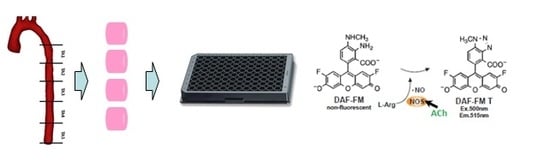
Development of High-Throughput Method for Measurement of Vascular Nitric Oxide Generation in Microplate Reader
Abstract
:1. Introduction
2. Results and Discussion
2.1. Effect of Dye Concentration
2.2. Effect of Buffer
2.3. Effect of Reaction Time and Fluorescence Stability
2.4. Method Validation
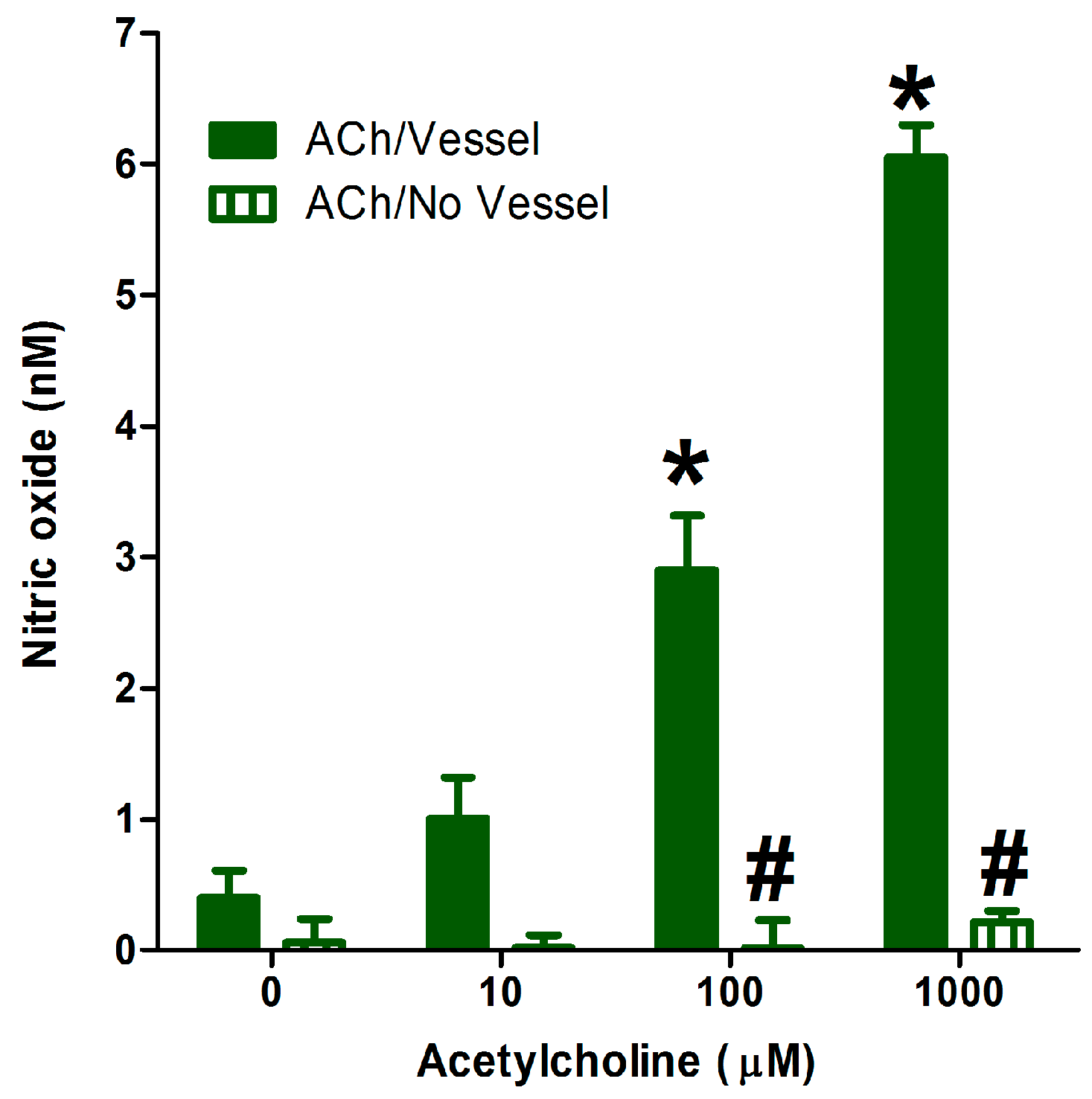
2.5. Application of the Method in Studying Endothelial NO Generation
3. Experimental Section
3.1. Apparatus
3.2. Reagents
3.3. Preparation of Solutions
3.4. Nitric Oxide Generation
3.4.1. Optimization of Excitation and Emission Wavelengths
3.4.2. Effect of Dye Concentration
3.4.3. Effect of Buffer
3.4.4. Effect of Reaction Time and Fluorescence Stability
3.5. Validation Study
3.6. Application of the Optimized Method in Measuring the Generation of Vascular Endothelial NO
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Poredos, P.; Jezovnik, M.K. Testing endothelial function and its clinical relevance. J. Atheroscler. Thromb. 2013, 20, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Moncada, S.; Erusalimsky, J.D. Does nitric oxide modulate mitochondrial energy generation and apoptosis? Nat. Rev. Mol. Cell Biol. 2002, 3, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, K.; Nagano, T.; Hayakawa, H.; Hirata, Y.; Hirobe, M. Detection of Nitric-Oxide Production from a Perfused Organ by a Luminol Hydrogen Peroxide System. Anal. Chem. 1993, 65, 1794–1799. [Google Scholar] [CrossRef] [PubMed]
- Ridnour, L.A.; Sim, J.E.; Hayward, M.A.; Wink, D.A.; Martin, S.M.; Buettner, G.R.; Spitz, D.R. A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal. Biochem. 2000, 281, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Wo, D.; Zhuang, P.; Xu, Z.G.; Xu, S.; Lu, Y.; Mao, H.M. A novel spectrophotometric method for indirect determination of nitric oxide (NO) in serum. Clin. Chim. Acta 2013, 424, 187–190. [Google Scholar] [CrossRef] [PubMed]
- Qiang, L.; Zhou, J. Determination of nitric oxide using horseradish peroxidase by UV second-order derivative spectrometry. Anal. Sci. 2009, 25, 1467–1470. [Google Scholar] [CrossRef] [PubMed]
- Malinski, T.; Taha, Z. Nitric oxide release from a single cell measured in situ by a porphyrinic-based microsensor. Nature 1992, 358, 676–678. [Google Scholar] [CrossRef] [PubMed]
- Balamurugan, M.; Madasamy, T.; Pandiaraj, M.; Bhargava, K.; Sethy, N.K.; Karunakaran, C. Electrochemical assay for the determination of nitric oxide metabolites using copper(II) chlorophyllin modified screen printed electrodes. Anal. Biochem. 2015, 478, 121–127. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.X.; Chen, J.B.; Guo, X.F.; Wang, H.; Zhang, H.S. Highly sensitive determination of nitric oxide in biologic samples by a near-infrared BODIPY-based fluorescent probe coupled with high-performance liquid chromatography. Talanta 2013, 116, 335–342. [Google Scholar] [CrossRef] [PubMed]
- El-Bagary, R.; Azzazy, H.M.; ElKady, E.F.; Farouk, F. Simultaneous determination of sildenafil citrate and some nitric oxide releasing drugs in human plasma using UPLC MS/MS. Clin. Biochem. 2014, 47, 654–656. [Google Scholar] [CrossRef] [PubMed]
- Leite, C.E.; Teixeira Ada, C.; Cruz, F.F.; Concatto, S.C.; Amaral, J.H.; Bonan, C.D.; Campos, M.M.; Morrone, F.B.; Battastini, A.M. Analytical method for determination of nitric oxide in zebrafish larvae: Toxicological and pharmacological applications. Anal. Biochem. 2012, 421, 534–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, H.; Liang, S.C.; Zhang, H.S. Spectrofluorimetric determination of nitric oxide at trace levels with 5,6-diamino-1,3-naphthalene disulfonic acid. Talanta 2002, 56, 499–504. [Google Scholar] [CrossRef]
- Semenova, A.D.; Glazachev, Y.I.; Slepneva, I.A.; Glupov, V.V. Quantitative determination of nitric oxide production in haemocytes: Nitrite reduction activity as a potential pathway of NO formation in haemolymph of Galleria mellonella larvae. Nitric Oxide 2014, 37, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.P.; Magalhaes, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L. Fully automatic flow method for the determination of scavenging capacity against nitric oxide radicals. Anal. Bioanal. Chem. 2010, 397, 3005–3014. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Nakatsubo, N.; Kikuchi, K.; Kawahara, S.; Kirino, Y.; Nagoshi, H.; Hirate, Y.; Nagano, T. Detection and imaging of nitric oxide with novel fluorescent indicators: Diaminofluoresceins. Anal. Chem. 1998, 70, 2446–2453. [Google Scholar] [CrossRef] [PubMed]
- Kojima, H.; Urano, Y.; Kikuchi, K.; Higuchi, T.; Hirata, Y.; Nagano, T. Fluorescent Indicators for Imaging Nitric Oxide Production. Angew. Chem. Int. Ed. Engl. 1999, 38, 3209–3212. [Google Scholar] [CrossRef]
- St. Laurent, C.D.; Moon, T.C.; Befus, A.D. Measurement of nitric oxide in mast cells with the fluorescent indicator DAF-FM diacetate. Methods Mol. Biol. 2015, 1220, 339–345. [Google Scholar] [PubMed]
- Namin, S.M.; Nofallah, S.; Joshi, M.S.; Kavallieratos, K.; Tsoukias, N.M. Kinetic analysis of DAF-FM activation by NO: Toward calibration of a NO-sensitive fluorescent dye. Nitric Oxide 2013, 28, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Jain, P.; David, A.; Bhatla, S.C. A Novel Protocol for Detection of Nitric Oxide in Plants. Methods Mol. Biol. 2016, 1424, 69–79. [Google Scholar] [PubMed]
- Winterbourn, C.C. The ability of scavengers to distinguish OH. production in the iron-catalyzed Haber-Weiss reaction: Comparison of four assays for OH. Free Radic. Biol. Med. 1987, 3, 33–39. [Google Scholar] [CrossRef]
- Hogg, N.; Darley-Usmar, V.M.; Wilson, M.T.; Moncada, S. Production of hydroxyl radicals from the simultaneous generation of superoxide and nitric oxide. Biochem. J. 1992, 281, 419–424. [Google Scholar] [CrossRef] [PubMed]
- Feelisch, M.; Ostrowski, J.; Noack, E. On the mechanism of NO release from sydnonimines. J. Cardiovasc. Pharmacol. 1989, 14 (Suppl. 11), S13–S22. [Google Scholar] [CrossRef] [PubMed]
- Govantes, C.; Rodriguez-Martinez, M.A.; Marin, J. Vasodilator effect and tolerance induced by the nitrocompound SIN-1 in rabbit femoral artery. Methods Find. Exp. Clin. Pharmacol. 1996, 18, 387–395. [Google Scholar] [PubMed]
- Munkholm, C.; Parkinson, D.R.; Walt, D.R. Intramolecular Fluorescence Self-Quenching of Fluoresceinamine. J. Am. Chem. Soc. 1990, 112, 2608–2612. [Google Scholar] [CrossRef]
- Sample Availability: Not available




| Parameter | SIN-1 (nM) | |
|---|---|---|
| Slope | 4.862 ± 0.3808 | |
| y-intercept | 8.432 ± 2.311 | |
| x-intercept | −1.734 | |
| Coefficient of determination | r2 | 0.9761 |
| p | 0.0002 | |
| LOD (nM) | 0.20 | |
| LOQ (nM) | 0.61 | |
| Precision % RSD (intra-day) | 2.45 | |
| % RSD (interday) | 3.56 | |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abd El-Hay, S.S.; Colyer, C.L. Development of High-Throughput Method for Measurement of Vascular Nitric Oxide Generation in Microplate Reader. Molecules 2017, 22, 127. https://doi.org/10.3390/molecules22010127
Abd El-Hay SS, Colyer CL. Development of High-Throughput Method for Measurement of Vascular Nitric Oxide Generation in Microplate Reader. Molecules. 2017; 22(1):127. https://doi.org/10.3390/molecules22010127
Chicago/Turabian StyleAbd El-Hay, Soad S., and Christa L. Colyer. 2017. "Development of High-Throughput Method for Measurement of Vascular Nitric Oxide Generation in Microplate Reader" Molecules 22, no. 1: 127. https://doi.org/10.3390/molecules22010127
APA StyleAbd El-Hay, S. S., & Colyer, C. L. (2017). Development of High-Throughput Method for Measurement of Vascular Nitric Oxide Generation in Microplate Reader. Molecules, 22(1), 127. https://doi.org/10.3390/molecules22010127