Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings
Abstract
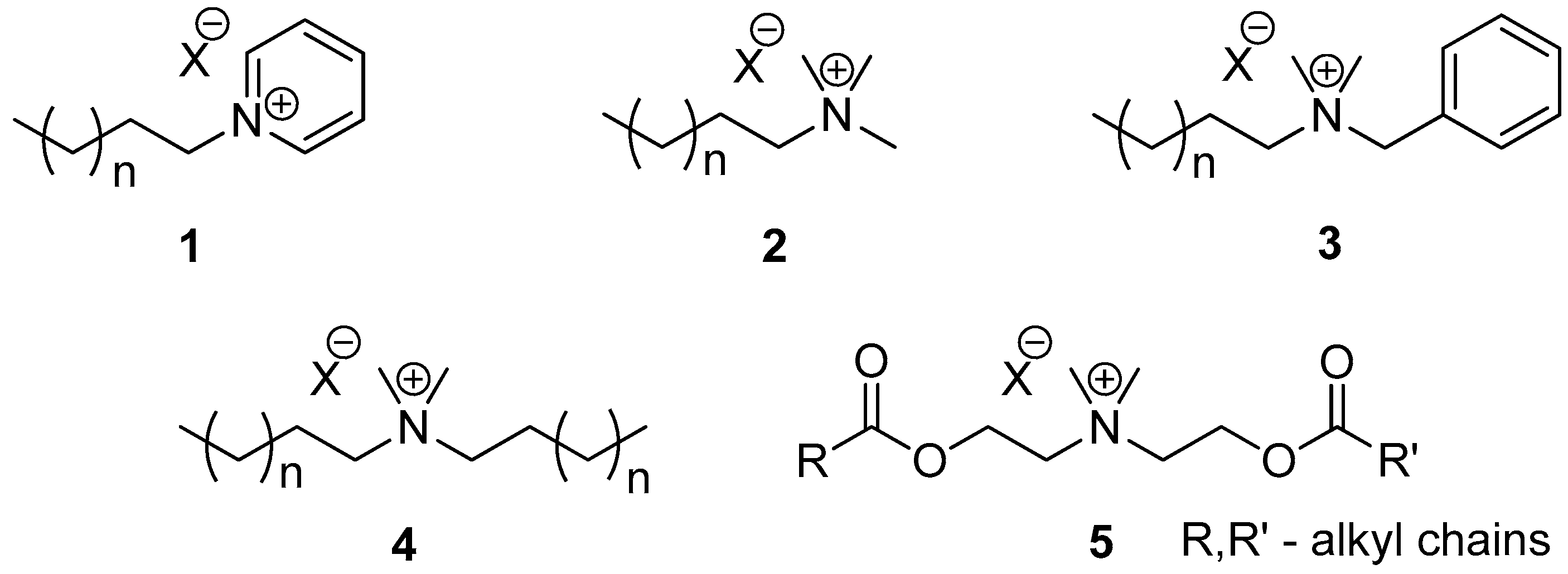
:1. Introduction
2. Results and Discussion
2.1. Spectroscopic Characterization
2.1.1. Nuclear Magnetic Resonance Spectra
2.1.2. Infrared Spectra
2.1.3. ESI-MS
2.2. Semiempirical Calculations
2.3. Aggregation Behavior
2.4. Antimicrobial Properties
3. Materials and Methods
3.1. General Information
3.2. Typical Procedure of Synthesis of N,N-Dimethyl-N-(4-methylpyridyl)-N-alkylammonium Chlorides P13–P18
3.3. Antimicrobial Properties Evaluation
3.4. Conductometric Study
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Espinoza, R. Multivesicular Emulsion Drug Delivery Systems. U.S. Patent US6709663 B2, 23 March 2004. [Google Scholar]
- Goredema, A.; Strong, A.; Odell, P.; Allen, C.G.; Birau, M.M. Low Molecular Weight Quaternary Ammonium Salt Dispersants. U.S. Patent US8101801 B2, 24 January 2012. [Google Scholar]
- Yamada, H.; Urata, C.; Higashitamori, S.; Aoyama, Y.; Yamauchi, Y.; Kuroda, K. Critical roles of cationic surfactants in the preparation of colloidal mesostructured silica nanoparticles: Control of mesostructure, particle size, and dispersion. ACS Appl. Mater. Interfaces 2014, 6, 3491–3500. [Google Scholar] [CrossRef] [PubMed]
- Igarashi, T.; Morita, N.; Okamoto, Y.; Nakamura, K. Elucidation of softening mechanism in rinse cycle fabric softeners. Part 1: Effect of hydrogen bonding. J. Surfactants Deterg. 2015, 19, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Okawa, H.; Hanabusa, K.; Suzuki, M.; Fukui, H.; Sakurai, S. Novel gemini compounds bearing an amide group show water solubility and useful functions as cosmetic ingredients. Int. J. Res. Cosmet. Sci. 2014, 4, 1–6. [Google Scholar]
- Jones, R.A. Quaternary Ammonium Salts: Their Use in Phase-Transfer Catalysis; Academic Press: Cambridge, MA, USA, 2000. [Google Scholar]
- Denmark, S.E.; Gould, N.D.; Wolf, L.M. A systematic investigation of quaternary ammonium ions as asymmetric phase-transfer catalysts. Application of quantitative structure activity/selectivity relationships. J. Org. Chem. 2011, 76, 4337–4357. [Google Scholar] [CrossRef] [PubMed]
- Zhi, D.; Zhang, S.; Cui, S.; Zhao, Y.; Wang, Y.; Zhao, D. The headgroup evolution of cationic lipids for gene delivery. Bioconjug. Chem. 2013, 24, 487–519. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Rajkowska, K.; Otlewska, A.; Piotrowska, M.; Kunicka-Styczyńska, A.; Brycki, B.; Nowicka-Krawczyk, P.; Kościelniak, M.; Gutarowska, B. Protection of historical wood against microbial degradation—Selection and application of microbiocides. Int. J. Mol. Sci. 2016, 17, 1364. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.C.; Minbiole, K.P.C.; Wuest, W.M. Quaternary ammonium compounds: An antimicrobial mainstay and platform for innovation to address bacterial resistance. ACS Infect. Dis. 2015, 1, 288–303. [Google Scholar] [CrossRef] [PubMed]
- Niu, L.; Zhang, H.; Wei, F.; Wu, S.; Cao, X.; Liu, P. Corrosion inhibition of iron in acidic solutions by alkyl quaternary ammonium halides: Correlation between inhibition efficiency and molecular structure. Appl. Surf. Sci. 2005, 252, 1634–1642. [Google Scholar] [CrossRef]
- Henry, K.M.; Hicks, K.D. Bis-Quaternary Ammonium Salt Corrosion Inhibitors. U.S. Patent US8999315 B2, 7 April 2015. [Google Scholar]
- Domagk, G. Eine neue Klasse von Desinfektionsmitteln. DMW Dtsch. Med. Wochenschr. 1935, 61, 829–832. [Google Scholar] [CrossRef]
- Block, S.S. Disinfection, Sterilization, and Preservation; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2001. [Google Scholar]
- Brycki, B. Gemini alkylammonium salts as biodeterioration inhibitors. Pol. J. Microbiol. 2010, 59, 227–231. [Google Scholar] [PubMed]
- Fraise, A.P.; Lambert, P.A.; Maillard, J.-Y. Russell, Hugo & Ayliffe’s Principles and Practice of Disinfection, Preservation & Sterilization; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Paulson, D.S. Handbook of Topical Antimicrobials: Industrial Applications in Consumer Products and Pharmaceuticals; CRC Press: Boca Raton, FL, USA, 2002. [Google Scholar]
- Keen, P.L.; Montforts, M.H.M.M. Antimicrobial Resistance in the Environment; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Ascenzi, J.M. Handbook of Disinfectants and Antiseptics; CRC Press: Boca Raton, FL, USA, 1995. [Google Scholar]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization: Types, Action, and Resistance; ASM Press: Washington, DC, USA, 2013. [Google Scholar]
- Tezel, U.; Pavlostathis, S.G. Quaternary ammonium disinfectants: Microbial adaptation, degradation and ecology. Curr. Opin. Biotechnol. 2015, 33, 296–304. [Google Scholar] [CrossRef] [PubMed]
- Mc Cay, P.H.; Ocampo-Sosa, A.A.; Fleming, G.T.A. Effect of subinhibitory concentrations of benzalkonium chloride on the competitiveness of Pseudomonas aeruginosa grown in continuous culture. Microbiology 2010, 156, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Tandukar, M.; Oh, S.; Tezel, U.; Konstantinidis, K.T.; Pavlostathis, S.G. Long-term exposure to benzalkonium chloride disinfectants results in change of microbial community structure and increased antimicrobial resistance. Environ. Sci. Technol. 2013, 47, 9730–9738. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.B.; Marshall, B. Antibacterial resistance worldwide: Causes, challenges and responses. Nat. Med. 2004, 10, S122–S129. [Google Scholar] [CrossRef] [PubMed]
- Bandyopadhyay, P.; Jha, S.; Imran Ali, S.K. Picolyl alkyl amines as novel tyrosinase inhibitors: Influence of hydrophobicity and substitution. J. Agric. Food Chem. 2009, 57, 9780–9786. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Fernandez, Y.; Foti, F.; Mangano, C.; Pallavicini, P.; Patroni, S.; Perez-Gramatges, A.; Rodriguez-Calvo, S. Micelles for the self-assembly of “off-on-off” fluorescent sensors for pH windows. Chemistry 2006, 12, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.P.; Saxena, N.; Tiwari, V.K.; Verma, S.S.; Chaturvedi, V.; Manju, Y.K.; Srivastva, A.K.; Gaikwad, A.; Sinha, S. Synthesis and antitubercular activity of substituted phenylmethyl- and pyridylmethyl-amines. Bioorg. Med. Chem. 2006, 14, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Sagara, T.; Uematsu, K.; Nagata, K. Dynamic phase change and adsorption/desorption of 4-pyridyl terminated amphiphiles possessing an amide functionality at a Au(111) electrode as tracked by electrochemical measurements. J. Electroanal. Chem. 2003, 550–551, 219–228. [Google Scholar] [CrossRef]
- Uematsu, K.; Sagara, T. Voltammetric study of adsorption layers of various 4-pyridyl terminated surfactants on a Au(111) electrode: Effects of electronic property of pyridyl group and intermolecular hydrogen bonding upon potential-driven phase changes. J. Electroanal. Chem. 2008, 623, 109–119. [Google Scholar] [CrossRef]
- Uematsu, K.; Sagara, T. Voltammetric study of insoluble 4-pyridyl-terminated surfactant binary-component films prepared by multiple horizontal touching method on a Au(1 1 1) electrode. Colloids Surf. Physicochem. Eng. Asp. 2008, 335, 43–49. [Google Scholar] [CrossRef]
- Oertling, H. 4-Alkyl Substituted Pyridines as Odiferous Substances. U.S. Patent US20100130624 A1, 27 May 2010. [Google Scholar]
- Pretsch, E.; Bühlmann, P.; Badertscher, M. Structure Determination of Organic Compounds: Tables of Spectral Data; Springer Science & Business Media: Berlin, Germany, 2009. [Google Scholar]
- Gottlieb, H.E.; Kotlyar, V.; Nudelman, A. NMR Chemical shifts of common laboratory solvents as trace impurities. J. Org. Chem. 1997, 62, 7512–7515. [Google Scholar] [CrossRef] [PubMed]
- Lundén, B.M. The crystal structure of n-dodecylammonium bromide. Acta Crystallogr. B 1974, 30, 1756–1760. [Google Scholar] [CrossRef]
- Rodier, N.; Dugué, J.; Céolin, R.; Baziard-Mouysset, G.; Stigliani, J.L.; Payard, M. Bromure de benzododécinium monohydraté. Acta Crystallogr. C 1995, 51, 954–956. [Google Scholar] [CrossRef]
- Mata, J.; Varade, D.; Bahadur, P. Aggregation behavior of quaternary salt based cationic surfactants. Thermochim. Acta 2005, 428, 147–155. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Cella, J.A.; Eggenberger, D.N.; Noel, D.R.; Harriman, L.A.; Harwood, H.J. The relation of structure and critical concentration to the bactericidal activity of quaternary ammonium salts. J. Am. Chem. Soc. 1952, 74, 2061–2062. [Google Scholar]
- González-Pérez, A.; Czapkiewicz, J.; del Castillo, J.L.; Rodrı́guez, J.R. Micellar properties of long-chain alkyldimethylbenzylammonium chlorides in aqueous solutions. Colloids Surf. Physicochem. Eng. Asp. 2001, 193, 129–137. [Google Scholar] [CrossRef]
- Ledbetter, J.W.; Bowen, J.R. Spectrophotometric determination of the critical micelle concentration of some alkyldimethylbenzylammonium chlorides using fluorescein. Anal. Chem. 1969, 41, 1345–1347. [Google Scholar] [CrossRef] [PubMed]
- Klevens, H.B. Structure and aggregation in dilate solution of surface active agents. J. Am. Oil Chem. Soc. 1953, 30, 74–80. [Google Scholar] [CrossRef]
- Hage, S.E.; Lajoie, B.; Stigliani, J.-L.; Furiga-Chusseau, A.; Roques, C.; Baziard, G. Synthesis, antimicrobial activity and physico-chemical properties of some n-alkyldimethylbenzylammonium halides. J. Appl. Biomed. 2014, 12, 245–253. [Google Scholar] [CrossRef]
- Bisacchi, G.S.; Sutton, J.C.; Slusarchyk, W.A.; Treuner, U.; Zhao, G. Beta Lactam Compounds and Their Use as Inhibitors of Tryptase. U.S. Patent US6335324 B1, 1 January 2002. [Google Scholar]
- Brycki, B.; Kowalczyk, I.; Kozirog, A. Synthesis, molecular structure, spectral properties and antifungal activity of polymethylene-α,ω-bis(N,N-dimethyl-N-dodecyloammonium bromides). Molecules 2011, 16, 319–335. [Google Scholar] [CrossRef] [PubMed]
- Koziróg, A.; Brycki, B. Monomeric and gemini surfactants as antimicrobial agents—Influence on environmental and reference strains. Acta Biochim. Pol. 2015, 62, 879–883. [Google Scholar] [CrossRef] [PubMed]
- Sample Availability: Samples of the compounds P13–P17 are available from the authors.

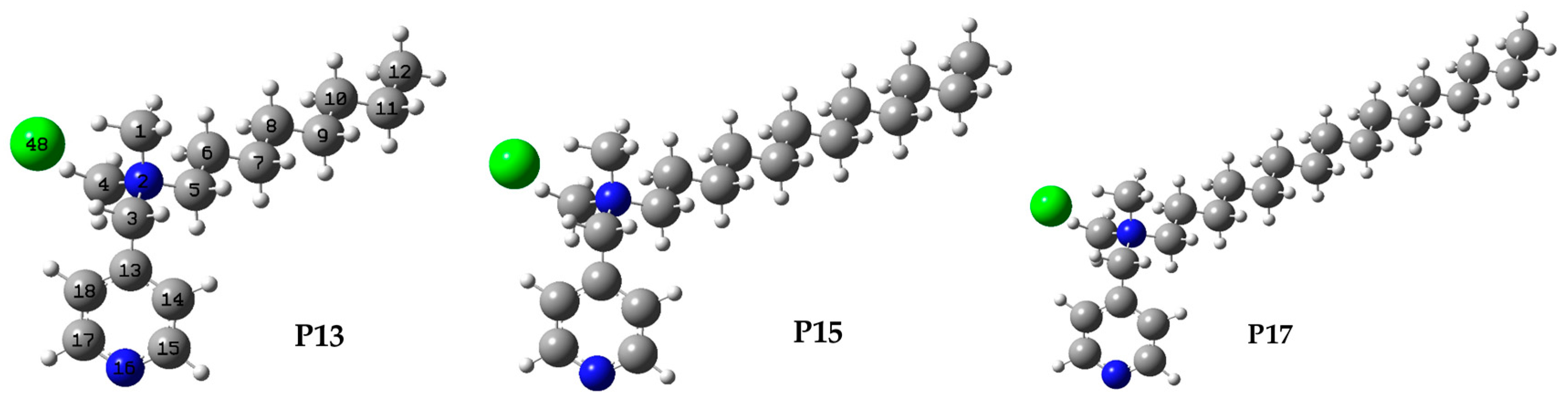





| Compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| P13 | P14 | P15 | P16 | P17 | P18 | BDDAC 1 | BDDAB 2 | BDDAB 3 | |
| (Calculated) | (Calculated) | (Crystal) | |||||||
| Heat of Formation [kcal/mol] | −14.766 | −25.638 | −36.512 | −47.387 | −58.264 | −69.141 | −44.786 | −37.285 | - |
| Distance [Å] | |||||||||
| N(2)...X− | 3.137 | 3.137 | 3.137 | 3.137 | 3.137 | 3.137 | 3.148 | 3.535 | 4.301 |
| N(2)–C(1) | 1.508 | 1.508 | 1.508 | 1.508 | 1.508 | 1.508 | 1.508 | 1.515 | 1.517 |
| N(2)–C(4) | 1.502 | 1.502 | 1.502 | 1.502 | 1.502 | 1.502 | 1.502 | 1.510 | 1.531 |
| N(2)–C(3) | 1.536 | 1.536 | 1.536 | 1.536 | 1.536 | 1.536 | 1.537 | 1.539 | 1.546 |
| N(2)–C(5) | 1.529 | 1.529 | 1.529 | 1.529 | 1.529 | 1.529 | 1.528 | 1.534 | 1.510 |
| C(5)–C(6) | 1.525 | 1.525 | 1.525 | 1.525 | 1.525 | 1.525 | 1.525 | 1.526 | 1.538 |
| C(3)–C(13) | 1.494 | 1.494 | 1.494 | 1.494 | 1.494 | 1.494 | 1.494 | 1.492 | 1.487 |
| C(13)–C(14) | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.402 |
| C(14)–C(15) | 1.394 | 1.394 | 1.394 | 1.394 | 1.394 | 1.394 | 1.390 | 1.389 | 1.393 |
| C(15)–N(16) | 1.353 | 1.353 | 1.353 | 1.353 | 1.353 | 1.353 | - | - | - |
| N(16)–C(17) | 1.352 | 1.352 | 1.352 | 1.352 | 1.352 | 1.352 | - | - | - |
| C(17)–C(18) | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.397 | 1.391 | 1.392 | 1.356 |
| C(13)–C(18) | 1.396 | 1.396 | 1.396 | 1.396 | 1.396 | 1.396 | 1.397 | 1.398 | 1.363 |
| Angle [°] | |||||||||
| C(1)–N(2)–C(4) | 108.556 | 108.558 | 108.558 | 108.560 | 108.558 | 108.558 | 108.556 | 109.668 | 109.560 |
| C(1)–N(2)–C(3) | 107.159 | 107.159 | 107.157 | 107.158 | 107.158 | 107.159 | 107.223 | 108.079 | 108.440 |
| C(1)–N(2)–C(5) | 110.034 | 110.035 | 110.034 | 110.034 | 110.035 | 110.035 | 109.989 | 108.868 | 110.100 |
| C(3)–N(2)–C(4) | 110.028 | 110.024 | 110.024 | 110.024 | 110.024 | 110.023 | 109.982 | 112.142 | 108.160 |
| C(3)–N(2)–C(5) | 110.391 | 110.393 | 110.395 | 110.392 | 110.393 | 110.393 | 110.446 | 109.140 | 112.530 |
| C(4)–N(2)–C(5) | 110.593 | 110.593 | 110.594 | 110.593 | 110.594 | 110.593 | 110.566 | 108.890 | 107.990 |
| N(2)–C(3)–C(13) | 114.561 | 114.562 | 114.564 | 114.562 | 114.562 | 114.561 | 114.413 | 113.849 | 116.070 |
| N(2)–C(5)–C(6) | 112.170 | 112.171 | 112.171 | 112.171 | 112.171 | 112.170 | 112.243 | 112.948 | 116.240 |
| Dihedral [°] | |||||||||
| N(2)–C(3)–C(13)–C(14) | 90.143 | 90.145 | 90.140 | 90.145 | 90.139 | 90.146 | 90.138 | 89.914 | 89.500 |
| N(2)–C(3)–C(13)–C(18) | −93.233 | −93.232 | −93.240 | −93.233 | −93.241 | −93.233 | −93.370 | −93.929 | −89.540 |
| C(4)–N(2)–C(3)–C(13) | 58.616 | 58.621 | 58.630 | 58.620 | 58.632 | 58.626 | 60.176 | 44.503 | 60.950 |
| C(5)–N(2)–C(3)–C(13) | −63.720 | −63.715 | −63.707 | −63.715 | −63.704 | −63.709 | −62.133 | −76.241 | −58.260 |
| C(1)–N(2)–C(3)–C(13) | 176.460 | 176.463 | 176.471 | 176.465 | 176.474 | 176.469 | 178.030 | 165.504 | 179.690 |
| CMC [mM] | Klevens Equation Parameters | |||||||
|---|---|---|---|---|---|---|---|---|
| N 1 | 10 | 12 | 14 | 16 | 18 | A | B | R 2 |
| P14–P18 | 87.12 | 20.17 | 4.87 | 1.14 | 0.310 | 1.9978 | 0.3073 | 0.9996 |
| TMAC | 68.0 2 | 20.0 2 | 4.50 2 | 1.50 3 | 0.35 3 | 1.6927 | 0.2851 | 0.9983 |
| BAC | 39.0 2 | 8.8 2 | 2.00 2 | 0.49 4 | 0.093 5 | 1.8487 | 0.325 | 0.9994 |
| Compound | MIC [mM] | ||||||
|---|---|---|---|---|---|---|---|
| A. niger | C. albicans | P. chrysogenum | S. aureus | B. subtilis | E. coli | P. aeruginosa | |
| P13 | - | 12.497 | - | 6.2485 | 6.2485 | 6.2485 | 6.2485 |
| P14 | 12.499 | 3.125 | 12.4999 | 1.5625 | 1.5625 | 1.5625 | 1.5625 |
| P15 | 1.5625 | 0.7182 | 1.5625 | 0.1953 | 0.1953 | 0.3906 | 0.7812 |
| P16 | 0.3906 | 0.1953 | 0.1953 | 0.0488 | 0.0977 | 0.1953 | 0.1953 |
| P17 | 0.1953 | 0.0976 | 0.1953 | 0.0244 | 0.0244 | 0.0488 | 0.09764 |
| N 1 | MIC [mM] | ||
|---|---|---|---|
| P. aeruginosa | A. niger | C. albicans | |
| 8 | 5 | 5 | 2.5 |
| 10 | 1.25 | 0.625 | 0.312 |
| 12 | 0.312 | 0.0781 | 0.039 |
| 14 | 0.0781 | 0.0195 | 0.0097 |
| 16 | 0.156 | 0.156 | 0.0195 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license ( http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Brycki, B.; Małecka, I.; Koziróg, A.; Otlewska, A. Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings. Molecules 2017, 22, 130. https://doi.org/10.3390/molecules22010130
Brycki B, Małecka I, Koziróg A, Otlewska A. Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings. Molecules. 2017; 22(1):130. https://doi.org/10.3390/molecules22010130
Chicago/Turabian StyleBrycki, Bogumił, Izabela Małecka, Anna Koziróg, and Anna Otlewska. 2017. "Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings" Molecules 22, no. 1: 130. https://doi.org/10.3390/molecules22010130
APA StyleBrycki, B., Małecka, I., Koziróg, A., & Otlewska, A. (2017). Synthesis, Structure and Antimicrobial Properties of Novel Benzalkonium Chloride Analogues with Pyridine Rings. Molecules, 22(1), 130. https://doi.org/10.3390/molecules22010130








