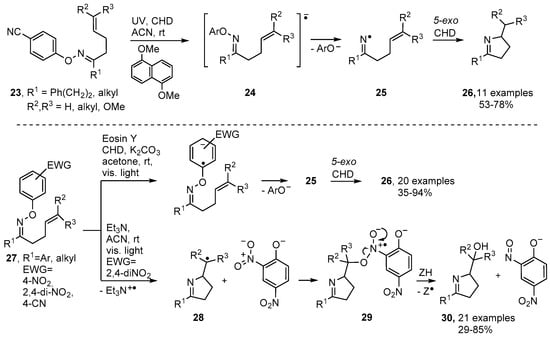
Scheme 5.
Photoredox catalytic routes from oxime ethers to dihydropyrroles [28,60].
Furthermore, Leonori and co-workers reported recently that the dye Eosin Y (as PC) catalysed dihydropyrrole formation, simply with light of visible wavelength, when oxime ethers with O-2,4-dinitroaryl substitution 27 were employed as reactants [28]. Remarkably, Et3N could replace Eosin Y: visible light irradiation of 27 (EWG = 2,4-di-NO2) with Et3N in CH3CN furnished imino-alcohols 31 in yields up to 85%. The complex of Et3N with the electron-poor ring of 27, on excitation with visible light, generated a radical anion that dissociated to give 2,4-dinitrophenoxide together with pyrrolidinylmethyl radical 28. The oxygen atom was believed to arise from an intermediate such as 29 that fragmented to nitrosophenoxide and a pyrrolidine-containing alkoxyl radical. The latter picked up an H-atom to deliver imino-alcohols 30 as the products (Scheme 5).
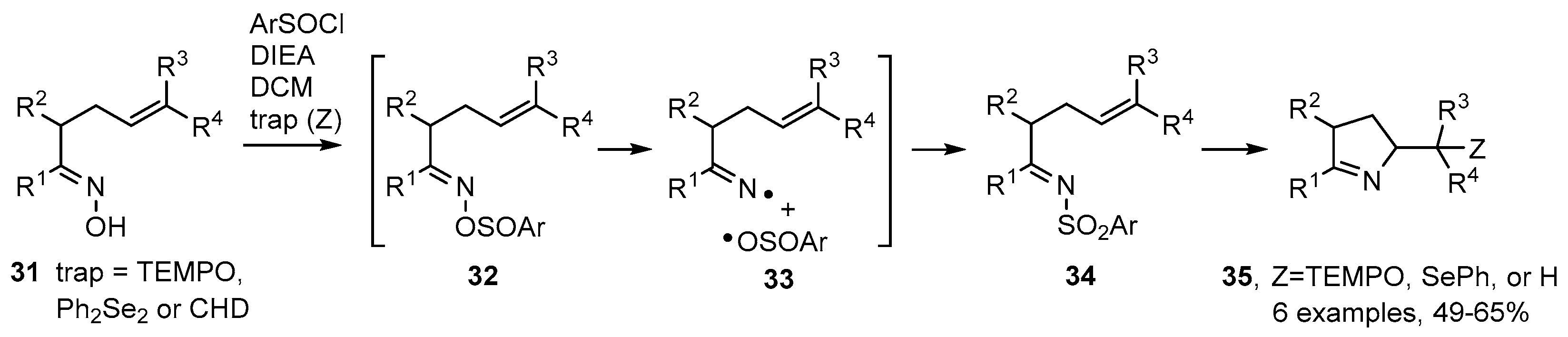
Weinreb and co-workers described an alternative strategy in which oximes 31 could be used directly when treated with 2,6-dimethylbenzenesulfinyl chloride in the presence of Hunig’s base (N,N-diisopropylethylamine, DIEA) and a radical trapping agent in dichloromethane (DCM) [61]. The best radical traps (Z) were found to be TEMPO and diphenyl diselenide; but CHD could also be used when in great excess (Scheme 6). Probably sulfinate esters 32 were first formed that dissociated to a caged iminyl/sulfonyl radical pair 33. The reformed N-sulfonylimines 34 then released iminyl radicals that cyclised and were trapped to afford functionalised dihydropyrroles 35 in moderate to good yields (Scheme 6).
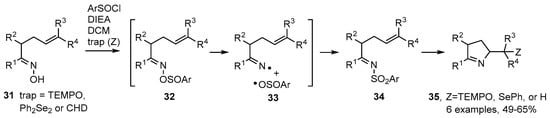
Scheme 6.
Preparation of dihydropyrroles from N-sulfonylimines [61].
3. Preparations of Pyridine, Quinoline, Phenanthridine and Related Aza-Arenes
Compounds containing the quinoline unit are hugely important to the well-being of society because they play essential roles across the areas of medicine, pharmacology, nutrition, dyes, and even electronics [62]. Bioactive natural products containing quinoline cores are widely distributed in many plants, marine plants, corals, sponges [63,64,65,66,67] and even in chestnut honey [68]. Much the same can be said for compounds containing phenanthridine units. They are noted for their ability to bind to DNA [69] and have antimicrobial, anti-inflammatory, and anti-tumor activities [70,71,72,73,74]. Because of this importance to the field of drug development, synthetic methods for molecules containing these structural units, particularly short, mild and atom-efficient ones, have attracted great attention.
3.1. Preparations of Aza-Arenes from Carbonyl Oximes
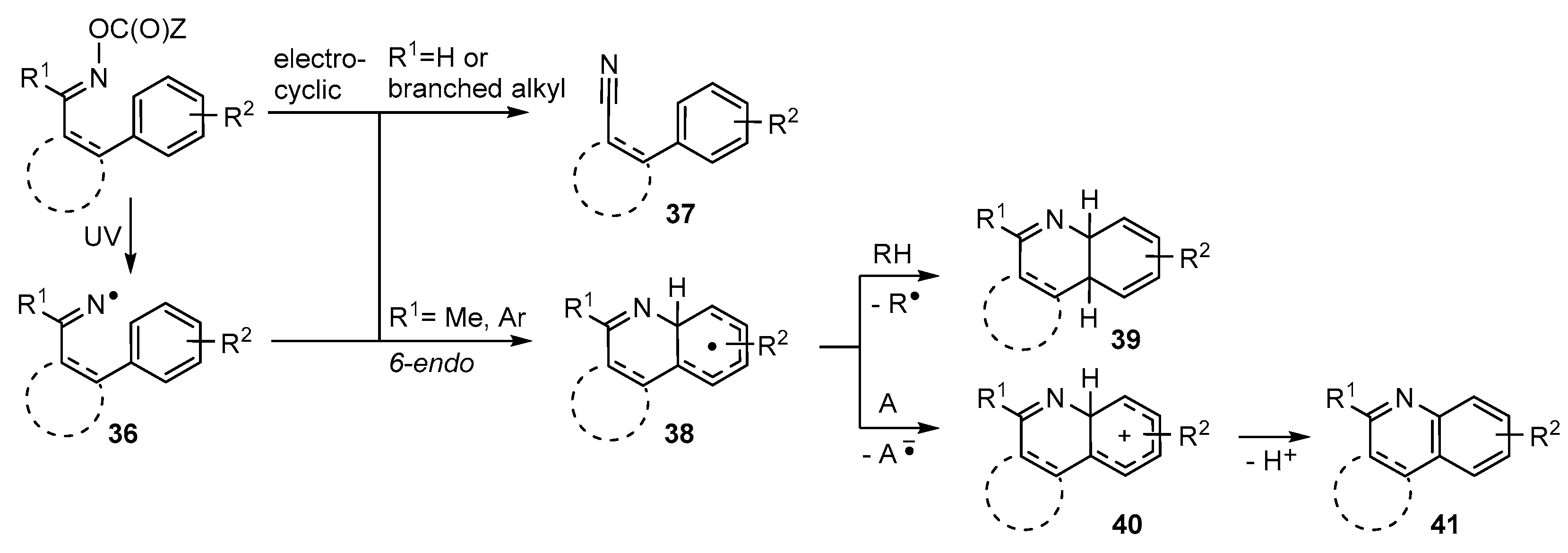
Iminyl radicals 36 containing suitably sited aromatic acceptor units usually ring close in 6-endo mode with formation of a 6-membered N-containing ring 38 (Scheme 7). This rule holds good for cyclisations onto a wide range of aromatic acceptors including benzene, naphthalene, furan, thiophene, indole, pyridine and analogous moieties. As would be expected for less-favoured 6-endo processes, the rate constants for these iminyl ring closures are significantly smaller than for 5-exo cyclisations [75]. Never-the-less practicable preparative protocols have been established for many mono- and poly-cyclic heterocycles.
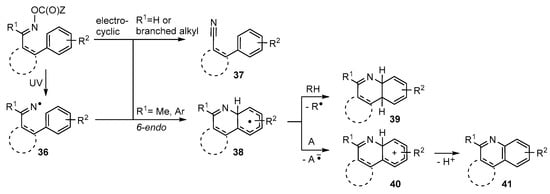
Scheme 7.
Iminyl radical 6-endo ring closures onto aromatic acceptors.
It is usually necessary to work with precursors having the second imine substituent R1 either Me or Ar. If R1 is H (or a branched alkyl group) formation of a nitrile 37 is either the main process, or 37 is an important by-product (Scheme 7). Nitrile formation may entail an electrocyclic process of the oxime ester; in which case the proportion may be diminished by suitable solvent choice. With several types of oxime esters cyclisations onto aromatic rings initially create cyclohexadienyl type radicals 38. If a suitable H-donor is present as solvent, or otherwise, H-atom transfer to radicals 38 yields cyclohexadienes 39 as a mixture of isomers. In practice this route has rarely been developed. Instead restoration of aromaticity to the acceptor ring has usually been observed as in quinoline derivative 41. This can result when some radical in the system abstracts the highly labile tertiary H-atom of the cyclohexadienyl ring in radical 38. However, this is a disproportionation that requires two radicals to meet and so is not favored because reactive radical concentrations are always very low. The alternative is that an acceptor molecule A is either added, or is adventitiously present, and single electron transfer (SET) occurs with production of the corresponding cyclohexadienyl cations 40. These rapidly deprotonate to yield cyclised products 41 with restored aromaticity. This oxidative process is facilitated by non-H-atom donor solvents such as PhCF3 or t-BuOH.
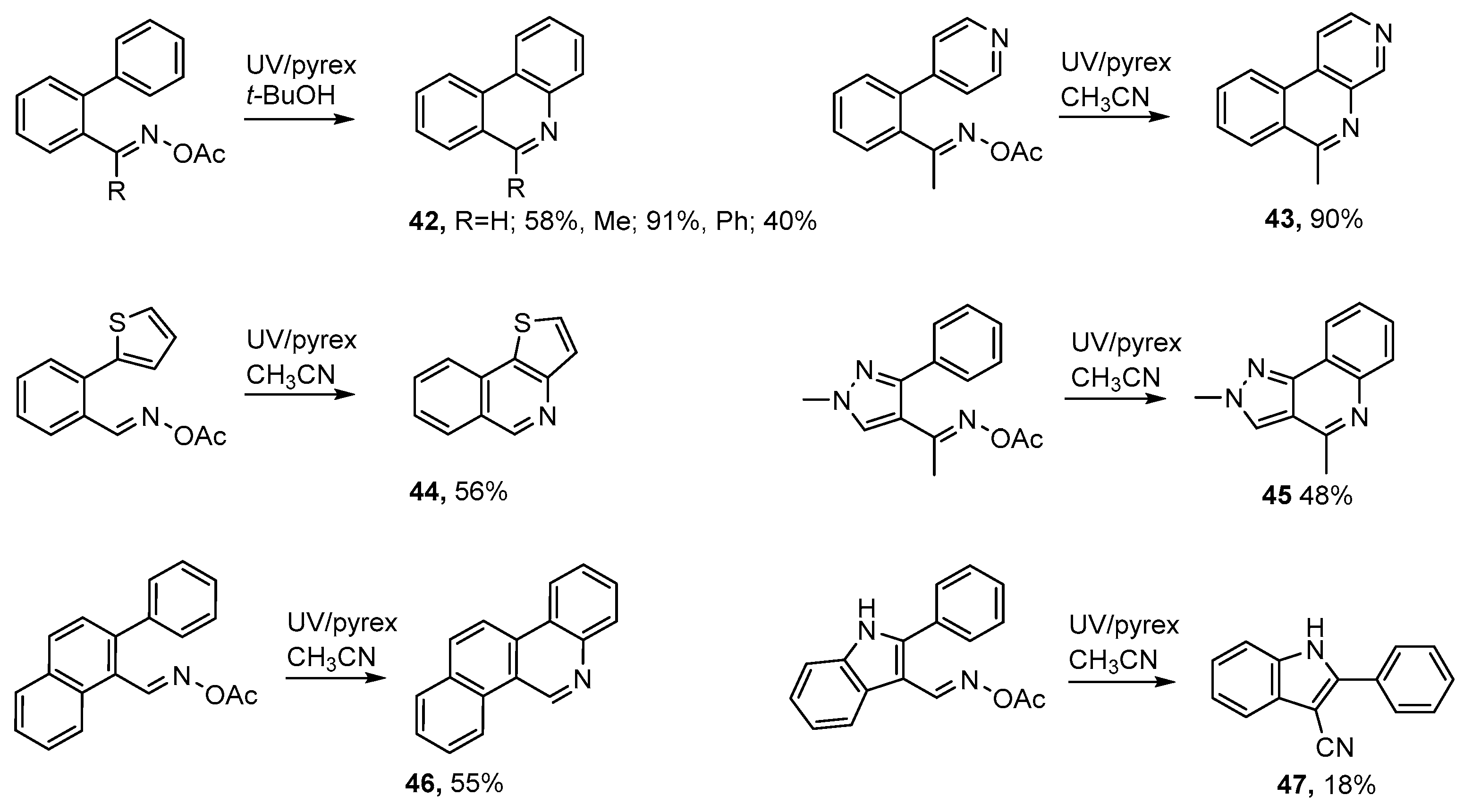
Formyl and acyl derivatives of biphenyl, 4-phenylpyridine, 2-phenylthiophene, 3-phenylpyrazole, 2-phenylnaphthalene, 2-phenylindole and analogous aromatics are readily accessible and can be converted to the corresponding acyl oximes without difficulty (see Scheme 8). UV photolyses of these precursors through Pyrex in acetonitrile or t-butanol solutions enabled several 6-substituted phenanthridines 42 to be prepared [50,52]. Similarly, benzo[c][1,7]naphthyridines 43, and thieno[3,2-c]isoquinolines 44 were conveniently prepared from the corresponding acyloximes.
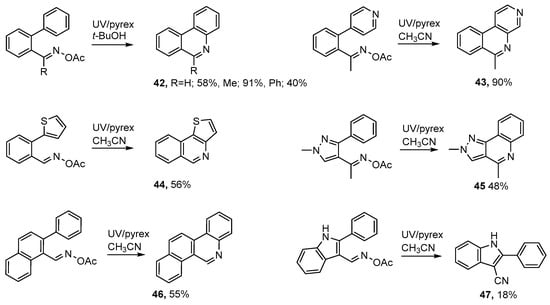
Scheme 8.
Preparations of tri- and tetra-cyclic heterocycles from acyl oximes [50,52].
Analogous photochemical preparations were described for 2,4-dimethyl-2H-pyrazolo[4,3-c]quinoline 45 and for benzo[i]phenanthridine 46. However, the aldoxime precursor from 2-phenylindole gave only 2-phenyl-1H-indole-3-carbonitrile 47 in low yield. Isoquinoline derivatives were also obtained in good yields from photolyses of mixtures of benzophenone acetyl oxime with alkynes in t-butanol [50].
Huang, Deng and co-workers recently described a novel method of making 2-arylpyridines by treatment of O-acyl oxime esters and acroleins with iodine and triethylamine in toluene at 120 °C [76]. The method had wide scope and yields ranged from moderate to excellent.
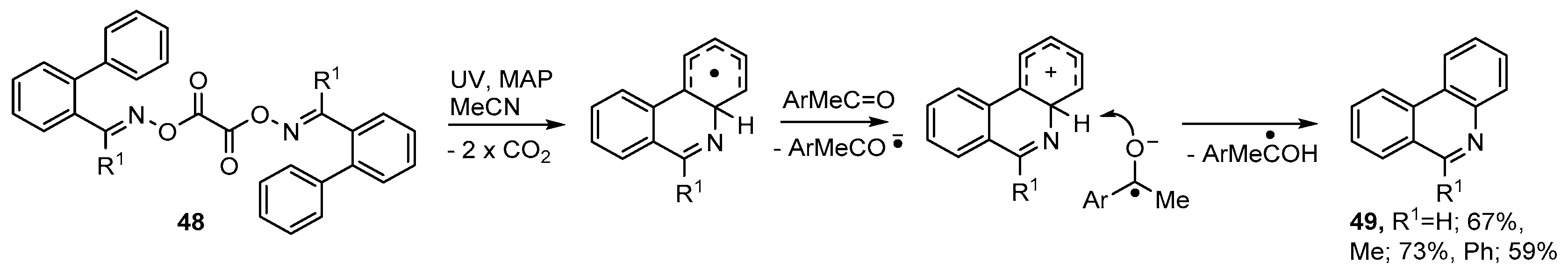
Symmetrical dioxime oxalates containing aromatic acceptors 48 were prepared in essentially quantitative yields, via the oxime oxalyl chlorides, from the corresponding oximes. On UV photolyses at ambient temperature in MeCN solution these returned phenanthridines 49 in clean and atom-efficient processes [54]. Best yields were obtained on inclusion of MAP (ArMeC=O) and it seems this acted as both a photosensitizer and as an electron acceptor to facilitate the final re-aromatization (see Scheme 9).
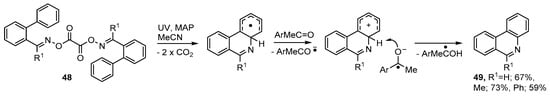
Scheme 9.
Preparation of phenanthridines from dioxime oxalates [54].
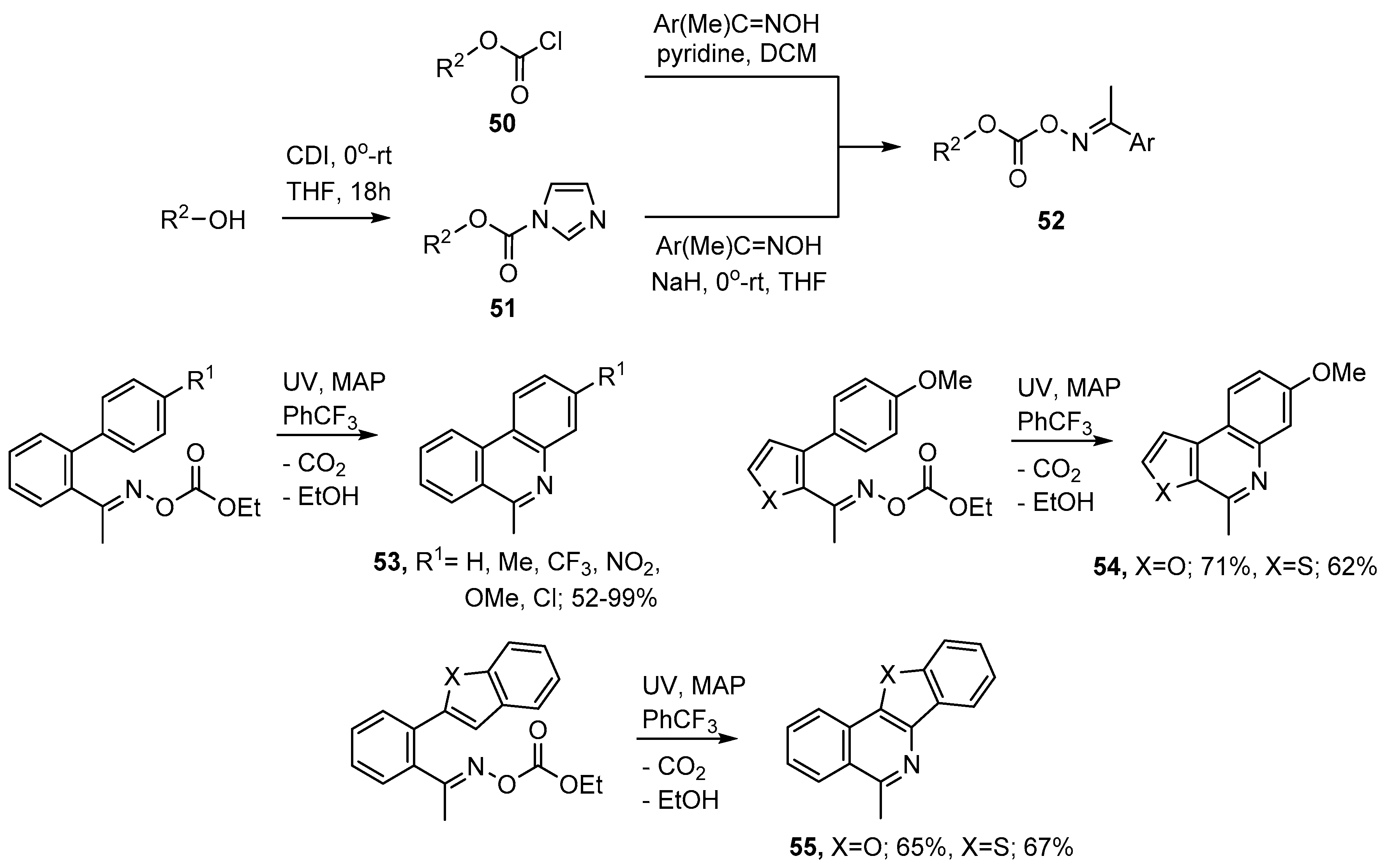
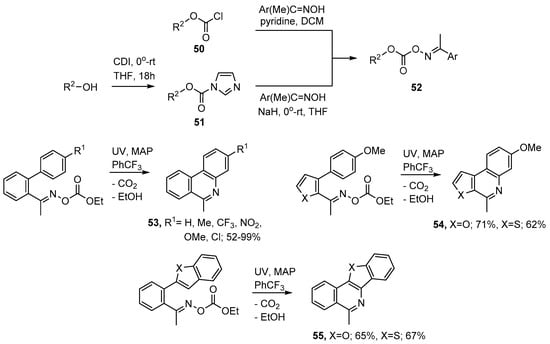
Oxime carbonates 52 can be prepared either by treatment of an oxime with a chloroformate 50 or via the 1H-imidazole-1-carboxylates 51. The latter can be obtained from reaction of an alcohol with carbonyldiimidazole (CDI) (see Scheme 10). For the preparation of aza-arenes commercial ethyl chloroformate (50, R2 = Et) was found to be very convenient because the main by-product (EtOH) is volatile and easily separated. UV photolyses of appropriately functionalised oxime carbonates, with MAP as additive, enabled phenanthridines 53, methylfuro- and methylthieno-[2,3-c]quinolines 54 to be prepared as well as the 5-methylbenzofuro- and 5-methylbenzo[4,5]thieno-[3,2-c]isoquinolines 55 in generally good yields [75,77].
Scheme 10.
Preparations of aza-arenes from oxime carbonates [75,77].
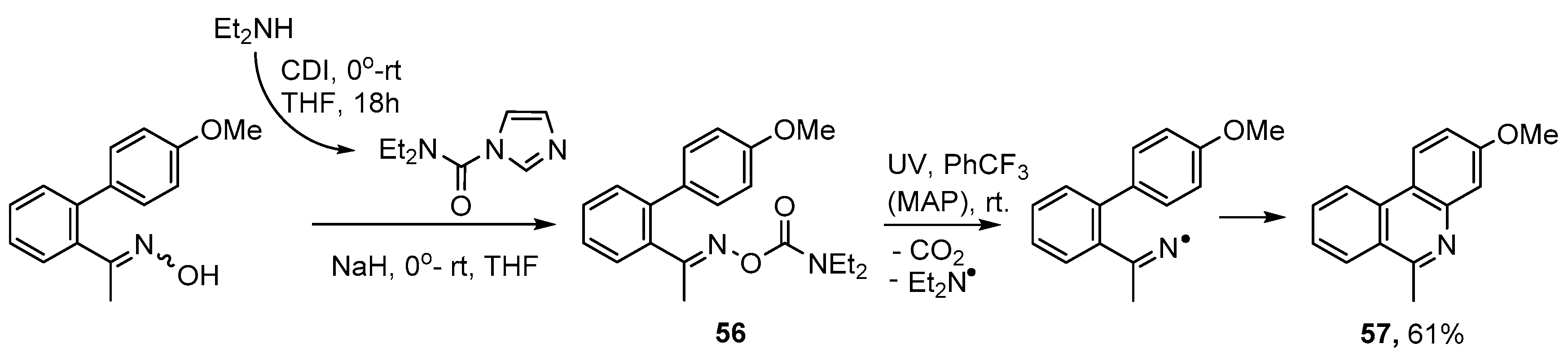
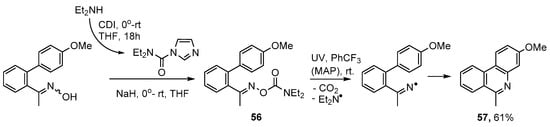
Oxime carbamates 56 offer another alternative for aza-arene syntheses but have not yet been much exploited. The Et2N aminyl group is sufficiently small that by-products derived from it volatilize away. UV irradiation of 56 in PhCF3 at room temperature and with MAP additive provided 3-methoxy-6-methylphenanthridine 57 in 60% yield (Scheme 11) [78]. A slightly higher yield (61%) was obtained in the absence of MAP. This was a worthwhile finding because residues of the photosensitizer can be troublesome to remove.
Scheme 11.
Preparation of phenanthridines from oxime carbamates [78].
3.2. Preparations of Aza-Arenes from Oxime Ethers
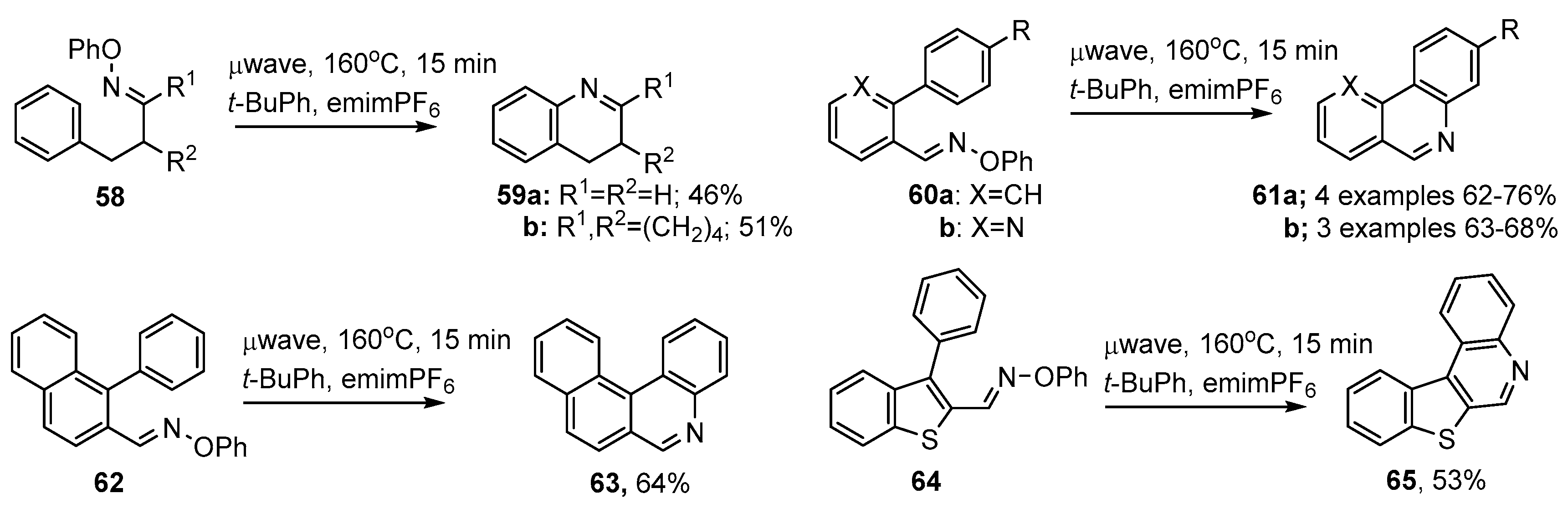
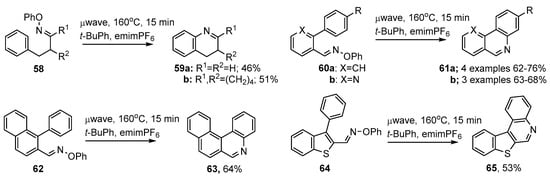
As mentioned above (Section 2.2) O-aryl oxime ethers, including those with appropriately placed aryl acceptor units, can easily be made from carbonyl compounds. The 3-phenylpropanone O-phenyl oxime ethers 58 were subjected to microwave irradiation at 160 °C in t-BuPh together with emimPF6 ionic liquid. Quinoline 59a and tetrahydroacridine 59b were thereby produced in moderate yields [56,57] (Scheme 12).
Scheme 12.
Microwave promoted preparations of aza-arenes from oxime ethers [56,57].
Similarly, biphenyl-2-carbaldehyde O-phenyl oximes 60a and 2-arylnicotinaldehyde O-phenyl oximes 60b, with either electron releasing or electron withdrawing substituents, yielded the corresponding phenanthridine 61a or benzo[h][1,6]naphthyridine derivatives 61b respectively. The scope of the method was easily extended such that benzo[k]phenanthridine 63 and benzo[b]thieno[2,3-c]quinoline 65 were prepared in a very few steps and in acceptable yields (Scheme 12).
3.3. Photoredox Catalyzed Preparations of Aza-Arenes from Oxime Derivatives
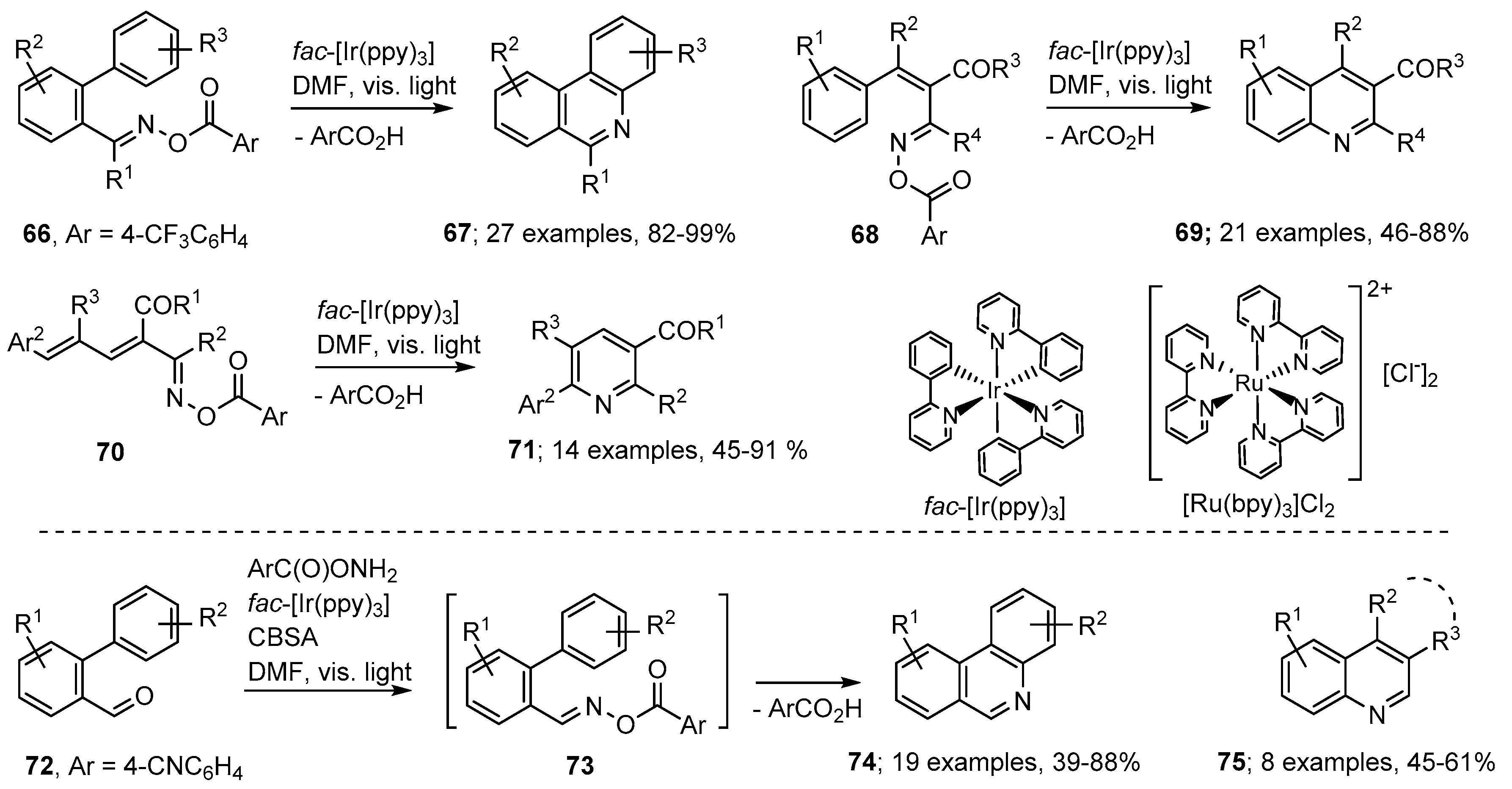
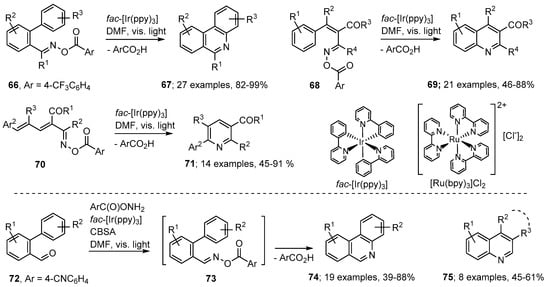
The advantages of photoredox catalyzed processes include that visible light, rather than more damaging UV radiation, is usually sufficient and that only catalytic quantities of the PC are required. Several such methods have been reported recently for aza-arene preparations with oxime derivatives as starting materials For example, Zhang, Yu and co-workers generated iminyl radicals from a wide range of aroyl oximes with fac-[Ir(ppy)3] (ppy = 2-phenylpyridine) as PC [79]. The most reliable and efficient aroyl group was shown to be 4-trifluoromethylbenzoyl; although other electron deficient benzoates succeeded to variable extents. Visible light irradiations of biphenyl aroyl oximes 66 with catalytic quantities of fac-[Ir(ppy)3] in DMF produced phenanthridine derivatives 67 in very impressive yields (Scheme 13). The PC catalysed the formation of a radical anion from the substrate that then released an iminyl radical together with the 4-trifluorobenzoate anion. The non-H-atom donor solvent DMF favoured production of the fully aromatic products shown. Using similar methodology, the precursors 68 yielded quinolines 69 with a good range of functionality. Furthermore, aroyl oxime esters 70, with butadienyl substituents, led to iminyl radicals that ring closed selectively in 6-endo mode with eventual production of pyridine derivatives 71, generally in very good yields.
Scheme 13.
Photoredox methods for preparations of aza-arenes from oxime derivatives [79,80].
Yu and An developed a convenient one-pot tactic that enabled aza-arenes to be prepared in one step from readily available carbonyl compounds [80]. They used O-(4-cyanobenzoyl)hydroxylamine [ArC(O)ONH2] in conjunction with biphenyl aldehydes 72. To promote aroyl oxime formation 4-chlorobenzenesulfonic acid (CBSA) was employed as additive. On irradiation with visible light from white LED strips, phenanthridines 74 were obtained directly without isolation of the intermediate oxime esters 73. A similar one-pot procedure, but employing blue LED strip illumination, was established for preparations of quinoline derivatives 75 from cinnamaldehyde type precursors.
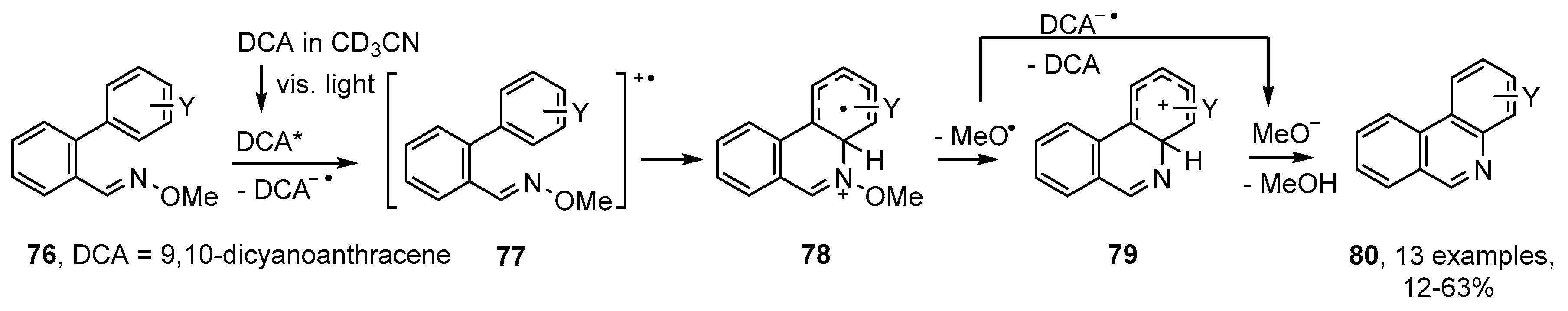
A somewhat related method was reported by de Lijser and co-workers who employed 2′-arylbenzaldehyde oxime ethers 76 together with 9,10-dicyanoanthracene (DCA) [29]. Photolyses at 420 nm in CD3CN solutions enabled a set of substituted phenanthridines 80 to be obtained. This process was believed to proceed via radical cation 77 that ring closed by nucleophilic attack of the aromatic unit onto the N-atom of the oxime ether 78 (Scheme 14). Subsequent loss of methoxyl radicals led to cyclohexadienyl cations 79 that underwent deprotonation to afford the product phenanthridines. Yields were variable with strongly electron-releasing substituents Y being deleterious. The main by-product was the volatile and innocuous MeOH and no nitrile formation was observed.
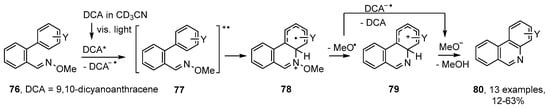
Scheme 14.
Photoredox production of phenanthridines from 2′-arylbiphenyl oxime ethers [29].
4. Preparations of Quinazoline, Quinoxaline and Related Heterocycles from Oxime Derivatives
Bioactivities of various kinds have been associated with numerous substances having structures incorporating quinazoline rings. A number are enzyme inhibitors [81], some display antiviral or anticancer activity [82,83]; others are antibacterial agents [84]. The growing library of approved drugs containing this structural unit, such as erlotinib (Tarceva®) [85], prazosin (Minipress®, Vasoflex®, Lentopres®, Hypovase® [86] and gefitinib (Iressa®) [87,88], demonstrates the transferable character of aspects of its properties. Steady progress has been made in the development of synthetic methods for quinazoline alkaloids in the last few decades [89,90,91]. Some useful microwave promoted procedures have also been described including those based on: aniline N-ethyl carbamates [92], cyano-aromatic compounds with anthranilonitrile [93], N-(2-cyanophenyl)-N,N-dimethylformamidine derivatives [94], N-arylamidines with various aldehydes [95] and 2-aminoaryl imines with aldehydes [96]. The common methods of making the quinazoline ring involve multi-step precursor preparations or special reagents. A pleasing and effective exception is the CuCl2-catalysed reaction of aldehydes with anthranilamide [97].
4.1. Preparations of Quinazoline Derivatives
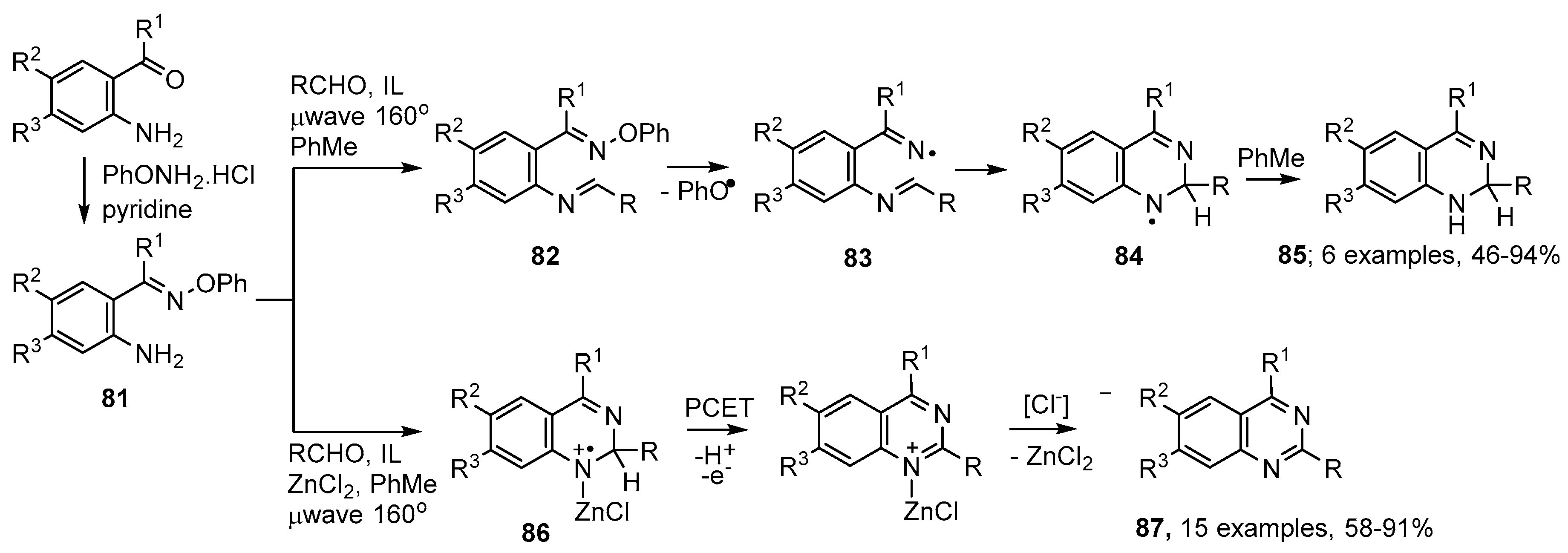
Aminoarylalkanone O-phenyl oximes 81 can be efficiently prepared from the corresponding ketones (Scheme 15). On mixing these starting materials with an aldehyde and microwave irradiating at 160 °C, in toluene as H-donor solvent, with emimPF6 as ionic liquid, imine intermediates 82 were formed. Dissociation to phenoxyl and iminyl radicals 83 took place in one pot, without the need to isolate the imine intermediates 82. Ring closure was exclusively in the 6-endo mode with production of aminyl radicals 84 that were reduced to 1,2-dihydroquinazolines 85 by H-atom donation from the solvent [98,99] (Scheme 15).
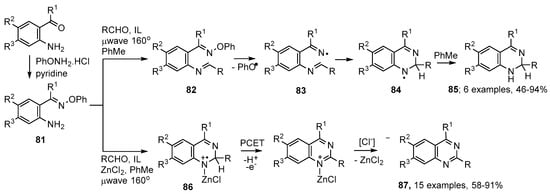
Scheme 15.
Microwave-assisted preparations of quinazoline derivatives from (2-aminoaryl)alkanone O-phenyl oximes [98,99].
This proved to be a rapid, mild, reproducible and efficient process with aliphatic, aromatic and heterocyclic aldehydes; the only by-product being phenol. Analogous methodology with 81 and ketones was not successful, except with a few ketones such as cyclohexanone. The dihydroquinazolines 85 slowly oxidized to the corresponding quinazolines in air or oxygen. Alternatively, they could be converted to the latter in high yields by μwave irradiation with DDQ (2,3-dichloro-5,6-dicyanobenzoquinone) at 100 °C in dichloromethane solvent.
Inclusion of ZnCl2, a known promoter of imine formation [100], in practice led directly to the corresponding fully aromatic quinazolines 87. Good to excellent yields were obtained with aliphatic aldehydes, aromatic aldehydes having either electron-withdrawing or electron releasing substituents and with heterocyclic aldehydes. The method was also tolerant of substituents in the aminoaryl rings. It’s likely that, in addition to encouraging imine formation, the ZnCl2 forms a complex with the iminyl N-atom in radical cation 86. The acidity of the H-atom adjacent to the cation would thereby be increased, thus facilitating proton-coupled electron transfer (PCET) and thence to the quinazolines 87 (Scheme 15).
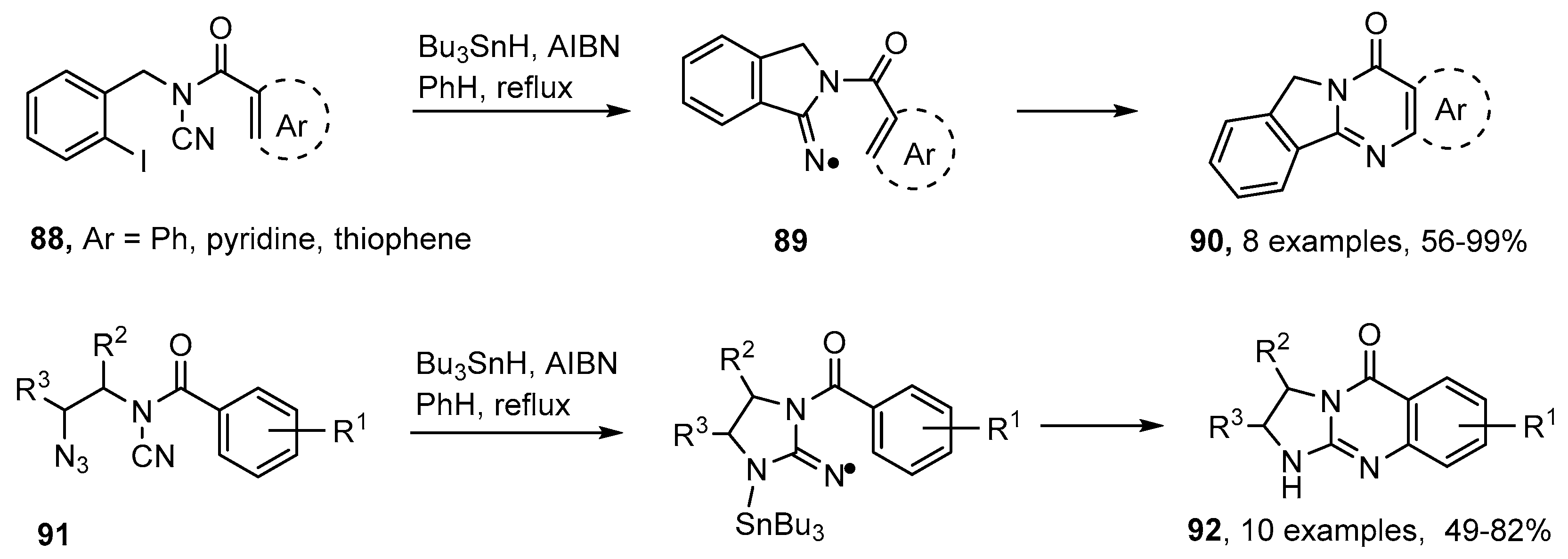
Conventional organic preparations of quinazolinones usually start with derivatives of anthranilic acid [89,101]. Several good Pd- and Cu-catalyzed methods for compounds containing this structure have also been published [102,103,104]. Malacria, and co-workers devised an ingenious radical cascade for making quinazolinones starting from N-acyl-N-(2-iodobenzyl)cyanamides 88 [105,106,107]. Iminyl radicals 89 were obtained by 5-exo-cyclisation onto the nitrile group of the first-formed aryl radicals. Subsequent ring closure took place efficiently onto a variety of aromatic and heteroaromatic rings yielding quinazolin-2-ones 90 (Scheme 16). The method was subsequently extended to the analogous 2-azidoalkyl-N-acylcyanamides 91 and these yielded guanidines 92 [108]. The disadvantages of this methodology were the need to use toxic cyanogen bromide in the syntheses of the precursors and the stoichiometric amounts of organotin hydride needed for the ring closures.
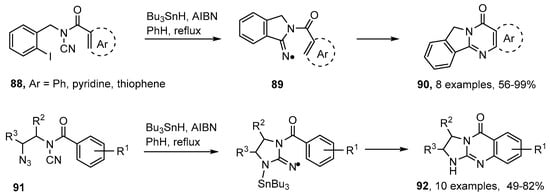
Scheme 16.
Cascade preparations of quinazolinones and guanidines [105,106,107,108].
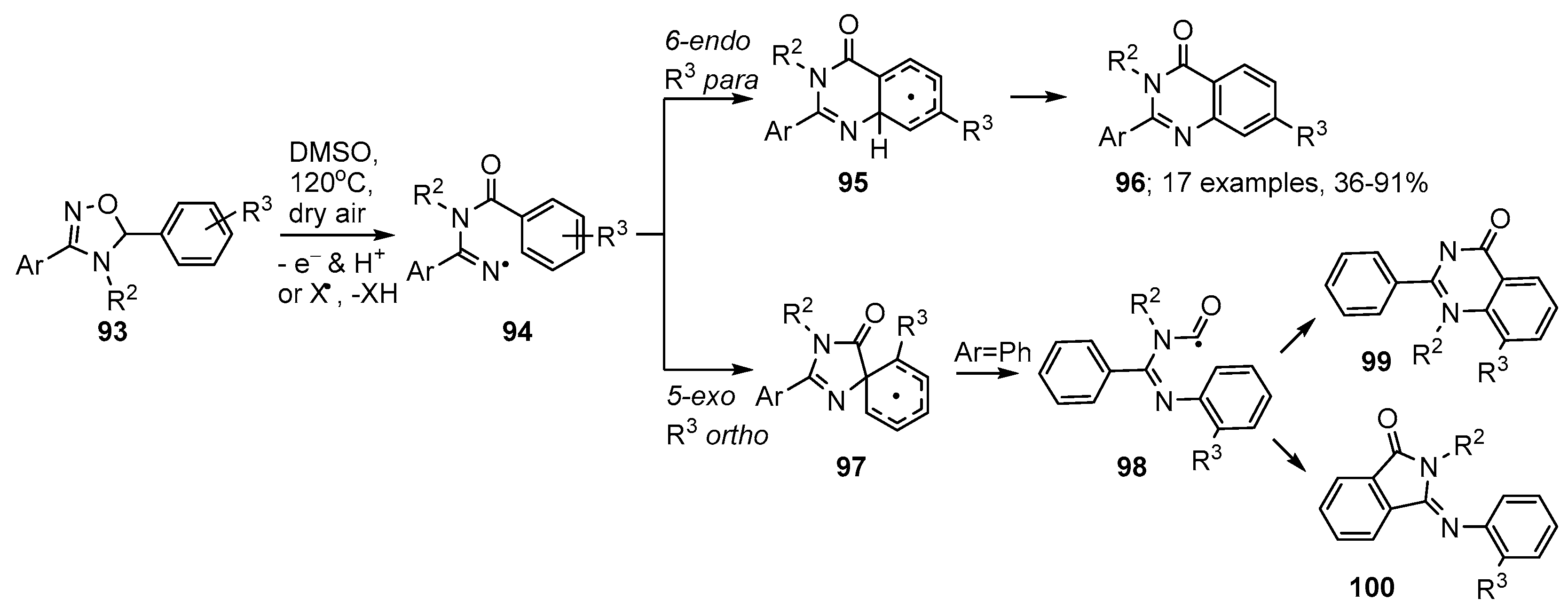
Aware that 5-aryl-4,5-dihydro-1,2,4-oxadiazoles 93 are readily available, either from 1,3-dipolar cycloadditions of nitrile oxides to aldimines, or from reactions of amidoximes with carbonyls [109,110], Chiba and co-workers adopted these as their iminyl radical precursors [111]. They discovered that on treatment in DMSO solution simply with dry air at 120 °C, oxidative rearrangements of 93 were set in motion and resulted in formation of quinazolinones 96 (Scheme 17). Iminyl radicals 94 were generated either via a SET and proton loss process, or by abstraction of the tertiary benzylic H-atom followed by β-scission (Scheme 17). The dominant ring closure was in the 6-endo mode yielding cyclohexadienyl radicals 95 that were oxidised to the quinazolinone products 96 usually in very good yields. The process was tolerant of electron withdrawing and electron releasing substituents, as well as 5-heteroaryl substituents. Substrates with ortho- or meta-substituents R3 gave rise to mixtures of isomeric quinazolinones (see structure 99).
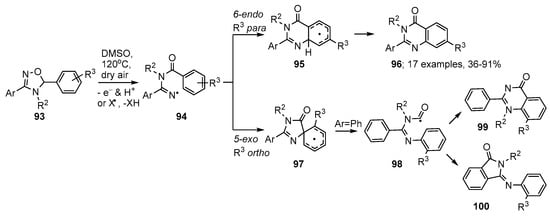
Scheme 17.
Preparations of quinazolinones from 4,5-dihydro-1,2,4-oxadiazoles [111].
Research with precursor 4,5-dihydro-1,2,4-oxadiazoles with ortho-R3, or containing 5-pyridine units, indicated that spiro-cyclisation to radicals 97 could compete. In this case an alternative ring opening to carbamoyl radicals 98 also took place and these species ring closed either to quinazolinone isomers 99 or in 5-exo-mode onto a 3-phenyl substituent with production of 3-(arylimino)isoindolin-1-one by-products 100.
4.2. Preparations of Quinoxaline Derivatives
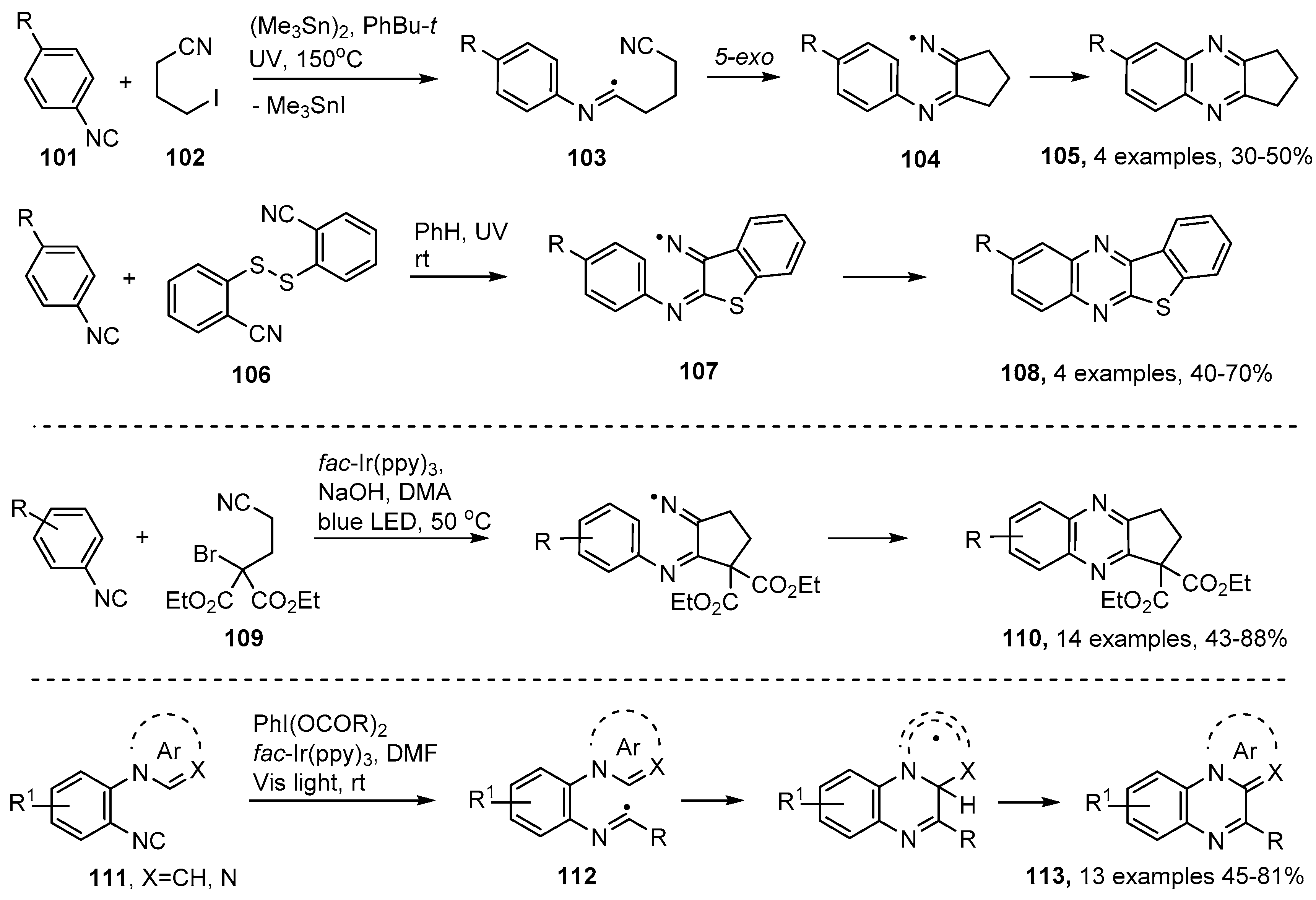
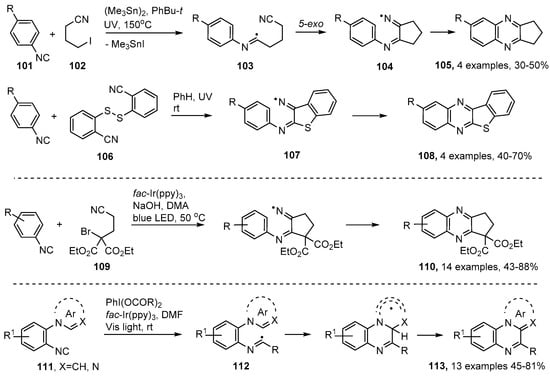
The quinoxaline and quinoxalin-2-one structural units are also associated with many natural products and biologically active substances [112,113,114,115]. These rings have been assembled in several ways based on initial addition of C-, or S-centered radicals to aromatic isonitriles 101. Nanni and co-workers generated cyano-substituted alkyl radicals by treatment of 4-iodobutanenitrile 102 with hexamethylditin in t-butylbenzene at 150 °C [116]. Additions to the isonitriles generated imidoyl radicals 103 that ring closed onto the pendant cyano group in 5-exo mode to afford cyclic iminyl radicals 104. The cascade continued with a further cyclisation on to the aromatic nucleus followed by oxidative re-aromatization to produce cyclopentaquinoxalines 105 in moderate yields (Scheme 18). Significantly, the 2-cyanophenyl disulfide 106 was employed in an analogous process that was free of organotin compounds. UV photolysis of 106, together with an isonitrile, in benzene solution at r.t. gave rise to benzo[4,5]thieno[2,3-b]quinoxalines 108 in fair yields via α-thioimidoyl radicals and polycyclic-iminyl radicals 107 (Scheme 18).
Scheme 18.
Preparations of quinoxalines via aromatic isonitrile insertion routes [116,117,119].
More recently, photoredox catalyzed versions of the isonitrile insertion process requiring only visible light, have been deployed for quinoxaline preparations. Sun et al. demonstrated that diethyl 2-bromo-2-(2-isocyanoethyl)malonate 109 was a suitable precursor for 3-cyano-alkyl radicals when irradiated by blue LED light in the presence of fac-Ir(ppy)3 as PC. Cyclopenta-iminyl radicals were obtained as intermediates and afforded cyclopentaquinoxalines 110 in moderate to excellent yields [117] (Scheme 18). Jamison and co-workers generated alkyl radicals from readily accessible phenyliodine(III) dicarboxylates [118] with visible light and fac-Ir(ppy)3 in DMF [119]. Their substrates were aromatic isonitriles with ortho-pyrrole or analogous heteroarene substituents 111. The alkyl radicals coupled with the isonitriles giving imidoyl radical intermediates 112 that ring closed to pyrrolo[1,2-a]quinoxalines (and related structures) 113 without the intermediacy of iminyl radicals (Scheme 18). The process was tolerant of many substituent types enabling a good range of polycyclic heteroarenes to be prepared. Efficiency was enhanced by their development of a continuous flow system that integrated isonitrile formation with the photo-redox cascade.
5. Iminyl Radical Mediated Preparations of Natural Products and Bioactive Compounds
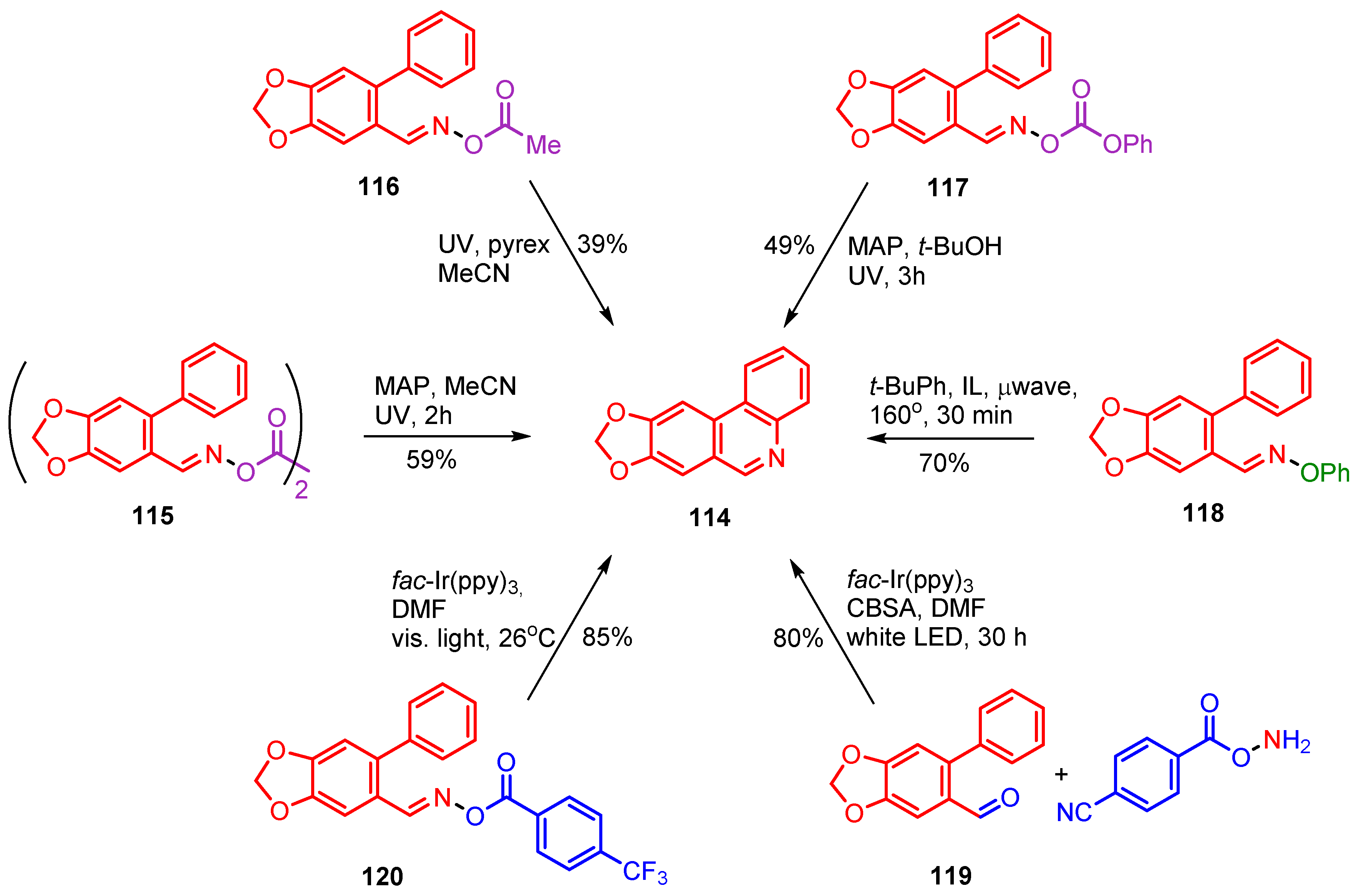
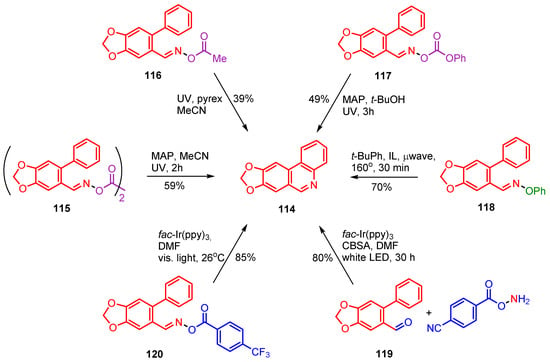
A significant number of iminyl radical based preparative methods have been developed for one or more stages in total or partial syntheses of natural products and bioactive compounds. Trispheridine 114 occurs naturally in flowering plants of the family Amaryllidaceae [120,121]. Trispheridine itself, as well as related compounds, are known to possess anti-tumor activity [122], high anti-retroviral activity [123] and neuro-protective activity [124]. At least six iminyl radical-mediated preparations of trispheridine have been devised and these are summarized in Scheme 19.
Scheme 19.
Iminyl radical mediated preparations of trispheridine.
Three of these rely on UVA irradiations; all of easily prepared precursors including dioxime oxalate 115 [54], O-acyl oxime ester 116 [52] and O-phenyl oxime carbonate 117 [77]. All three precursors were made in high yields, essentially in three steps, from commercially available 6-bromopiperonal. The highest reported yield (59%) was that from dioxime oxalate 115 but it is probable that yields from all three processes could be increased by optimization studies. The μwave promoted process from O-phenyl oxime ester 118 was short, clean and comparatively efficient (70%) [57]. Compound 118 was made in high yield in two steps from 6-bromopiperonal and the reaction conditions were clean and straightforward. The two photoredox catalyzed methods starting from aldehyde 119 [80] and trifluoromethylbenzoyl oxime ester 120 [79] had the highest reported yields and reaction conditions were benign (Scheme 19). The fac-Ir(ppy)3 catalyzed preparation, starting from aldehyde 119, took only two steps from 6-bromopiperonal. It should be noted, however, that the co-reactant O-(4-cyanobenzoyl)hydroxylamine is not commercial; although it can be made in two high-yielding steps from 4-cyanobenzoyl chloride.
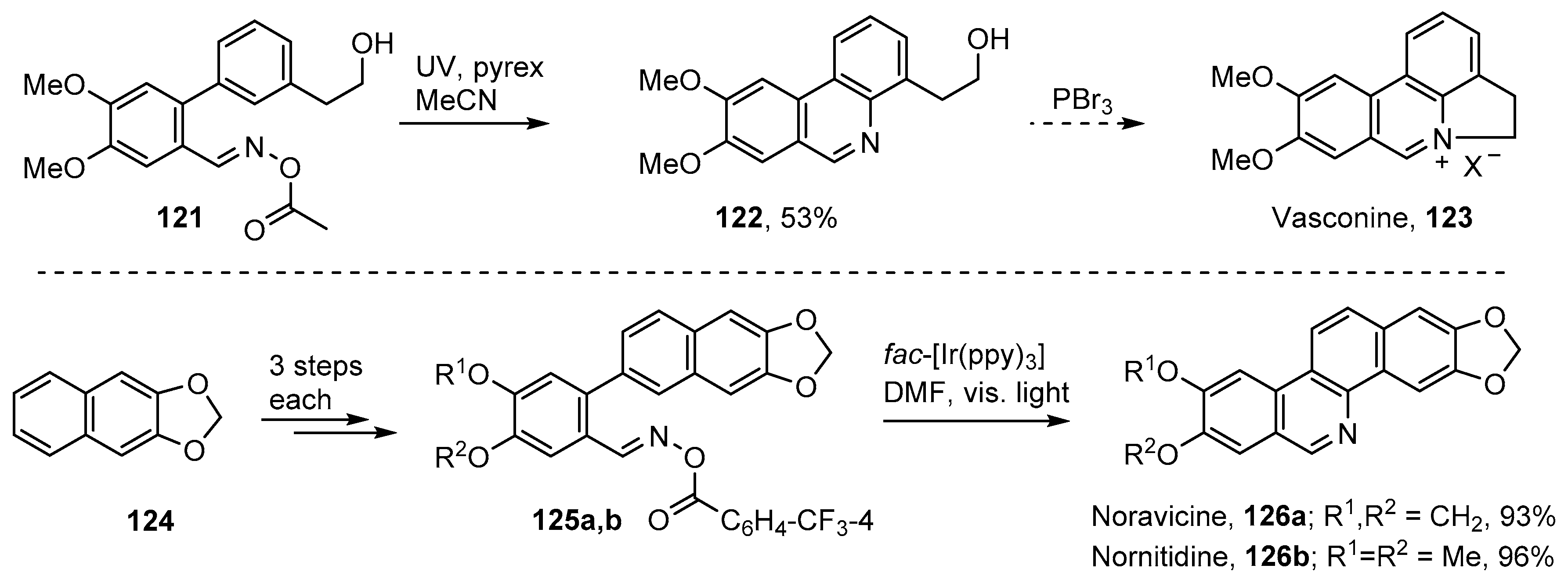
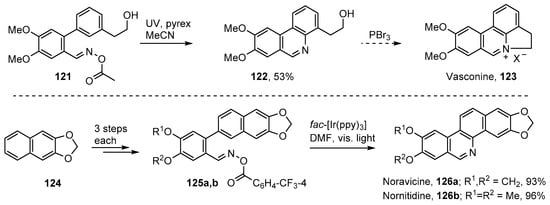
Vasconine 123, along with assoanine, oxoassoanine, pratosine and ismine, constitute another group of alkaloids, isolated from Narcissus plants of the Amaryllidaceae family, that contain phenanthridine rings [125,126]. The acyl oxime 121 was targeted as a key compound and prepared in six steps from commercial 2-(3-bromophenyl)ethanol [52]. UV irradiation of this through Pyrex in MeCN solution afforded phenanthridinylethanol derivative 122 in 53% yield (Scheme 20). Compound 122 can be converted to vasconine 123 by treatment with PBr3 and can also act as precursor for assoanine, oxoassoanine, and pratosine [125].
Scheme 20.
Iminyl radical strategies for vasconine [52], noravicine and nornitidine [79].
Noravicine 126a and nornitidine 126b are bioactive benzo[c]phenanthridine alkaloids with antitumor activity [127,128]. Zhang, Yu and co-workers devised short (4-steps from methylenedioxy-naphthalene 124) and high yielding total syntheses employing their photoredox strategy with oxime esters 125a and b [79] (Scheme 20).
Natural products that contain the indolizino[1,2-b]quinolin-9(11H)-one structure 130a include camptothecine, mappicine and the nothapodytines [129]. Camptothecine has been obtained from the bark of Camptotheca acuminata (Camptotheca, Happy tree, Tupelo family) [130] and mappicine from Mappia foetida miers (Olacaceae family) [131]. The high bioactivity of these compounds stimulated the production of many synthetic and semi-synthetic analogues [132,133]. Several pharmaceuticals containing this core have been licensed for use including irinotecan (Camptosar®, Campto®) which is used for the treatment of colon cancer and Topotecan (trade name Hycamtin®) which is approved for treatment of several cancers including ovarian and lung types [134]. A number of total syntheses, incorporating important radical-mediated steps, have been reported. Of particular note are those of Curran and co-workers, most of which have key steps involving amidoyl radicals obtained from additions to aromatic isonitriles [135,136,137,138,139,140,141].
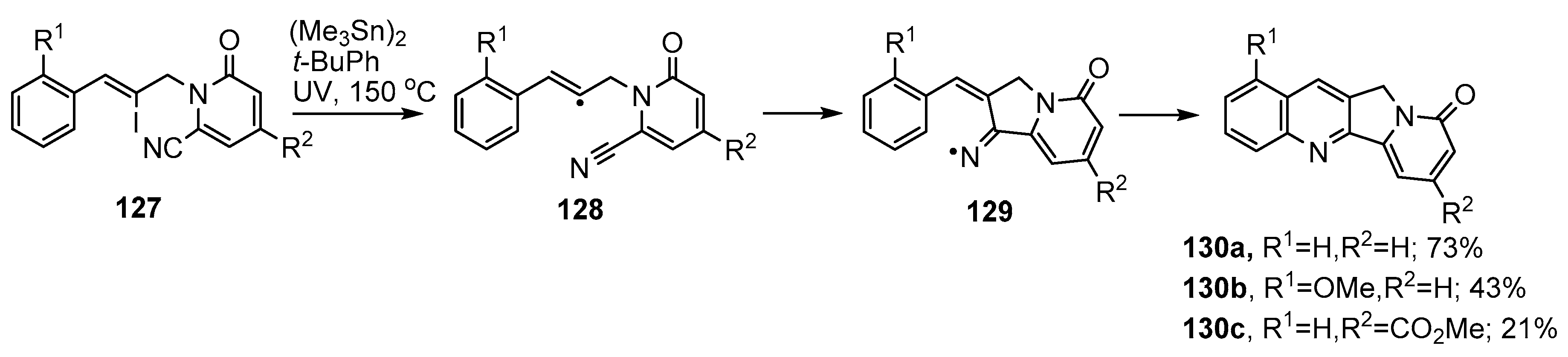
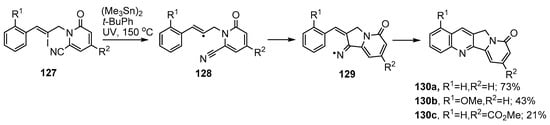
Bowman and co-workers implemented an iminyl radical cascade synthetic protocol for the tetracyclic rings A–D of heterocycles containing the indolizino[1,2-b]quinolin-9(11H)-one 130a unit [142,143] (Scheme 21). The starting vinyl iodides 127 were assembled in four steps from cinnamaldehyde derivatives and cyanopyridones and then UV irradiated with hexamethylditin in t-BuPh at 150 °C. The initial vinyl radicals 128 ring closed onto the nitrile group in 5-exo mode producing iminyl radicals 129 that underwent cyclisation and re-aromatization to afford tetracycles 130a–c.
Scheme 21.
Radical-mediated preparations of indolizino[1,2-b]quinolin-9(11H)-ones [142,143].
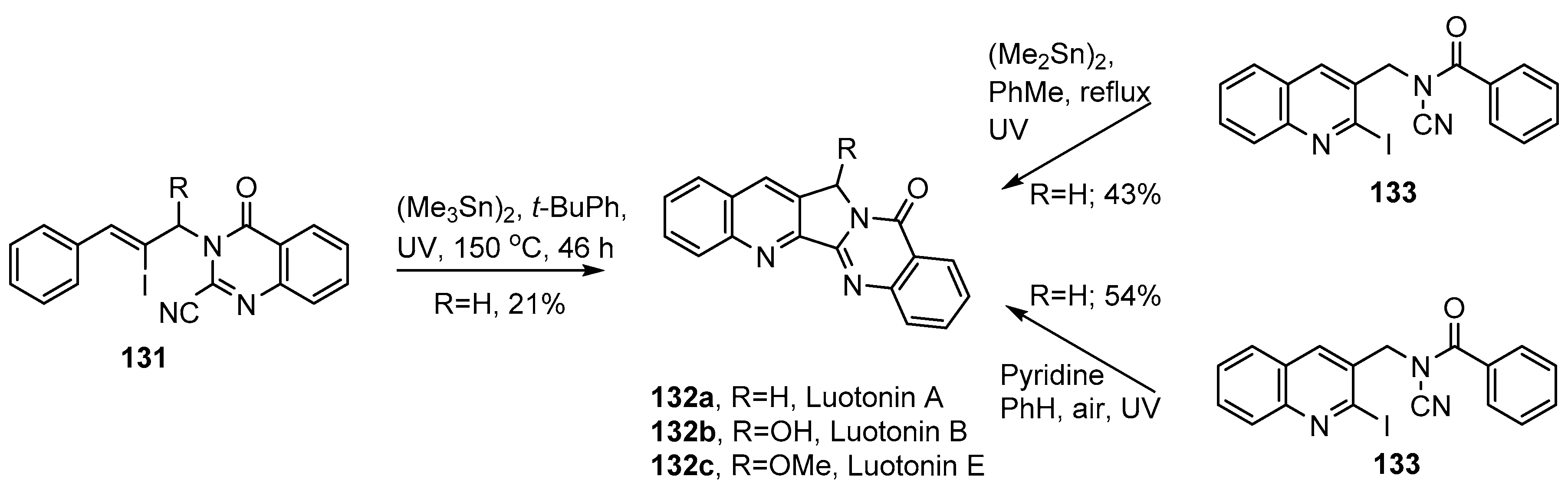
Luotonins A, B, E (132a–c) and congeners are structurally related to the mappicines and combine the 2,3-dihydro-1H-pyrrolo[3,4-b]quinoline ring system with the quinazolinone unit. They have been isolated from plant species of the genus Peganum found in China and are well-recognized as remedies in traditional Chinese medicine [144]. They are given for disorders including rheumatism, inflammation, influenza, hepatitis and leukemia and are known antagonists for human DNA topoisomerase I [145,146]. Structure/activity relationships have been investigated for heterocycles of this class [147]. Various non-radical syntheses have been devised [148,149,150,151]. Bowman and co-workers adapted their radical cascade protocol for a comparatively short synthesis of luotonin A 123a [143]. The vinyl iodide radical precursor 131 was made by alkylation of 4-oxo-3,4-dihydroquinazoline-2-carbonitrile with a substituted cinnamyl bromide. On UV irradiation of a solution of 131 and hexamethylditin in t-BuPh, luotonin A 132a was isolated in a very modest yield of 21% (Scheme 22). A slightly improved yield (30%) was obtained when the reaction was carried out in a sealed tube, at 120 °C, with added di-t-butyl peroxide.
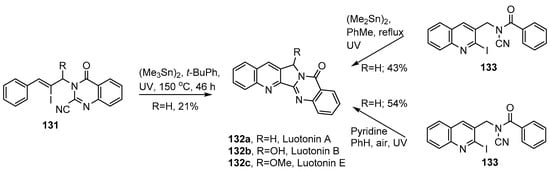
Scheme 22.
Iminyl radical mediated syntheses of luotonin A [105,107,143].
Courillon, Malacria and co-workers devised an alternative strategy in which the starting material was the N-acyl-N-(2-iodobenzyl)cyanamide 133 [105] (Scheme 22). For the luotonin synthesis, 3-(azidomethyl)-2-iodoquinoline was prepared in four steps from quinoline chlorcarbaldehyde and then reduced to the corresponding amine and benzoylated to give 133. UV irradiation of the latter, with hexamethylditin, in refluxing toluene for 6 h, gave a 43% yield of luotonin A 132a.
The same research team also discovered an organotin-free protocol employing the same N-acyl-N-(2-iodobenzyl)cyanamide 133 [107]. When this was UV photolyzed with pyridine (5 equiv.) in refluxing benzene, in air, luotonin A (132a) was obtained in an improved yield of 54%.
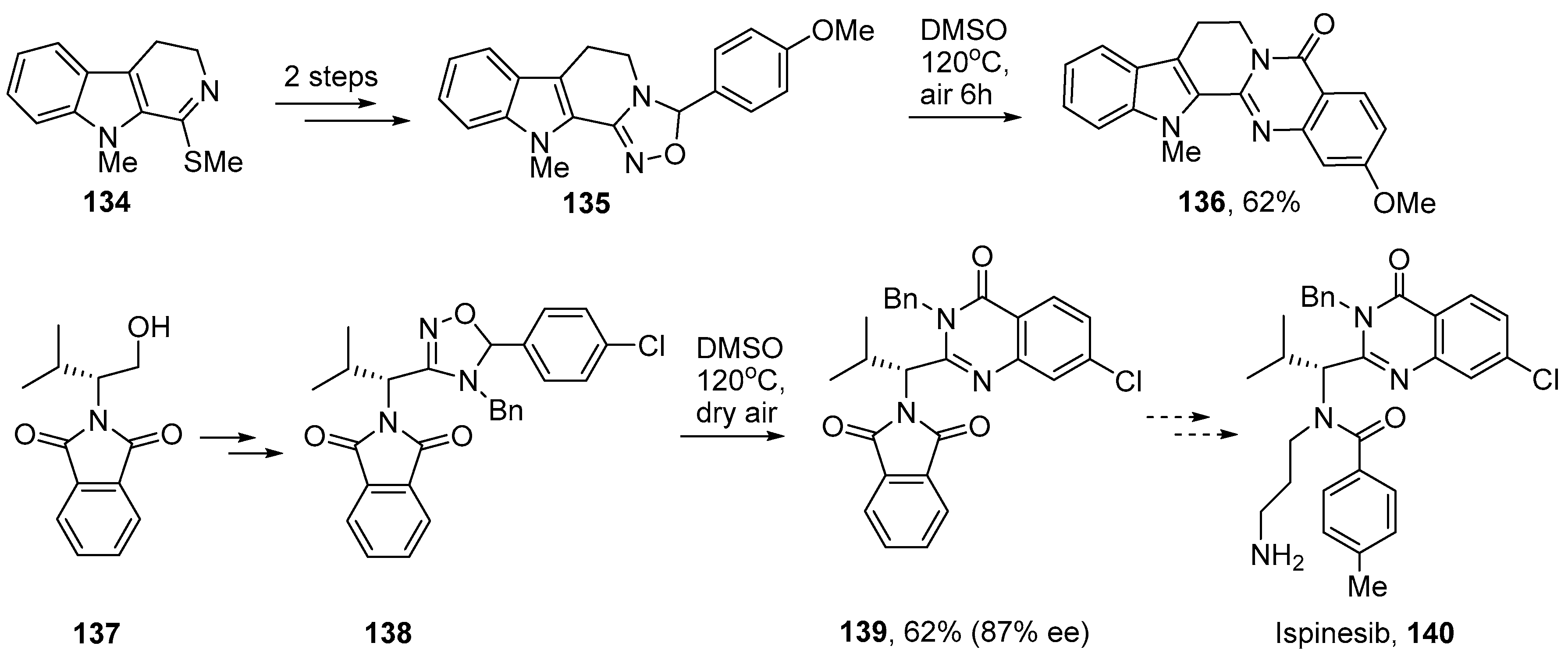
Chiba and co-workers successfully adapted their dihydro-1,2,4-oxadiazole method for preparations of several bio-active compounds. Rutaecarpine (sometimes rutecarpine) is an indoloquinazoline alkaloid that has been isolated from Euodia plants of the Rutaceae family and has non-steroidal anti-inflammatory drug (NSAID) activity [152]. The related 2-methoxy-13-methyl-rutaecarpine 136 has antimalarial activity and has been obtained from Araliopsis tabouensis, an African perennial tree [153]. In their synthesis, Chiba and co-workers prepared dihydro-1,2,4-oxadiazole 135 in two steps from indolyl imino methylthioether 134 [111]. Then on heating this as a DMSO solution in air the 2-methoxy-13-methylrutaecarpine 136 was obtained in 62% yield (Scheme 23).
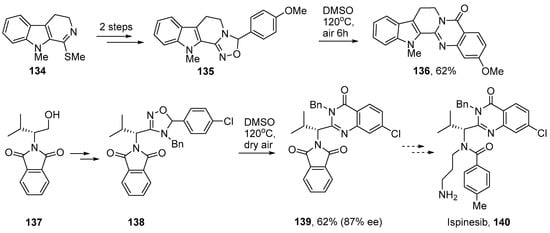
Scheme 23.
Syntheses of rutaecarpine and ispinesib derivatives via dihydro-1,2,4-oxadiazoles [111].
Ispinesib 140 is a synthetic quinazolinone that is under study as a potential anti-cancer agent [154,155]. A similar strategy was employed in a short iminyl radical-mediated synthesis [111]. Alkylated phthalimide 137 was prepared from L-valine and thence to dihydro-1,2,4-oxadiazole 138 as a mixture of diastereoisomers in three steps. On heating one stereoisomer in aerated DMSO the quinazoline derivative 139, which is a key precursor for ispinesib, was obtained in moderate yield though with some racemization [156]. These dihydro-1,2,4-oxadiazole rearrangements provide a benign, metal-free and atom-economical protocol with great promise as a general synthetic tool for the incorporation of quinazolinone units.
6. Conclusions
Five- and six-membered ring aza-arenes are hugely important in nutrition, medicine and pharmacology because of their many useful therapeutic properties. Short, mild and atom-efficient synthetic methods for compounds containing these units are clearly provided by iminyl radical mediated systems. Although iminyl radicals can be accessed by a variety of ways, carbonyl oximes and oxime ethers are particularly effective non-toxic precursors with long shelf lives. These starting materials lend themselves to ‘clean’ preparative protocols, free of harsh acid/base or redox reagents, and employing photolysis, μwave irradiation or photoredox catalysis. Iminyl radicals generated by these means and suitably substituted with acceptor units facilitate syntheses of a large variety of such compounds. Suitable acceptor groups include aromatics, heteroarenes, alkenes and alkynes. Reproducible and efficient syntheses of good scope and impressive generality have thereby been devised for diverse families of heterocycles. Significantly, iminyl radical based steps have been chosen for natural products and bioactive molecules containing phenanthridine, indole and quinazoline core units. It seems certain that further variations of these methods will be devised and find additional roles and applications. It can be reliably concluded that iminyl-radical based synthetic methods form a most valuable toolkit to expedite the exploration of ‘drug-like’ chemical space.
Acknowledgments
The author thanks EaStCHEM for financial support.
Conflicts of Interest
The author declares no conflict of interest.
References
- Reymond, J.-L. The chemical space project. Acc. Chem. Res. 2015, 48, 722–730. [Google Scholar] [CrossRef] [PubMed]
- Bon, R.S.; Waldemann, H. Bioactivity-guided navigation of chemical space. Acc. Chem. Res. 2010, 43, 1103–1114. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P. Cheminformatics analysis of organic substituents: Identification of the most common substituents, calculation of substituent properties, and automatic identification of drug-like bioisosteric groups. J. Chem. Inf. Comput. Sci. 2003, 43, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Bohacek, R.S.; McMartin, C.; Guida, W.C. The art and practice of structure-based drug design: A molecular modelling perspective. Med. Res. Rev. 1996, 16, 3–50. [Google Scholar] [CrossRef]
- Eigen, M. Self-organization of matter and the evolution of biological macromolecules. Naturwissenschaften 1971, 58, 465–523. [Google Scholar] [CrossRef] [PubMed]
- Ertl, P.; Jelfs, S.; Muehlbacher, J.; Schuffenhauer, A.; Selzer, P. Quest for the rings. In silico exploration of ring universe to identify novel bioactive heteroaromatic scaffolds. J. Med. Chem. 2006, 49, 4568–4573. [Google Scholar] [CrossRef] [PubMed]
- Renaud, P.; Sibi, M. (Eds.) Radicals in Organic Synthesis; Wiley: Weinheim, Germany, 2001; Volumes 1–2.
- Newcomb, M. Synthetic strategies & applications. In Encyclopedia of Radicals in Chemistry, Biology and Materials; Chatgilialoglu, C., Studer, A., Eds.; Wiley: New York, NY, USA, 2012; Volume 2. [Google Scholar]
- Baguley, P.A.; Walton, J.C. Flight from the tyranny of tin: the quest for practical radical sources free from metal encumbrances. Angew. Chem. Int. Ed. 1998, 110, 3072–3082. [Google Scholar] [CrossRef]
- Studer, A.; Amrein, S. Tin hydride substitutes in reductive radical chain reactions. Synthesis 2002, 835–849. [Google Scholar] [CrossRef]
- McCarroll, A.J.; Walton, J.C. Programming organic molecules: Design and management of organic syntheses through free-radical cascade processes. Angew. Chem. Int. Ed. 2001, 40, 2224–2248. [Google Scholar] [CrossRef]
- McCarroll, A.J.; Walton, J.C. Organic syntheses through free-radical annulations and related cascade sequences. J. Chem. Soc. Perkin Trans. 1 2001, 3215–3229. [Google Scholar] [CrossRef]
- Walton, J.C.; Studer, A. Evolution of functional cyclohexadiene-based synthetic reagents: The importance of becoming aromatic. Acc. Chem. Res. 2005, 38, 794–802. [Google Scholar] [CrossRef] [PubMed]
- Doni, E.; Mondal, B.; O’Sullivan, S.; Tuttle, T.; Murphy, J.A. Overturning established chemoselectivities: Selective reduction of arenes over malonates and cyanoacetates by photoactivated organic electron donors. J. Am. Chem. Soc. 2013, 135, 10934–10937. [Google Scholar] [CrossRef] [PubMed]
- Murphy, J.A. Discovery and development of organic super-electron-donors. J. Org. Chem. 2014, 79, 3731–3746. [Google Scholar] [CrossRef] [PubMed]
- Hanson, S.S.; Doni, E.; Traboulsee, K.T.; Coulthard, G.; Murphy, J.A.; Dyker, C.A. Pushing the limits of neutral organic electron donors: A tetra(iminophosphorano)-substituted bispyridinylidene. Angew. Chem. Int. Ed. 2015, 54, 11236–11239. [Google Scholar] [CrossRef] [PubMed]
- Darmency, V.; Renaud, P. Tin-free radical reactions mediated by organoboron compounds. Top. Curr. Chem. 2006, 263, 71–106. [Google Scholar]
- Luethy, M.; Darmency, V.; Renaud, P. Modified B-alkylcatecholboranes as radical precursors. Eur. J. Org. Chem. 2011, 2011, 547–552. [Google Scholar] [CrossRef]
- Ueng, S.-H.; Solovyev, A.; Yuan, X.; Geib, S.J.; Fensterbank, L.; Lacote, E.; Malacria, M.; Newcomb, M.; Walton, J.C.; Curran, D.P. N-Heterocyclic carbene boryl radicals: A new class of boron-centered radical. J. Am. Chem. Soc. 2009, 131, 11256–11262. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C. Linking borane with N-heterocyclic carbenes: Effective hydrogen-atom donors for radical reactions. Angew. Chem. Int. Ed. 2009, 48, 1726–1728. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C.; Brahmi, M.M.; Monot, J.; Fensterbank, L.; Malacria, M.; Curran, D.P.; Lacôte, E. Electron paramagnetic resonance and computational studies of radicals derived from boron-substituted N-heterocyclic carbene boranes. J. Am. Chem. Soc. 2011, 133, 10312–10321. [Google Scholar] [CrossRef] [PubMed]
- Kawamoto, T.; Geib, S.J.; Curran, D.P. Radical reactions of N-heterocyclic carbene boranes with organic nitriles: Cyanation of NHC-boranes and reductive decyanation of malononitriles. J. Am. Chem. Soc. 2015, 137, 8617–8622. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.P.; Ischay, M.A.; Du, J. Visible light photocatalysis as a greener approach to photochemical synthesis. Nat. Chem. 2010, 2, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Narayanam, J.M.R.; Stephenson, C.R.J. Visible light photoredox catalysis: Applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Zou, Y.-Q.; Chen, J.-R.; Xiao, W.-J. Homogeneous visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2013, 52, 11701–11703. [Google Scholar] [CrossRef] [PubMed]
- Prier, C.K.; Rankic, D.A.; MacMillan, D.W.C. Visible light photoredox catalysis with transition metal complexes: Applications in organic synthesis. Chem. Rev. 2013, 113, 5322–5363. [Google Scholar] [CrossRef] [PubMed]
- Davies, J.; Booth, S.G.; Essafi, S.; Dryfe, R.A.W.; Leonori, D. Visible-light-mediated generation of nitrogen-centered radicals: Metal-free hydroimination and iminohydroxylation cyclization reactions. Angew. Chem. Int. Ed. 2015, 54, 14017–14021. [Google Scholar] [CrossRef] [PubMed]
- Hofstra, J.L.; Grassbaugh, B.R.; Tran, Q.M.; Armada, N.R.; de Lijser, H.J.P. Catalytic oxidative cyclization of 2′-arylbenzaldehyde oxime ethers under photoinduced electron transfer conditions. J. Org. Chem. 2015, 80, 256–265. [Google Scholar] [CrossRef] [PubMed]
- Manley, D.W.; McBurney, R.T.; Miller, P.; Howe, R.F.; Rhydderch, S.; Walton, J.C. Unconventional titania photocatalysis: Direct deployment of carboxylic acids in alkylations and annulations. J. Am. Chem. Soc. 2012, 134, 13580–13583. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, N. Photocatalysis with TiO2 applied to organic synthesis. Aust. J. Chem. 2015, 68, 1621–1639. [Google Scholar] [CrossRef]
- Manley, D.W.; Walton, J.C. Preparative semiconductor photoredox catalysis: An emerging theme in organic synthesis. Beilstein J. Org. Chem. 2015, 11, 1570–1582. [Google Scholar] [CrossRef] [PubMed]
- Walton, J.C. Functionalised oximes: Emergent precursors for carbon-, nitrogen- and oxygen-centred radicals. Molecules 2016, 21, 63. [Google Scholar] [CrossRef] [PubMed]
- Zard, S.Z. Recent progress in the generation and use of nitrogen-centred radicals. Chem. Soc. Rev. 2008, 37, 1603–1618. [Google Scholar] [CrossRef] [PubMed]
- Gagosz, F.; Zard, S. Generation and capture of iminyl radicals from ketoxime xanthates. Synlett 1999, 1978–1980. [Google Scholar] [CrossRef]
- Nanni, D.; Calestani, G.; Leardini, R.; Zanardi, G. On the regioselectivity of imidoyl radical cyclisations. Eur. J. Org. Chem. 2000, 2000, 707–711. [Google Scholar] [CrossRef]
- Leardini, R.; McNab, H.; Minozzi, M.; Nanni, D.; Reed, D.; Wright, A.G. Reactions of 1-(2-alkoxyphenyl)alkaniminyl radicals. J. Chem. Soc. Perkin Trans. 1 2001, 2704–2710. [Google Scholar] [CrossRef]
- Minozzi, M.; Nanni, D.; Spagnolo, P. From azides to nitrogen-centered radicals: Applications of azide radical chemistry to organic synthesis. Chem. Eur. J. 2009, 15, 7830–7840. [Google Scholar] [CrossRef] [PubMed]
- Aldabbagh, F.; Bowman, W.R. Synthesis of heterocycles by radical cyclization. Contemp. Org. Synth. 1997, 4, 261–280. [Google Scholar] [CrossRef]
- Aldabbagh, F.; Bowman, W.R.; Mann, E.; Slawin, A.M.Z. Bu3SnH mediated oxidative radical cyclization onto imidazoles and pyrroles. Tetrahedron 1999, 55, 8111–8128. [Google Scholar] [CrossRef]
- Bowman, W.R.; Bridge, C.F.; Cloonan, M.O.; Leach, D.C. Synthesis of heteroarenes via radical cyclisation onto nitriles. Synlett 2001, 765–768. [Google Scholar] [CrossRef]
- Fallis, A.G.; Brinza, I.M. Free radical cyclizations involving nitrogen. Tetrahedron 1997, 53, 17543–17594. [Google Scholar] [CrossRef]
- Zhang, B.; Studer, A. Recent advances in the synthesis of nitrogen heterocycles via radical cascade reactions using isonitriles as radical acceptors. Chem. Soc. Rev. 2015, 44, 3505–3521. [Google Scholar] [CrossRef] [PubMed]
- Costantino, L.; Barlocco, D. Privileged structures as leads in medicinal chemistry. Curr. Med. Chem. 2006, 13, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Bellina, F.; Rossi, R. Synthesis and biological activity of pyrrole, pyrroline and pyrrolidine derivatives with two aryl groups on adjacent positions. Tetrahedron 2006, 62, 7213–7256. [Google Scholar] [CrossRef]
- Domagala, A.; Jarosz, T.; Lapkowski, M. Living on pyrrolic foundations - advances in natural and artificial bioactive pyrrole derivatives. Eur. J. Med. Chem. 2015, 100, 176–187. [Google Scholar] [CrossRef] [PubMed]
- Cullen, K.E.; Sharp, J.T. Reactions of diene-conjugated 1,3-dipolar intermediates: A versatile and efficient route to dibenz[c,e]azepines via benzonitrile o-arylbenzyl ylides. J. Chem. Soc. Perkin Trans. 1 1993, 2961–2967. [Google Scholar] [CrossRef]
- McCarroll, A.J.; Walton, J.C. Exploitation of aldoxime esters as radical precursors in preparative and EPR spectroscopic roles. J. Chem. Soc. Perkin Trans. 2 2000, 2399–2409. [Google Scholar] [CrossRef]
- McCarroll, A.J.; Walton, J.C. Enhanced radical delivery from aldoxime esters for EPR and ring closure applications. Chem. Commun. 2000, 351–352. [Google Scholar] [CrossRef]
- Alonso, R.; Campos, P.J.; Garcıa, B.; Rodrıguez, M.A. New light-induced iminyl radical cyclization reactions of acyloximes to isoquinolines. Org. Lett. 2006, 8, 3521–3423. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Campos, P.J.; Rodrıguez, M.A.; Sampedro, D. Photocyclization of iminyl radicals: Theoretical study and photochemical aspects. J. Org. Chem. 2008, 73, 2234–2239. [Google Scholar] [CrossRef] [PubMed]
- Alonso, R.; Caballero, A.; Campos, P.J.; Rodrıguez, M.A. Photochemistry of acyloximes: Synthesis of heterocycles and natural products. Tetrahedron 2010, 66, 8828–8831. [Google Scholar] [CrossRef]
- Jochims, J.C.; Hehl, S.; Herzberger, S. Preparation and Beckmann rearrangement of O-(chlorooxalyl)oximes. Synthesis 1990, 1128–1133. [Google Scholar] [CrossRef]
- Portela-Cubillo, F.; Lymer, J.; Scanlan, E.M.; Scott, J.S.; Walton, J.C. Dioxime oxalates; new iminyl radical precursors for syntheses of N-heterocycles. Tetrahedron 2008, 64, 11908–11916. [Google Scholar] [CrossRef]
- Blake, J.A.; Pratt, D.A.; Lin, S.; Walton, J.C.; Mulder, P.; Ingold, K.U. Thermolyses of O-phenyl oxime ethers. A new source of iminyl radicals and a new source of aryloxyl radicals. J. Org. Chem. 2004, 69, 3112–3120. [Google Scholar] [CrossRef] [PubMed]
- Portela-Cubillo, F.; Scott, J.S.; Walton, J.C. Microwave-assisted preparations of dihydropyrroles from alkenone O-phenyl oximes. Chem. Commun. 2007, 4041–4043. [Google Scholar] [CrossRef] [PubMed]
- Portela-Cubillo, F.; Scott, J.S.; Walton, J.C. Microwave-assisted syntheses of N-heterocycles using alkenone-, alkynone- and aryl-carbonyl O-phenyl oximes: Formal synthesis of neocryptolepine. J. Org. Chem. 2008, 73, 5558–5565. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Jalan, A.; Kubosumi, A.R.; Castle, S.L. Microwave-promoted tin-free iminyl radical cyclization with TEMPO trapping: A practical synthesis of 2‑acylpyrroles. Org. Lett. 2015, 17, 488–491. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-R.; Hu, X.-Q.; Lu, L.-Q.; Xiao, W.-J. Visible light photoredox-controlled reactions of N-radicals and radical ions. Chem. Soc. Rev. 2016, 45, 2044–2056. [Google Scholar] [CrossRef] [PubMed]
- Mikami, T.; Narasaka, K. Photochemical transformation of γ,δ-unsaturated ketone O-(p-cyanophenyl)oximes to 3,4-dihydro-2H-pyrrole derivatives. Chem. Lett. 2000, 29, 338–339. [Google Scholar] [CrossRef]
- Lin, X.; Artman, G.D.; Stien, D.; Weinreb, S.M. Development of efficient new methodology for generation, cyclization and functional trapping of iminyl and amidyl radicals. Tetrahedron 2001, 57, 8779–8791. [Google Scholar] [CrossRef]
- Madapa, S.; Tusi, Z.; Batra, S. Advances in the syntheses of quinoline and quinoline-annulated ring systems. Curr. Org. Chem. 2008, 12, 1116–1183. [Google Scholar] [CrossRef]
- Chung, P.-Y.; Bian, Z.-X.; Pun, H.-Y.; Chan, D.; Chan, A.S.-C.; Chui, C.-H.; Tang, J.C.-O.; Lam, K.-H. Recent advances in research of natural and synthetic bioactive quinolines. Future Med. Chem. 2015, 7, 947–967. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Ding, K.-M.; Zhang, L.; Cheng, X.-M.; Wang, C.-H.; Wang, Z.-T. Acetylcholinesterase and butyrylcholinesterase inhibitory activities of β-carboline and quinoline alkaloids derivatives from the plants of genus Peganum. J. Chem. 2013, 2013. [Google Scholar] [CrossRef]
- Cheng, P.; Gu, Q.; Liu, W.; Zou, J.-F.; Ou, Y.-Y.; Luo, Z.-Y.; Zeng, J.-G. Synthesis of quinolin-2-one alkaloid derivatives and their inhibitory activities against HIV-1 reverse transcriptase. Molecules 2011, 16, 7649–7661. [Google Scholar] [CrossRef] [PubMed]
- Byler, K.G.; Wang, C.; Setzer, W.N. Quinoline alkaloids as intercalative topoisomerase inhibitors. J. Mol. Model. 2009, 15, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Fujioka, H.; Kita, Y. Synthesis of the marine pyrroloiminoquinone alkaloids, discorhabdins. Mar. Drugs 2010, 8, 1394–1416. [Google Scholar] [CrossRef] [PubMed]
- Truchado, P.; Martos, I.; Bortolotti, L.; Sabatini, A.G.; Ferreres, F.; Tomas-Barberan, F.A. Use of quinoline alkaloids as markers of the floral origin of chestnut honey. J. Agric. Food Chem. 2009, 57, 5680–5686. [Google Scholar] [CrossRef] [PubMed]
- Tumir, L.-M.; Stojkovic, M.R.; Piantanida, I. Come-back of phenanthridine and phenanthridinium derivatives in the 21st century. Beilstein J. Org. Chem. 2014, 10, 2930–2954. [Google Scholar] [CrossRef] [PubMed]
- Dostal, J.; Slavik, J. Some aspects of the chemistry of quaternary benzo[c]phenanthridine alkaloids. Stud. Nat. Prod. Chem. 2002, 27, 155–184. [Google Scholar]
- Dvorak, Z.; Kuban, V.; Klejdus, B.; Hlavac, J.; Vicar, J.; Ulrichova, J.; Simanek, V. Quaternary benzo[c]phenanthridines sanguinarine and chelerythrine: A review of investigations from chemical and biological studies. Heterocycles 2006, 68, 2403–2422. [Google Scholar] [CrossRef]
- Hammerova, J.; Uldrijan, S.; Taborska, E.; Slaninova, I. Benzo[c]phenanthridine alkaloids exhibit strong anti-proliferative activity in malignant melanoma cells regardless of their p53 status. J. Dermatol. Sci. 2011, 62, 22–35. [Google Scholar] [CrossRef] [PubMed]
- Slaninova, I.; Pencikova, K.; Urbanova, J.; Slanina, J.; Taborska, E. Antitumour activities of sanguinarine and related alkaloids. Phytochem. Rev. 2014, 13, 51–68. [Google Scholar] [CrossRef]
- Cao, F.-J.; Yang, R.; Lv, C.; Ma, Q.; Lei, M.; Geng, H.-L.; Zhou, L. Pseudocyanides of sanguinarine and chelerythrine and their series of structurally simple analogues as new anticancer lead compounds: Cytotoxic activity, structure-activity relationship and apoptosis induction. Eur. J. Pharm. Sci. 2015, 67, 45–54. [Google Scholar] [CrossRef] [PubMed]
- McBurney, R.T.; Walton, J.C. Interplay of ortho- with spiro-cyclisation during iminyl radical closures onto arenes and heteroarenes. Beilstein J. Org. Chem. 2013, 9, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Cai, J.; Tang, L.; Wang, Z.; Li, F.; Deng, G.-J. Metal-free assembly of polysubstituted pyridines from oximes and acroleins. J. Org. Chem. 2016, 81, 1499–1505. [Google Scholar] [CrossRef] [PubMed]
- McBurney, R.T.; Slawin, A.M.Z.; Smart, L.A.; Yu, Y.; Walton, J.C. UV promoted phenanthridine syntheses from oxime carbonate derived iminyl radicals. Chem. Commun. 2011, 47, 7974–7976. [Google Scholar] [CrossRef] [PubMed]
- McBurney, R.T.; Walton, J.C. Dissociation or cyclization: Options for a triad of radicals released from oxime carbamates. J. Am. Chem. Soc. 2013, 135, 7349–7354. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; An, X.; Tong, K.; Zheng, T.; Zhang, Y.; Yu, S. Visible-light-promoted iminyl-radical formation from acyl oximes: A unified approach to pyridines, quinolines, and phenanthridines. Angew. Chem. Int. Ed. 2015, 54, 4055–4059. [Google Scholar] [CrossRef] [PubMed]
- An, X.-D.; Yu, S. Visible-light-promoted and one-pot synthesis of phenanthridines and quinolines from aldehydes and O‑acyl hydroxylamine. Org. Lett. 2015, 17, 2692–2695. [Google Scholar] [CrossRef] [PubMed]
- Fry, D.W.; Kraker, A.J.; McMichael, A.; Ambroso, L.A.; Nelson, J.M.; Leopold, W.R.; Connors, R.W.; Bridges, A.J. A specific inhibitor of the epidermal growth factor receptor tyrosine kinase. Science 1994, 265, 1093–1095. [Google Scholar] [CrossRef] [PubMed]
- Kunes, J.; Bazant, J.; Pour, M.; Waisser, K.; Slosarek, M.; Janota, J. Quinazoline derivatives with antitubercular activity. Farmaco 2000, 55, 725–729. [Google Scholar] [CrossRef]
- Foster, B.A.; Coffrey, H.A.; Morin, M.J.; Rastinejad, F. Pharmacological rescue of mutant p53 conformation and function. Science 1999, 286, 2507–2510. [Google Scholar] [CrossRef] [PubMed]
- Bedi, P.M.S.; Kumar, V.; Mahajan, M.P. Synthesis and biological activity of novel antibacterial quinazolines. Bioorg. Med. Chem. Lett. 2004, 14, 5211–5213. [Google Scholar] [CrossRef] [PubMed]
- Gundla, R.; Kazemi, R.; Sanam, R.; Muttineni, R.; Sarma, J.A.R.P.; Dayam, R.; Neamati, N. Discovery of novel small-molecule inhibitors of human epidermal growth factor receptor-2: Combined ligand and target-based approach. J. Med. Chem. 2008, 51, 3367–3377. [Google Scholar] [CrossRef] [PubMed]
- Mendes da Silva, J.F.; Walters, M.; Al-Damluji, S.; Ganellin, C.R. Molecular features of the prazosin molecule required for activation of transport-P. Bioorg. Med. Chem. 2008, 16, 7254–7263. [Google Scholar] [CrossRef] [PubMed]
- Rewcastle, G.W.; Palmer, B.D.; Bridges, A.J.; Showalter, H.D.H.; Sun, L.; Nelson, J.; McMichael, A.; Kraker, A.J.; Fry, D.W.; Denny, W.A. Tyrosine kinase inhibitors. 9. Synthesis and evaluation of fused tricyclic quinazoline analogs as ATP site inhibitors of the tyrosine kinase activity of the epidermal growth factor receptor. J. Med. Chem. 1996, 39, 918–928. [Google Scholar] [CrossRef] [PubMed]
- Luth, A.; Lowe, W. Syntheses of 4-(indol-3-yl)quinazolines—A new class of epidermal growth factor receptor tyrosine kinase inhibitors. Eur. J. Med. Chem. 2008, 43, 1478–1488. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.J.; Cusack, D.; O’Sullivan, T.P.; Guiry, P.J. Synthesis of quinazolinones and quinazolines. Tetrahedron 2005, 61, 10153–10202. [Google Scholar] [CrossRef]
- Besson, T.; Chosson, E. Microwave-assisted synthesis of bioactive quinazolines and quinazolinones. Comb. Chem. High Throughput Screen. 2007, 10, 903–917. [Google Scholar] [CrossRef] [PubMed]
- Michael, J.P. Quinoline, quinazoline and acridone alkaloids. Nat. Prod. Rep. 2003, 20, 476–493. [Google Scholar] [CrossRef] [PubMed]
- Chilin, A.; Marzaro, G.; Zanatta, S.; Guiotto, A. A microwave improvement in the synthesis of the quinazoline scaffold. Tetrahedron Lett. 2007, 48, 3229–3231. [Google Scholar] [CrossRef]
- Seijas, J.A.; Vazquez-Tato, M.P.; Martinez, M.M. Microwave-enhanced synthesis of 4-aminoquinazolines. Tetrahedron Lett. 2000, 41, 2215–2217. [Google Scholar] [CrossRef]
- Yoon, D.S.; Han, Y.; Stark, T.M.; Haber, J.C.; Gregg, B.T.; Stankovich, S.B. Efficient synthesis of 4-aminoquinazoline and thieno[3,2-d]pyrimidin-4-ylamine derivatives by microwave irradiation. Org. Lett. 2004, 6, 4775–4778. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Mohan, C.; Gupta, M.; Mahajan, M.P. A catalyst- and solvent-free selective approach to biologically important quinazolines and benzo[g]quinazoline. Tetrahedron 2005, 61, 3533–3538. [Google Scholar] [CrossRef]
- Maitraie, D.; Yakaiah, T.; Srinivas, K.; Reddy, G.V.; Ravikanth, S.; Narsaiah, B.; Rao, S.P.; Ravikumar, K.; Sridhar, B. Regioselective addition of Grignard reagents to 2,6-dicyanoanilines and cyclization to new quinazoline derivatives under thermal/microwave irradiation conditions. J. Fluor. Chem. 2006, 127, 351–359. [Google Scholar] [CrossRef]
- Abdel-Jalil, R.J.; Völter, W.; Saeed, M. A novel method for the synthesis of 4(3H)-quinazolinones. Tetrahedron Lett. 2004, 45, 3475–3476. [Google Scholar] [CrossRef]
- Portela-Cubillo, F.; Scott, J.S.; Walton, J.C. 2-(Aminoaryl)alkanone O-phenyl oximes: Versatile reagents for syntheses of quinazolines. Chem. Commun. 2008, 2935–2937. [Google Scholar] [CrossRef] [PubMed]
- Portela-Cubillo, F.; Scott, J.S.; Walton, J.C. Microwave-promoted syntheses of quinazolines and dihydroquinazolines from 2-aminoarylalkanone O-phenyl oximes. J. Org. Chem. 2009, 74, 4934–4942. [Google Scholar] [CrossRef] [PubMed]
- Pouilhes, A.; Langlois, Y.; Chiaroni, A. First synthesis of marine alkaloid (±)-bengacarboline. Synlett 2003, 1488–1490. [Google Scholar] [CrossRef]
- Patil, D.A.; Patil, P.O.; Patil, G.B.; Surana, S.J. Synthesis of 2,3-disubstituted-quinazolin-4-(3H)-ones. Mini-Rev. Med. Chem. 2011, 11, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Jiang, Y.; Ma, D. Synthesis of 3-substituted and 2,3-disubstituted quinazolinones via Cu-catalyzed aryl amidation. Org. Lett. 2012, 14, 1150–1153. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Wang, Y.; Peng, J.; Zhu, Q. Synthesis of quinazolin-4(3H)-ones via Pd(II)-catalyzed intramolecular C(sp2)-H carboxamidation of N-arylamidines. J. Org. Chem. 2011, 76, 6362–6366. [Google Scholar] [CrossRef] [PubMed]
- Giri, R.; Lam, J.K.; Yu, J.-Q. Synthetic applications of Pd(II)-catalyzed C-H carboxylation and mechanistic insights: Expedient routes to anthranilic acids, oxazolinones, and quinazolinones. J. Am. Chem. Soc. 2010, 132, 686–693. [Google Scholar] [CrossRef] [PubMed]
- Servais, A.; Azzouz, M.; Lopes, D.; Courillon, C.; Malacria, M. Radical cyclization of N-acylcyanamides: Total synthesis of luotonin A. Angew. Chem. Int. Ed. 2007, 46, 576–579. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, M.-H.; Courillon, C.; Ollivier, C.; Lacôte, E.; Malacria, M.; Fensterbank, L. Radical migration of substituents of aryl groups on quinazolinones derived from N-acyl cyanamides. J. Am. Chem. Soc. 2010, 132, 4381–4387. [Google Scholar] [CrossRef] [PubMed]
- Beaume, A.; Courillon, C.; Derat, E.; Malacria, M. Unprecedented aromatic homolytic substitutions and cyclization of amide-iminyl radicals: Experimental and theoretical study. Chem. Eur. J. 2008, 14, 1238–1252. [Google Scholar] [CrossRef] [PubMed]
- Larraufie, M.-H.; Ollivier, C.; Fensterbank, L.; Malacria, M. Radical synthesis of guanidines from N-acyl cyanamides. Angew. Chem. Int. Ed. 2010, 49, 2178–2181. [Google Scholar] [CrossRef] [PubMed]
- Alcaide, B.; Mardomingo, C.L.; Plumet, J.; Cativiela, C.; Mayoral, J.A. Orbital control in the 1,3-dipolar cycloaddition of benzonitrile oxide to benzylideneanilines. Can. J. Chem. 1987, 65, 2050–2056. [Google Scholar] [CrossRef]
- Srivastava, R.M.; Freire, M.V.S.; Chaves, A.S.S.C.; Beltrao, T.M.; Carpenter, G.B. Synthesis, spectroscopic studies and the mechanism of formation of 4,5-dihydro-1,2,4-oxadiazoles. J. Heterocycl. Chem. 1987, 24, 101–105. [Google Scholar] [CrossRef]
- Wang, Y.-F.; Zhang, F.-L.; Chiba, S. Oxidative radical skeletal rearrangement induced by molecular oxygen: Synthesis of quinazolinones. Org. Lett. 2013, 15, 2842–2845. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, E.J.; Stelzer, L.S.; TenBrink, R.E.; Belonga, K.L.; Carter, D.B.; Im, H.K.; Im, W.B.; Sethy, V.H.; Tang, A.H.; Zhong, W.Z.; et al. Piperazine imidazo[1,5-a]quinoxaline ureas as high-affinity GABAA ligands of dual functionality. J. Med. Chem. 1999, 42, 1123–1144. [Google Scholar] [PubMed]
- Lawrence, D.S.; Copper, J.E.; Smith, C.D. Structure-activity studies of substituted quinoxalinones as multiple-drug-resistance antagonists. J. Med. Chem. 2001, 44, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Padvi, P.A.; Mahale, G.H.; Pawar, D.E.; Falak, C.S.; Kendre, A.V. Synthesis and biological activity of quinoxaline derivatives. World J. Pharm. Res. 2015, 4, 1892–1900. [Google Scholar]
- Ferreira, S.R.A.; Franco, M.S.F.; Diniz, E.M.L.P.; Emery, F.D.S.; Clososki, G.C. Drug likeness and selective functionalization of quinoxalines. Curr. Org. Synth. 2015, 12, 714–729. [Google Scholar] [CrossRef]
- Camaggi, C.M.; Leardini, R.; Nanni, D.; Zanardi, G. Radical annulations with nitriles: Novel cascade reactions of cyano-substituted alkyl and sulfanyl radicals with isonitriles. Tetrahedron 1998, 54, 5587–5598. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Ni, Y.; Ren, D.; Hu, Z.; Yu, S. Synthesis of fused quinoline and quinoxaline derivatives enabled by domino radical triple bond insertions. Asian J. Org. Chem. 2014, 3, 1317–1325. [Google Scholar] [CrossRef]
- Togo, H.; Katohgi, M. Synthetic uses of organohypervalent iodine compounds through radical pathways. Synlett 2001, 565–581. [Google Scholar] [CrossRef]
- He, Z.; Bae, M.; Wu, J.; Jamison, T.F. Synthesis of highly functionalized polycyclic quinoxaline derivatives using visible-light photoredox catalysis. Angew. Chem. Int. Ed. 2014, 53, 14451–14455. [Google Scholar] [CrossRef] [PubMed]
- Viladomat, F.; Selles, M.; Codina, C.; Bastida, J. Alkaloids from Narcissus asturiensis. Planta Med. 1997, 63, 583. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Halim, O.B.; Morikawa, T.; Ando, S.; Matsuda, H.; Yoshikawa, M. New crinine-type alkaloids with inhibitory effect on induction of inducible nitric oxide synthase from Crinum yemense. J. Nat. Prod. 2004, 67, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Zupko, I.; Rethy, B.; Hohmann, J.; Molnar, J.; Ocsovszki, I.; Falkay, G. Antitumor activity of alkaloids derived from Amaryllidaceae species. In Vivo 2009, 23, 41–48. [Google Scholar] [PubMed]
- Szlavik, L.; Gyuris, A.; Minarovits, J.; Forgo, P.; Molnar, J.; Hohmann, J. Alkaloids from leucojum vernum and antiretroviral activity of amaryllidaceae alkaloids. Planta Med. 2004, 70, 871–873. [Google Scholar] [CrossRef] [PubMed]
- Cortes, N.; Posada-Duque, R.A.; Alvarez, R.; Alzate, F.; Berkov, S.; Cardona-Gomez, G.P.; Osorio, E. Neuroprotective activity and acetylcholinesterase inhibition of five amaryllidaceae species: A comparative study. Life Sci. 2015, 122, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.M.; Lobo, A.M.; Branco, P.S.; Prabhakar, S.; Sa-da-Costa, M. New syntheses of the Amaryllidaceae alkaloids vasconine, assoanine, oxoassoanine, pratosine and ismine by radical cyclization. Tetrahedron 1997, 53, 299–306. [Google Scholar] [CrossRef]
- Parnes, J.S.; Carter, D.S.; Kurz, L.J.; Flippin, L. A. Concise synthesis of narcissus pyrrolophenanthridine alkaloids: Vasconine, Assoanine and Oxoassoanine. J. Org. Chem. 1994, 59, 3497–3499. [Google Scholar] [CrossRef]
- Nakanishi, T.; Masuda, A.; Suwa, M.; Akiyama, Y.; Hoshino-Abe, N.; Suzuki, M. Synthesis of derivatives of NK109, 7-OH benzo[c]phenanthridine alkaloid, and evaluation of their cytotoxicities and reduction-resistant properties. Bioorg. Med. Chem. Lett. 2000, 10, 2321–2323. [Google Scholar] [CrossRef]
- Nakanishi, T.; Suzuki, M. Revision of the structure of Fagaridine based on comparison of UV and NMR data of synthetic compounds. J. Nat. Prod. 1998, 61, 1263–1267. [Google Scholar] [CrossRef] [PubMed]
- Potmesil, M.; Pinedo, H. Camptothecins: New Anticancer Agents; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- Li, S.; Zhang, Z.; Cain, A.; Wang, B.; Long, M.; Taylor, J. Antifungal activity of camptothecin, trifolin, and hyperoside isolated from camptotheca acuminata. J. Agric. Food Chem. 2005, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Tuticorin, R.G.; Kongovi, R.R.; Narayanan, V. Mappicine, a minor alkaloid from Mappia foetida miers. J. Chem. Soc. Perkin Trans. 1 1974, 1215–1217. [Google Scholar] [CrossRef]
- Zhao, X.-B.; Goto, M.; Song, Z.-L.; Morris-Natschke, S.L.; Zhao, Y.; Wu, D.; Yang, L.; Li, S.-G.; Liu, Y.-Q.; Zhu, G.-X.; et al. Design and synthesis of new 7-(N-substituted-methyl)-camptothecin derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2014, 24, 3850–3853. [Google Scholar] [CrossRef] [PubMed]
- Loza-Mejia, M.A.; Olvera-Vazquez, S.; Maldonado-Hernandez, K.; Guadarrama-Salgado, T.; Gonzalez-Sanchez, I.; Rodriguez-Hernandez, F.; Solano, J.D.; Rodriguez-Sotres, R.; Lira-Rocha, A. Synthesis, cytotoxic activity, DNA topoisomerase-II inhibition, molecular modeling and structure-activity relationship of 9-anilinothiazolo[5,4-b]quinoline derivatives. Bioorg. Med. Chem. 2009, 17, 3266–3277. [Google Scholar] [CrossRef] [PubMed]
- Liew, S.T.; Yang, L.-X. Design, synthesis and development of novel camptothecin drugs. Curr. Pharm. Des. 2008, 14, 1078–1097. [Google Scholar] [CrossRef] [PubMed]
- Gabarda, A.E.; Du, W.; Isarno, T.; Tangirala, R.S.; Curran, D.P. Asymmetric total synthesis of (20R)-homocamptothecin, substituted homocamptothecins and homosilatecans. Tetrahedron 2002, 58, 6329–6341. [Google Scholar] [CrossRef]
- Dom, B.; Curran, D.P.; Kruszewski, S.; Zimmer, S.G.; Strode, J.T.; Kohlhagen, G.; Du, W.; Chavan, A.J.; Fraley, K.A.; Bingcang, A.L.; et al. The novel silatecan 7-tert-butyldimethylsilyl-10-hydroxycamptothecin displays high lipophilicity, improved human blood stability, and potent anticancer activity. J. Med. Chem. 2000, 43, 3970–3980. [Google Scholar]
- Josien, H.; Bom, D.; Curran, D.P.; Zheng, Y.-H.; Chou, T.-C. 7-Silylcamptothecins (silatecans): A new family of camptothecin antitumor agents. Biorg. Med. Chem. Lett. 1997, 7, 3189–3194. [Google Scholar] [CrossRef]
- Curran, D.P.; Liu, H.; Josien, H.; Ko, S.-B. Tandem radical reactions of isonitriles with 2-pyridonyl and other aryl radicals: Scope and limitations, and a first generation synthesis of (±)-camptothecin. Tetrahedron 1996, 52, 11385–11404. [Google Scholar] [CrossRef]
- Curran, D.P.; Ko, S.-B.; Josien, H. Cascade radical reactions of isonitriles: A second-generation synthesis of (20S)-camptothecin, topotecan, irinotecan, and GI-147211C. Angew. Chem. Int. Ed. 1995, 34, 2683–2684. [Google Scholar] [CrossRef]
- Liu, H.; Curran, D.P. New 4 + 1 radical annulations. A formal total synthesis of (±)-camptothecin. J. Am. Chem. Soc. 1992, 114, 5863–5864. [Google Scholar]
- Zhang, W.; Luo, Z.; Chen, C.H.-T.; Curran, D.P. Solution-phase preparation of a 560-compound library of individual pure mappicine analogues by fluorous mixture synthesis. J. Am. Chem. Soc. 2002, 124, 10443–10450. [Google Scholar] [CrossRef] [PubMed]
- Bowman, W.R.; Bridge, C.F.; Brookes, P.; Cloonan, M.O.; Leach, D.C. Cascade radical synthesis of heteroarenes via iminyl radicals. J. Chem. Soc. Perkin Trans. 1 2002, 58–68. [Google Scholar] [CrossRef]
- Bowman, W.R.; Cloonan, M.O.; Fletcher, A.J.; Stein, T. Synthesis of heteroarenes using cascade radical cyclisation via iminyl radicals. Org. Biomol. Chem. 2005, 3, 1460–1467. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.-Z.; Hano, Y.; Nomura, T.; Chen, Y.-J. Two new pyrroloquinazolinoquinoline alkaloids from Peganum nigellastrum. Heterocycles 1997, 46, 541–546. [Google Scholar]
- Cagir, A.; Jones, S.H.; Gao, R.; Eisenhauer, B.M.; Hecht, S.M.; Luotonin, A. A naturally occurring human DNA topoisomerase I poison. J. Am. Chem. Soc. 2003, 125, 13628–13629. [Google Scholar] [CrossRef] [PubMed]
- Mhaske, S.B.; Argade, N.P. Regioselective quinazolinone-directed ortho lithiation of quinazolinoylquinoline: Practical synthesis of naturally occurring human DNA topoisomerase I poison Luotonin A and Luotonins B and E. J. Org. Chem. 2004, 69, 4563–4566. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.S.; Park, J.G.; Kim, S.I.; Jahng, Y. Synthesis and properties of luotonin A homologues and their aza-analogues. Heterocycles 2006, 68, 151–158. [Google Scholar]
- Ma, Z.-Z.; Hano, Y.; Nomura, T.; Chen, Y.-J. Synthesis of the cytotoxic pyrroloquinazolinoquinoline alkaloid, luotonin A. Heterocycles 1999, 51, 1593–1596. [Google Scholar]
- Toyota, M.; Komori, C.; Ihara, M. Three-step total synthesis of pyrroloquinazolinoquinoline alkaloid, luotonin A, by intramolecular hetero Diels-Alder reaction. Heterocycles 2002, 56, 101–103. [Google Scholar] [CrossRef]
- Yadav, J.S.; Reddy, B.V.S. Microwave-assisted rapid synthesis of the cytotoxic alkaloid luotonin A. Tetrahedron Lett. 2002, 43, 1905–1907. [Google Scholar] [CrossRef]
- Dallavalle, S.; Merlini, L. A new synthesis of the cytotoxic alkaloid Luotonine A. Tetrahedron Lett. 2002, 43, 1835–1837. [Google Scholar] [CrossRef]
- Ahn, H.; Nam, J.-W.; Seo, E.-K.; Mar, W. Induction of NAD(P)H: Quinone reductase by rutaecarpine isolated from the fruits of Evodia rutaecarpa in the murine hepatic Hepa-1c1c7 cell line. Planta Med. 2008, 74, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Christopher, E.; Bedir, E.; Dunbar, C.; Khan, I.A.; Okunji, C.O.; Schuster, B.M.; Iwu, M.M. Indoloquinazoline alkaloids from Araliopsis tabouensis. Helv. Chim. Acta 2003, 86, 2914–2918. [Google Scholar] [CrossRef]
- Sheth, P.R.; Shipps, G.W., Jr.; Seghezzi, W.; Smith, C.K.; Chuang, C.C.; Sanden, D.; Basso, A.D.; Vilenchik, L.; Gray, K.; Annis, D.A.; et al. Novel benzimidazole inhibitors bind to a unique site in the kinesin spindle protein motor domain. Biochemistry 2010, 49, 8350–8358. [Google Scholar] [CrossRef] [PubMed]
- Theoclitou, M.E.; Aquila, B.; Block, M.H.; Brassil, P.J.; Castriotta, L.; Code, E.; Collins, M.P.; Davies, A.M.; Deegan, T.; Ezhuthachan, J.; et al. Discovery of (+)-N-(3-aminopropyl)-N-[1-(5-benzyl-3-methyl-4-oxo-[1,2]thiazolo[5,4-d]pyrimidin-6-yl)-2-methylpropyl]-4-methylbenzamide (AZD4877), a kinesin spindle protein inhibitor and potential anticancer agent. J. Med. Chem. 2011, 54, 6734–6750. [Google Scholar] [CrossRef] [PubMed]
- Sorbera, L.A.; Bolós, J.; Serradell, N.; Bayés, M. Ispinesib mesilate. Drugs Future 2006, 31, 778–787. [Google Scholar] [CrossRef]
© 2016 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).